Abstract
Greig cephalopolysyndactyly (GCPS) syndrome is an autosomal dominant disorder with high penetrance in majority of cases, characterized by a triad of polysyndactyly, macrocephaly and hypertelorism. GCPS is known to be caused by mutations in the transcription factor GLI3 gene (7p13) which results in functional haploinsufficiency of this gene. The present study reports a large multiplex family having 12 members affected with GCPS in 3 generations and several unaffected members showing autosomal dominant pattern of inheritance with complete penetrance. Interestingly an affected member of the family had unusual features including thumb which is although biphalangeal (confirmed with X-ray) but morphologically looks like finger and a unilateral tiny bony outgrown (externally indistinguishable) on the distal phalanx of the first toe of the left foot. This member also presented with mild ichthyosis. Although it is also possible that one or more of these features are coincidentally present in this member and might not be part of GCPS. Resequencing of the GLI3 gene detected a novel frame-shift mutation c.750delC in heterozygous state transmitting in the family and co-segregating with the disorder suggesting it to be the causal for the GCPS phenotype in the family. In silico analysis suggests that this mutation creates a truncated GLI3 protein resulting in its haploinsufficiency leading to GCPS syndrome. Furthermore, genotype-phenotype correlation is supported by the mutation as it lies in the amino terminal domain of the protein.
Keywords: Cephalopolysyndactyly, GCPS, GLI3, India, Limb defects
Highlights
-
•
The present manuscript report a novel single nucleotide deletion mutation in the GLI3 gene leading to frame-shift truncation in the protein.
-
•
The uniqueness of this mutation is that along with the typical GCPS phenotypes it also caused some very distinct unusual clinical features.
Introduction
Greig cephalopolysyndactyly (GCPS) is a rare (estimated frequency 1–9/1,000,000) multiple congenital anomaly syndrome characterized by the preaxial polydactyly together with at least one feature among syndactyly, macrocephaly and hypertelorism (Jamsheer et al., 2012, Balk and Biesecker, 2008, Johnston et al., 2005, Biesecker, 2008). Phenotypes may vary among patients even within the family (Jamsheer et al., 2012, Debeer et al., 2003). GLI3 is the major gene known for GCPS (Jamsheer et al., 2012, Balk and Biesecker, 2008, Johnston et al., 2005, Biesecker, 2008, Debeer et al., 2003, Johnston et al., 2010). So far many GLI3 mutations have been reported in GCPS (Johnston et al., 2010, Vortkamp et al., 1991, Elson et al., 2002, Wild et al., 1997). GLI3 shows allelic heterogeneity as mutations in this gene, besides GCPS, are also associated with Pallister-Hall syndrome (PHS; 146510) and to less frequently, other phenotypes such as acrocallosal syndrome (200990), non-syndromic polydactyly (174700,174200), trigonocephaly with craniosynostosis and polydactyly and some types of oral–facial-digital syndrome (Johnston et al., 2010, McDonald-McGinn et al., 2010).
The GLI3 protein is a zinc finger transcription factor expressed during early development and is a downstream mediator of sonic hedgehog (SHH) pathway (Cohen, 2010). The SHH/GLI3 pathway is involved in specifying anterior-posterior polarity of limb bud, dorsal-ventral polarity of the developing neural tube, craniofacial structures, lung and many others (Cohen, 2010). GLI3 is a bifunctional SHH mediator protein that functions as a repressor or activator for the transcription of downstream target genes. In the absence of SHH, GLI3 is cleaved to produce a repressor that down-regulates target genes, whereas, in the presence of SHH, full-length GLI3 up-regulates target genes. GLI3 harbors a repressor domain, five highly conserved zinc finger domains that bind to DNA in a sequence-specific manner and a trans-activation domain (Cohen, 2010, Biesecker, 2006, Ruppert et al., 1988). There exists a strong correlation between position of mutation and phenotype manifestation. Truncating mutations in the middle third of the gene generally associated with PHS whereas large deletions or truncating mutations elsewhere in the gene (one third part at the amino terminal-encoding or carboxy-terminal) cause GCPS (Jamsheer et al., 2012, Johnston et al., 2005, Ruppert et al., 1988). Truncation mutations in the one third part of the gene at the amino-terminal end cause the loss of the zinc finger DNA binding domain whereas those in one third part at the carboxy-terminal are predicted to cause the loss of a transactivation domain of GLI3, both leading to GCPS (Jamsheer et al., 2012, Johnston et al., 2005, Ruppert et al., 1988, Krauß et al., 2009, Shin et al., 1999). In the current study, we report a novel frameshift truncation mutation that causes GCPS in an Indian family. This truncation mutation lies in the amino terminal end of the protein causing GCPS along with some unusual features.
Patients and methods
Ethics statement
The study was approved by the ethical committee of the Faculty of Science, Banaras Hindu University. Written consent was obtained from the guardian of the family for the use of their samples, photographs and clinical details for the study.
A large multiplex family with GCPS was recruited from Swami Harshankaranandji Hospital, Varanasi (Fig. 1). Clinical information, photographs, X-rays of the hands and feet and blood samples were collected from 10 affected and 5 unaffected individuals. Genomic DNA was isolated from 2–3 ml peripheral blood lymphocytes according to the standard protocol. All of the exons as well as the exon-intron boundaries were PCR amplified followed by direct resequencing of the GLI3 gene on ABI-3130 genetic analyzer following protocol as recommended by the manufacturer (ABI, CA, USA). Primer sequences used for PCR amplification and resequencing were taken from Jamsheer et al. (2012). The database of single nucleotide polymorphisms was used for mutation analysis (dbSNP; http://www.ncbi.nlm.nih.gov/projects/SNP/). MutationTaster program was used to predict the effect of the mutations (Schwarz et al., 2010). The Exome variant server was used to determine if the sequence alterations were present in unaffected individual (http://evs.gs.washington.edu/EVS/).
Fig. 1.
Pedigree of the family with GCPS. Individuals for whom samples were collected are marked with an asterisk (*).
Results
Case report
Here we report a large multiplex family of GCPS from Varanasi city of Uttar Pradesh state of India. This family includes 12 affected and 13 unaffected members in 3 generations. In the present study 11 affected and 5 unaffected members were recruited (Fig. 1). Affected members show phenotypes similar to GCPS but there are high degrees of phenotypic variations among family members including some unreported features in GCPS (Fig. 2). Following are the general phenotypic description of the disorder in the family whereas Table 1 illustrates individual specific phenotypes.
Fig. 2.
Photographs of the patients. (A, B, C) Photographs of an affected member representing typical features of GCPS in the family, displaying craniofacial abnormalities (hypertelorism, low set ear, flat and wide nose, frontal bossing), bilateral syndactyly of hand and bilateral preaxial polysyndactyly of feet. (D, E, F) Photographs of the affected individual (II-11) with unusual features in the GCPS family having mild ichthyosis, sparse hair and low set ears, finger like thumb of upper limb, and bilateral syndactyly of lower limb with no visible polydactyly. (G) X-ray of hand showing biphalangeal finger like thumbs in patient II-11. (H) X-ray of feet showing a tiny unilateral outgrowth (arrow) on the distal phalanx of great toe of left foot in patient II-11.
Table 1.
Clinical features observed in the affected members.
| Individual | I-1 | II-4 | II-6 | II-11 | II-13 | III-2 | III-3 | III-5 | III-6 | III-7 | III-8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Craniofacial | |||||||||||
| High, broad forehead | - | - | - | - | - | - | - | - | - | - | - |
| Frontal bossing | - | - | - | - | - | - | + | - | + | + | + |
| Hypertelorism | - | - | - | - | - | - | + | - | + | + | + |
| Broad nasal bridge | + | + | + | - | - | - | + | - | + | + | + |
| Hands | |||||||||||
| Preaxial polydactyly | + | - | - | - | - | - | - | - | - | - | - |
| Postaxial polydactyly | + | + | + | - | - | - | - | - | + | - | + |
| Broad thumbs | - | - | + | - | - | - | - | - | - | + | + |
| Syndactyly | - | - | - | - | + | - | + | - | + | + | - |
| Feet | |||||||||||
| Preaxial polydactyly | + | + | + | - | + | + | + | + | + | + | + |
| Postaxial polydactyly | - | - | - | - | - | - | - | - | - | - | - |
| Broad halluces | - | - | - | + | - | - | - | - | - | - | - |
| Syndactyly | + | + | + | + | + | + | + | + | + | + | + |
| Variable features | |||||||||||
| Low set ears | Finger like biphalangeal thumbs, a unilateral tiny bony outgrowth on distal phalanx of first toe of left foot, dry skin, sparse hair, low set ears | Low set ears | Low set ear, improper long philtrum | Strabismus | Long philtrum, sandle gap, low set ears | Low set ears, sandle gap | |||||
Craniofacial abnormalities
High degree of craniofacial variations was present among family members. Macrocephaly (a characteristic feature of GCPS) was not noticed in any affected member but hypertelorism and frontal bossing were present in 4 affected members. Broad nasal bridge was also present in 7 affected members and was also present in 4 unaffected members.
Hand malformations
Both preaxial and postaxial polydactyly of hand were observed in this family. Postaxial polydactyly was more common than preaxial polydactyly. Syndactyly and broad thumb were also noticed in the affected members. Syndactyly of all 5 digits was observed in 4 affected members while one with fusion of digits III and IV. Interestingly an affected member of the family had unusual features including thumbs which are although biphalangeal (confirmed with X-rays) but morphologically looks like a finger (see Fig. 2E and G).
Foot malformations
All affected members except one had preaxial polydactyly and none with postaxial polydactyly. Syndactyly was observed in all affected members with a variable degree of clinical presentation. All affected members had individual nails and a complete syndactyly between the duplicated halluces and toes 2 and 3 were present. Syndactyly was consistently present in all affected members mostly with preaxial polydactyly. One affected member (II-11) had syndactyly of hallux and toes 2 and 3 but no polydactyly was observed externally but on radiological examination a unilateral tiny outgrowth was observed on the distal phalanx of the first toe of the left foot which might represent a mild form of polydactyly (see Fig. 2F and H).
Non typical GCPS features
We found some variable clinical features that are not typical for GCPS. II-11 had finger like thumbs of the hands, mild ichthyosis, sparse hair, and low set ear. Low set ear was also present in 4 other affected members. Long philtrum and strabismus were also noticed in some affected members of the family.
GLI3 mutation analysis
A novel single nucleotide deletion (g.42085059delG) (Fig. 3) in heterozygous state was identified in all the affected individuals and none of the unaffected individuals of the family. This framshifting mutation (p.Y251Mfs59) truncates the protein at N-terminal that creates a truncated protein of 309 amino acids (Fig. 4).
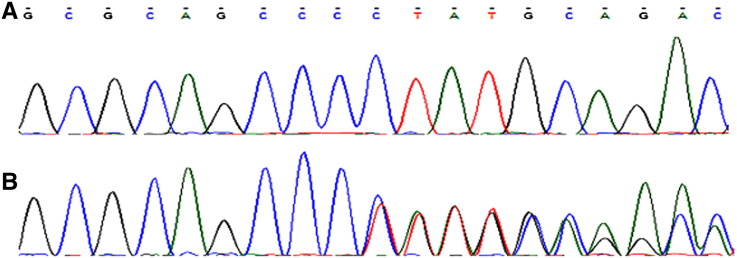
Fig. 3.
Sequence electropherograms of exon 7 of GLI3 gene. (A) DNA sequence of an unaffected member (II-8), (B) Frameshift deletion of the single nucleotide C in an affected member (III-8) of the family.
Fig. 4.
(A) A part of normal (1581 amino acid) GLI3 protein and (B) a part of the truncated GLI3 protein sequence of 309 amino acids. Letters in red color indicate abnormal amino acid sequence generated due to frameshift caused by 750delC single nucleotide deletion.
Discussion
In the present study a multiplex family is reported with typical features of GCPS in most of the members along with some unusual features in an affected member. A single mutation (c.750delC) in GLI3 gene is identified as the cause of GCPS in this family. Despite that the same mutation is present in all of the affected members; there exist a high degree of phenotypic variability among them.
One affected member (II-11) of the family with the same GLI3 mutation had syndactyly and no macrocephaly. Interestingly this member had radiologically a unilateral tiny outgrowth (but not clearly a polydactyly) on the distal phalanx of the first toe of the left foot (see Fig. 2H). This feature is not so far reported in GCPS suggesting that polydactyly in GCPS may be present as minor as a tiny bony outgrowth (without an external intervention) and also without being bilateral. This member has another novel feature; finger like biphalangeal thumb morphologically similar to finger. It is also possible that these features are coincidentally present and might not be part of GCPS.
The presence of the unusual features as well as high phenotypic variability in the family suggest that there exist some modifiers which affect the phenotypic manifestation of the mutations to a significant level.
Based on guideline suggested by Johnston et. al., mutations from nucleotides 1 to 1997 as well as after nucleotide 3481 of the open reading frame cause GCPS, whereas mutations from nucleotides 1998 to 3481 generate primarily PHS. Phenotypic variability is reported among GCPS but there is no correlation for the position of mutation and severity of phenotype within GCPS. One study suggests that the 3′ frameshifting GCPS mutations may cause hypertelorism less often than 5′ frameshifting mutations (Johnston et al., 2005).
This frameshift mutation introduces a premature stop codon within its N-terminal repressor domain which causes the truncation of the GLI3 protein synthesis. Due to this truncation, the mutant proteins lack all downstream functionally important domains, including highly conserved zinc-finger domain (ZFD) (which is crucial for DNA binding capacity in a sequence-specific manner), cleavage site, CBP-binding module and C-terminal transactivation domain (Ruppert et al., 1988, Krauß et al., 2009, Vortkamp et al., 1995).
According to the previously reported genotype–phenotype correlation for GLI3, truncating mutations (i.e. frameshift and nonsense), those lying in the N-terminal and C-terminal third of the protein result in GCPS due to haploinsufficiency of GLI3, whereas alterations affecting the middle third cause PHS (Johnston et al., 2005, Johnston et al., 2010, Biesecker, 2006). In the present report, the identified novel GLI3 c.750delC mutation that lies in the N-terminal domain of GLI3 protein also causes GCPS, which further support the genotype–phenotype correlation as described by other reports (Jamsheer et al., 2012, Balk and Biesecker, 2008, Johnston et al., 2005). In this family, the truncated GLI3 product may be the direct reason causing the haploinsufficiency for full length GLI3, or the nonsense-mediated decay may lead to its insufficiency which implies that the dose or the ratio of normal to mutated gene product might also have an effect on the phenotype diversity (Pettigrew et al., 1991).
Acknowledgments
The authors are grateful to all members of the patient family who participated in the study and to Mr. Chandrabhan Singh and Mrs. Kusum for the help in sample collection. The authors are highly grateful to Prof. Rajiva Raman for his valuable suggestions. The present work is funded by a young scientist grant from SERB, DST, New Delhi (grant no. SB/YS/LS-46/2013) to Dr Akhtar Ali.
Contributor Information
Rashmi Patel, Email: rashmi008patel@gmail.com.
Fanish Mani Tripathi, Email: fmtripathiims@gmail.com.
Subodh Kumar Singh, Email: singhsubodh@satyam.net.in.
Anjali Rani, Email: anjaliraani@yahoo.com.
Visweswar Bhattacharya, Email: visweswar1@rediffmail.com.
Akhtar Ali, Email: akhtar_genetics@yahoo.co.in, akhtar@bhu.ac.in.
References
- Balk K., Biesecker L.G. The clinical atlas of Greig cephalopolysyndactyly syndrome. Am. J. Med. Genet. A. 2008;146A:548–557. doi: 10.1002/ajmg.a.32167. [DOI] [PubMed] [Google Scholar]
- Biesecker L.G. What you can learn from one gene: GLI3. J. Med. Genet. 2006;43:465–469. doi: 10.1136/jmg.2004.029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker L.G. The Greig cephalopolysyndactyly (review) Orphanet J. Rare Dis. 2008;3:10–15. doi: 10.1186/1750-1172-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.M., Jr. Hedgehog signalling update. Am. J. Med. Genet. A. 2010;152A:1875–1914. doi: 10.1002/ajmg.a.32909. [DOI] [PubMed] [Google Scholar]
- Debeer P., Peeters H., Driess S., De Smet L., Freese K. Variable phenotype in Greig cephalopolysyndactyly syndrome: clinical and radiological findings in 4 independent families and 3 sporadic cases with identified GLI3 mutations. Am. J. Med. Genet. 2003;120A:49–58. doi: 10.1002/ajmg.a.20018. [DOI] [PubMed] [Google Scholar]
- Elson E., Perveen R., Donnai D., Wall S., Black G.C. De novo GLI3 mutation in acrocallosal syndrome: broadening the phenotypic spectrum of GLI3 defects and overlap with murine models. J. Med. Genet. 2002;39:804–806. doi: 10.1136/jmg.39.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamsheer A., Sowińska A., Trzeciak T., Jamsheer-Bratkowska M., Geppert A., Latos-Bieleńska A. Expanded mutational spectrum of the GLI3 gene substantiates genotype–phenotype correlations. J. Appl. Genet. 2012;53:415–422. doi: 10.1007/s13353-012-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J.J., Olivos-Glander I., Killoran C., Elson E., Turner J.T. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am. J. Hum. Genet. 2005;76:609–622. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J.J., Sapp J.C., Turner J.T., Amor D., Aftimos S. Molecular analysis expands the spectrum of phenotypes associated with GLI3 mutations. Hum. Mutat. 2010;31:1142–1154. doi: 10.1002/humu.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauß S., So J., Hambrock M., Ko¨hler A., Kunath M. Point mutations in GLI3 lead to misregulation of its subcellular localization. PLoS ONE. 2009;4:e7471. doi: 10.1371/journal.pone.0007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn D.M., Feret H., Nah H.D., Bartlett S.P., Whitaker L.A. Metopic craniosynostosis due to mutations in GLI3: a novel association. Am. J. Med. Genet. A. 2010;152A:1654–1660. doi: 10.1002/ajmg.a.33495. [DOI] [PubMed] [Google Scholar]
- Pettigrew A.L., Greenberg F., Caskey C.T., Ledbetter D.H. Greig syndrome associated with an interstitial deletion of 7p: confirmation of the localization of Greig syndrome to 7p13. Hum. Genet. 1991;87:452–456. doi: 10.1007/BF00197167. [DOI] [PubMed] [Google Scholar]
- Ruppert J.M., Kinzler K.W., Wong A.J., Bigner S.H., Kao F.T. The GLI-Kruppel family of human genes. Mol. Cell. Biol. 1988;8:3104–3113. doi: 10.1128/mcb.8.8.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., R€odelsperger C., Schuelke M., Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- Shin S.H., Kogerman P., Lindstrom E., Toftgard R., Biesecker L.G. GLI3 mutations in human disorders mimic Drosophila cubitus interruptus protein functions and localization. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2880–2884. doi: 10.1073/pnas.96.6.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A., Gessler M., Grzeschik K.H. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–540. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Gessler M., Grzeschik K.H. Identification of optimized target sequences for the GLI3 zinc finger protein. DNA Cell Biol. 1995;14:629–634. doi: 10.1089/dna.1995.14.629. [DOI] [PubMed] [Google Scholar]
- Wild A., Kalff-Suske M., Vortkamp A., Bornholdt D., Konig R., Grzeschik K.H. Point mutations in human GLI3 cause Greig syndrome. Hum. Mol. Genet. 1997;6:1979–1984. doi: 10.1093/hmg/6.11.1979. [DOI] [PubMed] [Google Scholar]