Summary
Therapeutic application of human embryonic stem cells (hESCs) requires precise control over their differentiation. However, spontaneous differentiation is prevalent, and growth factors induce multiple cell types; e.g., the mesoderm inducer BMP4 generates both mesoderm and trophoblast. Here we identify endogenous WNT signals as BMP targets that are required and sufficient for mesoderm induction, while trophoblast induction is WNT independent, enabling the exclusive differentiation toward either lineage. Furthermore, endogenous WNT signals induce loss of pluripotency in hESCs and their murine counterparts, epiblast stem cells (EpiSCs). WNT inhibition obviates the need to manually remove differentiated cells to maintain cultures and improves the efficiency of directed differentiation. In EpiSCs, WNT inhibition stabilizes a pregastrula epiblast state with novel characteristics, including the ability to contribute to blastocyst chimeras. Our findings show that endogenous WNT signals function as hidden mediators of growth factor-induced differentiation and play critical roles in the self-renewal of hESCs and EpiSCs.
Graphical Abstract
Highlights
-
•
BMP induces WNT-dependent and -independent differentiation pathways in hESCs
-
•
Modulating WNT and BMP directs differentiation toward mesoderm or trophoblast
-
•
WNT inhibition returns epiblast stem cells to a chimera-competent pregastrula state
-
•
WNT inhibition prevents spontaneous differentiation of hESCs and epiblast stem cells
ten Berge and colleagues show that BMP signals direct the differentiation of human and mouse pluripotent cells via the induction of endogenous WNT signals. These determine trophoblast or primitive streak lineage specification. Furthermore, inhibition of endogenous WNT signals prevents spontaneous differentiation in both hESCs and mouse epiblast stem cells (EpiSCs) and maintains EpiSCs in a pregastrula, chimera-competent state.
Introduction
Pluripotent stem cells can generate all cell types of the body and hold great potential for transplantation medicine and the study of early development. Pluripotency arises in the inner cell mass of blastocyst-stage embryos during formation of the epiblast, and both human and mouse blastocysts can give rise to pluripotent embryonic stem cells (ESCs). Differentiation of the pluripotent epiblast toward the primary germ layers occurs after implantation of the embryo during the process of gastrulation. Signaling proteins belonging to the BMP and WNT families are key gastrulation factors that mediate induction of the primitive streak in the embryo and can induce primitive streak derivatives in human ESCs (hESCs) and mouse ESCs (mESCs) (Bakre et al., 2007; Blauwkamp et al., 2012; Davidson et al., 2012; Drukker et al., 2012; Gadue et al., 2006; Lako et al., 2001; Lindsley et al., 2006; Nostro et al., 2008; Sumi et al., 2008; ten Berge et al., 2008). However, BMP4 additionally induces trophoblast (Drukker et al., 2012; Xu et al., 2002), complicating efforts to obtain single lineages. Furthermore, other reports show that both BMP and WNT signals support the self-renewal of mESCs instead (Hao et al., 2006; Ogawa et al., 2006; Singla et al., 2006; ten Berge et al., 2011; Ying et al., 2003). These conflicting reports may reflect the action of BMP and WNT signals on different pluripotent states, as the epiblast of post implantation mouse embryos can also give rise to a pluripotent cell type, the epiblast stem cell (EpiSC) (Brons et al., 2007; Tesar et al., 2007). EpiSCs are developmentally more advanced than mESCs and possess different morphology, growth factor requirements, gene expression profile, and epigenetic state (Brons et al., 2007; Tesar et al., 2007). They can generate teratomas, a measure of pluripotency, but unlike mESCs are not competent to contribute to blastocyst chimeras.
EpiSCs express many differentiation factors present in the primitive streak (Brons et al., 2007; Tesar et al., 2007) and were found to comprise heterogeneous populations of cells with distinct potency (Bernemann et al., 2011; Tsakiridis et al., 2014). This suggests that EpiSCs are to some extent prespecified, and their pluripotent state has therefore been designated “primed,” as opposed to the unspecified “naïve” pluripotent state of mESCs (Nichols and Smith, 2009). Similar observations were made for hESCs, consistent with them occupying a primed pluripotent state (Blauwkamp et al., 2012; Davidson et al., 2012; Drukker et al., 2012; Stewart et al., 2006). Interestingly, for both EpiSCs and hESCs, it has been shown that endogenous WNT proteins, produced by the cells themselves, drive prespecification of the cells (Blauwkamp et al., 2012; Frank et al., 2012; Sumi et al., 2013; Tsakiridis et al., 2014).
Here we address the consequences of endogenous WNT signals for directed differentiation and self-renewal of human and mouse pluripotent cells. We show that endogenous WNT signals mediate differentiation decisions in response to BMP signals and furthermore that they are the main cause of spontaneous differentiation in both hESCs and EpiSCs.
Results
BMP4-Induced Differentiation of EpiSCs Is Mediated by WNT Signals
Both WNT and BMP signals are implicated in the initiation of gastrulation and induction of the primitive streak. To monitor these processes in vitro, we established EpiSCs carrying the T-GFP reporter for the primitive streak marker Brachyury by differentiating T-GFP ESCs (Fehling et al., 2003) into EpiSCs by culture in FGF2 and ACTIVIN. We included IWP2, a small molecule inhibitor that blocks the biosynthesis of mature WNT proteins (Chen et al., 2009), to increase the efficiency of differentiation (ten Berge et al., 2011). Phenotypic and functional assays verified the complete differentiation (Figures S1A–S1D available online).
Treatment of the T-GFP EpiSCs with either WNT3A or BMP4, in the presence of ACTIVIN and FGF2, strongly induced reporter expression, followed by loss of the pluripotency marker SSEA1 (Figure 1A). However, in the BMP4-treated cells, these events were delayed, possibly because BMP4 may act indirectly, by inducing WNT signals in EpiSCs (Figure 1A). Indeed, BMP4 protein strongly induced a reporter for WNT signaling in Axin2-LacZ EpiSCs (ten Berge et al., 2011) (Figure 1B). This was due to the induction of endogenous WNT proteins as reporter expression was inhibited by IWP2 (Figure 1B). Importantly, IWP2 prevented not only the induction of T-GFP but also the loss of SSEA1 in response to BMP4 (Figure 1A), suggesting that BMP4-mediated exit from pluripotency requires the activation of WNT. Indeed, while both WNT3A and BMP4 induced expression of differentiation markers and loss of EpiSC markers, IWP2 prevented the gain or loss of these markers in response to BMP4, demonstrating that it relied on the induction of WNT signals (Figures 1C and S1E). IWP2 did not interfere with WNT signal transduction or differentiation per se since it did not block the effects of WNT3A (Figure S1F).
Figure 1.
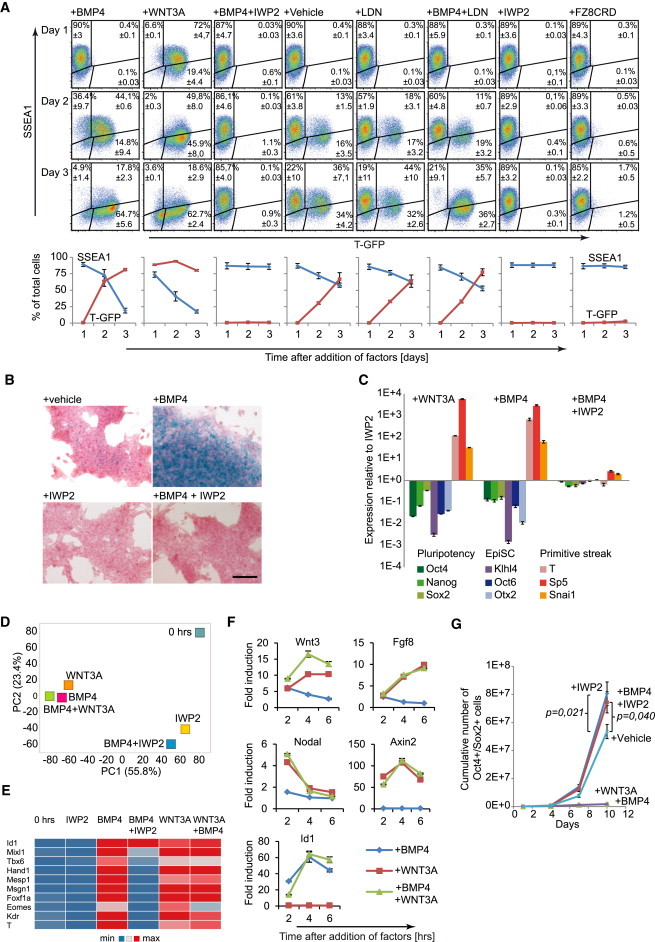
BMP4-Induced Differentiation of EpiSCs Depends on WNT Signals
(A) Flow cytometry plots of T-GFP EpiSCs treated with the indicated factors and analyzed for T-GFP and SSEA1. The cells were maintained in the presence of IWP2 prior to the experiment. Line plots indicate the mean of three independent experiments ± SEM.
(B) Axin2-LacZ EpiSCs treated for 3 days with the indicated factors and stained for LACZ (blue).
(C) RT-PCR gene expression profiles of GFP9 EpiSCs treated for 2 days with the indicated factors, plotted relative to EpiSCs maintained in the presence of IWP2 (n = 3, mean ± SEM).
(D) Principal component analysis of transcriptomes of GFP9 EpiSCs treated for 48 hr with the indicated factors or untreated (0 hr). The percentage of variance explained by the principal components is indicated between parentheses.
(E) Heat map of selected gene expression levels in GFP9 EpiSCs treated for 48 hr with the indicated factors, determined by RNA-Seq.
(F) Time course RT-PCR analysis of indicated genes in GFP9 EpiSCs following treatment with the indicated factors (n = 3, mean ± SEM).
(G) Plot showing the expansion of OCT4- and SOX2-positive 129S2C1a EpiSCs in the indicated conditions (three independent experiments, mean ± SEM).
The scale bar represents 200 μm. See also Figure S1.
We used RNA-Seq to analyze the interactions between BMP4 and WNT in EpiSCs treated for 48 hr in the presence of ACTIVIN and FGF2. Principal component analysis showed that the BMP4- and/or WNT3A-treated samples separated from all other samples along the first component, whereas the IWP2-treated samples clustered together, regardless of the presence of BMP4 (Figure 1D). Interestingly, the WNT3A-treated samples clustered together with the BMP4-treated sample and induced the same mesodermal markers, such as Kdr, Mesp1, and Tbx6 (Figures 1D and 1E). BMP4 was unable to induce these markers in the presence of IWP2 despite inducing the canonical BMP target Id1, showing that IWP2 did not interfere with BMP signal transduction (Figure 1E). These data show that the gene-expression changes induced by BMP4 in EpiSCs are to a large extent secondary to activation of WNT proteins.
Feedback loops between signaling factors are an important element of gastrulation (Ben-Haim et al., 2006; Tortelote et al., 2013). We therefore analyzed the short-term (2–6 hr) induction of the gastrulation factors Nodal, Wnt3, and Fgf8 by BMP4 and/or WNT3A. While BMP4 induced Wnt3, WNT3A induced all three factors, and the highest induction of Wnt3 was obtained using both signals (Figure 1F). These observations explain why BMP signals are not required for primitive streak induction once WNT signals have been activated. No induction of the WNT target Axin2 by BMP4 or the BMP-target Id1 by WNT3A was observed within the 6 hr timeframe (Figure 1F). However, Id1 is somewhat induced in the 48 hr WNT3A-only condition, suggesting that the differentiating cells activate endogenous BMP signals (Figure 1E). Nonetheless, T-GFP induction was not suppressed by the BMP inhibitor LDN193189, indicating that it did not require BMP signals (Figure 1A). Finally, IWP2 prevented the loss of both OCT4- and SOX2-positive as well as SSEA1-positive EpiSCs in response to BMP4 (Figures 1G and S1G). Combined, these data indicate that induction of EpiSC differentiation by BMP4 is mediated by the induction of WNT signals.
Endogenous WNT Proteins Induce Differentiation and Loss of Pluripotency in EpiSCs
In the course of our studies, we found that T-GFP EpiSCs spontaneously induced a significant GFP-positive population (Figure 1A, +vehicle). This induction was suppressed either by IWP2 or by the WNT antagonist FZ8CRD, a soluble domain of the WNT receptor that binds and sequesters WNT proteins, indicating that it was due to endogenous WNT proteins (Figure 1A). The presence of endogenous WNT activity was further confirmed by the spontaneous LACZ activity evident in Axin2-LacZ EpiSCs, which was also suppressed by IWP2 (Figure 1B). Moreover, multiple Wnt genes were expressed in EpiSCs, in particular Wnt3 (Figure S2A). These observations are in line with a recent report showing that endogenous WNT signals specify a fraction of EpiSCs toward primitive-streak lineages (Tsakiridis et al., 2014). Clonal assays showed that these specified cells were not committed to differentiation and maintained a pluripotent phenotype (Tsakiridis et al., 2014).
However, we noticed that the GFP-positive cells showed a shift to lower SSEA1 expression, suggesting that some of them lost pluripotency (Figure 1A). We therefore sorted the cells based on T-GFP intensity and assessed their potential to establish colonies or to form embryoid bodies (EBs), both measures of pluripotency. A clear negative correlation was visible between the level of GFP and potential to establish NANOG-positive colonies (Figures 2A, S2B, and S2C). Likewise, cells with higher levels of GFP produced smaller EBs, while the cells with the highest level failed to form EBs at all (Figures 2B and S2D). Moreover, these cells downregulated SOX2 and OCT4 (Figure 2B). These data show that the T-GFP-positive population is enriched for cells that have lost pluripotency.
Figure 2.
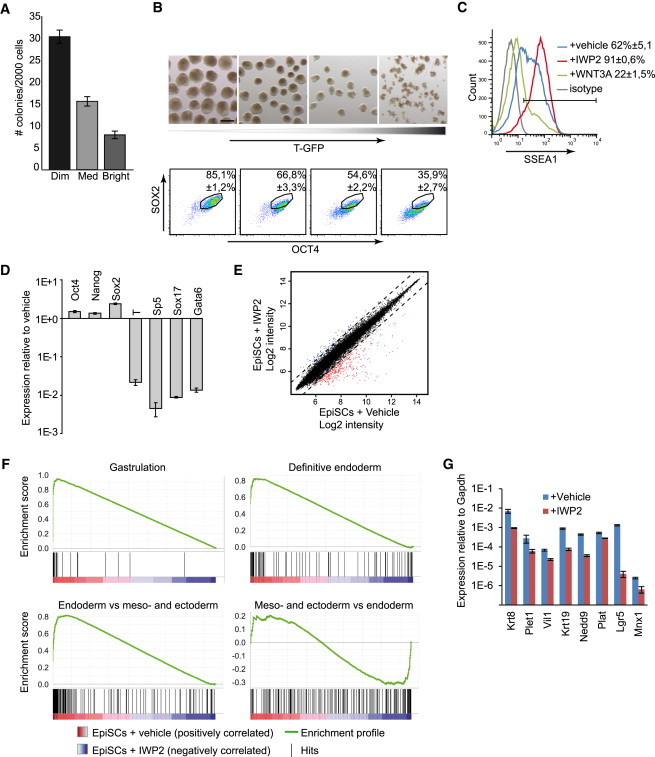
Endogenous WNT Proteins Induce Loss of Pluripotency in EpiSCs
(A) T-GFP EpiSCs were sorted into three categories based on GFP and assayed for their ability to establish NANOG-positive colonies (three independent experiments, mean ± SEM).
(B) T-GFP EpiSCs were sorted into four categories based on GFP and assayed for their ability to establish EBs (top) or analyzed by flow cytometry for SOX2 and OCT4 (bottom, three independent experiments, mean ± SEM).
(C) Flow cytometry histogram showing T-GFP EpiSCs treated for 3 days with the indicated factors and analyzed for SSEA1 (three independent experiments, mean ± SEM).
(D) Real-time RT-PCR gene expression profiles of 129S2C1a EpiSCs cells treated for 3 days with IWP2, plotted relative to untreated EpiSCs (three independent experiments, mean ± SEM).
(E) Scatter plot comparing the global gene expression levels of GFP9 EpiSCs cultured in the presence or absence of IWP2. The dotted lines delineate the boundaries of 2-fold difference in gene expression levels. Genes expressed more than 2-fold higher or lower in the presence of IWP2 are plotted in blue or red, respectively.
(F) Gene set enrichment analysis plots demonstrating the enrichment of the indicated gene sets in EpiSCs cultured in the absence versus the presence of IWP2.
(G) RT-PCR analysis for definitive endoderm genes in EpiSCs, in response to IWP2 (three biological replicates using 129S2C1a, Axin2LacZ, and GFP9 EpiSCs, mean ± SEM).
Scale bar represents 200 μm. See also Figure S2.
We next tested whether WNT inhibition would prevent this loss of pluripotency. When analyzed for SSEA1, multiple EpiSC lines all displayed substantial levels of SSEA1-negative cells, indicating significant differentiation (Figures 2C and S2E, vehicle). However, in the presence of IWP2, more than 90% of the cells expressed SSEA1 (Figures 2C and S2E). In addition, RT-PCR and immunostaining showed that IWP2 not only repressed primitive streak markers but also raised the level of the pluripotency markers Oct4, Nanog, and Sox2 (Figures 2D and S1E). Moreover, IWP2 substantially enhanced the expansion of OCT4- and SOX2-positive or SSEA1-positive cells (Figures 1G and S1G). In line with a recent report (Sumi et al., 2013), suppression of endogenous WNT signals also greatly enhanced the derivation of novel EpiSC lines from 25% (four lines from 16 E5.5 embryos) to 79% (15 of 19). These data show that endogenous WNT signals induce loss of pluripotency in a subset of EpiSCs, and WNT inhibition suppresses this spontaneous differentiation, greatly enhancing their self-renewal and derivation efficiency. In fact, certain cell lines, e.g., the T-GFP EpiSCs, could essentially not be maintained in the absence of IWP2 as they progressively accumulated differentiated cells (Figure 1A, +vehicle).
To identify the differentiation pathways induced by endogenous WNT signals we compared the transcriptomes of EpiSCs maintained in the presence or absence of IWP2. Most differences were due to a set of genes that was repressed by IWP2 (Figure 2E), with 321 genes downregulated and 87 genes upregulated in response to IWP2 (Table S1). Using gene set enrichment analysis (Subramanian et al., 2005), we found that a set of 29 genes first expressed around the start of gastrulation (Pfister et al., 2007) was strongly enriched in conventional EpiSCs when compared with EpiSCs treated with IWP2 (Figure 2F; Table S2). We next looked for signatures of more committed cell types that derive from the primitive streak. A set of 98 genes expressed in committed human- and mouse-definitive endoderm and endoderm precursors (Hou et al., 2007; McLean et al., 2007; Ogaki et al., 2011; Tada et al., 2005) was highly enriched in conventional EpiSCs (Figure 2F; Table S3). Since some of these genes are also expressed in mesoderm progenitors, we created a gene set consisting of 154 genes specifically expressed in E7.5 endoderm versus mesoderm and ectoderm (Gu et al., 2004) and found strong enrichment of this set in conventional EpiSCs (Figure 2F; Table S4). Furthermore, a panel of genes associated with the committed endoderm state showed consistent repression in response to IWP2 (Figure 2G). In contrast, a set of 155 genes expressed in E7.5 mesoderm and ectoderm versus endoderm (Gu et al., 2004) showed no enrichment (Figure 2F; Table S5) and committed mesoderm markers such as Mesp1, Meox1, Kdr, Hand1, Msgn1, Foxf1a, Tlx2, or Tbx6 ranked low in the comparison (Table S5). Anterior neurectoderm genes did not increase in response to IWP2 (Figure S2F), indicating that endogenous WNT signals were not required to inhibit neural differentiation. These findings show that endogenous WNT signals induce a committed definitive endoderm state in a subset of EpiSCs, explaining the loss of pluripotency in response to endogenous WNT signals.
WNT Inhibition Maintains EpiSCs in a Pregastrula Epiblast Stage
Despite their origin from the pregastrula epiblast, transcriptome comparisons indicate that EpiSCs are more similar to the late-gastrula-stage epiblast (Kojima et al., 2014). To test whether WNT inhibition maintains EpiSCs in a state closer to that of the pregastrula epiblast, we compared their transcriptomes with those of epiblasts derived from embryos ranging from the cavity (Cav) stage to the prestreak (PS), late mid streak (LMS), late streak (LS), no bud (OB), early bud (EAB), and late bud (LB) stage, obtained using Illumina bead-chip arrays (Kojima et al., 2014). A normalization procedure matched the distribution of the expression values from our Affymetrix to the Illumina platform, and the genes that were at least 1.5-fold differentially expressed in response to IWP2 (1,066 gene identifications shared between both platforms) were analyzed using principal component analysis. As observed before (Kojima et al., 2014), the first component separated the embryo-derived samples from the EpiSCs, whereas the second component separated the embryo-derived samples according to their developmental stage (Figure 3A). Importantly, while the regular EpiSCs aligned at the early bud stage, EpiSCs maintained in the presence of IWP2 aligned between the prestreak and early-streak stages, showing that their transcriptome is indeed more similar to that of the pregastrula epiblast (Figure 3A).
Figure 3.
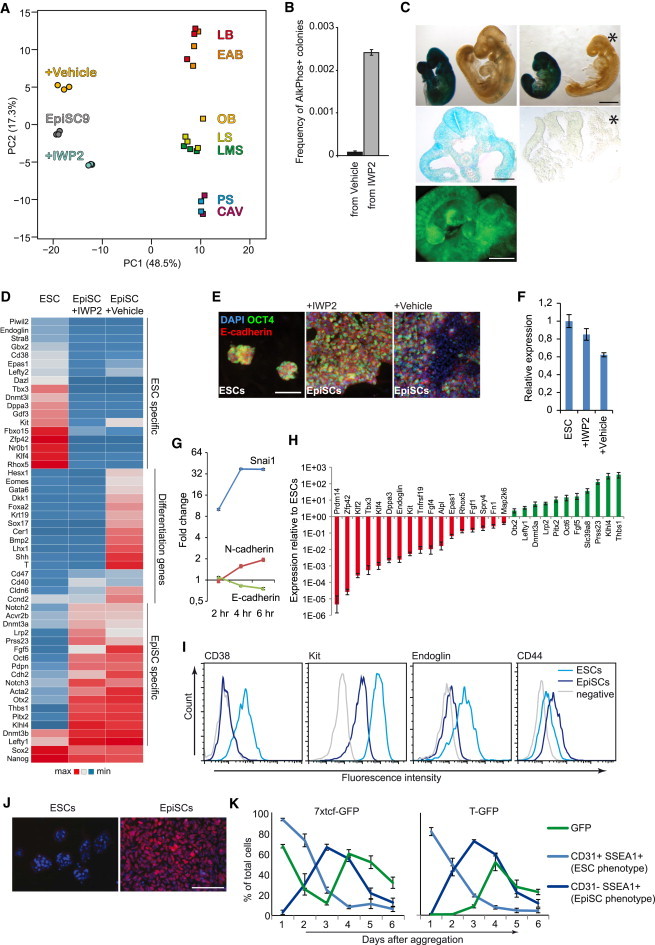
WNT Inhibition Maintains EpiSCs in a Pregastrula Epiblast Stage
(A) Transcriptomes from primary epiblasts dissected from embryos ranging from cavity to late bud stages and from the EpiSC line EpiSC9 were obtained from GEO (GSE46227) and combined with six microarray gene expression data sets from GFP9 EpiSCs cultured with and without IWP2 and analyzed by principal component analysis. The percentage of variance explained by the principal components is indicated between parentheses.
(B) Reversal efficiency of T-GFP EpiSCs. Prior to start of the reversal experiment, the cells were maintained in the presence or absence of IWP2 as indicated (n = 3, mean ± SEM).
(C) (Upper) X-gal stained chimeras derived from blastocyst injections of passage 5 Rosa26-LacZ EpiSCs. (Middle) Sections to indicate EpiSC contribution (blue). Asterisk indicates a nonchimeric littermate. (Lower) Chimera derived from blastocyst injections of passage 5 GFP9 EpiSCs. Green fluorescence indicates EpiSC contribution.
(D) Heat map of selected gene expression levels of ESCs and GFP9 EpiSCs cultured in the presence or absence of IWP2 and analyzed by microarray.
(E) E-CADHERIN and OCT4 immunofluorescence images of EpiSCs and ESCs.
(F) RT-PCR for E-cadherin in EpiSCs and ESCs (three biological replicates using 129S2C1a, Axin2LacZ, and GFP9 EpiSCs, mean ± SEM).
(G) Time course RT-PCR analysis of indicated genes in GFP9 EpiSCs following treatment with WNT3A (n = 3, mean ± SEM).
(H) Real-time RT-PCR gene expression analysis of FVB EpiSCs relative to ESCs for a range of genes found by microarray to be differentially expressed between EpiSCs and ESCs (n = 3, mean ± SEM).
(I) Flow cytometry histograms showing surface markers distinguishing ESCs (CD38, KIT, and ENDOGLIN) and EpiSCs (CD44).
(J) OCT6 immunostaining (red) of 129S2C1a EpiSCs and ESCs (blue, DAPI).
(K) The indicated ESC lines were aggregated into EBs and analyzed daily by flow cytometry for expression of reporter and the indicated cell surface markers (three independent experiments, mean ± SEM).
Scale bar represents 1 mm (C, embryos), 200 μm (C, section), 100 μm (E and J). See also Figure S3.
We tested the pregastrula state of EpiSCs maintained with IWP2 using two functional assays. First, a small percentage of EpiSCs can revert to the ESC state when transferred to ESC conditions (Greber et al., 2010), and we found that IWP2 treatment strongly raised this reversal efficiency (Figure 3B). This indicates that IWP2 caused many more cells to occupy a state of pluripotency sufficiently close to that of ESCs to make the transition. Second, in contrast to epiblast from the gastrula, the pregastrula epiblast can contribute to chimeras upon blastocyst injection (Gardner et al., 1985). However, EpiSCs rarely contribute to blastocyst chimeras but rather, corresponding to their late-gastrula stage character, can integrate when introduced into the primitive streak (Huang et al., 2012; Kojima et al., 2014). We derived EpiSCs from E6.5 transgenic embryos carrying either a Rosa26-LacZ or Actin-GFP reporter in the presence of IWP2, cultured the cells for five passages, and performed blastocyst injections. We obtained 3 chimeras out of 14 E10.5 embryos from the Rosa26-LacZ EpiSCs, and 1 chimera out of 14 embryos from the Actin-GFP-derived EpiSC line GFP9 (Figure 3C). X-gal and immunostainings demonstrated integration into multiple tissues, including the neural tube, somite, nephrogenic cord, body wall, splanchnopleure, and parts of the gut tube (Figure S3A). Together, these tests strongly support the pregastrula character of EpiSCs shielded from WNT signals. Moreover, they indicate that the ability to contribute to blastocyst chimeras does not distinguish naive from primed pluripotent cells.
We considered several explanations for the blastocyst compatibility of IWP2-treated EpiSCs. First, some cells may be reprogrammed to the naive state. However, IWP2 induced no increase in Tbx3, Dppa3, Zfp42, Klf4, Dppa5, or other naive markers (Figure 3D; Table S1). Second, IWP2 may stabilize a minor fraction of EpiSCs that contributes to blastocyst chimeras, marked by the Oct4-GFP reporter GOF18 (Han et al., 2010). IWP2 did however not enhance the GFP-positive fraction and sorted GFP-positive cells lost GFP expression regardless of the presence of IWP2 (Figures S3B and S3C). Third, E-CADHERIN overexpression allows EpiSCs to participate in blastocyst chimeras (Ohtsuka et al., 2012). We observed higher E-cadherin expression and strong E-CADHERIN staining throughout the cultures in the presence of IWP2, similar in strength as in ESCs, whereas staining was faint and patchy in regular EpiSCs (Figures 3E and 3F). Furthermore, WNT3A induced the E-cadherin repressor Snai1 and N-cadherin and downregulated E-cadherin in EpiSCs (Figure 3G). These observations indicate that endogenous WNT proteins repress E-cadherin in EpiSCs, thereby reducing their ability to integrate in the pregastrulation epiblast.
WNT inhibition repressed multiple differentiation genes, including Eomes, Foxa2, Gata6, Lefty2, and Sox17, to the same level as in ESCs, indicating that they are not EpiSC markers (Figure 3D). We screened our gene expression data for potential markers for genuine EpiSCs, maintained in the presence of WNT inhibition (Figure 3H), and identified CD38, CD107/KIT, CD105/ENDOGLIN, and CD44 as cell surface markers suitable to separate ESCs and EpiSCs by flow cytometry (Figure 3I) and OCT6 as a nuclear marker for genuine EpiSCs (Figure 3J).
Next, we addressed whether the differentiation-inducing effect of WNT on EpiSCs explains the conflicting reports on the role of WNT in ESCs. While we previously demonstrated that endogenous WNT signals support ESC self-renewal by inhibiting their differentiation into EpiSCs (ten Berge et al., 2011), we and others also demonstrated that WNT signals induce differentiation of ESCs in EBs (Nostro et al., 2008; ten Berge et al., 2008). However, a transient EpiSC signature has been detected in differentiating EBs (Zhang et al., 2010). This could be the result of a shutdown of the WNT pathway in EBs, which would induce differentiation of ESCs into EpiSCs. When we generated EBs from ESCs carrying the 7xTcf-GFP reporter for WNT signaling (ten Berge et al., 2008), we observed rapid downregulation of the WNT reporter, followed by loss of the ESC marker CD31 (Figure 3K). However, the cells maintained expression of the pluripotency marker SSEA1, suggesting they converted into EpiSCs. Following this transition, the WNT reporter was induced while SSEA1 was lost (Figure 3K), suggesting that endogenous WNTs now acted as differentiation signals. Indeed, using T-GFP ESCs, we observed induction of the differentiation reporter following the transition of the ESCs into EpiSCs (Figure 3K). Thus, EBs first mediate the conversion of ESCs into EpiSCs; only then are endogenous WNT signals activated that induce their differentiation.
Inhibition of Endogenous WNT Signals Prevents the Accumulation of Differentiated Cells in hESC Cultures
It is thought that hESCs occupy a state of primed pluripotency like that of mouse EpiSCs, rather than the naive pluripotency of mESCs (Nichols and Smith, 2009). hESC cultures experience substantial spontaneous differentiation and require frequent manual removal of accumulations of differentiated cells. We investigated whether commitment to differentiation could be prevented by inhibition of endogenous WNT signals, similar to what we showed for EpiSCs.
In the presence of IWP2 or FZ8CRD, both H1 and H9 hESCs established flatter, sharper edged colonies with very little evidence of differentiated cells, whether cultured on mouse embryo fibroblasts (MEFs) or in mTESR1, a serum- and feeder-free medium (Figures 4A and S4A). When cultured in standard conditions, both H1 and H9 hESCs formed patches of BRACHYURY- and GATA4-positive cells (Figures S4B and S4C), and significant proportions of the cells lacked NANOG, OCT4, or SOX2 (Figures 4B and S4D). In contrast, no BRACHYURY or GATA4 was visible when the cells were cultured in the presence of IWP2 (Figures S4B and S4C), and the proportion of cells lacking the pluripotency factors was strongly reduced (Figures 4B and S4D). A recently introduced defined medium, E8, performed better yet IWP2 significantly improved the proportion of cells expressing the pluripotency factors (Figure 4B). RT-PCR analysis confirmed that IWP2 repressed multiple markers of mesendodermal differentiation and enhanced expression of pluripotency markers, while neurectodermal markers either showed minor changes or were downregulated (Figures 4C and S4E). These data suggest that WNT inhibition prevents the spontaneous mesendodermal differentiation of hESCs, while not increasing neurectodermal differentiation.
Figure 4.
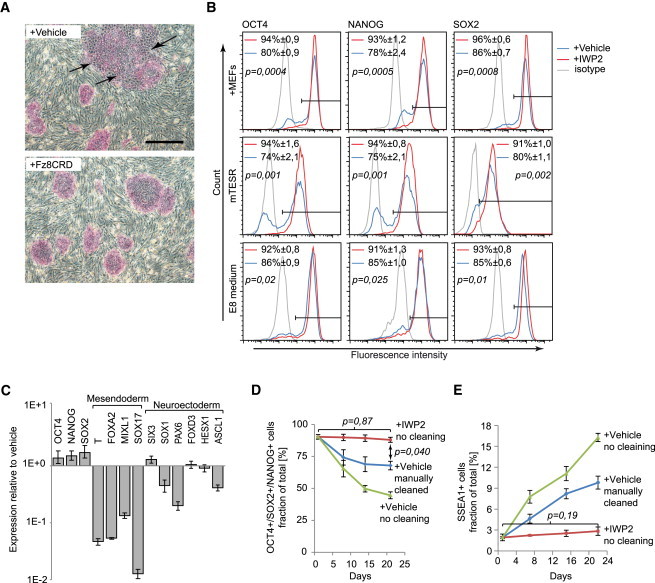
Inhibition of Endogenous WNT Signals Prevents Accumulation of Differentiated Cells in hESC Cultures
(A) H1 hESCs cultured for 5 days at the indicated conditions and stained for alkaline phosphatase (red). Arrows indicate differentiating areas of the colonies.
(B) Flow cytometry histograms showing H1 hESCs cultured for 7 days in the presence or absence of IWP2 and analyzed for NANOG, OCT4, and SOX2 (three independent experiments, mean ± SEM).
(C) Real-time RT-PCR gene expression profiles of H1 hESCs cultured for 6 days in the presence of IWP2, plotted relative to untreated cells (n = 3, mean ± SEM).
(D) Percentage of H1 hESCs triple positive for NANOG, OCT4, and SOX2 in the indicated conditions and procedures (three independent experiments, mean ± SEM).
(E) Percentage of H1 hESCs expressing SSEA1 in the indicated conditions and procedures (three independent experiments, mean ± SEM).
Scale bar represents 500 μm. See also Figure S4.
We next tested whether WNT inhibition obviated the need for manual removal of differentiated cells during routine culture of hESCs. With manual cleaning, both H1 and H9 hESCs maintained persistent populations of cells lacking one or more of the pluripotency factors, while in the absence of cleaning this population progressively increased (Figures 4D, S4F, and S4H). Strikingly, IWP2 maintained pluripotency factor expression in most cells in the absence of cleaning (Figures 4D, S4F, and S4H). Furthermore, IWP2 prevented the accumulation of cells expressing the hESC differentiation marker SSEA1, which otherwise rapidly accumulated (Figures 4E, S4G, and S4H). Finally, both H1 and H9 cells cultured for 10 passages in IWP2 efficiently formed teratomas, indicating that they retained their pluripotency (Figure S4I). Combined, these data show that inhibition of endogenous WNT signals prevents the accumulation of differentiated cells in hESC cultures and obviates the need for their manual removal.
BMP4 Induces Both WNT-Dependent and WNT-Independent Differentiation Pathways in hESCs
We next investigated whether WNT signals mediate BMP-induced differentiation in hESCs. Similar to the observations with mouse EpiSCs, both WNT3A and BMP4 protein induced BRACHYURY and GATA4 in H1 hESCs, with concomitant loss of OCT4 (Figure S5A). Flow cytometry indicated a strong induction of the mesoderm marker ROR2 (Drukker et al., 2012), together with suppression of pluripotency factors (Figures 5A, S5B, and S5C). RT-PCR analysis showed induction of additional primitive streak and mesoderm markers (Figure 5B). Furthermore, induction of WNT3 in response to BMP4 suggested that in hESCs too mesodermal induction was mediated by endogenous WNT (Figure 5C). Indeed, IWP2 prevented mesodermal induction in response to BMP4 (Figures 5A, 5B, and S5A). IWP2 did not abolish the induction of WNT3, indicating that it did not directly interfere with BMP4 signaling (Figure 5C). Importantly, IWP2 reduced but did not prevent the loss of the pluripotency markers in response to BMP4 (Figures 5D and S5C). These data show that BMP4 induces mesodermal lineages in hESCs indirectly, via induction of WNT proteins, but also suggest that it induces an alternative differentiation pathway that is WNT independent.
Figure 5.
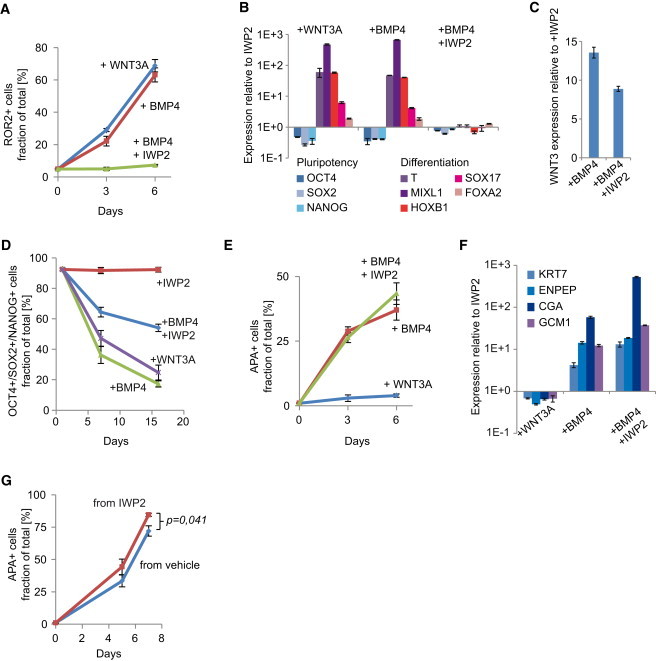
BMP4 Induces Both WNT-Dependent and WNT-Independent Differentiation Pathways in hESCs
(A) H1 hESCs were cultured with the indicated factors for 3 or 6 days and analyzed by flow cytometry for the mesodermal marker ROR2 (three independent experiments, mean ± SEM).
(B) Real-time RT-PCR gene expression profiles of H1 hESCs cultured for 3 days in the indicated factors, plotted relative to cells maintained in the presence of IWP2 (n = 3, mean ± SEM).
(C) WNT3 expression level 6 hr after induction of H1 hESCs with the indicated factors, plotted relative to cells maintained in IWP2 (n = 3, mean ± SEM).
(D) H1 hESCs cultured in the presence of the indicated factors and analyzed at several time points by flow cytometry for NANOG, OCT4, and SOX2. Plotted is the percentage of cells positive for all three markers (three independent experiments, mean ± SEM).
(E) H1 hESCs cultured with the indicated factors for 3 or 6 days and analyzed by flow cytometry for the trophoblast marker APA (three independent experiments, mean ± SEM).
(F) Real-time RT-PCR gene expression profiles of H1 hESCs cultured for 4 days in the indicated factors, plotted relative to cells maintained in the presence of IWP2 (n = 3, mean ± SEM).
(G) H1 hESCs maintained in the presence or absence of IWP2 prior to the experiment were differentiated with BMP4 in the presence of IWP2 and analyzed by flow cytometry for the trophoblast marker APA (three independent experiments, mean ± SEM).
See also Figure S5.
In addition to mesodermal lineages, hESCs have the ability to differentiate into trophoblast (Pera et al., 2004; Xu et al., 2002), and it has recently been shown that a mixture of mesoderm- and trophoblast-committed cells emerge in response to BMP4 (Drukker et al., 2012). In agreement with this study, both flow cytometry for the trophoblast surface marker APA (Drukker et al., 2012), and RT-PCR for the trophoblast markers KRT7, ENPEP, CGA, and GCM1 show that BMP4 induces the emergence of trophoblast progenitors (Figures 5E, 5F, and S5D). Importantly, WNT3A did not induce trophoblast differentiation, nor was the induction of the trophoblast markers by BMP4 inhibited by IWP2 (Figures 5E and 5F). Combined, these data show that BMP4 can induce the emergence of trophoblast-committed cells from hESCs in a WNT-independent manner, whereas the induction of mesoderm-committed cells requires the action of WNT proteins, either induced endogenously by BMP4 or added directly to the cells.
Finally, a reasonable assumption would be that hESCs maintained in the presence of IWP2 are better substrates for differentiation as they contribute fewer undesired lineages to the population. Indeed, when differentiated toward trophoblast by BMP4+IWP2, H1 hESCs that were maintained in the presence of IWP2 produced more APA+ cells than regular H1 cells (Figures 5G and S5E).
Discussion
This work shows that endogenous WNT signals are major hidden factors in the differentiation of hESCs and EpiSCs and affect the outcome of directed differentiation protocols in hitherto unappreciated ways. We show that WNT signals induce the main gastrulation factors Nodal, Wnt3, and Fgf8 and are required and sufficient for the induction of mesoderm by the commonly used mesoderm inducer BMP4. A surprising finding is that BMP4 induces both mesoderm as well as trophoblast-committed cells from hESCs, but only mesoderm induction requires the activation of WNT genes by BMP4.
We further show that endogenous WNT signals interfere with self-renewal of hESCs and mEpiSCs. Endogenous WNTs push the aggregate developmental phenotype of EpiSCs to that reminiscent of late-gastrula stage epiblast, consisting of a mixture of genuine EpiSCs with cells in various stages of differentiation, including cells committed to the definitive endoderm lineage. WNT inhibition prevents the induction of differentiation genes and commitment to endoderm, thereby maintaining a high percentage of genuine EpiSCs displaying their pregastrula phenotype, as evidenced by their contribution to blastocyst chimeras. A similar process takes place in hESCs, where we show that WNT inhibition is so effective in suppressing differentiation that it obviates the need for manual removal of differentiated cells during routine culture.
These findings are summarized in Figure 6, and they have obvious ramifications for the guided differentiation of hESCs. For instance, to induce trophoblast one should stimulate with BMP4 in the presence of a WNT inhibitor to avoid induction of mesoderm. Conversely, mesoderm is best obtained using WNT3A in lieu of BMP4 to avoid trophoblast induction. Furthermore, WNT-inhibited hESCs differentiate more efficiently to the trophoblast lineage, suggesting that genuine EpiSCs and hESCs, maintained as homogeneous undifferentiated populations by WNT inhibition, are superior substrates for differentiation as they contribute fewer undesired lineages to the population. We also find that different cell lines and culture media display various tendencies for endogenous WNT-induced differentiation, affecting their suitability for specific purposes such as mesoderm or neural differentiation. This may also influence to what extent WNT inhibition supports their self-renewal or improves their subsequent differentiation. Another interesting observation is that spontaneous endogenous WNT signals induce endoderm in EpiSCs, consistent with the finding that low levels of WNT3A (25 ng/ml) induce definitive endoderm (D’Amour et al., 2006), whereas high levels of WNT3A (250 ng/ml) induce mesoderm. This may reflect a later function for WNT in redirecting primitive streak-specified cells from endoderm to mesoderm (Loh et al., 2014). Finally, ESCs are commonly aggregated into EBs for the derivation of mesendodermal lineages. We now show that EBs mediate the transition of mESCs into EpiSCs, which is followed by activation of an endogenous WNT gradient and primitive streak induction (ten Berge et al., 2008). A more controlled way of inducing mesendodermal lineages would be by directly inducing genuine EpiSCs with defined levels of WNT signals.
Figure 6.
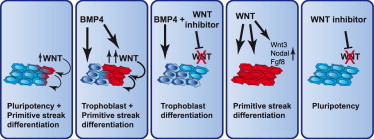
BMP and Endogenous WNT Signals Act Sequentially to Induce Shared and Distinct Differentiation Pathways in hESCs
(Left to right) Endogenous WNT proteins induce differentiation toward primitive streak lineages (red), reducing self-renewal. BMP4 induces both trophoblast (dark blue) and, mediated by endogenous WNTs, primitive streak lineages. In the presence of WNT inhibitors, BMP4 induces only trophoblast and no primitive streak lineages. WNT signals upregulate gastrulation factors and induce primitive streak lineages. WNT inhibitors block spontaneous differentiation, obviating the need to remove differentiated cells during culture.
The sequential action of BMP and WNT signals that we uncover here is consistent with embryological findings: Bmp4 is expressed prior to gastrulation in the extraembryonic ectoderm (Waldrip et al., 1998), both Wnt3 and active beta-catenin have been detected in the prestreak epiblast bordering this Bmp4-expressing region (Mohamed et al., 2004; Rivera-Pérez and Magnuson, 2005), and WNT pathway activation in the epiblast is required for primitive streak induction (Haegel et al., 1995; Huelsken et al., 2000; Liu et al., 1999). The spatiotemporal expression patterns of Bmp4 and Wnt3 are therefore consistent with a role for BMP4 in inducing Wnt3 in the primitive streak-forming region. This is supported by the observations that BMP4 induces Wnt3 expression in epiblast explants (Ben-Haim et al., 2006), and Bmp4 loss of function mutants fail to initiate gastrulation (Winnier et al., 1995). Since we find that WNT signals induce the essential gastrulation factors Wnt3, Nodal, and Fgf8 in the epiblast, this suggests that, upon WNT3 induction, the primitive streak can continue to expand distally because of continuous induction of the gastrulation factors, including WNT3 itself, by WNT3.
A recent study shows that a subpopulation of EpiSCs expressing a transgenic Brachyury reporter displays reversible primitive streak characteristics, i.e., while biased toward mesoderm and endoderm fates, these cells retain their pluripotency (Tsakiridis et al., 2014). In contrast, we find that a significant fraction of the cells labeled by our Brachyury reporter has lost pluripotency, as indicated by the inability to establish EpiSC colonies or to contribute to EBs. In support of this, our gene expression data indicate the presence of cells committed to a definitive endoderm fate in EpiSC cultures. The differences in our results may be explained by the Brachyury reporters used; the T-GFP reporter is targeted into the endogenous Brachyury locus and faithfully replicates its expression (Fehling et al., 2003). In contrast, the Tps/tb-RED reporter used by Tsakiridis et al. (2014) fails to recapitulate Brachyury expression in the anterior streak region which, importantly, is the source of definitive endoderm precursors (Clements et al., 1996). Therefore, while the T-GFP-labeled population would include the cells that have lost pluripotency because they committed to a definitive endoderm state, the Tps/tb-RED reporter would not identify this population.
Recently, there has been debate about the nature of the trophoblast-committed cells induced by BMP4 in hESCs, with one study reporting that these cells represent a subpopulation of mesodermal cells that go through a BRACHYURY-positive state (Bernardo et al., 2011). However, the absence of BRACHYURY and other mesendodermal and mesodermal markers in our BMP4+IWP2-differentiated trophoblast cells argues against a mesodermal character.
Experimental Procedures
Statistics
All data are presented as mean ± SEM. Technical replicates are meant unless further specified; p values < 0.05 determined using Student’s t test were considered significant.
Cell Culture
EpiSCs were cultured on gelatin and fetal calf serum-coated plates in N2B27 supplemented with 20 ng/ml ACTIVIN A and 12 ng/ml FGF2 (Peprotech). H1 and H9 hESCs were cultured on MEFs in Dulbecco’s modified Eagle’s medium/F12 supplemented with 20% knockout serum replacement and 10 ng/ml human FGF2 (Millipore). Feeder free culture was done on Matrigel (BD) in mTeSR1 medium (StemCell Technologies). Media, recombinant proteins, and small molecules were changed daily.
Animal Experiments
All animal experiments were conducted after approval by the Erasmus MC animal ethical committee.
Transcriptome Analysis
Total RNA from GFP9 EpiSCs was prepared using TriPure (Roche), converted to biotin-labeled cRNA, hybridized to Affymetrix Mouse Genome 430 2.0 Arrays, and analyzed with the Affymetrix GeneChip Scanner 3000. RNA-Seq was performed at the Erasmus MC Center for Biomics using the Illumina HiSeq platform. We combined Illumina BeadArray gene expression data (Kojima et al., 2014) with our microarray data using a similar approach as described (Heider and Alt, 2013). Microarray (GSE62155) and RNA-Seq (GSE62205) data are available in Gene Expression Omnibus (GEO). Further details are provided in the Supplemental Experimental Procedures.
Acknowledgments
129S2C1a EpiSCs were donated by L. Vallier, GOF18 EpiSCs by H.R. Schöler, and T-GFP ESCs by G. Keller. DtB was supported by NWO ECHO.10.B1.064, TI Pharma D5-402, Marie Curie FP7-PEOPLE-2009-RG-256560, ZonMW 911-09-036 and FES NIRM (Dutch Innovation Award), HvdW by NGI Zenith 93511036, and SP by LSBR 1040, ZonMW TOP 40-00812-98-12128, and EU fp7 THALAMOSS 306201.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
This table uses a threshold value of 0.05 for false discovery rate-adjusted p value and a minimum fold change of 2. The list contains 515 probe sets representing 408 unique gene symbols.
References
- Bakre M.M., Hoi A., Mong J.C., Koh Y.Y., Wong K.Y., Stanton L.W. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J. Biol. Chem. 2007;282:31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N., Lu C., Guzman-Ayala M., Pescatore L., Mesnard D., Bischofberger M., Naef F., Robertson E.J., Constam D.B. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev. Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Bernardo A.S., Faial T., Gardner L., Niakan K.K., Ortmann D., Senner C.E., Callery E.M., Trotter M.W., Hemberger M., Smith J.C. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernemann C., Greber B., Ko K., Sterneckert J., Han D.W., Araúzo-Bravo M.J., Schöler H.R. Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells. 2011;29:1496–1503. doi: 10.1002/stem.709. [DOI] [PubMed] [Google Scholar]
- Blauwkamp T.A., Nigam S., Ardehali R., Weissman I.L., Nusse R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M.E., Tang W., Lu J., Ma Z., Fan C.W., Wei S., Hao W., Kilgore J., Williams N.S. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements D., Taylor H.C., Herrmann B.G., Stott D. Distinct regulatory control of the Brachyury gene in axial and non-axial mesoderm suggests separation of mesoderm lineages early in mouse gastrulation. Mech. Dev. 1996;56:139–149. doi: 10.1016/0925-4773(96)00520-5. [DOI] [PubMed] [Google Scholar]
- D’Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G., Moorman M.A., Kroon E., Carpenter M.K., Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Davidson K.C., Adams A.M., Goodson J.M., McDonald C.E., Potter J.C., Berndt J.D., Biechele T.L., Taylor R.J., Moon R.T. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M., Tang C., Ardehali R., Rinkevich Y., Seita J., Lee A.S., Mosley A.R., Weissman I.L., Soen Y. Isolation of primitive endoderm, mesoderm, vascular endothelial and trophoblast progenitors from human pluripotent stem cells. Nat. Biotechnol. 2012;30:531–542. doi: 10.1038/nbt.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling H.J., Lacaud G., Kubo A., Kennedy M., Robertson S., Keller G., Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- Frank S., Zhang M., Schöler H.R., Greber B. Small molecule-assisted, line-independent maintenance of human pluripotent stem cells in defined conditions. PLoS ONE. 2012;7:e41958. doi: 10.1371/journal.pone.0041958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Paddison P.J., Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R.L., Lyon M.F., Evans E.P., Burtenshaw M.D. Clonal analysis of X-chromosome inactivation and the origin of the germ line in the mouse embryo. J. Embryol. Exp. Morphol. 1985;88:349–363. [PubMed] [Google Scholar]
- Greber B., Wu G., Bernemann C., Joo J.Y., Han D.W., Ko K., Tapia N., Sabour D., Sterneckert J., Tesar P., Schöler H.R. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6:215–226. doi: 10.1016/j.stem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Gu G., Wells J.M., Dombkowski D., Preffer F., Aronow B., Melton D.A. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development. 2004;131:165–179. doi: 10.1242/dev.00921. [DOI] [PubMed] [Google Scholar]
- Haegel H., Larue L., Ohsugi M., Fedorov L., Herrenknecht K., Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Han D.W., Tapia N., Joo J.Y., Greber B., Araúzo-Bravo M.J., Bernemann C., Ko K., Wu G., Stehling M., Do J.T., Schöler H.R. Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell. 2010;143:617–627. doi: 10.1016/j.cell.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Hao J., Li T.G., Qi X., Zhao D.F., Zhao G.Q. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Heider A., Alt R. virtualArray: a R/bioconductor package to merge raw data from different microarray platforms. BMC Bioinformatics. 2013;14:75. doi: 10.1186/1471-2105-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Charters A.M., Lee S.C., Zhao Y., Wu M.K., Jones S.J., Marra M.A., Hoodless P.A. A systematic screen for genes expressed in definitive endoderm by Serial Analysis of Gene Expression (SAGE) BMC Dev. Biol. 2007;7:92. doi: 10.1186/1471-213X-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Osorno R., Tsakiridis A., Wilson V. In Vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2012;2:1571–1578. doi: 10.1016/j.celrep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Huelsken J., Vogel R., Brinkmann V., Erdmann B., Birchmeier C., Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Kaufman-Francis K., Studdert J.B., Steiner K.A., Power M.D., Loebel D.A., Jones V., Hor A., de Alencastro G., Logan G.J. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell. 2014;14:107–120. doi: 10.1016/j.stem.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Lako M., Lindsay S., Lincoln J., Cairns P.M., Armstrong L., Hole N. Characterisation of Wnt gene expression during the differentiation of murine embryonic stem cells in vitro: role of Wnt3 in enhancing haematopoietic differentiation. Mech. Dev. 2001;103:49–59. doi: 10.1016/s0925-4773(01)00331-8. [DOI] [PubMed] [Google Scholar]
- Lindsley R.C., Gill J.G., Kyba M., Murphy T.L., Murphy K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu P., Wakamiya M., Shea M.J., Albrecht U., Behringer R.R., Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Loh K.M., Ang L.T., Zhang J., Kumar V., Ang J., Auyeong J.Q., Lee K.L., Choo S.H., Lim C.Y., Nichane M. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14:237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean A.B., D’Amour K.A., Jones K.L., Krishnamoorthy M., Kulik M.J., Reynolds D.M., Sheppard A.M., Liu H., Xu Y., Baetge E.E., Dalton S. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- Mohamed O.A., Clarke H.J., Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev. Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Cheng X., Keller G.M., Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogaki S., Harada S., Shiraki N., Kume K., Kume S. An expression profile analysis of ES cell-derived definitive endodermal cells and Pdx1-expressing cells. BMC Dev. Biol. 2011;11:13. doi: 10.1186/1471-213X-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Nishinakamura R., Iwamatsu Y., Shimosato D., Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem. Biophys. Res. Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Ohtsuka S., Nishikawa-Torikai S., Niwa H. E-cadherin promotes incorporation of mouse epiblast stem cells into normal development. PLoS ONE. 2012;7:e45220. doi: 10.1371/journal.pone.0045220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M.F., Andrade J., Houssami S., Reubinoff B., Trounson A., Stanley E.G., Ward-van Oostwaard D., Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Pfister S., Steiner K.A., Tam P.P. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr. Patterns. 2007;7:558–573. doi: 10.1016/j.modgep.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rivera-Pérez J.A., Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev. Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Singla D.K., Schneider D.J., LeWinter M.M., Sobel B.E. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem. Biophys. Res. Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Stewart M.H., Bossé M., Chadwick K., Menendez P., Bendall S.C., Bhatia M. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat. Methods. 2006;3:807–815. doi: 10.1038/nmeth939. [DOI] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T., Tsuneyoshi N., Nakatsuji N., Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Sumi T., Oki S., Kitajima K., Meno C. Epiblast ground state is controlled by canonical Wnt/β-catenin signaling in the postimplantation mouse embryo and epiblast stem cells. PLoS ONE. 2013;8:e63378. doi: 10.1371/journal.pone.0063378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada S., Era T., Furusawa C., Sakurai H., Nishikawa S., Kinoshita M., Nakao K., Chiba T., Nishikawa S. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- ten Berge D., Koole W., Fuerer C., Fish M., Eroglu E., Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R.K., Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Tortelote G.G., Hernández-Hernández J.M., Quaresma A.J., Nickerson J.A., Imbalzano A.N., Rivera-Pérez J.A. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev. Biol. 2013;374:164–173. doi: 10.1016/j.ydbio.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridis A., Huang Y., Blin G., Skylaki S., Wymeersch F., Osorno R., Economou C., Karagianni E., Zhao S., Lowell S., Wilson V. Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development. 2014;141:1209–1221. doi: 10.1242/dev.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip W.R., Bikoff E.K., Hoodless P.A., Wrana J.L., Robertson E.J. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Winnier G., Blessing M., Labosky P.A., Hogan B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Xu R.H., Chen X., Li D.S., Li R., Addicks G.C., Glennon C., Zwaka T.P., Thomson J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Li L., Huang C., Shen C., Tan F., Xia C., Liu P., Rossant J., Jing N. Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development. 2010;137:2095–2105. doi: 10.1242/dev.049494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table uses a threshold value of 0.05 for false discovery rate-adjusted p value and a minimum fold change of 2. The list contains 515 probe sets representing 408 unique gene symbols.


