Abstract
Source memory, the ability to identify the context in which a memory occurred, is impaired in schizophrenia and has been related to clinical symptoms such as hallucinations. The neurobiological underpinnings of this deficit are not well understood. Twenty-five patients with recent onset schizophrenia (within the first 4.5 years of treatment) and twenty-four healthy controls completed a source memory task. Participants navigated through a 3D virtual city, and had 20 encounters of an object with a person at a place. Functional magnetic resonance imaging was performed during a subsequent forced-choice recognition test. Two objects were presented and participants were asked to either identify which object was seen (new vs. old object recognition), or identify which of the two old objects was associated with either the person or the place being presented (source memory recognition). Source memory was examined by contrasting person or place with object. Both patients and controls demonstrated significant neural activity to source memory relative to object memory, though activity in controls was much more widespread. Group differences were observed in several regions, including the medial parietal and cingulate cortex, lateral frontal lobes and right superior temporal gyrus. Patients with schizophrenia did not differentiate between source and object memory in these regions. Positive correlations with hallucination proneness were observed in the left frontal and right middle temporal cortices and cerebellum. Patients with schizophrenia have a deficit in the neural circuits which facilitate source memory, which may underlie both the deficits in this domain and be related to auditory hallucinations.
Keywords: Source memory, Schizophrenia, Associative memory, First episode, Virtual reality, Hallucinations
1. Introduction
Patients with schizophrenia (SCZ) exhibit a wide variety of cognitive deficits, particularly with memory (Barch and Ceaser, 2012; Ranganath et al., 2008). These deficits are predictive of overall functional outcome and clinical remission (Bodnar et al., 2008; Green, 2006; Green et al., 2004; Kahn and Keefe, 2013), and it has recently been suggested that schizophrenia should be viewed primarily as a cognitive disorder (Kahn and Keefe, 2013). Understanding the nature of the memory deficits is therefore a critical goal when moving towards improved clinical interventions in SCZ.
When considering episodic memory, one area in which patients with SCZ have a substantial deficit is with source monitoring (identifying the context in which a stimulus was encountered; Johnson et al., 1993). For successful source monitoring it is necessary to bind elements of a memory together into a memory trace, along with their context (spatial context, temporal context, etc.). Patients demonstrate source monitoring deficits even when stimulus recognition is preserved (Danion et al., 1999; Vinogradov et al., 1997). This deficit in source monitoring is in many ways not surprising given that patients with SCZ also demonstrate difficulties with relational or associative memory (binding items together during memory encoding, and later recalling which stimuli were presented together), while object memory is not as severely impaired (Achim et al., 2007; Luck et al., 2009). Most typically, source monitoring problems in SCZ are considered in the context of attributing events from internal (self) to external sources. Patients with predominant hallucinations and thought disorder have a greater tendency or bias to attribute internally generated events to an external source, while still being able to correctly identify externally generated stimuli (Brunelin et al., 2006; Keefe et al., 2002). This bias for SCZ to misattribute internal sources has been observed in the absence of recognition memory deficits or false positive responses (Fisher et al., 2008). Interestingly, in a repetitive magnetic stimulation trial of low frequency (1 Hz, inhibitory) stimulation to the left temporal parietal junction over 5 consecutive days not only improved auditory hallucinations but also resulted in an improvement in source monitoring compared to sham stimulation (Brunelin et al., 2006). The improvement in auditory hallucination was marginally correlated with the improvement in source monitoring, further suggesting the relationship between source monitoring and hallucinations.
Patients with schizophrenia have also been found to have a deficit in source memory (remembering the context in which experimental stimuli occurred; Brebion et al., 2002; Brebion et al., 2012; Diaz-Asper et al., 2008; Waters et al., 2004). The deficit in source memory has been related to deficits in binding contextual cues together into a holistic memory representation (Diaz-Asper et al., 2008; Waters et al., 2004), which is an essential component of source memory. Source memory errors are present in SCZ even for short-term recognition (when source information is tested immediately, minimizing the need to recollect information stored in long-term memory), suggesting that source memory deficits in SCZ may be related to encoding errors rather than problems in recognition (Achim et al., 2011). Brebion et al. (2009) performed a list learning task in SCZ and found that patients who hallucinate had more intrusions (indicating a word was part of the memory set when it was not) than non-hallucinating patients. This finding was related to source misattribution (patients attributing an internally generated stimulus to an external source, the original memory set). Overall, SCZ appears to have a noteworthy deficit in source memory which is likely intricately related to other memory and cognitive processes, such as contextual binding and episodic memory.
Within healthy controls, source memory involves a range of cortical regions known to be involved in episodic memory, including the medial temporal lobes, prefrontal cortex, and parietal cortex. Increased hippocampal activity has been related to trials in which the source was correctly identified (Davachi et al., 2003; Ranganath et al., 2004), probably due to the role of the hippocampus in relational binding (Davachi, 2006). One of the first fMRI studies to examine source memory found greater left prefrontal activity for source memory and for old–new recognition (Nolde et al., 1998), with subsequent studies finding activity in the left lateral prefrontal cortex associated with source memory for a variety of stimuli types (Mitchell and Johnson, 2009). Prefrontal lesions have been found to disrupt the self-initiation of processes which promote feature binding (Stuss and Benson, 1986), and left prefrontal damage is associated with deficits in source monitoring (Duarte et al., 2005). Prefrontal activity during source recognition may be more involved in the evaluation of source information (e.g. “does this stimuli fit with source X”) rather than retrieving source information per se (Mitchell et al., 2004). Activity in medial parietal areas (intraparietal sulcus and precuneus) has been suggested to be present regardless of the type of source information being assessed (Uncapher et al., 2006), while activity in lateral parietal areas may be more dependent on how well the information has been encoded (Wheeler and Buckner, 2004) and/or to attentional processes (Cabeza, 2008). Examining declines in source memory with age has proven fruitful for examining structural and functional correlates of source memory, with evidence that age-related decline in source memory is related to decreased activity mainly in the prefrontal and medial temporal lobes (see Mitchell and Johnson, 2009, for review).
Few studies have examined the neural correlates of source monitoring in SCZ. Ragland et al. (2006) examined source monitoring in SCZ using a level-of-processing framework. Patients were presented words with either deep (semantic) or shallow (orthographic) encoding instructions. During recognition, when participants successfully identified a previously encountered stimuli they were asked under which encoding condition the word was encountered (the source memory aspect being recalling the context in which the word was encountered, in this case, which encoding condition). When contrasting correct vs. incorrect source, both patients and controls activated areas of prefrontal and parietal cortices. Patients showed activity in the middle and superior temporal gyrus, thalamus, and parahippocampal gyrus, which was correlated with more severe positive and negative symptoms independent of memory performance. Other neuroimaging studies of source monitoring in SCZ have focused on deficits related to attributing stimuli as self-generated or externally-generated (reality monitoring). Deficits in reality monitoring appear to involve the medial prefrontal cortex (Subramaniam et al., 2012; Vinogradov et al., 2008; Wang et al., 2011). Following computerized training to improve source monitoring, activity was found to be increased in the medial prefrontal cortex (Subramaniam et al., 2012).
The purpose of this study was to examine the neural correlates of source memory in schizophrenia. While most previous studies of source memory (in both controls and schizophrenia) have used less ecologically valid task (e.g. identifying the color of the stimuli during encoding), we utilized a paradigm involving encounters (with a person and an object in a specific place) within a realistic 3D environment (Burgess et al., 2001; King et al., 2005), which may better evaluate source memory networks used in everyday life. Source memory was then examined during a recognition test inside the MRI. We examined participants with early schizophrenia (within the first 4.5 years of treatment, with 75% of patients within the first 2 years) thus minimizing potential confounds associated with prolonged illness such as cognitive decline, social isolation and long-term medication effects. During fMRI scanning a source recognition memory task was employed, which was contrasted with an object memory task. By directly comparing source memory to object memory, we can identify regions in the cortex which are specific to source memory compared to object memory and determine if any deficits observed in schizophrenia are source-memory specific. We hypothesized that patients would show deficits in source memory relative to object memory and may show compensatory activation in source memory retrieval contrasts. Furthermore, as deficits in source memory have been associated with auditory hallucinations (Woodward et al., 2007) we expected to observe relationships between hallucinations and the neural activity of source memory retrieval.
2. Methods
2.1. Participants
All participants with SCZ were treated at the Douglas Mental Health University Institute in Montreal, Canada, at the Prevention and Early Intervention Program for Psychoses, a specialized service providing treatment to individuals aged 14–35 years from a local catchment area. Individuals with an IQ > 70 who had not taken antipsychotic medication for more than 1 month were consecutively admitted as in- or out-patients. Patients were assessed with the Scale for Assessment of Positive Symptoms (SAPS) (Andreasen, 1984) and the Scale for Assessment of Negative Symptoms (SANS) (Andreasen, 1983) at numerous time-points following clinic admission (baseline; at 1, 2, 3, 6, 9, and 12 months; and every 6 months thereafter). See Malla et al. (2003) or visit http://www.douglas.qc.ca/pages/view?section_id=165 for more details.
For the neuroimaging study, only individuals aged 18–30 years with no previous history of neurological disease, head trauma causing loss of consciousness, or lifetime diagnosis of substance dependence were eligible. Twenty-five people with schizophrenia spectrum disorders were recruited, diagnosed according to the Structured Clinical Interview for DSM-IV (First et al., 1997) and confirmed between two senior research psychiatrists (A.M. and R.J.). Twenty-four healthy controls were recruited through advertisements in local newspapers and were included only if they had no current or previous history of any Axis I disorders, neurological diseases, or head trauma causing loss of consciousness, and no first-degree family members with schizophrenia or related schizophrenia-spectrum disorders. All patients provided written informed consent, and the study was approved by the research ethics boards of the Douglas Hospital Research Centre and the Montreal Neurological Institute. One patient and one control were subsequently removed due to excessive movement during scanning.
2.2. Experimental task
Participants performed an encoding task (outside the MRI) using a modified version of the virtual city developed by Burgess and colleagues (Burgess et al., 2001; King et al., 2005), created using 3Ds Max (3Ds Max, 2011) and Unity software (Unity, 2011). Scenes were displayed using Unity web player. Participants navigated through a 3D virtual city, following a path indicated by green arrows to the site of an encounter (an encoding trial, a character at a location). After approaching within five virtual meters of the character, the participant's view was frozen and the character stepped aside, and a life-sized object appeared on a small table displayed to the right. The object appeared to glow to enhance its visibility. Participants were instructed to pay careful attention to the object, character and location, and try to remember for a later memory test. After a 5 s study delay the person and object disappeared, and participants then followed the arrows to the next encounter. There were a total of 20 encounters, each with a unique person, location and object.
A recognition task was then performed inside the MRI scanner. A total of 80 recognition trials were administered, in which participants were shown an image consisting of a typical viewpoint encountered within the virtual city (in one of 20 places where encounters occurred), with a person in the center of the screen, and two objects, one on each side of the person. Participants were then asked one of four possible recognition questions: (1) Person (which object was paired with this person), (2) Place (which object did you view in this location), (3) Object (which object was viewed in the city; the other object was new), and (4) Bright (which object is brighter in appearance). Participants responded on an MRI-compatible button box to indicate which item (left or right) was selected. For the Person condition, the place was not associated with either object, while in the Place condition the person was not associated with either object. The Person and Place conditions were designed to access source memory (in what context was an object encountered) while the Object condition assesses object memory (old vs. new). Including two source memory conditions allows us to better understand if the observed activity is modality specific. The bright condition was not considered in this analysis as we were specifically interested in source vs. object memory. Images (with encoding question) were presented on the screen for 8000 ms, with a 2000–8000 ms ISI (in 100 ms increments), with an average trial length of 13 s. Stimuli were presented and results were recorded through E-Prime 1.0 software. The virtual city and examples of a recognition trial are shown in Fig. 1.
Fig. 1.
(A) Aerial view showing the layout of the virtual city, with green dashes indicating the path participants followed for the study. (B) An example of an encounter with a character paired with an object at a location during encoding (prior to fMRI scanning). (C) An example of a recognition trial from the “Person” condition during fMRI scanning.
A practice route was designed to allow participants to become familiar with the arrow key and mouse, and to practice following the arrows and encounter two characters with objects in independent locales. Participants also practiced answering two of each of the four forced-choice recognition questions regarding the objects collected.
2.3. fMRI scanning parameters
Echo-planar images were collected on a Siemens 3 T Tim trio MRI (TR = 2000 ms, TE = 30 ms, flip angle = 90, 36 slices of 4 mm thick, 64 × 64 voxel plane with an FOV of 256 mm 4 mm slices). Each BOLD run was preceded by 4 volumes that were later discarded to allow a magnetic steady state. The functional run included 530 whole brain volumes and lasted 17.67 min. The anatomical scan was an MPRAGE (TR = 2300 ms, TE = 2.98 ms, FOV 256, 1 × 1 × 1 mm voxels, flip angle = 9) and lasted 5.21 min.
2.4.1. fMRI data analysis: general linear model
Data analysis was conducted using SPM 8 (Wellcome Department of Cognitive Neurology, London, UK). Data was motion corrected by realigning to the 3rd TR, normalized to the ICBM template (and resampled at 2 × 2 × 2 mm voxel size) and smoothed with an 8 mm isotropic Gaussian kernel. The general linear model was implemented by convolving a standard hemodynamic response function and its first temporal derivative and dispersion. Events were defined based upon recognition question (Person, Place, Object, or Bright), with incorrect answers modeled as distinct event types and excluded from further analysis. Accuracy data was not available for three controls and four SCZ patients due to technical problems during initial data collection. For these participants, all events were included in the analysis. Reanalysis excluding these participants showed a very similar pattern of results. Motion parameters were included as regressors of no interest. Contrasts were Person vs. Object and Place vs. Object (both performed bidirectionally) to identify voxels which are differentially activated by source or object memory. A second level analysis was performed separately for each group (controls or SCZ) using a one sample t-test. Corrections for multiple comparisons were performed at the cluster level using an individual voxel threshold of p < 0.001 (uncorrected). A Monte-Carlo simulation of 1000 iterations (Slotnick et al., 2003) resulted in a cluster extent threshold of 49 resampled voxels. To test for differences between groups, an independent-samples t-test was performed, using the contrast β value derived for each participant from the above contrasts. Given that between-group differences often have smaller effect sizes, we used a slightly more liberal single-voxel threshold of p < 0.005, but corrected to p < 0.01 at the cluster level (resulting in an extent threshold of 97 voxels).
2.4.2. fMRI data analysis: regression with positive symptoms
A regression analysis in patients was performed to examine the relationship between source memory and clinical symptoms. As patients were stabilized and undergoing treatment at the time of scanning, few patients were actively experiencing positive symptoms as of the assessment closest to the date of scanning. As a result, the data did not possess sufficient variability for a regression analysis (the majority of cases had global scores of 0 or 1 on the SAPS at closest assessment). Furthermore, positive symptoms are often highly responsive to treatment (Malla et al., 2006; Robinson et al., 1999). As such any patient displaying continued positive symptoms may represent treatment resistant patients or those with a more severe illness, rather than relate to the symptoms themselves per se. Instead, we utilized scores from the assessment at the initial visit to the clinic, prior to treatment onset. The presence of specific symptoms at initial clinical assessment was considered as a proxy of how prone to those symptoms each patient may be. We propose that the pre-treatment ratings best represent the underlying neurobiology and clinical characteristics of individual patients, as they show their symptom characteristics in an untreated state of illness. While it is not possible to make several such assessments prior to treatment onset (which would best capture the potential symptom profile of each patient), such an approach may allow for a data exploration which separates patients who are prone to certain symptoms (such as hallucinations) from those who have experienced less or never experienced those symptoms while in an untreated state. As such, this can be conceptualized as a ‘trait’ based approach to symptom evaluation. SAPS scores for global hallucinations and global thought disorder were entered into a whole-brain regression model with the Person > Object and Place > Object contrasts. The global delusion score was not included as most patients were highly delusional at baseline. In order to account for differences in time since initial diagnosis, the interval between baseline assessment and MRI scan (in days) was entered as a covariate in the second-level regression analysis. Cluster threshold for the regression was 49 voxels at p < 0.001 uncorrected (voxel threshold).
3. Results
3.1. Sociodemographic and clinical results
Demographic characteristics of the control and schizophrenia groups are presented in Table 1. There were no significant differences in age or gender distribution between groups, though patients had a marginally significant lower parental SES and significantly fewer years of education.
Table 1.
Demographic data; mean ± std.
| Controls | Schizophrenia | ||
|---|---|---|---|
| Age | 24.4 ± 3.9 | 24.4 ± 4.1 | p = 0.99 |
| Gender (M:F) | 19:5 | 22:4 | p = 0.95 |
| Education | 13.5 ± 2.0 | 11.4 ± 1.8 | p < 0.001 |
| Parental SES | 47.3 ± 17.1 | 38.7 ± 14.0 | p = 0.078 |
| Handedness (L/Amb/R) | 20/2/2 | 23/1/2 | |
| Time in treatment (years) | 1.4 ± 1.4 (0.12–4.5) | ||
| Total SAPS | 10.6 ± 12.1 (0–44) | ||
| Total SANS | 19.4 ± 18.5 (0–66) | ||
| Calgary Depression Scale | 1.1 ± 1.99 (0–7) | ||
| Hamilton Anxiety Rating Scale | 3.1 ± 4.4 (0–21) | ||
L = left. R = right. Amb = ambidextrous. SAPS = Scale for Assessment of Positive Symptoms, SANS = Scale for Assessment of Negative Symptoms. Clinical scores from assessment closest to scanning date. Total SANS excludes attention (items 23–25). Calgary Depression Scale and HARS were not available for two patients.
3.2. Behavioral results
Accuracy in the Person, Place and Object conditions was assessed using independent samples t-test. Schizophrenia patients had significantly lower accuracy in the Person condition, t(40) = 2.247, p = 0.03, but not for Place, t(40) = −0.44, p = 0.66, or Object, t(40) = −0.07, p = 0.95. Accuracy results for the recognition tasks are presented in Table 2.
Table 2.
Behavioral performance on the forced-choice recognition task; mean percent correct ± std.
| Controls | Schizophrenia | p-Value | |
|---|---|---|---|
| Person | 79 ± 14 | 70 ± 14 | p = 0.03 |
| Place | 75 ± 17 | 78 ± 14 | p = 0.66 |
| Object | 92 ± 13 | 91 ± 13 | p = 0.95 |
Note: p-Value for an independent samples t-test between groups, t(40).
3.3. fMRI results
3.3.1. Controls
In controls, numerous cortical regions showed significant increases in neural activity for retrieval of source memory over retrieval of object memory, similar to the pattern observed in previous studies using a similar paradigm (Burgess et al., 2001; King et al., 2005). Activated regions in the source memory contrasts (Person > Object and Place > Object) included bilateral activity around the parietal occipital sulcus (including the precuneus and retrosplenial cortex) extending into the superior parietal cortex, left inferior frontal gyrus (VLPFC), fusiform gyrus bilaterally, and the occipital cortex. Activity in the Person > Object contrast included the head of the caudate nucleus, the right inferior frontal (VLPFC), and the medial aspect of the superior frontal gyrus. Object > Place showed widespread activity in the medial frontal cortex and parietal cortices (supramarginal gyrus bilaterally) and smaller clusters in the frontal and occipital cortices. For the Object > Person contrast, activity was observed in the left and right angular gyrus. Activations observed in control subjects are summarized in Table 3 and illustrated in Fig. 2.
Table 3.
fMRI activity in the control group.
| Volume | Peak t | X | Y | Z | BA | Location | |
|---|---|---|---|---|---|---|---|
| Person > Object | |||||||
| 4846 | 9.58 | −30 | −74 | 36 | 7, 23, 30 | Parietal occipital sulcus | |
| 2920 | 9.22 | 10 | 4 | −8 | 25 | Head of the caudate and subgenual area | |
| 366 | 7.09 | 4 | −28 | −2 | Superior colliculus | ||
| 833 | 6.17 | −6 | 22 | 46 | 32, 6 | Medial superior frontal gyrus | |
| 1415 | 5.94 | −40 | 14 | 22 | 44, 45, 6 | Left inferior frontal gyrus, VLPFC, and midfrontal gyrus | |
| 2217 | 5.79 | 16 | −104 | 14 | 17, 19 | Right occipital | |
| 162 | 5.68 | −40 | −42 | −22 | 37 | Left fusiform cortex | |
| 1398 | 5.59 | −34 | −82 | −6 | 17, 19 | Left occipital | |
| 120 | 5.16 | 8 | −54 | −40 | Cerebellum | ||
| 155 | 4.69 | −28 | 24 | −6 | Left anterior Insula | ||
| 78 | 4.50 | 12 | −74 | −24 | Cerebellum | ||
| 85 | 4.39 | 46 | 28 | 16 | 45 | Right inferior frontal gyrus, VLPFC | |
| Place > Object | |||||||
| 6753 | 10.73 | 18 | −54 | 20 | 17, 7, 30, 23 | Parietal occipital sulcus | |
| 722 | 7.61 | 22 | −82 | −4 | 18,17 | Right occipital | |
| 287 | 6.99 | 34 | −36 | −16 | 37 | Right fusiform gyrus | |
| 1089 | 6.94 | −42 | 16 | 20 | 44, 45, 6 | Left inferior frontal gyrus, VLPFC, and midfrontal gyrus | |
| 338 | 6.74 | −30 | −40 | −14 | 37 | Left fusiform gyrus | |
| 577 | 6.03 | −18 | −92 | −8 | 18,17 | Left occipital | |
| 49 | 4.27 | 8 | −52 | −44 | cerebellum | ||
| Object > Person | |||||||
| 236 | 6.37 | −56 | −56 | 36 | Left angular gyrus | ||
| 873 | 6.26 | 64 | −40 | 44 | Right angular gyrus | ||
| Object > Place | |||||||
| 1614 | 9.94 | −60 | −32 | 32 | 40 | Left supramarginal gyrus | |
| 5804 | 9.13 | 18 | 10 | 64 | 6, 24, 23, 4, 5 | Medial superior frontal, cingulate, and superior parietal | |
| 4889 | 8.99 | 66 | −32 | 36 | 40, 21, 22 | Right supramarginal gyrus, superior temporal, anterior insula | |
| 1005 | 8.00 | 26 | 46 | 22 | 46 | Right mid frontal gyrus, DLPFC | |
| 604 | 7.24 | 44 | 44 | 0 | 47 | Right inferior frontal gyrus, VLPFC | |
| 887 | 6.83 | −48 | 6 | 2 | 44 | Left frontal operculum, anterior insula | |
| 419 | 5.80 | −34 | 44 | 36 | 46 | Left midfrontal gyrus | |
| 272 | 5.26 | −16 | −82 | 10 | 17,18 | Left occipital, calcarine sulcus | |
| 86 | 4.79 | 32 | −30 | 14 | Right posterior insula | ||
Note: X ,Y, and Z are in MNI coordinates. Volume is the number of resampled voxels (2 × 2 × 2 mm) in the cluster. BA = Brodmann's Area, VLPFC = ventrolateral prefrontal cortex, DLPFC = dorsolateral prefrontal cortex.
Fig. 2.
Activations in memory contrasts (Person vs. Object or Place vs. Object) for both controls and SCZ patients. Areas showing greater activity for source memory are shown on the left, while regions showing greater activity to object memory are shown on the right.
3.3.2. Schizophrenia
When examining source vs. object retrieval contrasts in SCZ, a smaller number of significantly activated voxels were observed relative to the activity maps of controls. In the Person > Object contrast only smaller clusters in the head of the caudate and occipital cortex were significant. In Place > Object, mainly posterior activity was observed (e.g. parietal occipital sulcus and fusiform cortex). In both the Object > Person and Object > Place, SCZ showed a right inferior frontal (VLPFC) activity which was not observed in controls. Full details of activations for SCZ are shown in Table 4 and Fig. 2.
Table 4.
fMRI activity in the SCZ group.
| Volume | Peak t | X | Y | Z | BA | Location | |
|---|---|---|---|---|---|---|---|
| Person > Object | |||||||
| 394 | 5.22 | −6 | 10 | −2 | Head of the caudate nucleus (bilateral) | ||
| 386 | 5.15 | 12 | −98 | 22 | 18 | Right occipital | |
| Place > Object | |||||||
| 1272 | 6.63 | −10 | −60 | 14 | 17, 30, 23 | Left parietal occipital sulcus (precuneus) | |
| 325 | 6.23 | 48 | −78 | 28 | 39 | Right angular gyrus | |
| 646 | 5.54 | −6 | 10 | 0 | head of the caudate nucleus (bilateral) | ||
| 556 | 5.50 | 14 | −50 | 10 | 17, 30, 23 | Right parietal occipital sulcus (precuneus) | |
| 339 | 5.35 | −28 | −38 | −16 | 37 | Left fusiform gyrus | |
| 230 | 5.03 | 12 | −86 | −2 | 17 | Right medial occipital | |
| Object > Person | |||||||
| 114 | 4.53 | 48 | 44 | 16 | 45 | Right inferior frontal gyrus, VLPFC | |
| 76 | 4.29 | 50 | −50 | 54 | 40 | Left supramarginal gyrus | |
| Object > Place | |||||||
| 113 | 5.49 | 46 | 46 | 18 | 45 | Right inferior frontal gyrus, VLPFC | |
| 592 | 5.36 | 52 | −40 | 42 | 40 | Left supramarginal gyrus | |
| 57 | 4.24 | 62 | −20 | 30 | 2 | Right post-central gyrus | |
Note: X ,Y, and Z are in MNI coordinates. Volume is the number of resampled voxels (2 × 2 × 2 mm) in the cluster. BA = Brodmann's Area, VLPFC = ventrolateral prefrontal cortex.
3.3.3. SCZ vs. controls
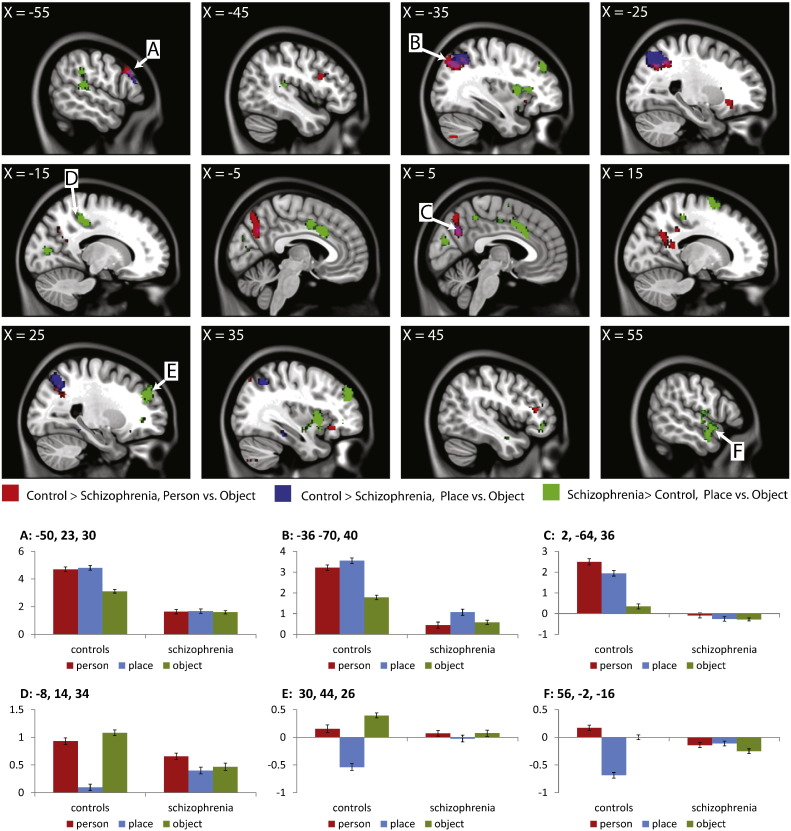
When comparing between groups, controls demonstrated regions of greater differences between conditions than SCZ for Person > Object condition (including bilaterally in the precuneus and superior parietal, and left and right inferior frontal in the VLPFC) and for Place > Object (bilaterally in the superior parietal, and the left precuneus). Schizophrenia patients had greater differences between conditions than controls when considering Place > Object across a wide range of areas. Group differences are summarized in Table 5 and Fig. 3.
Table 5.
Group differences in activity between SCZ and controls.
| Volume | Peak t | X | Y | Z | BA | Location | |
|---|---|---|---|---|---|---|---|
| Controls > schizophrenia, Person vs. Object | |||||||
| 1153 | 3.99 | 14 | −44 | 16 | 29, 30, 23, 7 | Bilateral parietal occipital sulcus and right superior parietal | |
| 592 | 3.9 | −36 | −76 | 44 | 7 | Left superior parietal | |
| 169 | 3.69 | −44 | 12 | 24 | 44 | Left inferior frontal gyrus, VLPFC | |
| 98 | 3.63 | −28 | 26 | −8 | 44 | Left frontal operculum, anterior insula | |
| 127 | 3.38 | 40 | 34 | 16 | 45 | Right inferior frontal gyrus, VLPFC | |
| Controls > schizophrenia, Place vs. Object | |||||||
| 964 | 4.13 | −32 | −60 | 48 | 7 | Left superior parietal | |
| 370 | 3.74 | 24 | −66 | 50 | 7 | Right superior parietal | |
| 165 | 3.67 | 2 | −64 | 36 | 7 | Left parietal occipital sulcus (precuneus) | |
| Schizophrenia > controls, Person vs. Object | |||||||
| No activations | |||||||
| Schizophrenia > controls, Place vs. Object | |||||||
| 302 | 4.69 | 20 | 8 | 64 | 6 | Right superior frontal gyrus | |
| 285 | 4.49 | 66 | −30 | 38 | 40 | Right supramarginal gyrus | |
| 285 | 4.49 | −16 | −36 | 46 | 31 | Left precuneus | |
| 400 | 4.32 | 42 | 10 | 10 | Right anterior insula | ||
| 415 | 4.31 | 30 | 44 | 26 | 46 | Right mid frontal gyrus, DLPFC | |
| 681 | 4.3 | −8 | 14 | 34 | 24 | Bilateral anterior cingulate gyrus | |
| 218 | 4.23 | −34 | 10 | 8 | Left anterior insula | ||
| 162 | 4.18 | −64 | −38 | 30 | 40 | Left supramarginal gyrus | |
| 155 | 3.96 | 64 | −52 | 4 | 21 | Right mid temporal gyrus (posterior) | |
| 397 | 3.93 | 56 | −2 | −16 | 21, 22 | Right middle and superior temporal gyrus (anterior) | |
| 135 | 3.79 | 6 | −42 | 48 | 31 | Right precuneus | |
| 109 | 3.67 | −34 | 42 | 40 | 9 | Left mid frontal gyrus, DLPFC | |
| 205 | 3.65 | −50 | −36 | 12 | 41, 42 | Left anterior transverse temporal gyrus | |
| 296 | 3.63 | 2 | −82 | 22 | 18 | Medial occipital | |
| 141 | 3.41 | 30 | 40 | 0 | 47 | Left inferior frontal gyrus, VLPFC | |
Note: X ,Y, and Z are in MNI coordinates. Volume is the number of resampled voxels (2 × 2 × 2 mm) in the cluster. BA = Brodmann's Area, VLPFC = ventrolateral prefrontal cortex, DLPFC = dorsolateral prefrontal cortex.
Fig. 3.
Results of the group analysis comparing SCZ patients and controls. β values were plotted for selected regions, labeled by letter (error bars represent standard error of the β values). Regions A, B, and C are areas in which controls showed larger β values for both Person vs. Object and Place vs. Object in the between group contrasts, while D, E, and F show areas in which SCZ patients showed larger β values than controls. Patients with schizophrenia show a pattern of not differentiating between object memory and source memory, even in regions in which we observed SCZ > controls. This apparent increase in activity in SCZ appears to be driven by a decrease in activity which is present in controls but not in SCZ patients.
The group comparison was run using contrast β values, which can be positive (e.g. if Place > Object) or negative (e.g. if Object > Place). Thus, while we observed greater activity in SCZ than control in the Place > Object contrast, it is not clear from the activation maps if such a difference is due to changes in activity in either Object or Place. In order to visualize the results for each condition, β values were plotted for a range of clusters. Beta values were extracted for an ROI of 11 voxels (9 in-plane voxels surrounding the selected voxel and one above and one below). ROIs were selected to represent a range of representative patterns of activity across the brain (e.g. regions in which controls showed differences from SCZ in both Person > Object and Place > Object, and regions in which SCZ showed “greater” activity in Place > Object). Results are presented in Fig. 2. For regions in which controls had greater differences between conditions than SCZ, controls are showing increased activity to the source memory conditions (Person and/or Place) relative to object, while β values in SCZ do not show any such differentiation. Interestingly, in regions in which we observed “greater” differences between conditions (in this case, Place > Object) in SCZ than controls, we again observe little differences between source memory and object memory in SCZ. This “increase” is actually driven by a decrease in activity for Place in controls. Thus, it is not that patients with SCZ are showing greater activity in the Place condition, but that they are failing to modulate brain activity in the same way as control participants.
3.3.4. SCZ regression with positive symptoms
Results of the regression analysis with positive symptoms are presented in Table 6 and Fig. 4. No significant relationships were observed between source retrieval contrasts and baseline global thought disorder. We observed significant negative correlations with the global hallucination score from the SAPS (at first assessment) and Place > Object contrast, in the right midtemporal gyrus, left prefrontal cortex, and right cerebellum (in a region noted in at least one lesional case study to be associated with source memory deficits; Tamagni et al., 2010). In order to examine the relationship between recognition memory performance and neural activity, Spearman's correlations were run on global hallucination score, memory performance in the Place and Object conditions, and β values from the three clusters. While hallucinations did not significantly correlate with performance in either condition (Place, Rho = .228, p = 0.32; Object, Rho = −0.28, p = 0.22), there was a marginally significant correlation between performance in the Object condition and β value in the DLPFC cluster (Rho = 0.38, p = 0.088).
Table 6.
Significant clusters in regression analysis with global hallucinations (SAPS) and Place > Object.
| Global hallucinations, Place > Object |
|||||||
|---|---|---|---|---|---|---|---|
| Volume | Peak t | X | Y | Z | BA | Location | |
| 121 | −5.51 | 48 | −22 | −14 | 20 | Right midtemporal gyrus | |
| 50 | −5.3 | −48 | 40 | 22 | 45 | Left inferior/middle frontal gyrus | |
| 80 | −4.93 | 20 | −42 | −26 | Right cerebellum | ||
Fig. 4.
Significant clusters in the regression analysis, global hallucinations vs. Place > Object. Graphs show calculated β values for Place > Object plotted against global hallucination score (item 7 on the SAPS, rated from 0 (none) to 5 (severe)). All coordinates are in MNI space, k = number of active voxels in the cluster.
4. Discussion
This study examined differences in the neural correlates of source memory in patients with schizophrenia and controls. We examined this issue using a virtual reality paradigm, which has improved ecological validity when assessing the “source” of a memory, as compared to other studies which have used less ecologically valid tasks. Furthermore, we examined a group of patients, who were within the first 4.5 years of treatment avoiding potential issues associated with illness chronicity such as cognitive decline, prolonged exposure to medications (although medication exposure remains an issue), social isolation, and sedentary lifestyle (Pelletier et al., 2005). While patients with SCZ demonstrated some activation in the source memory contrasts, the extent and magnitude of activity were substantially less than those in controls. Even in regions in which the difference appeared to be in the directions of SCZ > controls, it seems to be the case that controls differentiate between source and object memory while patients do not. This suggests widespread deficits in source memory processing in SCZ, with only “core” regions of the source memory network differentiating between source and object memory. As discussed below, this finding may be true of other forms of memory, and may therefore be representative of the underlying deficit across a range of memory subtypes. More specifically, patients with SCZ may fail to activate cortical regions which facilitate elaborative memory processes. This is consistent with previous findings of relatively intact object memory in SCZ (Achim et al., 2007; Luck et al., 2009), but deficits in source memory (Johnson et al., 1993) and associative memory (Achim and Lepage, 2003).
We did not observe source memory deficits in patients for the Place condition. It may be that the VR environment minimizes these behavioral differences, in that the Place condition may be easier than Person as locations were more distinct from each other relative to the 3D rendered characters. Alternatively, our recent-onset sample may have better preserved function than the more enduring schizophrenia samples often examined. However, this lack of behavioral difference has a positive aspect, in that it removes performance as a potentially major confound in the fMRI analysis. Had patients with schizophrenia demonstrated profound deficits in performance, it would beg the question if any observed neural activity differences were due to disease pathology or simply related to poor performance (and as such would be similar to poor performing healthy controls). We did observe a performance difference in the Person condition, and interestingly very little significant activity in the schizophrenia group for Person > Object. However, some activations observed in the group analysis, particularly in the posterior regions, were present in both Person > Object and Place > Object, suggesting that these differences were not at all modality specific.
Negative correlations were observed between the difference in neural activity in Place > Object and global hallucinations, measured at intake baseline using the SAPS. This suggests that the propensity of an individual to experience hallucination may modulate differences between source and object memory in these areas. We did not observe significant correlations with global thought disorder, which is not surprising as hallucinations but not thought disorder are generally associated with source memory (Woodward et al., 2007). While these regions did not directly overlap activity observed in the healthy control group, the left frontal cluster was proximal to significant activity in Place > Object in controls, while the middle-temporal cluster was proximal to significant voxels in the Object > Place clusters in controls, suggesting that these regions are at least similar to those observed in healthy controls. However, the lack of direct overlap and given the nature of the result (greater difference with more hallucinations), it is possible that these regions represent malfunctioning cortical regions which are more active in patients who have experienced hallucinations as part of their disorder. This over-activation within these regions when considering source information may play an important role in generating hallucinations, which are essentially the misattribution of internally generated stimuli to an external source. This hypothesis will require more systematic testing for confirmation.
At least one study has found relationships between the left prefrontal cortex and the right temporal cortex while patients are actively experiencing hallucinations (Hoffman et al., 2011), and a meta-analysis suggests that these regions among others are frequently active during hallucination (Jardri et al., 2011). As such, the regions found to be significantly active in our regression are consistent with the existing literature on the neurobiology of hallucinations. While many studies examining hallucinations have utilized either general ‘state’ based measurements (approximately how much the participant is hallucinating in general at the time) or direct analysis of overall activity during hallucinations, we have attempted to utilize a ‘trait’ based approach of looking at pre-treatment hallucinations. Our sample of patients early in treatment makes such an approach possible with minimal confounding for chronicity (which we attempted to control for by including length of treatment as a covariate in the regression). Our results overall suggest a relationship between mal-adaptive neural activity related to source memory retrieval and how prone participants are to hallucinatory symptoms, further verifying the relationship between source memory and hallucinations (Woodward et al., 2007). However, some limitations must be considered as well. While we have attempted to use baseline scores as a ‘trait’ measure of how prone an individual is to hallucinations, it is well known that clinical symptoms can vary across time. As such, the appropriate method for evaluating ‘trait’ symptoms is to take repeated symptom measurements over time (Mathalon and Ford, 2012). It would be preferable to have several assessments of pre-treatment symptoms to have a complete picture of the symptom profile of a given patient, but this is not possible as it would require delaying treatment. As such we utilized the best available measure to assess pre-treatment symptom profile, which produced results which are consistent with the existing literature on those symptoms. However, it is important to remain considerate of the limitations of our measures when considering these results.
4.2. Under-activation of extended regions as a model for memory deficits in SCZ
Source memory can be considered a form of associative/relational memory, as participants are binding elements together during encoding and storing these elements together as part of a larger whole. A deficit in source memory can be viewed as a failure to associate a memory item with its context. In the case of this study in particular, our source memory paradigm is not far removed from studies examining associative memory (Achim et al., 2007; Achim and Lepage, 2005; Murray and Ranganath, 2007), which is known to be more impaired in SCZ than object memory (Achim and Lepage, 2003). Some studies have suggested that patients with schizophrenia do not properly differentiate between associative and item memory (Achim et al., 2007; Ragland et al., 2012), in keeping with suggestions that source memory impairments are part of associative memory impairments (Achim et al., 2011).
Our present study focused on source retrieval, and as such we cannot definitively determine if the differences observed in our contrasts are driven by deficits in encoding (in that the information was not properly moved into memory) or retrieval (in that the memories may have been encoded but are not properly accessed), or a combination of both (which seems likely given the plurality of evidence for memory deficits in SCZ). It is noteworthy, however, that Achim et al. (2011) found deficits in source memory even when using short term recall, minimizing the need for retrieval of information from long-term memory. This suggests that at least part of the source memory deficit in SCZ is related to problems with encoding. Our finding of an overall pattern of lack of differentiation between conditions may be a fairly consistent finding in the cognitive neuroscience of memory in SCZ, regardless if one considers activity during encoding or retrieval/recognition. We suggest the following hypothesis: Activity in ‘core’ regions required for task performance may be relativity intact in SCZ, while deficits in activity will be observed in ‘supporting’ regions which facilitate improved task performance. That is to say, the deficits in memory (and possibly cognition in general) are related to a lack of engagement of extended cortical regions. These extended regions are not critically required for minimal task performance, but instead serve to enhance performance (such as regions involved in cognitive control processes). Healthy controls will tend to utilize such areas automatically, while patients with schizophrenia will fail to do so.
Such an interpretation is consistent with the notion of a deficit in ‘cognitive control’ systems which facilitate memory (Ranganath et al., 2008). For example, patients with SCZ have been shown to have impairments in initiating elaborative encoding processes which may be beneficial during associative encoding (Brebion et al., 1997). However, when patients are specifically instructed to utilize effective encoding strategies they show an improvement in memory performance, demonstrating that patients with SCZ can utilize such encoding strategies when specifically instructed but fail to do so spontaneously (Brebion et al., 1997). This pattern of results is similar to that in patients with prefrontal cortical lesions (Alexander et al., 2003). Bonner-Jackson et al. (2008) examined memory strategies in schizophrenia by contrasting intentional but unstructured encoding (simply instructing participant to memorize words) with an externally imposed deep encoding strategy (having participants perform an abstract/concrete judgement on words, deep semantic encoding which facilitates memory encoding; Craik and Lockhart, 1972). They observed group × encoding interactions in several regions, including the left inferior frontal gyrus and precuneus, with the most common finding being a difference between controls and SCZ in the incidental, unstructured encoding condition. For example, in the left inferior frontal cortex, SCZ patients showed no activity for unstructured encoding, but substantial activity for deep encoding. That is to say, when SCZ patients were provided a structured encoding strategy they activated this region, but failed to do so spontaneously. Likewise, controls showed significantly better memory performance than SCZ patients for the deep encoding condition. Further support for the importance in strategic and/or cognitive control comes from findings of changes and/or normalization of activity in SCZ following cognitive training (Hooker et al., 2012; Penades et al., 2013).
Within both encoding and recognition studies in schizophrenia, the most prevalent finding is a decrease in the extent or magnitude of activity in SCZ patients relative to controls, although many studies also report findings in the direction of SCZ > controls (Ragland et al., 2009). While it is likely that SCZ often shows compensatory networks or inefficient over-activation during cognitive tasks, it can be difficult to judge from many of the published studies on cognition in SCZ. Many papers report differences in group activities without also reporting the parameter estimates which accompany those changes. For example, if we had done so in this study (by only presenting the activation maps in Fig. 3 and not the Beta values) we may have concluded that SCZ showed greater activity in some regions for Place vs. Object and concluded that this was compensatory of maladaptive over-activation. However, when examining the Beta values, we realize that this is not the case but instead these are regions where controls show greater activity for Object over Place (often in the form of a decrease in activity for Place). This highlights the importance of carefully examining parameter estimates when performing between-group comparisons. It is possible that in at least some cases in the existing literature, the so-called ‘compensatory’ activity may instead be a lack of activity relative to the control group, such as was the case in this study. Such ambiguity and misinterpretation can be avoided if studies fully report parameter estimates for contrasts which differ between groups.
5. Conclusions
We have reported the results of a study looking at source memory retrieval in SCZ using a virtual reality environment on a group of patients relatively early into treatment. While controls activated a large, extensive network for source relative to object retrieval, SCZ showed a marked reduction in activity. This reduction was borne out in the group comparison, and appears to be related to the fact that patients with SCZ are failing to activate these regions or differentiate between object and source retrieval. Despite the large-scale group differences, patients were still able to perform the source retrieval tasks, suggesting that at least some of the ‘core’ system involved in source retrieval is intact. Instead, we propose that the observed differences are related to ‘supporting’ regions which are not critical to task performance but instead facilitate source retrieval. This pattern may be related to the overall cognitive deficits observed in schizophrenia. That is, patients may generally fail to engage extended cortical networks which facilitate task performance and facilitate overall cognitive functioning.
Acknowledgments
This work was funded through a grant from the Canadian Institutes of Health Research (CIHR). CH was supported by a Banting Post-Doctoral Fellowship from the Canadian Institutes of Health Research (CIHR). LB was supported by a Vanier Graduate Scholarship from CIHR.
References
- Achim A.M., Bertrand M.C., Montoya A., Malla A.K., Lepage M. Medial temporal lobe activations during associative memory encoding for arbitrary and semantically related object pairs. Brain Research. 2007;1161:46–55. doi: 10.1016/j.brainres.2007.05.046. 17604009 [DOI] [PubMed] [Google Scholar]
- Achim A.M., Bertrand M.C., Sutton H., Montoya A., Czechowska Y., Malla A.K., Joober R., Pruessner J.C., Lepage M. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Archives of General Psychiatry. 2007;64(9):999–1014. doi: 10.1001/archpsyc.64.9.999. 17768265 [DOI] [PubMed] [Google Scholar]
- Achim A.M., Lefèbvre A.A., Cellard C., Bouchard R.H., Roy M.A., Tremblay S. The role of recollection in source memory: an examination of schizophrenia patients and their first-degree relatives. Brain and Cognition. 2011;75(2):147–153. doi: 10.1016/j.bandc.2010.11.002. 21134707 [DOI] [PubMed] [Google Scholar]
- Achim A.M., Lepage M. Is associative recognition more impaired than item recognition memory in schizophrenia? A meta-analysis. Brain and Cognition. 2003;53(2):121–124. doi: 10.1016/s0278-2626(03)00092-7. 14607130 [DOI] [PubMed] [Google Scholar]
- Achim A.M., Lepage M. Neural correlates of memory for items and for associations: an event-related functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2005;17(4):652–667. doi: 10.1162/0898929053467578. 15829085 [DOI] [PubMed] [Google Scholar]
- Alexander M.P., Stuss D.T., Fansabedian N. California verbal learning test: performance by patients with focal frontal and non-frontal lesions. Brain: A Journal of Neurology. 2003;126(6):1493–1503. doi: 10.1093/brain/awg128. 12764068 [DOI] [PubMed] [Google Scholar]
- Andreasen N.C. The Scale for Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen N.C. Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City: 1984. [Google Scholar]
- Barch D.M., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in Cognitive Sciences. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. 22169777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar M., Malla A., Joober R., Lepage M. Cognitive markers of short-term clinical outcome in first-episode psychosis. British Journal of Psychiatry: the Journal of Mental Science. 2008;193(4):297–304. doi: 10.1192/bjp.bp.107.040410. 18827291 [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A., Yodkovik N., Csernansky J.G., Barch D.M. Episodic memory in schizophrenia: the influence of strategy use on behavior and brain activation. Psychiatry Research. 2008;164(1):1–15. doi: 10.1016/j.pscychresns.2007.12.012. 18790618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brébion G., Amador X., Smith M.J., Gorman J.M. Mechanisms underlying memory impairment in schizophrenia. Psychological Medicine. 1997;27:383–393. doi: 10.1017/s0033291796004448. 9089831 [DOI] [PubMed] [Google Scholar]
- Brébion G., David A.S., Bressan R.A., Ohlsen R.I., Pilowsky L.S. Hallucinations and two types of free-recall intrusion in schizophrenia. Psychological Medicine. 2009;39(6):917–926. doi: 10.1017/S0033291708004819. 19079808 [DOI] [PubMed] [Google Scholar]
- Brébion G., Gorman J.M., Amador X., Malaspina D., Sharif Z. Source monitoring impairments in schizophrenia: characterisation and associations with positive and negative symptomatology. Psychiatry Research. 2002;112(1):27–39. doi: 10.1016/s0165-1781(02)00187-7. 12379448 [DOI] [PubMed] [Google Scholar]
- Brébion G., Ohlsen R.I., Bressan R.A., David A.S. Source memory errors in schizophrenia, hallucinations and negative symptoms: a synthesis of research findings. Psychological Medicine. 2012;42(12):2543–2554. doi: 10.1017/S003329171200075X. 22716666 [DOI] [PubMed] [Google Scholar]
- Brunelin J., Combris M., Poulet E., Kallel L., D'Amato T., Dalery J., Saoud M. Source monitoring deficits in hallucinating compared to non-hallucinating patients with schizophrenia. European Psychiatry: the Journal of the Association of European Psychiatrists. 2006;21(4):259–261. doi: 10.1016/j.eurpsy.2006.01.015. 16545546 [DOI] [PubMed] [Google Scholar]
- Brunelin J., Poulet E., Bediou B., Kallel L., Dalery J., D'Amato T., Saoud M. Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophrenia Research. 2006;81(1):41–45. doi: 10.1016/j.schres.2005.10.009. 16314076 [DOI] [PubMed] [Google Scholar]
- Burgess N., Maguire E.A., Spiers H.J., O'Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14:439–453. doi: 10.1006/nimg.2001.0806. 11467917 [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46(7):1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. 18439631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F.I.M., Lockhart R.S. Levels of processing: a framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Danion J.-M., Rizzo L., Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Archives of General Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. 10401510 [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. 17097284 [DOI] [PubMed] [Google Scholar]
- Davachi L., Mitchell J.P., Wagner A.D. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. 12578977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Asper C., Malley J., Genderson M., Apud J., Elvevåg B. Context binding in schizophrenia: effects of clinical symptomatology and item content. Psychiatry Research. 2008;159(3):259–270. doi: 10.1016/j.psychres.2007.02.018. 18442860 [DOI] [PubMed] [Google Scholar]
- Duarte A., Ranganath C., Knight R.T. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2005;25(36):8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. 16148241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M. Structured Clinical Interview For DSM-IV Axis I Disorders. APA.; Washington: 1997. [Google Scholar]
- Fisher M., McCoy K., Poole J.H., Vinogradov S. Self and other in schizophrenia: a cognitive neuroscience perspective. American Journal of Psychiatry. 2008;165(11):1465–1472. doi: 10.1176/appi.ajp.2008.07111806. 18708487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. Journal of Clinical Psychiatry. 2006;67(Suppl. 9):3–8. [Discussion 36–42] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Heaton R.K. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. 15531406 [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Pittman B., Constable R.T., Bhagwagar Z., Hampson M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. British Journal of Psychiatry: the Journal of Mental Science. 2011;198(4):277–283. doi: 10.1192/bjp.bp.110.086835. 21972276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Bruce L., Fisher M., Verosky S.C., Miyakawa A., Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophrenia Research. 2012;139(1–3):53–59. doi: 10.1016/j.schres.2012.05.009. 22695257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: s coordinate-based meta-analysis. American Journal of Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. 20952459 [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Hashtroudi S., Lindsay D.S. Source monitoring. Psychological Review. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Keefe R.S. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. 23925787 [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Arnold M.C., Bayen U.J., McEvoy J.P., Wilson W.H. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophrenia Research. 2002;57(1):51–67. doi: 10.1016/s0920-9964(01)00306-1. 12165376 [DOI] [PubMed] [Google Scholar]
- King J.A., Hartley T., Spiers H.J., Maguire E.A., Burgess N. Anterior prefrontal involvement in episodic retrieval reflects contextual interference. Neuroimage. 2005;28:256–267. doi: 10.1016/j.neuroimage.2005.05.057. 16027012 [DOI] [PubMed] [Google Scholar]
- Luck D., Montoya A., Menear M., Achim A.M., Lal S., Lepage M. Selective pair recognition memory impairment with no response bias in schizophrenia. Psychiatry Research. 2009;169(1):39–42. doi: 10.1016/j.psychres.2008.06.037. 19622416 [DOI] [PubMed] [Google Scholar]
- Malla A., Norman R., McLean T., Scholten D., Townsend L. A Canadian programme for early intervention in non-affective psychotic disorders. Australian and New Zealand Journal of Psychiatry. 2003;37(4):407–413. doi: 10.1046/j.1440-1614.2003.01194.x. 12873324 [DOI] [PubMed] [Google Scholar]
- Malla A., Norman R., Schmitz N., Manchanda R., Béchard-Evans L., Takhar J., Haricharan R. Predictors of rate and time to remission in first-episode psychosis: a two-year outcome study. Psychological Medicine. 2006;36(5):649–658. doi: 10.1017/S0033291706007379. 16515734 [DOI] [PubMed] [Google Scholar]
- Mathalon D.H., Ford J.M. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Frontiers in Human Neuroscience. 2012;6:136. doi: 10.3389/fnhum.2012.00136. 22654745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K.J., Johnson M.K. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135(4):638–677. doi: 10.1037/a0015849. 19586165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K.J., Johnson M.K., Raye C.L., Greene E.J. Prefrontal cortex activity associated with source monitoring in a working memory task. Journal of Cognitive Neuroscience. 2004;16(6):921–934. doi: 10.1162/0898929041502724. 15298780 [DOI] [PubMed] [Google Scholar]
- Murray L.J., Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27(20):5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. 17507573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolde S.F., Johnson M.K., D'Esposito M. Left prefrontal activation during episodic remembering: an event- related fMRI study. Neuroreport. 1998;9(15):3509–3514. doi: 10.1097/00001756-199810260-00032. 9855308 [DOI] [PubMed] [Google Scholar]
- Pelletier M., Achim A.M., Montoya A., Lal S., Lepage M. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;74(2–3):233–252. doi: 10.1016/j.schres.2004.08.017. 15722003 [DOI] [PubMed] [Google Scholar]
- Penadés R., Pujol N., Catalán R., Massana G., Rametti G., García-Rizo C., Bargalló N., Gastró C., Bernardo M., Junque C. Brain effects of cognitive remediation therapy in schizophrenia: a structural and functional neuroimaging study. Biological Psychiatry. 2013;73(10):1015–1023. doi: 10.1016/j.biopsych.2013.01.017. 23452665 [DOI] [PubMed] [Google Scholar]
- Ragland J.D., Blumenfeld R.S., Ramsay I.S., Yonelinas A., Yoon J., Solomon M., Carter C.S., Ranganath C. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. Neuroimage. 2012;59(2):1719–1726. doi: 10.1016/j.neuroimage.2011.08.055. 21907293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Laird A.R., Ranganath C., Blumenfeld R.S., Gonzales S.M., Glahn D.C. Prefrontal activation deficits during episodic memory in schizophrenia. American Journal of Psychiatry. 2009;166(8):863–874. doi: 10.1176/appi.ajp.2009.08091307. 19411370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Valdez J.N., Loughead J., Gur R.C., Gur R.E. Functional magnetic resonance imaging of internal source monitoring in schizophrenia: recognition with and without recollection. Schizophrenia Research. 2006;87(1–3):160–171. doi: 10.1016/j.schres.2006.05.008. 16814525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Minzenberg M.J., Ragland J.D. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biological Psychiatry. 2008;64(1):18–25. doi: 10.1016/j.biopsych.2008.04.011. 18495087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Yonelinas A.P., Cohen M.X., Dy C.J., Tom S.M., D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42(1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. 14615072 [DOI] [PubMed] [Google Scholar]
- Robinson D., Woerner M.G., Alvir J.M., Bilder R., Goldman R., Geisler S., Koreen A., Sheitman B., Chakos M., Mayerhoff D., Lieberman J.A. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Archives of General Psychiatry. 1999;56(3):241–247. doi: 10.1001/archpsyc.56.3.241. 10078501 [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J., Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Research. Cognitive Brain Research. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. 12763194 [DOI] [PubMed] [Google Scholar]
- Stuss D.T., Benson D.F. The Frontal Lobes. Raven; New York: 1986. [Google Scholar]
- Subramaniam K., Luks T.L., Fisher M., Simpson G.V., Nagarajan S., Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. 22365555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagni C., Mondadori C.R., Valko P.O., Brugger P., Schuknecht B., Linnebank M. Cerebellum and source memory. European Neurology. 2010;63(4):234–236. doi: 10.1159/000282277. 20299799 [DOI] [PubMed] [Google Scholar]
- Uncapher M.R., Otten L.J., Rugg M.D. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52(3):547–556. doi: 10.1016/j.neuron.2006.08.011. 17088219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S., Luks T.L., Schulman B.J., Simpson G.V. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cerebral Cortex (New York, N.Y.: 1991) 2008;18(11):2532–2539. doi: 10.1093/cercor/bhn028. 18321870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S., Willis-Shore J., Poole J.H., Marten E., Ober B.A., Shenaut G.K. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. American Journal of Psychiatry. 1997;154:1530–1537. doi: 10.1176/ajp.154.11.1530. 9356560 [DOI] [PubMed] [Google Scholar]
- Wang L., Metzak P.D., Woodward T.S. Aberrant connectivity during self–other source monitoring in schizophrenia. Schizophrenia Research. 2011;125(2–3):136–142. doi: 10.1016/j.schres.2010.11.012. 21147519 [DOI] [PubMed] [Google Scholar]
- Waters F.A., Maybery M.T., Badcock J.C., Michie P.T. Context memory and binding in schizophrenia. Schizophrenia Research. 2004;68(2–3):119–125. doi: 10.1016/S0920-9964(03)00221-4. 15099596 [DOI] [PubMed] [Google Scholar]
- Wheeler M.E., Buckner R.L. Functional–anatomic correlates of remembering and knowing. NeuroImage. 2004;21(4):1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. 15050559 [DOI] [PubMed] [Google Scholar]
- Woodward T.S., Menon M., Whitman J.C. Source monitoring biases and auditory hallucinations. Cognitive Neuropsychiatry. 2007;12(6):477–494. doi: 10.1080/13546800701307198. 17978935 [DOI] [PubMed] [Google Scholar]