Abstract
Little is known about the effect of migraine on neural cognitive networks. However, cognitive dysfunction is increasingly being recognized as a comorbidity of chronic pain. Pain appears to affect cognitive ability and the function of cognitive networks over time, and decrements in cognitive function can exacerbate affective and sensory components of pain. We investigated differences in cognitive processing and pain–cognition interactions between 14 migraine patients and 14 matched healthy controls using an fMRI block-design with two levels of task difficulty and concurrent heat (painful and not painful) stimuli. Across groups, cognitive networks were recruited in response to a difficult cognitive task, and a pain–task interaction was found in the right (contralateral to pain stimulus) posterior insula (pINS), such that activity was modulated by decreasing the thermal pain stimulus or by engaging the difficult cognitive task. Migraine patients had less task-related deactivation within the left dorsolateral prefrontal cortex (DLPFC) and left dorsal anterior midcingulate cortex (aMCC) compared to controls. These regions have been reported to have decreased cortical thickness and cognitive-related deactivation within other pain populations, and are also associated with pain regulation, suggesting that the current findings may reflect altered cognitive function and top-down regulation of pain. During pain conditions, patients had decreased task-related activity, but more widespread task-related reductions in pain-related activity, compared to controls, suggesting cognitive resources may be diverted from task-related to pain-reduction-related processes in migraine. Overall, these findings suggest that migraine is associated with altered cognitive-related neural activity, which may reflect altered pain regulatory processes as well as broader functional restructuring.
Keywords: fMRI, Pain–cognition interaction, Chronic pain, Cognitive networks, Posterior insula, Dorsolateral prefrontal cortex
Highlights
-
•
Migraine patients had blunted task-related deactivations in DLPFC, aMCC, and cerebellum in the absence of pain, vs. controls.
-
•
Unlike in healthy controls, these task-related deactivations were not modulated by the presence of an acute pain stimulus.
-
•
Migraine patients had less task-related activity during pain, compared to controls.
-
•
Acute pain disturbs cognitive processing more in patients than controls.
1. Introduction
Migraine is a central nervous system disease traditionally characterized by acute painful attacks (Goadsby et al., 2002). This definition, however, does not capture the effects of migraine that extend beyond episodic attack (ictal) periods (Goadsby et al., 2002; Stewart et al, 1992), including lasting alterations in brain structure and function (Lakhan et al., 2013; Noseda & Burstein, 2013; Sprenger & Borsook, 2012) that closely mirror the effects of chronic pain conditions. More broadly, migraine also appears to share physiological (Boyer et al., 2014) and clinical profiles (Blumenfeld et al., 2011; Buse et al., 2010; Yoon et al., 2013) with other chronic pain conditions. Similar to other chronic pain conditions (Apkarian et al., 2004a; McCracken & Iverson, 2001), migraine has been linked to impaired cognitive function. Pain demands and depletes cognitive resources, which is thought to contribute to cognitive impairments in chronic pain broadly defined (Eccleston & Crombez, 1999; Nes et al., 2009). The behavioral effect of migraine on cognitive function, however, has been debated within the literature, largely due to mixed results (Calandre et al., 2002; Pearson et al., 2006; Suhr & Seng, 2012; Waldie et al., 2002; Zeitlin & Oddy, 1984). A recent review noted that significant effects of migraine on cognitive function depended largely on study recruitment, such that patients recruited from neurology clinics show mild impairment across several cognitive domains, whereas migraine patients recruited from the community were less likely to show cognitive differences from healthy controls (de Araújo et al., 2012). Therefore, cognitive impairment in migraine may be dependent on disease severity (e.g. disease duration, migraine frequency, migraine pain intensity).
Pain–cognition interactions in neural response have been demonstrated among young healthy populations (Petrovic et al., 2000; Seminowicz & Davis, 2007a), such that cognitive engagement typically reduces pain-related activity. Cognitive-related activity, on the other hand, does not tend to be altered by simultaneous pain stimuli among healthy volunteers (Seminowicz & Davis, 2007a). However, whether these patterns are preserved among people with migraine is not known. Chronic pain appears to alter the functional organization of brain networks involved in pain and cognitive processes (Farmer et al., 2012). Initial evidence suggesting that cognitive modulation of pain processing may be altered or weakened in migraine patients indicated that, compared to healthy controls, patients with chronic and episodic migraine show deficient cognitive task-related suppression of evoked potential amplitude during acute pain stimulation (de Tommaso et al., 2003).
The effect of migraine on cognitive neural networks remains largely unexplored. Recently, the association between migraine and brain white matter lesion load (Kurth & Tzourio, 2009; Swartz & Kern, 2004) has supported the hypothesis that prolonged suffering with migraine may lead to cognitive decline (Paemeleire, 2009). However, a longitudinal study did not find such a relationship (Rist & Kurth, 2013). Still, others have found correlations between structural differences between migraine patients and controls, and impairments on some cognitive tasks (Schmitz et al., 2008) lending support to the hypothesis that cognitive decline in migraine may be due to structural brain changes over time. While little is known about functional neural response to cognitive challenge among migraine patients, hypotheses can be drawn from evidence of abnormal brain activity in response to cognitive tasks within other chronic pain populations including chronic low back pain (Seminowicz et al., 2011) and temporomandibular disorder (Weissman-Fogel et al., 2011). Additionally, studies have reported abnormal functional resting state connectivity in migraine patients between brain regions associated with affective pain processing and cognitive networks (Schwedt et al., 2013; Xue et al., 2012). One recent study demonstrated that migraine patients also display greater evoked pain-related activity in areas thought to be associated with cognitive aspects of pain processing, including the contralateral premotor cortex (PM), right dorsolateral prefrontal cortex (DLPFC), and ipsilateral hippocampus (Schwedt et al., 2014). Taken together, these findings suggest that changes in functional cognitive brain activity may be due to integration of pain and cognitive networks and pain–cognition interactions.
In the current study we examine neural response to cognitive challenge, and pain–cognition interactions during concurrent pain stimulation, among migraine patients and healthy volunteers. We hypothesized that there would be group differences in task-related neural response, where migraine patients would have to recruit greater brain resources to perform the cognitive task equally well as healthy controls, and that task-related suppression of acute pain-related activity (pain–cognition interactions) would also be altered among migraine patients.
2. Methods
2.1. Participants
Fourteen migraine patients (11 females, mean 40.8 years old (SD = 11.9)) and 14 healthy controls (11 females, mean 38.9 years old (SD = 12.5)) participated in this study and were compensated for their time. Participant groups were also matched for education and handedness. Healthy controls were recruited at the University of Maryland, Baltimore (UMB). Migraine patients were recruited from the Johns Hopkins University (JHU) campuses and local headache clinics, and through community advertisements. Migraine patients also participated in a subsequent longitudinal study, but the procedures described here were all completed at baseline, before any intervention. We specifically recruited participants who reported more headache days than headache free days. Evaluation of prospective 3-month headache diaries revealed that ten patients met diagnosis criteria for chronic migraine (Range: 14–28 headache days per 28 days) and four for episodic migraine (Range: 7.8–13.7 headache days per 28 days). Diagnosis was determined by the study physician (M.G.) using the International Classification of Headache Disorders-II (ICHD-II) criteria (Headache Classification Subcommittee of the International Headache Society, 2004; Olesen & Steiner, 2004). We did not exclude any patients in our primary analysis for a number of reasons. First, all patients were recruited based on reported chronic migraine. Additionally, within our migraine group we examined migraine frequency as a continuous variable. Though separable diagnostic criteria, chronic migraine and episodic migraine have a common underlying pathophysiology (Pietrobon & Moskowitz, 2013). Clinical and behavioral differences between chronic and episodic migraine patients have been demonstrated in factors that vary linearly with increasing frequency, and the demarcation created for diagnostic purposes is somewhat arbitrary (Borsook et al., 2012; Katsarava et al., 2012; Silberstein et al., 1996). Furthermore, others have examined chronic and episodic migraine patients together, which has led to a number of recent advances in the understanding of migraine (e.g. Anttila et al., 2013; Hamedani et al., 2013; Hunter et al., 2014). Nonetheless, to ensure that our findings were not dependent on the episodic migraine patients, we also report the main results excluding the four episodic migraine patients (Supplementary Results). Patients had migraine for an average of 10.9 years (SE = 2.0, Range: 1–27 years) and rated their average migraine pain intensity during the last month as a 5.4 on a 0 (no pain) to 10 (worst pain imaginable) scale (SE = 0.6, Range: 2–10). Patients had an average of 20.1 headache days per 28 day month according to prospective headache diaries (SE = 2.0, Range: 7.8–28 days) and retrospectively reported an average migraine frequency of 9.6 in the previous month (SE = 2.1, Range: 2–30 migraines). Exclusion criteria included an unstable psychiatric disorder, pregnancy, illicit drug use and severe alcoholism. Healthy controls were pain-free. This study was approved by the UMB and JHU Institutional Review Boards, and informed written consent was obtained from each participant prior to any study procedures.
2.2. Stimuli
The cognitive task and thermal stimuli were administered simultaneously according to a full 2 (task difficulty) × 3 (thermal stimulus intensity) factorial design, such that while participants completed either the difficult or easy cognitive task, they experienced a concurrent moderately painful, mildly painful, or non-painful thermal stimulus [i.e., (difficult/moderate pain (T1P2), difficult/mild pain (T1P1), difficult/no pain (T1P0), easy/moderate pain (T0P2), easy/mild pain (T0P1), easy/no pain (T0P0)].
2.2.1. Cognitive task
Participants performed a modified Attentional Network Test (ANT) (Fan et al., 2002), which involved identifying the direction of a central arrow, while ignoring the direction of flanking arrows. Difficult tasks involved distracting, incongruent flankers, easy tasks involved congruent flankers. During both the pre-scan practice session and the MRI scan sessions, participants indicated the direction of the arrow using a simple button press with their right hand.
2.2.2. Thermal stimuli
An fMRI-compatible thermal stimulator delivered thermal stimuli via a contact probe (30 × 30 mm Medoc Pathway ATS Peltier device; Medoc Advanced Medical Systems Ltd., Ramat Yishai, Israel) to the left volar surface of participants' forearms. The peak temperature (moderate pain, P2) used throughout the study was determined at the beginning of the pre-scan session – using a simple ramp-and-hold procedure – as the temperature at which participant rated a 5–6 on a 0 (no pain) to10 (most intense pain imaginable) pain intensity scale. The mild pain (P1) stimulus was defined as 1° less than moderate pain (P2-1°C). The no pain stimulus (P0) was 37 °C for all participants. Stimuli were presented for a variable duration of 8–12 s with a ramp time of 1.6 s and variable ramp rate based on peak stimulus temperature. Stimuli were separated by intervals (4–8 s) of baseline temperature (32 °C). The same temperature was used for each participant throughout the pre-scan and MRI scan sessions with the exception of one patient who required the peak stimulus temperature to be lowered 1 °C for the second functional run.
2.3. Procedure
2.3.1. Pre-scan session
Prior to scanning, participants were first familiarized with the cognitive task and thermal stimuli. Then, the individualized peak thermal stimulus (P2) was determined, and participants were trained to a point where they performed with >90% accuracy on the cognitive task. Participants then performed the full experimental paradigm, with concurrent task and thermal stimulation, identical to that experienced in the MRI session. Pain intensity and unpleasantness ratings were obtained on a 0–10 numerical rating scale for each trial, and reaction time and accuracy were recorded. Participants also completed retrospective reports of migraine frequency and migraine pain intensity and the Pain Catastrophizing Scale (PCS) (Sullivan et al., 1995). Pain catastrophizing is a cognitive coping strategy associated with increased rumination, magnification, and helplessness toward pain. We included this measure because there is some evidence that migraine patients catastrophize more than healthy controls (Hassinger et al., 1999), and pain catastrophizing increases preferential attention to pain (Sullivan et al., 1995). High catastrophizers, therefore, may be less able to redirect their attention away from their pain, which may inhibit task-related suppression of pain.
2.3.2. MRI session
The scan session included two functional runs (Run 1: 9 min 20 s, Run 2: and 9 min 32.5 s) containing 27 trials each. There were six trial types consisting of all possible combinations of task difficulty and thermal stimulus intensity: difficult/moderate pain (T1P2), difficult/mild pain (T1P1), difficult/no pain (T1P0), easy/moderate pain (T0P2), easy/mild pain (T0P1), and easy/no pain (T0P0). Participants provided a real-time response to the cognitive task during each trial using a fiber optic MRI-compatible button box in the scanner. Presentation of visual stimuli for the cognitive task was delivered via a desktop computer running E-Prime 2.0 (Psychology Software Tools, http://www.pstnet.com). Visual stimuli were back-projected onto a translucent screen positioned behind the scanner bore and viewed from an angled mirror mounted on the head coil positioned at subject's eye-level.
Images were acquired using a Siemens 3T Tim Trio MRI scanner equipped with a 12-channel head coil. Participants completed a high-resolution T1-weighted MPRAGE anatomical scan [144 slices, repetition time (TR) 2500 ms, echo time (TE) 3.44 ms, flip angle 9.0°, FOV 230 mm, resolution 0.9 × 0.9 mm, matrix size 256 × 256 mm, slice thickness 1 mm, no gap] and two functional runs using multi-slice T2*-weighted, echo planar imaging sequences [spin-echo, 224 (Run 1)/229 (Run 2) volumes, 36 slices, TR 2500 ms; TE 30 ms; flip angle 90°; FOV 230 mm, resolution 1.8 × 1.8, matrix size 128 × 128 mm, slice thickness 4 mm, no gap, oblique slices].
2.4. Analyses
Only the cognitive task and pain–task interaction results are presented here. The main effect of pain and group effects on pain processing are reported and discussed in a separate manuscript.
2.4.1. Behavioral analyses
A 2 (group: patients, controls) × 2 (task difficulty: easy, difficult) × 3 (thermal stimulus intensity: no pain, mild pain, moderate pain) repeated-measures ANOVA was used to analyze all behavioral data.
2.4.2. fMRI preprocessing and analyses
Functional images were preprocessed and analyzed using SPM8 software (Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab (Mathworks, Cherborn, MA, USA). Images were slice time corrected and motion corrected. Then, the high-resolution anatomical image was coregistered onto the mean functional image and segmented into CSF, white matter, and gray matter. All volumes were normalized to MNI (Montreal Neurological Institute) space and then spatially smoothed with an 8 mm Gaussian kernel.
After preprocessing, a general linear model was defined for each participant separately. Each model included six regressors of interest: difficult/moderate pain (T1P2), difficult/mild pain (T1P1), difficult/no pain (T1P0), easy/moderate pain (T0P2), easy/mild pain (T0P1), and easy/no pain (T0P0). In order to model only the period where cognitive load and thermal stimulation were stable and concurrent within each trial, the first and last portions (1.4 s off each end of the cognitive task trial, and corresponding, but variable tails of the thermal stimulus trial) of each trial were excluded. Resultant trial durations (5.7–11.8 s), reaction times (parametric modulator), and six motion parameters (covariates of no interest) were modeled independently for each subject. Reaction times were not available for one patient and one control.
Average task-related neural response was determined using contrast images [difficult > easy, across thermal stimulus intensity levels, T1 > T0] and [difficult > easy in the absence of pain, T1P0 > T0P0] for each participant. To evaluate group differences in task-related activity, independent sample t-tests were also conducted on these contrasts. The full task–pain interaction [task (difficult − easy) + pain] − [task (difficult − easy) + no pain]: [(T1P2 − T0P2) + (T1P1 − T0P1)] − 2[T1P0 − T0P0] was examined across and within groups. Additionally, we further probed this interaction by examining task-related activations during pain [pain (mild + moderate) + difficult task] > [pain (mild + moderate) + easy task]: [(T1P1 + T1P2) − (T0P1 + T0P2)] as well as task-related deactivations during pain [pain (mild + moderate) + easy task] > [pain (mild + moderate) + difficult task]: [(T0P1 + T0P2) − (T1P1 + T1P2)]. The latter was of particular interest as it may reflect task-related reductions in pain activity. Finally, we also examined pain-related activations during the difficult task [difficult task + no pain] > [difficult task + pain (mild + moderate)]: [2T1P0 − (T1P1 + T1P2)] to examine the effects of cognitive load on pain-related activity. Across analyses, an initial extent threshold of p < .005, cluster size k > 25 voxels was used, corrected for multiple comparisons at the cluster level (p < .05), unless otherwise stated. More stringent thresholds were used when necessary to identify separable clusters for subsequent extraction.
We used MarsBar (Brett et al., 2002) to extract beta values from regions of interest (ROIs), defined as significant clusters identified by planned contrasts. Extracted values were used in 2 (group: patients, controls) × 2 (task difficulty: easy, difficult) × 3 (thermal stimulus intensity: no pain, mild pain, moderate pain) repeated-measures ANOVAs to probe task–pain interactions and group differences in task-related activity.
3. Results
3.1. Behavioral results
3.1.1. Pre-scan session
3.1.1.1. Individualized pain stimulus temperatures
Migraine patients had marginally higher individualized peak stimulus (P2) temperatures (Mean = 47.5 °C, SE = .3) compared to healthy controls (Mean = 46.4 °C, SE = .5), t(26) = 2.0, p = .06.
3.1.1.2. Reaction time
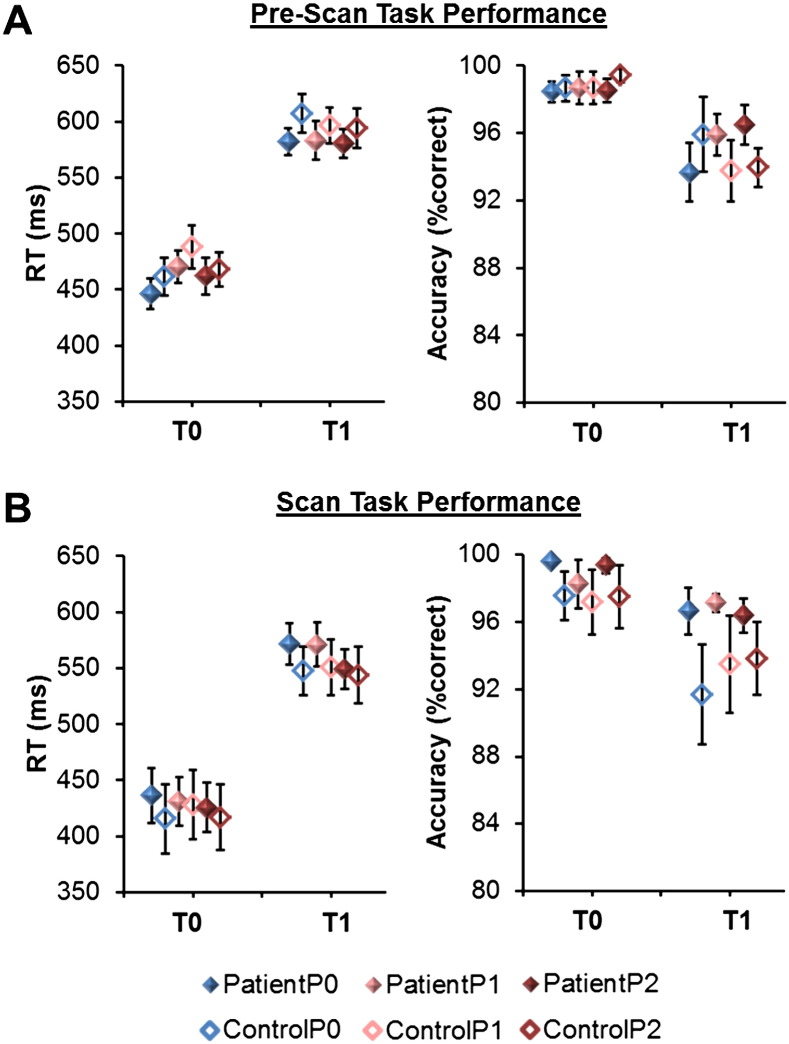
As expected (Seminowicz & Davis, 2007a) a main effect of task difficulty F(1,26) = 195.3, p < .001, and a marginal effect of pain F(2,52) = 2.7, p = .08, were found on reaction times. On average, participants were slower to respond during the difficult, relative to easy task t(27) = 14.2, p < .001, and during the mild (P1) pain condition relative to the moderate (P2; t(27) = 1.8, p = .08) and no pain (P0; t(27) = 2.0, p = .06) conditions. A significant task–pain interaction F(2,52) = 4.7, p = .01 was also seen in reaction time, such that the effect of pain on reaction time was larger during the easy task, and not significant during the difficult task (Fig. 1A). No other main effects or interactions were found.
Fig. 1.
Behavioral results. Patients and controls did not differ in their task performance (all between group comparisons, NS). Markers depict means and standard errors by condition. Solid markers represent patients, hollow markers represent controls. A) Pre-scan reaction time (RT) and accuracy. B) Reaction time and accuracy during scan. Reaction time (RT), no pain (P0), mild pain (P1) and moderate pain (P2), and easy task (T0) and difficult task (T1).
3.1.1.3. Accuracy
There was a main effect of task difficulty F(1,26) = 25.1, p < .001 on accuracy, such that participants had lower accuracy in the difficult, relative to easy task, t(27) = 5.0, p < .001 (Fig. 1B). No other main effects or interactions were found.
3.1.1.4. Group differences in task performance
Notably, there were no main effects of group on reaction time or accuracy, suggesting that patients performed the task as well as controls. When performance was examined within each of the six individual conditions, there were still no observable differences in performance between groups (Fig. 1A, all ps > .10).
3.1.1.5. Pain catastrophizing
Patients (Mean = 17.7, SE = 2.3) had significantly higher pain catastrophizing scores than controls (Mean = 6.9, SE = 2.1), t(26) = 3.5, p = .002.
3.1.2. MRI session
Reaction times and accuracy were not obtained for one patient and one control. Therefore these participants were not included in the following analyses. An additional two patients and one control have partially missing reaction time and accuracy data. For these participants, all available data were included.
3.1.2.1. Reaction time
A main effect of task difficulty F(1,24) = 183.7, p < .001, and a main effect of pain F(2,48) = 3.6, p = .04 were found on reaction time, such that participants were faster to respond during the easy, relative to difficult task t(25) = 13.8, p < .001, and during moderate (P2) relative to the no pain (P0; t(25) = 2.0, p = .06) and mild pain (P1; t(25) = 2.6, p = .02) conditions. No other main effects or interactions were significant.
3.1.2.2. Accuracy
A main effect of task difficulty F(1,24) = 23.4, p < .001 was also found on task accuracy, such that participants had lower accuracy in the difficult, relative to easy conditions, t(25) = 4.7, p < .001. No other effects were significant and there were no group differences in performance in any of the six individual conditions (Fig. 1B, all ps > .10).
3.2. fMRI results
3.2.1. Task-related activity across participants
3.2.1.1. Task-related activity across pain conditions
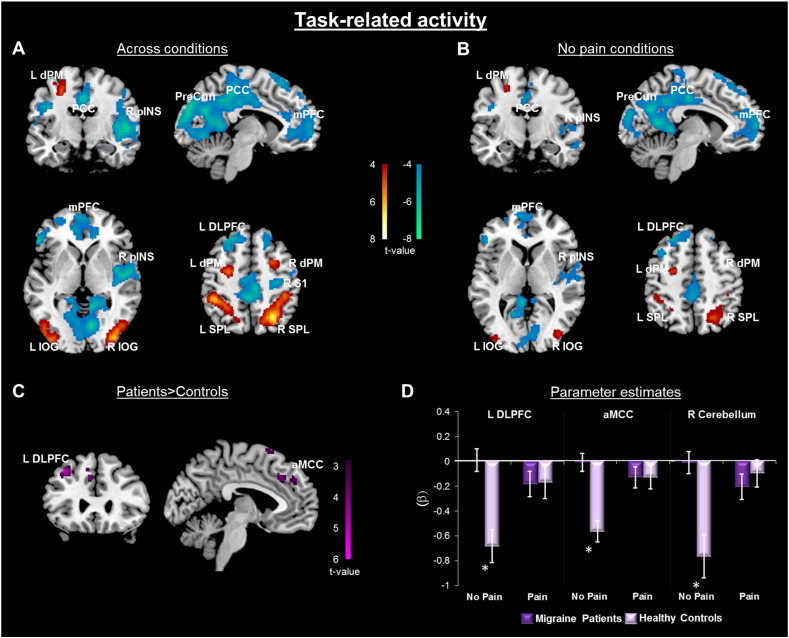
Across moderate, mild, and no pain conditions, task difficulty was associated with neural activity within regions known to be associated with cognitive processing (Table 1, Fig. 2A). All participants showed task-related activations in bilateral regions of the inferior occipital gyrus (IOG), superior parietal lobule (SPL) and PM cortices, and task-related deactivations within midline regions, including the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC), and the R pINS. In order to isolate smaller regions of interest (Task ROIs) from large suprathreshold clusters, we increased the cluster forming threshold to p < .001, k > 25 for activations, and p < .0001, k > 25 for deactivations.
Table 1.
Task-related activations and deactivations.
| Contrast | Region | Peak (MNI) |
Cluster p | Cluster k | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Task-related activations and deactivations across stimulus conditions | ||||||
| All participants Task-related Activation [Difficult (T1) > Easy (T0)] *Adjusted threshold: p < .001, k > 25 |
||||||
| R SPL/Precuneus extending to IOG | 32 | −66 | 38 | <.001 | 4252 | |
| L IOG | −44 | −72 | −8 | <.001 | 1575 | |
| L SPL/Precuneus | −44 | −40 | 50 | <.001 | 1523 | |
| R dorsal PM (dPM) | 26 | 2 | 54 | .002 | 265 | |
| L dPM | −24 | −6 | 52 | <.001 | 429 | |
| R vPM | 44 | 4 | 36 | .004 | 225 | |
| All participants Task-related Deactivation [Easy(T0) > Difficult(T1)] *Adjusted threshold: p < .0001, k > 25 |
||||||
| R S1 extending to PCC/Precuneus/R Lingual Gyrus | 20 | −34 | 62 | <.001 | 7851 | |
| L Amygdala | −28 | −2 | −22 | .002 | 136 | |
| L posterior Middle Temporal Gyrus (MTG) | −48 | −64 | 24 | <.001 | 503 | |
| L DLPFC | −16 | 44 | 32 | <.001 | 1010 | |
| R pINS | 52 | −10 | 4 | <.001 | 1090 | |
| L Parahippocampal Gyrus (PHG) | −22 | −32 | −16 | .005 | 108 | |
| R Cerebellum | 40 | −66 | −44 | <.001 | 354 | |
| L IFG | −54 | 32 | 2 | .004 | 117 | |
| R Middle Frontal Gyrus (MFG) | 20 | 24 | 42 | .001 | 148 | |
| R DLPFC | 16 | 52 | 34 | .02 | 75 | |
| mPFC | 2 | 60 | 8 | <.001 | 427 | |
| L Primary Motor (M1) | −40 | −12 | 32 | .003 | 125 | |
| L superior Frontal Gyrus(sFG)/lateral PFC | −22 | 62 | 14 | .002 | 142 | |
| L S1 | −24 | −32 | 60 | .01 | 82 | |
| L MTG | −64 | −20 | −14 | .03 | 56 | |
| L IFG/OFC | −28 | 30 | −16 | .01 | 83 | |
| subgenual ACC | 10 | 32 | −4 | .05 | 44 | |
| Controls > Patients Task-related Activation [Difficult (T1) > Easy (T0)] p < .005, k > 25 |
L vPM | −52 | 6 | 38 | .05 | 172 |
| Task-related activations and deactivations during no pain | ||||||
| All participants Task-related Activation (no pain) [Difficult (T1P0) > Easy (T0P0)] p < .005, k > 25 |
||||||
| R IOG extending to R SPL | 38 | −84 | −4 | <.001 | 3104 | |
| L IPL extending to L SPL | −42 | −46 | 60 | <.001 | 930 | |
| L dPM | −24 | −8 | 52 | .03 | 266 | |
| L IOG | −38 | −90 | 12 | <.001 | 932 | |
| R dPM | 24 | 2 | 50 | .1 | 140 | |
| All participants Task-related Deactivation (no pain) *Adjusted threshold: p <.001, k > 25 |
PCC/Precuneus/Cuneus | −2 | −20 | 42 | <.001 | 7944 |
| L PHG | −24 | −28 | −16 | .005 | 252 | |
| L sFG extending to mPFC/L DLPFC | −24 | 34 | 50 | <.001 | 2959 | |
| L posterior MTG | −48 | −60 | 22 | <.001 | 821 | |
| L IFG | −52 | 34 | −2 | .01 | 210 | |
| L middle Frontal Gyrus (mFG) | −40 | 14 | 46 | .02 | 166 | |
| R pINS | 44 | −30 | 16 | <.001 | 726 | |
| L superior Temporal Gyrus(STG) | −46 | 18 | −36 | .08 | 87 | |
| R Cerebellum | 40 | −66 | −46 | <.001 | 517 | |
| R posterior MTG | 52 | −70 | 30 | .1 | 71 | |
| R DLPFC | 16 | 50 | 20 | .07 | 93 | |
| R PHG | 20 | −30 | −10 | .07 | 94 | |
| L M1 | −44 | −10 | 30 | .09 | 80 | |
| R sFG | 20 | 32 | 46 | .04 | 117 | |
| Patients > Controls Task-related Activation (no pain) [Difficult(T1P0) > Easy(T0P0)] p < .005, k > 25 |
||||||
| L DLPFC | −34 | 30 | 46 | .05 | 206 | |
| L aMCC | −6 | 28 | 38 | .01 | 353 | |
| R Cerebellum | 24 | −80 | −40 | .03 | 234 | |
| R pINS | 42 | −12 | 4 | .14 | 102 | |
| L d sFG | −2 | 16 | 68 | .14 | 103 | |
Italicized ROIs are suprathreshold clusters that did not survive cluster-level correction, but are reported because they are marginally significant (p < .10) at the cluster-level threshold, or because they were regions of interest based on other analyses.
Fig. 2.
Task-related neural response. The task was a modified attention network test (ANT). A) Whole-brain contrast [difficult (T1) > easy (T0)] across all pain conditions (P0, P1, P2) and all participants (n = 28). Activation and deactivation clusters displayed at p < .001, cluster-level correction p < .05. B) Whole-brain contrast across all participants (n = 28) in the absence of pain [difficult (T1P0) > easy (T0P0)]. Activation and deactivation clusters displayed at p < .001, cluster-level correction p < .05. C) Patients > controls on relative task-related activation [difficult (T1P0) > easy (T0P0)], p < .005, k > 25 (R cerebellum not shown). D) Extracted activity from clusters depicted in (C) demonstrates that group difference in task-related activity is due to less deactivation among migraine patients, particularly in the absence of thermal pain. Average response across pain conditions (P1 and P2) is plotted against no pain (P0). Dorsal premotor cortex (dPM), posterior cingulate cortex (PCC), posterior insula (pINS), precuneus (PreCun), medial prefrontal cortex (mPFC), inferior occipital gyrus (IOG), dorsolateral prefrontal cortex (DLPFC), primary somatosensory cortex (S1), superior parietal lobule (SPL), anterior midcingulate cortex (aMCC).
3.2.1.2. Task-related activity in the absence of painful thermal stimuli
When the effect of task was examined only within the no pain condition, similar patterns of activation were seen in bilateral regions of the IOG, and PM cortices, as well as in the left inferior parietal lobule (IPL), and similar task-related deactivations were seen within midline regions, including the mPFC and PCC, as well as the R pINS (Table 1, Fig. 2B). Here, a more stringent threshold was used to isolate separable deactivation clusters (p < .001, k > 25).
3.2.2. Group differences in task-related activity
3.2.2.1. Task-related activity across pain conditions
Controls displayed greater activity within a left ventral region of the PM (vPM) cortex compared with patients (Table 1). Patients did not reveal any activity greater than controls that survived cluster-level correction (see Supplementary Table 1 for suprathreshold clusters that did not survive correction).
3.2.2.2. Task-related activity in the absence of pain stimuli
In the absence of the pain stimulus, migraine patients show less deactivation than controls within the L DLPFC as well as regions in the dorsal aMCC and cerebellum during the difficult relative to easy task (Group difference task ROIs, Table 1, Fig. 2C–D). The reverse contrast did not reveal any suprathreshold clusters. Patients who fell within the episodic migraine diagnostic criteria range were not outliers in this analysis (Supplementary Fig. 1), and the significant interaction between group and task-related deactivations remained when those with episodic migraine were excluded (L DLPFC: F(1,22) = 14.6, p = .001; aMCC: F(1,22) = 26.0, p < .001; R cerebellum: F(1,22) = 11.9, p = .002).
To ensure that this group difference was not driven by structural abnormalities, we extracted gray matter volume (GMV) from one of these regions – the DLPFC – and found no difference in mean GMV between migraine patients (M = .50, SE = .02) and controls (M = .49, SE = .02; t(26) = .55, p = .6). We also found that the task-related group difference in DLPFC deactivation remained after controlling for individual GMV (task difficulty × group interaction: F(1,25) = 17.74, p < .001). This group difference was also not affected by migraine diagnosis type.
3.2.2.3. Group differences in task-related ROIs
3.2.2.3.1. Task ROIs
Marginal group effects are reported in Supplementary Table 2.
3.2.2.3.2. Group difference task ROIs
A significant task–group interaction was found in the R aMCC group difference task ROI (defined as significant clusters from the patients > controls contrast in the absence of pain, Fig. 2C). Patients reveal a blunted pattern of responsiveness within the R aMCC such that they have decreased activation during the easy task, and decreased deactivation during the difficult task, compared to controls. However, the simple effect of group within task condition was not significant. Marginal effects are reported in Supplementary Table 2.
3.2.3. Task–pain interactions
3.2.3.1. Whole-brain task–pain interactions
3.2.3.1.1. Across participants
No activation or deactivation clusters exceeded the cluster-level corrected threshold in the full task–pain interaction.
3.2.3.1.2. Group differences
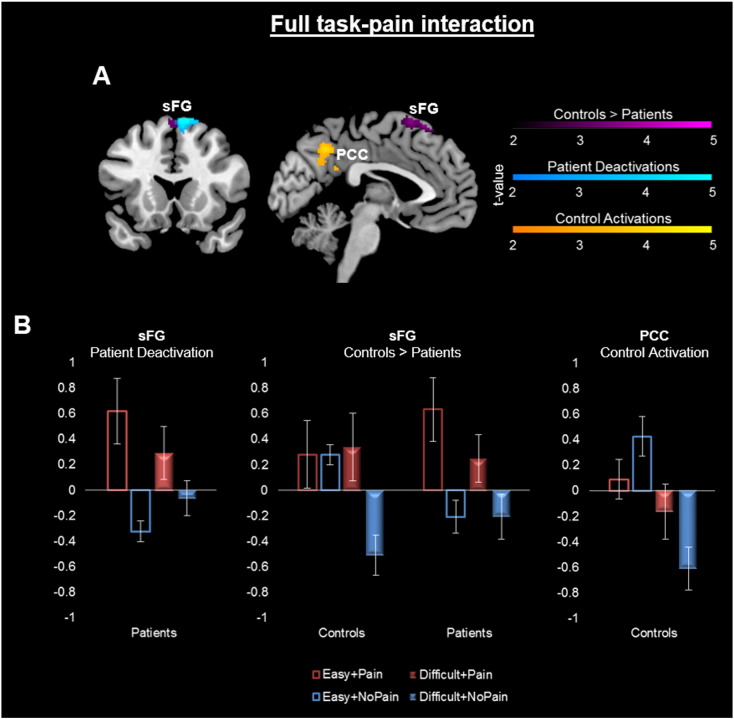
In the full task–pain interaction, controls showed greater activity within the dorsal superior frontal gyrus (sFG) compared to patients (Table 2, Fig. 3). Patients had no suprathreshold activity greater than controls.
Table 2.
Task–pain interactions.
| Contrast | Region | Peak (MNI) |
Cluster p | Cluster k | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Controls > Patients Activation |
||||||
| L medial sFG | −2 | 28 | 62 | .03 | 200 | |
| R Cerebellum | 24 | −82 | −44 | .05 | 149 | |
| Patients Deactivation |
||||||
| R medial sFG | 8 | 20 | 66 | .01 | 152 | |
| L Cerebellum | −22 | −46 | −14 | .02 | 130 | |
| L Lingual Gyrus | −14 | −62 | 0 | .03 | 110 | |
| Controls Activation |
||||||
| Precuneus/PCC | −4 | −56 | 44 | .007 | 387 | |
p < .005, cluster-level correction p < .05.
Fig. 3.
Full task–pain interaction A. Group differences in the whole-brain full task–pain interaction. [Task (difficult − easy) + pain] – [task (difficult − easy) + no pain]: [(T1P2 − T0P2) + (T1P1 − T0P1)] − 2[T1P0 − T0P0]. Clusters displayed at p < .005, cluster-corrected p < .05, for activations and deactivations. Purple clusters depict regions of greater interaction-related activity in controls relative to patients, blue clusters are regions of significant deactivation among patients alone, and yellow clusters are regions of significant activation among controls alone. Cerebellar and lingual clusters were also significant (Table 2), but not depicted in these slices. B. Activity extracted from clusters depicted in (A). Posterior cingulate cortex (PCC), superior frontal gyrus (sFG).
Within sample analyses showed that patients had significant task–pain interaction-related deactivations within the right dorsal medial sFG, which partially overlaps with the dorsal sFG identified by the two-sample analysis above. Among patients, activity within the primary somatosensory cortex (S1) was associated with the task–pain interactions, but this did not survive cluster-level correction (Supplementary Table 1). Among controls, activation within a precuneus/PCC cluster appears to best reflect the interaction. This suggests a different pattern of interaction-related activity between groups.
3.2.3.2. Whole-brain task-related deactivations during pain
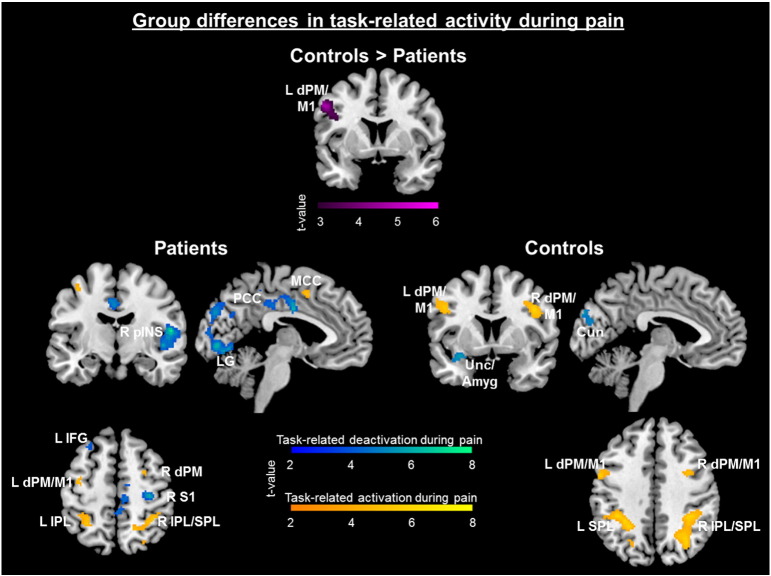
Across participants, task-related deactivations during pain [pain (mild + moderate) + easy task] > [pain (mild + moderate) + difficult task] were seen across regions associated with the sensory (e.g. R pINS, aMCC, S1), cognitive (e.g. mPFC, dorsal mPFC, L ventral lateral PFC), and affective (e.g. L amygdala, L anterior insula (aINS), L orbitofrontal cortex (OFC)) components of pain (Table 3), suggesting that this may reflect task-related reduction of pain activity. A more stringent threshold was used in this cross-participant analysis in order to isolate separable deactivation clusters (p < .001, k > 25 — for all but the two sample test). Patients revealed greater task-related deactivations during pain in the L posterior PM/primary motor (M1) cortex compared to controls (Table 3). Within group analyses suggest that this was largely due to greater activity among controls as seen in the reverse contrast within subjects (task-related activity while in pain) [pain (mild + moderate) + difficult task] > [pain (mild + moderate) + easy task]. Overall, more widespread reductions were observed among patients relative to controls (Fig. 4), whereas more robust task-related activity was seen among controls compared to patients.
Table 3.
Task-related brain response in the presence of pain.
| Contrast | Region | Peak (MNI) |
Cluster p | Cluster k | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Task-related deactivations during pain | ||||||
| All participants *Adjusted threshold: p < .001, k > 25 |
||||||
| Bilateral PCC/Cuneus/Precuneus | −10 | −90 | 22 | <.001 | 9787 | |
| R pINS/mINS/STG | 48 | −12 | −4 | <.001 | 1381 | |
| L Amygdala/aINS | −26 | 0 | −22 | <.001 | 384 | |
| L IFG/OFC | −28 | 30 | −16 | .01 | 146 | |
| L dmPFC | −10 | 48 | 40 | <.001 | 564 | |
| R dmPFC | 20 | 24 | 46 | .001 | 279 | |
| L caudal MTG | −44 | −64 | 24 | .008 | 157 | |
| L S1 | −22 | −30 | 56 | .05 | 79 | |
| R pINS | 30 | −24 | 22 | .05 | 80 | |
| R subgenual ACC | 14 | 42 | −4 | .02 | 112 | |
| L subgenual ACC | −4 | 34 | −4 | .03 | 105 | |
| mPFC | 2 | 60 | 8 | .01 | 146 | |
| L M1 | −54 | −4 | 16 | .05 | 74 | |
| Patients > Controls p < .005, k > 25 |
L posterior PM/M1 | −52 | 4 | 38 | .01 | 269 |
| Patients only *Adjusted threshold: p < .001, k > 25 |
||||||
| R M1/pINS | 56 | −6 | 10 | <.001 | 983 | |
| Bilateral posterior Lingual Gyrus | 20 | −76 | −4 | <.001 | 2923 | |
| L IFG | −46 | 26 | 6 | .001 | 158 | |
| R PHG | 20 | −40 | −6 | <.001 | 215 | |
| R subgenual ACC | 12 | 24 | −10 | .04 | 54 | |
| PCC | 0 | −26 | 34 | <.001 | 616 | |
| R M1/S1 | 32 | −22 | 52 | <.001 | 253 | |
| L Amygdala/uncus | −24 | 2 | −22 | .05 | 47 | |
| Precuneus/PCC | 0 | −40 | 50 | .04 | 49 | |
| L IFC/OFC | −30 | 26 | −22 | .05 | 47 | |
| R Precuneus | 6 | −54 | 34 | .04 | 53 | |
| Controls only *Adjusted threshold: p < .001, k > 25 |
L Cuneus | −10 | −94 | 30 | .001 | 231 |
| R Precuneus/Paracentral Lobule | 16 | −36 | 52 | .02 | 109 | |
| L Amygdala | −28 | −2 | −22 | .004 | 163 | |
| R PCC | 14 | −50 | 8 | .003 | 171 | |
| Task-related activations during pain | ||||||
| Patients only *Adjusted threshold: p < .001, k > 25 |
||||||
| R IPL/SPL | 36 | −44 | 52 | <.001 | 354 | |
| L IPL | −30 | −50 | 56 | .007 | 100 | |
| R dPM | 28 | −2 | 50 | .04 | 51 | |
| R mOG | 40 | −80 | 6 | .04 | 52 | |
| Controls only *Adjusted threshold: p < .001, k > 25 |
||||||
| R SPL/Precuneus/IPL | 16 | −68 | 60 | <.001 | 1274 | |
| R Cerebellum | 24 | −62 | −30 | .05 | 68 | |
| R dPM/M1 | 42 | 4 | 32 | .001 | 236 | |
| L Precuneus | −26 | −64 | 28 | .002 | 209 | |
| L SPL | −32 | −56 | 62 | <.001 | 723 | |
| R IOG | 44 | −74 | −6 | <.001 | 648 | |
| L dPM/M1 | −46 | 6 | 32 | .006 | 150 | |
| L IOG/MOG | −36 | −88 | 10 | <.001 | 556 | |
p < .005, cluster-level correction p < .05.
Fig. 4.
Task-related brain response in the presence of pain, shown for each group separately. The two sample comparison revealed that controls had greater task-related activations during pain within the LdPM/M1 compared to patients. Within group analyses revealed that patients had greater task-related deactivations [pain (mild + moderate) + easy task] > [pain (mild + moderate) + difficult task]: [(T0P1 + T0P2) − (T1P1 + T1P2)] shown in the cool colors, and controls had greater task-related activations [difficult task + no pain] > [difficult task + pain (mild + moderate)]: [2T1P0 − (T1P1 + T1P2)] shown in warm colors. Clusters displayed at p < .001 cluster-corrected p < .05. Several other clusters are also significant but not depicted here (Table 3). Amygdala (Amyg), cuneus (Cun), dorsal premotor cortex (dPM), inferior frontal gyrus (IFG), inferior parietal lobule (IPL), lingual gyrus (LG), midcingulate cortex (MCC), posterior cingulate cortex (PCC), posterior insula (pINS), primary motor cortex (M1), superior parietal lobule (SPL), uncus (Unc).
3.2.3.3. Whole-brain pain-related modulation of task-related activity
There were no significant pain-related reductions in task activity [difficult task + no pain] > [difficult task + pain (mild + moderate)].
3.2.3.4. Task–pain interactions within ROIs
3.2.3.4.1. Pain-related modulation of Task ROIs
3.2.3.4.1.1. Task ROIs
Effects of pain were seen across Task ROIs (defined as significant clusters from the difficult > easy contrast across participants and conditions, Fig. 2A). Inferential statistics are reported in Supplementary Table 2.
A main effect of pain was found in all task-related activation ROIs as well as some of the task-related deactivation clusters (R S1/PCC, R pINS, R DLPFC, L M1, L lateral PFC, L S1, L IFG/OFC), such that pain was associated with more activity than no pain.
A significant task–pain interaction was also found in the R pINS task-related deactivation ROI.
3.2.3.4.1.2. Group difference task ROIs
A main effect of pain was also found in all of the group difference task ROIs (defined as significant clusters from the patients > controls contrast in the absence of pain, Fig. 2C).
3.2.3.4.2. Task-related modulation of Pain ROIs
Pain ROIs were defined as significant clusters from the pain (P1 + P2) > no pain (P0) contrast across participants and conditions (Supplementary Fig. 2A). R pINS was the only pain ROI to show significant task-related modulation (Supplementary Table 2). A significant pain–task interaction was found in the R pINS, such that across participants there was deactivation during task (difficult compared to easy) in the no pain (t(27) = 2.4, p = .03) and moderate pain (t(27) = 4.0, p < .001) conditions, but no task difference in the mild pain condition (p > .10) (Supplementary Fig. 2B). A main effect of task was found in the R pINS such that the difficult task was related to less pain-related activity compared to the easy task. In the R thalamus, pain-related activity was greater during the difficult task than the easy task.
3.2.4. Task–pain–group interactions
A three way interaction was seen in two of the Group difference task ROIs (i.e., L DLPFC and R cerebellum). Controls show a greater decrease in activity within these regions in the difficult, relative to easy task compared to patients, particularly in the no pain condition (Fig. 2B). When we controlled for differences in pre-scan pain ratings, all of the task–pain–group interactions remained significant.
3.2.5. Pain catastrophizing, clinical pain, and task-related activity
Inferential statistics are reported in Supplementary Table 2, and correlations are plotted in Supplementary Fig. 3.
3.2.5.1. Task ROIs
Pain catastrophizing was not correlated with activity in Task ROIs across participants (patients and controls). Among patients, migraine pain intensity over the last month was positively correlated with activity in the R vPM task-ROI (Supplementary Fig. 3B), indicating that migraine pain intensity was associated with enhanced task-related activation in this cluster. Migraine frequency over the last month was positively correlated with three task deactivation ROIs (L middle temporal gyrus (MTG), L amygdala, L posterior MTG, Supplementary Fig. 3C), suggesting that increased migraine frequency was associated with abnormal task-related deactivations and may be associated with blunted deactivations observed among patients in task-related activity. Disease duration controlling for age was negatively correlated with the L S1 and L amygdala task deactivation ROIs, indicating that the longer patients have had migraines, the larger the magnitude of task-related deactivations in these regions (Supplementary Fig. 3D).
3.2.5.2. Group difference task ROIs
Pain catastrophizing, migraine frequency, migraine pain intensity, and disease duration controlling for age, were not correlated with task-related activity in the group difference task ROIs. When we did not control for age, disease duration was negatively correlated with the L DLPFC, such that the longer patients have had migraines, the more they deactivate this area during the difficult task (Supplementary Fig. 3E). Patients show a similar relationship between L DLPFC activity and age as they do with disease duration. However, controls show no such relationship with age (Supplementary Fig. 3F), suggesting that age may not fully explain the relationship between disease duration and task-related activity in L DLPFC among migraine patients.
4. Discussion
Here, we characterize brain activity associated with cognitive task performance and pain–cognition interactions among patients with migraine. Overall differences in cognitive networks primarily demonstrated that acute pain-related modulation of task-related deactivations present in controls is absent in migraine patients, and that migraine patients show reduced task-related deactivations and altered task–pain interaction activity patterns involving multiple brain areas compared to controls.
4.1. Task-related activity and task–pain interactions across participants
Robust task-related activations in task-positive (visual, sensorimotor, and executive) networks and deactivations in regions of the default mode network were observed across migraine patients and healthy controls. Task difficulty increased activity within regions previously shown to increase as a function of cognitive load (Owen et al., 2005; Seminowicz & Davis, 2007a). Also consistent with prior literature, greater task difficulty was associated with greater deactivation of midline regions of the default mode network, including the mPFC and PCC (Buckner et al., 2008). Additionally, the difficult task was associated with less activity within the pINS. This overall pattern of activations and deactivations was similar in the presence or absence of acute thermal pain.
Across participants, a significant pain–task interaction was found in the R pINS, such that task difficulty was associated with R pINS deactivation, and pain intensity was associated with R pINS activation. This was true whether we defined the R pINS region based on the pain contrast (i.e., task-related modulation of pain activity) or based on the task contrast (i.e., pain-related modulation of cognitive activity). This interaction pattern within the R pINS has been demonstrated previously in healthy controls (Seminowicz & Davis, 2007a), and suggests that the pINS may be an important site of task–pain integrations.
While no full interaction [task (difficult − easy) + pain] − [task (difficult − easy) + no pain] clusters survived in the whole-brain analysis, task-related reduction during pain [pain (mild + moderate) + easy task] > [pain (mild + moderate) + difficult task] was observed at the level of the whole-brain analysis across brain regions associated with the sensory, cognitive, and affective components of pain. Examination of task-related reduction in pain-related regions of interest revealed significant reduction in R pINS, which is consistent with prior research (Bantick et al., 2002; Frankenstein et al., 2001; Petrovic et al., 2000; Peyron et al., 1999; Seminowicz & Davis, 2007a; Shackman et al., 2011; Wiech et al., 2005; Wiech et al., 2008), and may reflect cognitive modulation of pain. However, pain intensity and unpleasantness ratings were not significantly attenuated by task-difficulty. Activity within a region of the thalamus identified in the pain contrast was increased during the difficult task, demonstrating an opposite pattern than was observed in the R pINS and MCC. The present findings are consistent with prior studies that have found posterior and mid-thalamic activity to be positively associated with pain modulation, displaying increased activation in response to concomitant pain stimulus and cognitive task (Peyron et al., 1999; Valet et al., 2004).
Pain-related modulation of cognitive activity was not observed at the level of whole-brain analyses. This is consistent with a previous study in healthy controls that reported no pain-related modulation of cognitive activity when univariate analyses were used (Seminowicz & Davis, 2007a). However, pain was associated with greater task-related activations and decreased task-related deactivations across most cognitive regions of interest, consistent with previous findings (Seminowicz & Davis, 2007b). As both pain and cognitive tasks engage cognitive processes (Eccleston & Crombez, 1999), it is possible that this increase is due to the increasing attentional demands when both the difficult task and pain are present. This is supported by evidence that increased cognitive load is often associated with greater task-related deactivation (Mckiernan et al., 2003). Thus, the current findings generally support previous work on brain activity associated with pain–cognition interactions.
4.2. Abnormal task-related activity and task–pain interactions in migraine patients
Overall, aberrant task-related activity among migraine patients emerged as blunted task-related activations and deactivations. Across conditions, patients revealed a blunted task-related response within cognitive regions of interest as well as at the whole brain level of analysis.
During task performance in the absence of pain, migraine patients revealed blunted task-related deactivation in the L DLPFC, aMCC, and cerebellum compared to controls. Analysis of activity within these clusters across conditions revealed that while controls show greater deactivation in these regions during the no pain condition, patients show no significant difference in activity within these regions across the pain and no pain conditions, suggesting that effects of acute pain on reducing task-related deactivation are absent among patients. In other words, patients show a pattern of task-related activity within these regions during no pain that is similar to activity in the presence of an external pain stimulus. These regions, therefore, may be important sites of alteration in cognitive processing in migraine.
Importantly, taken together with prior research, these results suggest that the DLPFC, in particular, may be an important site of alteration in chronic pain. Prior work also found decreased L DLPFC task-related deactivations among chronic low back patients, relative to healthy controls, in the absence of a pain stimulus (Seminowicz et al., 2011). Importantly, effective pain treatment was shown to normalize L DLPFC activity, such that deactivations became stronger and more like those seen among healthy controls (Seminowicz et al., 2011). Furthermore, decreased DLPFC cortical thickness has been demonstrated within other pain populations (Apkarian et al., 2004b; Seminowicz et al., 2011), and is also partially reversible with cognitive interventions that reduce pain and pain catastrophizing (Seminowicz et al., 2013). The blunted deactivation in L DLPFC task-related activity found here may reflect relatively constant engagement of the DLPFC across conditions – and perhaps chronically – among migraine patients. DLPFC is involved in pain modulation (Lorenz et al., 2003), and among chronic pain populations may be chronically upregulated to increase descending modulation of pain (Seminowicz et al., 2013).
Patients also reveal different patterns of activity underlying task–pain interactions. PCC activity was associated with the full task–pain interaction among controls, whereas deactivations in the dorsal sFG were associated with interactions among patients. To further probe this interaction, we examined whole-brain task-related activations and deactivations during pain, among patients and controls. Patients revealed greater and more widespread task-related deactivations within regions associated with pain processing, but less task-related activations in task-positive regions during pain, compared to controls. Taken together, this suggests that cognitive effects may be more related to pain reduction in patients, and task performance in controls, and that the presence of pain appears to be disturbing cognitive processing to a greater extent in patients than it does in healthy controls. Among healthy populations, attention to a task is associated with both cognitive task-specific and anti-nociceptive activations (Kucyi et al., 2013). However, among migraine patients, allocation of cognitive resources to pain suppression may be prioritized over task-specific processes.
4.3. Modulation by pain catastrophizing and disease severity
Migraine pain severity was associated with enhanced R vPM task-related activity. Disease duration was associated with greater L S1 and L amygdala task-related deactivation, even after controlling for patient age, such that the longer patients have had migraines, the more task-related deactivation is seen in the L S1 and L amygdala. A plausible interpretation of this result is that patients have higher baseline levels of activity within these regions, resulting in greater task-related decreases, and this baseline activity increases with disease duration. Migraine frequency was associated with blunted deactivation in the L MTG, L amygdala, and L pMTG. This suggests that increased migraine frequency may be leading to the abnormal task-related deactivations seen among migraine patients, and is consistent with a previous finding that cognitive impairment in migraine was related to migraine frequency (Calandre et al., 2002). Pain catastrophizing was not significantly associated with task-related activity or pain–task interactions.
4.4. Limitations and considerations for future research
The limitations of the current study include not strictly controlling the level of ongoing headache pain during scanning, the small sample size, and the inclusion of four patients with high frequency episodic migraine. Studies with larger samples powered to examine differences between clinical subtypes of migraine, including episodic migraine and chronic migraine, and the potential effects of medication overuse (Grazzi et al., 2010), will be informative. Future research should also examine the effects of migraine on cognitive networks over time, as symptom severity changes, as well as potential treatment effects on these alterations.
4.5. Conclusions
Taken together, these findings suggest that migraine patients have blunted cognitive-related neural activity in brain regions associated with cognitive processing and altered patterns of activity related to pain–cognition interactions. Specifically, healthy controls have strong task-related deactivations in the DLPFC, aMCC, and cerebellum that are decreased with acute pain, but patients have blunted task-related deactivations that are not modulated by acute pain. While no behavioral cognitive decrements were observed, blunted neural response may be reflective of broader functional restructuring as has been shown in other chronic pain populations (Baliki et al., 2008; Seminowicz et al., 2011), as well as altered pain regulatory processes. These changes may be related to migraine pain frequency, but were not associated with pain catastrophizing or migraine pain intensity in this sample. Furthermore, differential activity underlying pain–cognition interactions suggests that allocation of cognitive resources to pain suppression may be prioritized over task-specific processes in migraine.
Acknowledgments
We would like to thank the University of Maryland Magnetic Resonance Research Center, Dr. Rao Gullapalli, and Mr. George Makris for their contributions to data collection. We also thank Janell Payano Sosa for the helpful suggestions on the manuscript. This research was supported by funds from NIH/NCCAM 5R01AT007176 and the Department of Neural and Pain Sciences, University of Maryland School of Dentistry to Dr. Seminowicz, and NIH/NCRR1KL2RR025006-01 and the Society of General Internal Medicine Founders Award to Dr. Goyal. Dr. Mathur was supported by the NIH Ruth L. Kirschstein National Research Service Award T32 NS070201. All authors declare that they have no conflicts of interest.
Footnotes
Supplementary results can be found online at http://dx.doi.org/10.1016/j.nicl.2015.01.003.
Appendix A. Supplementary results
Supplementary material.
References
- Anttila V., Winsvold B.S., Gormley P., Kurth T., Bettella F., McMahon G., Kallela M., Malik R., de Vries B., Terwindt G., Medland S.E., Todt U., McArdle W.L., Quaye L., Koiranen M., Ikram M.A., Lehtimäki T., Stam A.H., Ligthart L., Wedenoja J. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat. Genet. 2013;45(8):912–917. doi: 10.1038/ng.2676. 23793025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Krauss B.R., Thomas P.S., Fredrickson B.E., Levy R.E., Harden R.N., Chialvo D.R. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1–2):129–136. doi: 10.1016/j.pain.2003.12.015. 15109516 [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Sonty S., Levy R.M., Harden R.N., Parrish T.B., Gitelman D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. 15548656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. 18256259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick S.J., Wise R.G., Ploghaus A., Clare S., Smith S.M., Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(2):310–319. doi: 10.1093/brain/awf022. 11844731 [DOI] [PubMed] [Google Scholar]
- Blumenfeld A.M., Varon S.F., Wilcox T.K., Buse D.C., Kawata A.K., Manack A., Goadsby P.J., Lipton R.B. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31(3):301–315. doi: 10.1177/0333102410381145. 20813784 [DOI] [PubMed] [Google Scholar]
- Borsook D., Maleki N., Becerra L., McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73(2):219–234. doi: 10.1016/j.neuron.2012.01.001. 22284178 [DOI] [PubMed] [Google Scholar]
- Boyer N., Dallel R., Artola A., Monconduit L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain. 2014;155(7):1196–1205. doi: 10.1016/j.pain.2014.03.001. 24631586 [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2) [Abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2-6, 2002, Sendai, Japan. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. 18400922 [DOI] [PubMed] [Google Scholar]
- Buse D.C., Manack A., Serrano D., Turkel C., Lipton R.B. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J. Neurol. Neurosurg. Psychiatry. 2010;81(4):428–432. doi: 10.1136/jnnp.2009.192492. 20164501 [DOI] [PubMed] [Google Scholar]
- Calandre E.P., Bembibre J., Arnedo M.L., Becerra D. Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia. 2002;22(4):291–302. doi: 10.1046/j.1468-2982.2002.00370.x. 12100092 [DOI] [PubMed] [Google Scholar]
- De Araújo C.M., Barbosa I.G., Lemos S.M.A., Domingues R.B., Teixeira A.L. Cognitive impairment in migraine. A systematic review. Dementia & Neuropsychologia. 2012;6(2):74–79. doi: 10.1590/S1980-57642012DN06020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tommaso M., Valeriani M., Guido M., Libro G., Specchio L.M., Tonali P., Puca F. Abnormal brain processing of cutaneous pain in patients with chronic migraine. Pain. 2003;101(1–2):25–32. doi: 10.1016/s0304-3959(02)00299-3. 12507697 [DOI] [PubMed] [Google Scholar]
- Eccleston C., Crombez G. Pain demands attention: a cognitive–affective model of the interruptive function of pain. Psychol. Bull. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. 10349356 [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. 11970796 [DOI] [PubMed] [Google Scholar]
- Farmer M.A., Baliki M.N., Apkarian A.V. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012;520(2):197–203. doi: 10.1016/j.neulet.2012.05.001. 22579823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenstein U.N., Richter W., McIntyre M.C., Rémy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. NeuroImage. 2001;14(4):827–836. doi: 10.1006/nimg.2001.0883. 11554801 [DOI] [PubMed] [Google Scholar]
- Goadsby P.J., Lipton R.B., Ferrari M.D. Migraine — current understanding and treatment. N. Engl. J. Med. 2002;346(4):257–270. doi: 10.1056/NEJMra010917. 11807151 [DOI] [PubMed] [Google Scholar]
- Grazzi L., Chiapparini L., Ferraro S., Usai S., Andrasik F., Mandelli M.L., Bruzzone M.G., Bussone G. Chronic migraine with medication overuse pre–post withdrawal of symptomatic medication: clinical results and fMRI correlations. Headache. 2010;50(6):998–1004. doi: 10.1111/j.1526-4610.2010.01695.x. [DOI] [PubMed] [Google Scholar]
- Hamedani A.G., Rose K.M., Peterlin B.L., Mosley T.H., Coker L.H., Jack C.R., Knopman D.S., Alonso A., Gottesman R.F. Migraine and white matter hyperintensities: the ARIC MRI study. Neurology. 2013;81(15):1308–1313. doi: 10.1212/WNL.0b013e3182a8235b. 23975874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinger H.J., Semenchuk E.M., O'Brien W.H. Appraisal and coping responses to pain and stress in migraine headache sufferers. J. Behav. Med. 1999;22(4):327–340. doi: 10.1023/a:1018722002393. 10495966 [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl. 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. 14979299 [DOI] [PubMed] [Google Scholar]
- Hunter L., Peterson J., Cortez M., Theriot J., Brennan K.C. Characterization of photosensitivity and pupil oscillation rate in migraine. Neurology. 2014;82(10 Suppl.):I9–I12. [Google Scholar]
- Katsarava Z., Buse D.C., Manack A.N., Lipton R.B. Defining the differences between episodic migraine and chronic migraine. Curr. Pain Headache Rep. 2012;16(1):86–92. doi: 10.1007/s11916-011-0233-z. 22083262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A., Salomons T.V., Davis K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. 24167282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth T., Tzourio C. Migraine and cerebral infarct-like lesions on MRI: an observation, not a disease. JAMA. 2009;301(24):2594–2595. doi: 10.1001/jama.2009.933. 19549979 [DOI] [PubMed] [Google Scholar]
- Lakhan S.E., Avramut M., Tepper S.J. Structural and functional neuroimaging in migraine: insights from 3 decades of research. Headache. 2013;53(1):46–66. doi: 10.1111/j.1526-4610.2012.02274.x. 23094683 [DOI] [PubMed] [Google Scholar]
- Lorenz J., Minoshima S., Casey K.L. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(5):1079–1091. doi: 10.1093/brain/awg102. 12690048 [DOI] [PubMed] [Google Scholar]
- McCracken L.M., Iverson G.L. Predicting complaints of impaired cognitive functioning in patients with chronic pain. J. Pain Symptom Manag. 2001;21(5):392–396. doi: 10.1016/s0885-3924(01)00267-6. 11369160 [DOI] [PubMed] [Google Scholar]
- Mckiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. 12729491 [DOI] [PubMed] [Google Scholar]
- Noseda R., Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl. 1):S44–S53. doi: 10.1016/j.pain.2013.07.021. 23891892 [DOI] [PubMed] [Google Scholar]
- Olesen J., Steiner T.J. The international classification of headache disorders, 2nd edn (ICDH-II) J. Neurol. Neurosurg. Psychiatry. 2004;75(6):808–811. doi: 10.1136/jnnp.2003.031286. 15145989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. 15846822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paemeleire K. Brain lesions and cerebral functional impairment in migraine patients. J. Neurol. Sci. 2009;283(1–2):134–136. doi: 10.1016/j.jns.2009.02.333. 19268310 [DOI] [PubMed] [Google Scholar]
- Pearson A.J., Chronicle E.P., Maylor E.A., Bruce L.A. Cognitive function is not impaired in people with a long history of migraine: a blinded study. Cephalalgia. 2006;26(1):74–80. doi: 10.1111/j.1468-2982.2005.01001.x. 16396669 [DOI] [PubMed] [Google Scholar]
- Petrovic P., Petersson K.M., Ghatan P.H., Stone-Elander S., Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85(1–2):19–30. doi: 10.1016/s0304-3959(99)00232-8. 10692599 [DOI] [PubMed] [Google Scholar]
- Peyron R., García-Larrea L., Grégoire M.C., Costes N., Convers P., Lavenne F., Mauguière F., Michel D., Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(9):1765–1780. doi: 10.1093/brain/122.9.1765. 10468515 [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Moskowitz M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. 23190076 [DOI] [PubMed] [Google Scholar]
- Rist P.M., Kurth T. Migraine and cognitive decline: a topical review. Headache. 2013;53(4):589–598. doi: 10.1111/head.12046. 23405909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N., Arkink E.B., Mulder M., Rubia K., Admiraal-Behloul F., Schoonman G.G., Kruit M.C., Ferrari M.D., van Buchem M.A. Frontal lobe structure and executive function in migraine patients. Neurosci. Lett. 2008;440(2):92–96. doi: 10.1016/j.neulet.2008.05.033. 18556120 [DOI] [PubMed] [Google Scholar]
- Schwedt T.J., Chong C.D., Chiang C.C., Baxter L., Schlaggar B.L., Dodick D.W. Enhanced pain-induced activity of pain-processing regions in a case–control study of episodic migraine. Cephalalgia. 2014 doi: 10.1177/0333102414526069. 24627432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Schlaggar B.L., Mar S., Nolan T., Coalson R.S., Nardos B., Benzinger T., Larson-Prior L.J. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53(5):737–751. doi: 10.1111/head.12081. 23551164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D.A., Davis K.D. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb. Cortex. 2007;17(6):1412–1422. doi: 10.1093/cercor/bhl052. 16908493 [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Davis K.D. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J. Neurophysiol. 2007;97(5):3651–3659. doi: 10.1152/jn.01210.2006. 17314240 [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Shpaner M., Keaser M.L., Krauthamer G.M., Mantegna J., Dumas J.A., Newhouse P.A., Filippi C.G., Keefe F.J., Naylor M.R. Cognitive–behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain. 2013;14(12):1573–1584. doi: 10.1016/j.jpain.2013.07.020. 24135432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., Jarzem P., Bushnell M.C., Shir Y., Ouellet J.A., Stone L.S. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. 21593339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. 21331082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein S.D., Lipton R.B., Sliwinski M. Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurol. 1996;47(4):871–875. doi: 10.1212/wnl.47.4.871. 8857711 [DOI] [PubMed] [Google Scholar]
- Sprenger T., Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr. Opin. Neurol. 2012;25(3):252–262. doi: 10.1097/WCO.0b013e3283532ca3. 22487570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W.F., Lipton R.B., Celentano D.D., Reed M.L. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64–69. 1727198 [PubMed] [Google Scholar]
- Suhr J.A., Seng E.K. Neuropsychological functioning in migraine: clinical and research implications. Cephalalgia. 2012;32(1):39–54. doi: 10.1177/0333102411430265. 22174355 [DOI] [PubMed] [Google Scholar]
- Sullivan M.J.L., Bishop S.R., Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess. 1995;7(4):524–532. [Google Scholar]
- Swartz R.H., Kern R.Z. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch. Neurol. 2004;61(9):1366–1368. doi: 10.1001/archneur.61.9.1366. 15364681 [DOI] [PubMed] [Google Scholar]
- Solberg Nes L., Roach A.R., Segerstrom S.C. Executive functions, self-regulation, and chronic pain: a review. Ann. Behav. Med. 2009;37(2):173–183. doi: 10.1007/s12160-009-9096-5. 19357933 [DOI] [PubMed] [Google Scholar]
- Valet M., Sprenger T., Boecker H., Willoch F., Rummeny E., Conrad B., Erhard P., Tolle T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain — an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. 15157701 [DOI] [PubMed] [Google Scholar]
- Waldie K.E., Hausmann M., Milne B.J., Poulton R. Migraine and cognitive function: a life-course study. Neurology. 2002;59(6):904–908. doi: 10.1212/wnl.59.6.904. 12297575 [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I., Moayedi M., Tenenbaum H.C., Goldberg M.B., Freeman B.V., Davis K.D. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152(2):384–396. doi: 10.1016/j.pain.2010.10.046. 21167644 [DOI] [PubMed] [Google Scholar]
- Wiech K., Ploner M., Tracey I. Neurocognitive aspects of pain perception. Trends Cogn. Sci. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. 18606561 [DOI] [PubMed] [Google Scholar]
- Wiech K., Seymour B., Kalisch R., Stephan E., Koltzenburg K., Driver M., Dolan R.J. Modulation of pain processing in hyperalgesia by cognitive demand. NeuroImage. 2005;27(1):59–69. doi: 10.1016/j.neuroimage.2005.03.044. 15978845 [DOI] [PubMed] [Google Scholar]
- Xue T., Yuan K., Zhao L., Yu D., Zhao L., Dong T., Cheng P., von Deneen K.M., Qin W., Tian J. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLOS ONE. 2012;7(12):e52927. doi: 10.1371/journal.pone.0052927. 23285228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M.S., Manack A., Schramm S., Fritsche G., Obermann M., Diener H.C., Moebus S., Katsarava Z. Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German Headache Consortium Study. Pain. 2013;154(3):484–492. doi: 10.1016/j.pain.2012.12.010. 23375162 [DOI] [PubMed] [Google Scholar]
- Zeitlin C., Oddy M. Cognitive impairment in patients with severe migraine. Br. J. Clin. Psychol. 1984;23(1):27–35. doi: 10.1111/j.2044-8260.1984.tb00623.x. 6697026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.