Abstract
Context:
Reliable thyroglobulin (Tg) autoantibody (TgAb) detection before Tg testing for differentiated thyroid cancer (DTC) is critical when TgAb status (positive/negative) is used to authenticate sensitive second-generation immunometric assay (2GIMA) measurements as free from TgAb interference and when reflexing “TgAb-positive” sera to TgAb-resistant, but less sensitive, Tg methodologies (radioimmunoassay [RIA] or liquid chromatography-tandem mass spectrometry [LC-MS/MS]).
Objective:
The purpose of this study was to assess how different Kronus (K) vs Roche (R) TgAb method cutoffs for “positivity” influence false-negative vs false-positive serum TgAb misclassifications that may reduce the clinical utility of reflex Tg testing.
Methods:
Serum Tg2GIMA, TgRIA, and TgLC-MS/MS measurements for 52 TgAb-positive and 37 TgAb-negative patients with persistent/recurrent DTC were compared. A total of 1426 DTC sera with TgRIA of ≥1.0 μg/L had false-negative and false-positive TgAb frequencies determined using low Tg2GIMA/TgRIA ratios (<75%) to indicate TgAb interference.
Results:
TgAb-negative patients with disease displayed Tg2GIMA, TgRIA, and TgLC-MS/MS serum discordances (% coefficient of variation = 24 ± 20%, range, 0%–100%). Of the TgAb-positive patients with disease, 98% had undetectable/lower Tg2GIMA vs either TgRIA or TgLC-MS/MS (P < .01), whereas 8 of 52 (15%) had undetectable Tg2GIMA + TgLC-MS/MS associated with TgRIA of ≥1.0 μg/L. Receiver operating characteristic curve analysis reported more sensitivity for TgAb method K vs R (81.9% vs 69.1%, P < .001), but receiver operating characteristic curve cutoffs (>0.6 kIU/L [K] vs >40 kIU/L [R]) had unacceptably high false-negative frequencies (22%–32%), whereas false positives approximated 12%. Functional sensitivity cutoffs minimized false negatives (13.5% [K] vs 21.3% [R], P < .01) and severe interferences (Tg2GIMA, <0.10 μg/L) (0.7% [K] vs 2.4% [R], P < .05) but false positives approximated 23%.
Conclusions:
Reliable detection of interfering TgAbs is method and cutoff dependent. No cutoff eliminated both false-negative and false-positive TgAb misclassifications. Functional sensitivity cutoffs were optimal for minimizing false negatives but have inherent imprecision (20% coefficient of variation) that, exacerbated by TgAb biologic variability during DTC monitoring, could cause TgAb status to fluctuate for patients with low TgAb concentrations, prompting unnecessary Tg method changes and disrupting Tg monitoring. Laboratories using reflexing should limit Tg method changes by considering a patient's Tg + TgAb testing history in addition to current TgAb status before Tg method selection.
Serum thyroglobulin (Tg) is the primary biochemical tumor marker used to detect recurrence in patients with differentiated thyroid cancers (DTCs) (1). Unfortunately, the thyroglobulin autoantibodies (TgAbs) present in 25% to 30% of patients with DTCs can interfere with Tg measurement (2–11). Automated (second-generation) immunometric assays (2GIMAs) are rapidly becoming the standard of care because they have superior functional sensitivity (FS) (0.05–0.10 μg/L) for detecting basal Tg without recombinant human TSH stimulation (9, 12–18). However, TgAb interference with Tg2GIMAs causes underestimated (falsely low/undetectable) Tg2GIMA (10, 11, 19). In contrast, the radioimmunoassay (RIA) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) classes of Tg methods resist TgAb interference but have an order of magnitude inferior FS (0.5–1.0 μg/L), lack automation, and are not universally available (4, 6, 7, 9, 11, 12, 17, 19, 20).
Laboratories often reflex Tg measurement to RIA or LC-MS/MS when the serum TgAb concentration is above a fixed cutoff set to define TgAb “positivity.” This strategy is designed to maximize clinical sensitivity by restricting Tg2GIMA measurement to “TgAb-negative” sera, while minimizing interference by reflexing “TgAb-positive” sera to a TgAb-resistant Tg methodology (RIA or LC-MS/MS) (6, 11, 19, 20). Clearly, the sensitivity and specificity of the TgAb method has a critical impact on the reliability of this reflex strategy, because false-negative TgAb tests can lead to inappropriately low/undetectable Tg2GIMA that can mask disease, whereas false-positive TgAb tests may prompt unnecessary reflexing to a less sensitive methodology that may fail to detect low Tg disease (21–27). Guidelines caution against unnecessarily changing Tg methods because of wide disparities in numeric Tg values reported by different methods for the same serum (9, 12, 17, 18, 28–30).
Studies use concordance between TgAb methods to assess the reliability of TgAb detection (2, 10, 31–35). This study directly evaluated the effects of interfering TgAbs on Tg measurement in terms of a low ratio (<75%) between values reported by a TgAb-sensitive Tg2GIMA and a TgAb-resistant TgRIA (2, 10, 11, 17, 36, 37). The Kronus TgAb method was selected for testing because this semiautomated radioassay predates current automated TgAb tests and has provided stable TgAb values for more than 2 decades (4). The Roche TgAb method was selected because laboratories adopted this method (38) after our previous study (10) found it to be more sensitive than 2 other automated TgAb tests (Beckman and Siemens) compared with Kronus as the reference.
Sensitivity differences between TgAb methods reflect the assay design, the specificity of the TgAb test reagents, and the cutoff selected to define a “positive” TgAb result. Previously, we reported that manufacturer-recommended cutoffs (MCOs) for TgAbs were set too high to reliably detect interfering TgAbs and were more appropriate for diagnosing thyroid autoimmunity (10). The goals of the current study were to assess whether lower cutoffs could reduce false-negative and minimize false-positive TgAb misclassifications that could have a negative impact on DTC monitoring when a fixed TgAb cutoff value was used to reflex Tg testing to different methods.
Materials and Methods
Tg methods
Both Tg methods were standardized against the International Reference Preparation CRM-457.
TgRIA
This TgRIA, developed by the USC Endocrine Laboratory, University of Southern California, Los Angeles (4, 10, 28, 39) had first-generation FS (0.5 μg/L) established by guidelines (Tg assay FS being the lowest Tg concentration measured with 20% between-run coefficient of variation [CV] in human serum over 6–12 months [clinical interval typical for DTC monitoring] employing ≥2 different reagent lots and calibrators [30]). Between-run precisions for TgAb-positive human sera measured over 1 year were 14.6%, 7.6%, 6.5%, 9.3%, and 7.8% for Tg concentrations of 0.75, 3.2, 12.0, 20.3, and 34.8 μg/L, respectively. Within-run precisions were 8.4%, 7.1%, 1.5%, and 5.3% at Tg concentrations of 0.94, 2.0, 16.1, and 31.6 μg/L, respectively.
Tg2GIMA
This Tg2GIMA method was the Access immunochemiluminometric method (Beckman Coulter). The FS established for TgAb-negative human serum was 0.05 μg/L. However, this study considered values of <0.10 μg/L “undetectable” in accord with other reports (15). Between-run precisions for TgAb-negative human sera measured over a 14-month period were 13.0%, 6.5%, 4.2%, and 4.0% for Tg concentrations of 0.13, 0.59, 7.0, and 54 μg/L, respectively. Within-run precisions were 4.1%, 3.2%, 1.7%, and 1.9% at Tg concentrations of 0.15, 0.76, 7.0, and 106 μg/L, respectively.
TgLC-MS/MS
TgLC-MS/MS measurements were made by the Mayo Medical Laboratories (40). The limit of quantitation (LOQ) (20% CV between runs made over 1 month) was 0.5 μg/L (6, 9).
Tg2GIMA/TgRIA ratios: used to indicate TgAb interference
A low Tg2GIMA/TgRIA ratio (<75%) was considered to indicate TgAb interference as previously established (2, 10, 36, 37). Sera with TgAb concentrations below any specific cutoff displaying a low Tg2GIMA/TgRIA ratio were considered false negative. Conversely, sera with TgAb values more than or equal to the cutoff without a low Tg2GIMA/TgRIA ratio were considered false positive. Sera with severe interference had unequivocally detectable TgRIA (≥1.0 μg/L) and undetectable (<0.10 μg/L) Tg2GIMA.
TgAb assays
Both TgAb assays claimed standardization against the World Health Organization International Reference Preparation IRP 65/93 and were performed according to the manufacturers' protocols.
Kronus/RSR TgAb (K)
Method K was a semiautomated radioassay (Kronus also known as RSR) that uses 125I-labeled Tg to bind TgAb in a diluted (1:21) serum specimen. The TgAb-Tg 125I-labeled complex is precipitated with protein A. Between-run precisions for human sera measured over 1 year were 14.3%, 9.5%, 8.7%, and 11.1% for TgAb concentrations of 0.7, 2.6, 5.2, and 11.0 kIU/L, respectively. TgAb concentrations of >17 kIU/L were remeasured at appropriate dilutions. Within-run precisions of TgAb measured in human sera were 5.7%, 3.1%, 1.9%, and 3.1% at concentrations of 0.8, 3.0, 6.2, and 14.6 kIU/L, respectively. The LOD (within-run precision of zero matrix [41]) was 0.3 kIU/L. FS, determined as for Tg (30) was 0.4 kIU/L. The MCO for a positive TgAb was 1.0 kIU/L.
Roche Elecsys TgAb (R)
Method R was the automated electrochemiluminescent competitive immunometric assay method (Roche Diagnostics) whereby serum TgAb competes for biotinylated human Tg with ruthenium-labeled TgAb. The Tg-TgAb complexes form and bind streptavidin-coated microparticles and are magnetically captured onto the surface of an electrode. Between-run precisions for human sera measured over 1 year were 22.5%, 20.1%, 13.1%, 9.3%, 14.5%, 8.9%, and 16.7% for concentrations of 13, 17, 27, 35, 87, 146, and 1329 kIU/L, respectively. Within-run precisions were 4.9%, 5.1%, and 5.6% at concentrations of 63, 115, and 2894 kIU/L, respectively. Values of >4000 kIU/L were not diluted. The LOD was 10 kIU/L, and the FS of 22 kIU/L used by other laboratories (38) was confirmed. The MCO for a positive TgAb was 115 kIU/L.
Serum specimen groups
Group A comprised 89 sera from patients with DTC (88 papillary and 1 Hurtle cell) drawn a median of 8 days (range, 0–167 days) before detection of persistent/recurrent disease by biopsy or anatomic imaging. Group A was used to compare the different classes of the Tg methods (Tg2GIMA, TgRIA, and TgLC-MS/MS). Of the patients, 37 were TgAb negative (below method K and R FSs), and 52 were classified as TgAb positive by both methods. Group B comprised 1426 sera from 1110 patients with DTC selected for sufficient volume for Tg2GIMA and TgAb (both K and R) with unequivocally detectable TgRIA (range, 1.0–40 μg/L), allowing Tg2GIMA/TgRIA ratio calculations. Group B sera were selected to cover a range of TgAb values from the LOD to very high TgAb concentrations. Group C comprised 607 sequential DTC sera received for routine Tg + TgAb testing that had K and R TgAb measurements and were used to establish the range and frequency of TgAb values typical of clinical practice.
Serum TgAb analyses using different cutoffs for TgAb positivity
Methods K and R TgAb data for groups B and C were analyzed as subgroups covering the measurement range. Cutoffs for TgAb positivity represented those frequently used by laboratories: LOD, FS, receiver operating characteristic (ROC) curve, and MCO. For method K, these cutoffs were 0.3 (LOD), 0.4(FS), 0.5, 0.6, 0.7 (ROC curve), 0.8, 0.9, 1.0 (MCO), 1.2, 1.5, 2, 3, 5, 10, 25, 50, 100, and ≥ 250 kIU/L, and for method R the cutoffs were 10 (LOD), 15, 18, 20, 22 (FS), 25, 30, 35, 41(ROC curve), 50, 60, 80, 115 (MCO), 200, 400, 600, and ≥1000 kIU/L). ROC curve analysis on group B subgroups used low Tg2GIMA/Tg RIA ratios (<75%) to detect interfering TgAb.
Statistical analyses
ROC curve analysis was performed using MedCalc 12.3.0 (Mariakerke) software. A true-positive serum had TgAb values more than or equal to the selected cutoff with an abnormally low Tg2GIMA/TgRIA ratio (<75%) indicating TgAb interference (2, 10, 17, 36, 37). A false-positive serum had TgAb values more than or equal to the selected cutoff without a low Tg2GIMA/TgRIA ratio (≥75%) indicating the absence of TgAb interference. A true-negative serum had TgAb below the cutoff without a low Tg2GIMA/TgRIA ratio. A false-negative serum had TgAb below the cutoff with a low Tg2GIMA/TgRIA ratio. Statistical analyses were performed with XLSTAT and Student t tests using SPSS software (version 13.0). Statistical significance was set at a value of P < .05.
Results
Figure 1 shows comparative serum Tg2GIMA, TgLC-MS/MS, and TgRIA measurements for the group A patients with documented disease. Of these patients, 37 had no TgAb detected (below the FS of both methods K and R) (Figure 1A), and 52 had TgAb detected by both methods (Figure 1B). TgAb-negative patients had comparable Tg2GIMA/TgRIA vs Tg2GIMA/TgLC-MS/MS ratios (median [range]: 81 [1%–182%] vs 91 [1%–221%], respectively, not significant [NS]). Despite correlations between the methods (Tg2GIMA = 0.94TgRIA − 3.1, r = 0.98; Tg2GIMA = 1.05TgLC-MS/MS − 2.1, r =0.98; and TgLC-MS/MS = 0.88TgRIA − 0.4, r =0.98), between-method % CVs for the 3 measurements made on individual sera were high (mean ± SD, 24 ± 20, range, 0%–100%). Of the patients, 17 of 37 (46%) displayed >20% between-method discordances, and in 5 of 37 (14%) discordances exceeded 30% (indicated in Figure 1A as solid red circles). Serum Tg values for the highly discordant patients were <0.10/3.5/2.9, 16.6/39.4/25.4, 2.0/6.1/1.8, 14.1/6.4/39.6, and 0.30/0.9/0.8 μg/L for Tg2GIMA, TgLC-MS/MS, and TgRIA, respectively. These between-method discordances exceeded the <10% CV expected for repetitive measurements made with a single method. One TgAb-negative patient with disease had no Tg detected by any method. The ratios for the TgAb-positive patients with disease were comparable irrespective of whether TgRIA or TgLC-MS/MS was used as the denominator (mean ± SD [range] 41 ± 29 [3%–99%] vs 50 ± 26 [5%–115%] for Tg2GIMA/TgRIA vs Tg2GIMA/TgLC-MS/MS ratios, respectively, NS). Both the Tg2GIMA/TgRIA and Tg2GIMA/TgLC-MS/MS ratios for group A TgAb-positive patients were significantly lower than those for group A TgAb-negative patients (P < .001). The group A TgAb-positive sera also displayed correlations between the methods: Tg2GIMA = 0.58TgRIA − 0.99, r =0.95; Tg2GIMA = 0.60TgLC-MS/MS − 0.35, r =0.97; and TgLC-MS/MS = 0.96TgRIA − 1.10, r =0.98. A total of 51 of 52 (98%) Tg2GIMA values for TgAb-positive patients were either undetectable (20 of 52, 38%) or lower than either TgLC-MS/MS (P < .02) or TgRIA (P < .01). TgLC-MS/MS was below the LOQ in 12 patients (23%) (indicated by red squares in Figure 1B). Six of 12 sera (solid squares) had unequivocally undetectable TgLC-MS/MS (no peak detected), whereas 6 of 12 sera had marginal TgLC-MS/MS in the 0.3 to 0.5 μg/L range. Two patients with undetectable TgLC-MS/MS also had undetectable Tg2GIMA and TgRIA, and 2 with marginal TgLC-MS/MS had some Tg detected by both Tg2GIMA (0.16 and 0.19 μg/L) and TgRIA (0.9 and 2.8 μg/L), whereas the remaining 8 with no Tg detected by either Tg2GIMA or TgLC-MS/MS had TgRIA of 1.0, 1.0, 1.7, 2.5, 2.6, 2.7, 3.5, and 10.5 μg/L.
Figure 1.
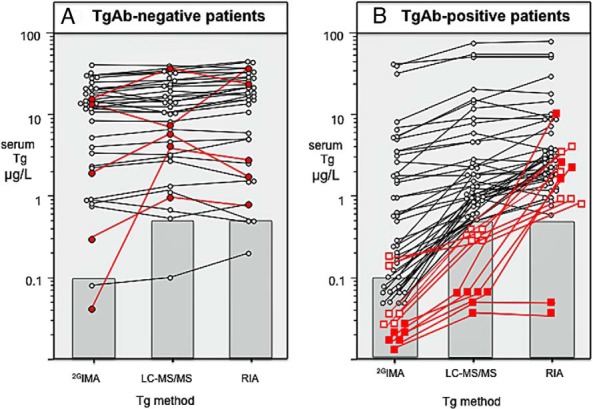
Comparative data for Tg measurements made by 2GIMA, RIA, and LC-MS/MS for the group A sera from patients with persistent/recurrent DTC. Sera with Tg values below detectability are shown in the shaded areas, indicating the respective sensitivity limits of the methods: <0.10 μg/L (FS) for Tg2GIMA, <0.5 μg/L (LOQ) for TgLC-MS/MS, and < 0.5 μg/L (FS) for TgRIA. A, 37 patients with disease who had no TgAb detected by either method K or method R (below FS limits). The solid red symbols indicate 5 patients displaying >30% CV between-method discordances. B, Serum Tg values for 52 TgAb-positive patients with disease. The squares show the method comparisons for 12 sera with Tg below the LOQ of the LC-MS/MS. A distinction is made between sera with unequivocally undetectable TgLC-MS/MS values (no peak = solid red squares) and sera with marginally detectable TgLC-MS/MS values in the 0.3 to 0.5 μg/L range (open red squares) (6, 9, 40).
Figure 2 shows relationships between TgRIA and Tg2GIMA for group B sera without (Figure 2A) vs with (Figure 2B) TgAb detected according to the FS cutoffs of both methods K and R. Group B TgAb-negative sera displayed a strong correlation between TgRIA and Tg2GIMA (Tg2GIMA = 0.96TgRIA − 0.3, r =0.95). Although the Tg2GIMA/TgRIA ratios for group A TgAb-negative sera were variable, overall they were comparable to those for group B TgAb-negative sera (101 ± 19% vs 83 ± 32%, group B vs group A, respectively, NS). When TgAb was detected, Tg2GIMA was frequently lower than TgRIA, and 25% of sera exhibited severe interference (Tg2GIMA of <0.10 μg/L) (Figure 2B). The table in Figure 2 shows that the relationship between TgRIA and severe interference was independent of the TgAb concentration and occurred with higher frequency (39%) at low TgRIA (1.0–2.5 μg/L) than at high (>10 μg/L) TgRIA (4%).
Figure 2.
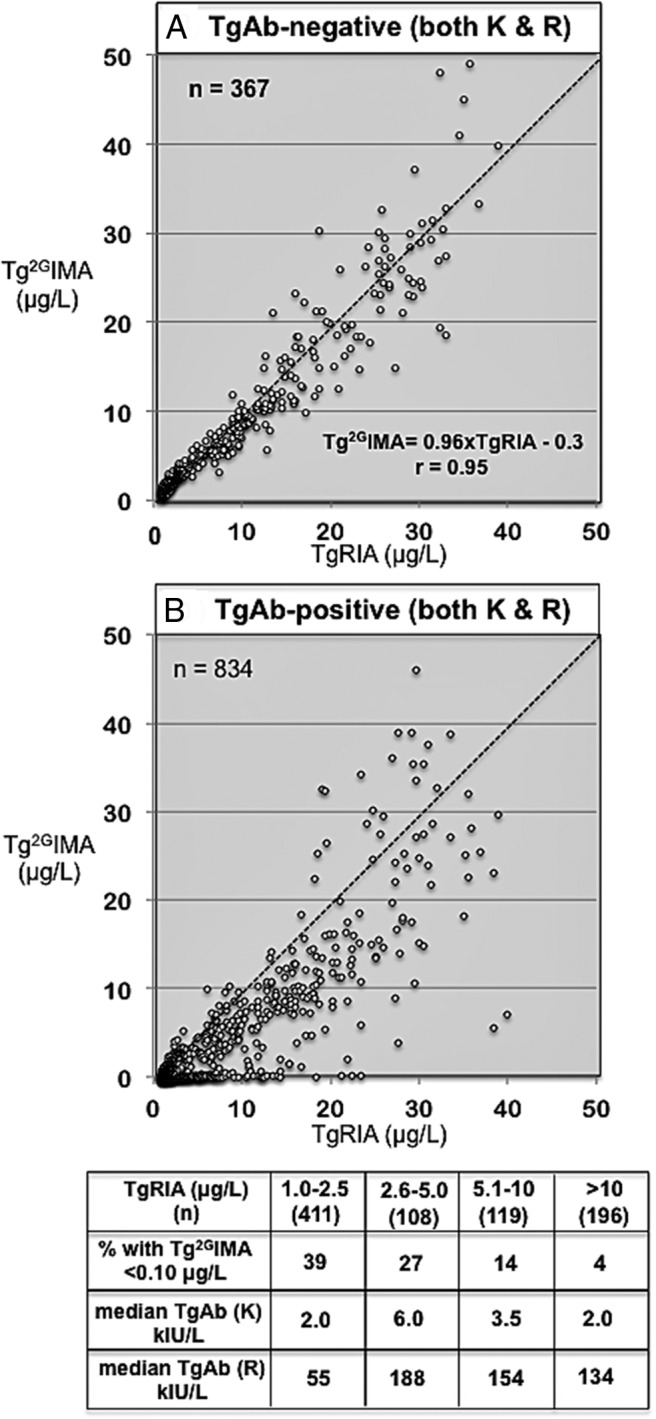
A, Relationship between TgRIA (abscissa) and Tg2GIMA (ordinate) for the 367 group B sera that were classified as TgAb negative according to the FS cutoff of both methods K and R. B, Relationship between TgRIA (abscissa) and Tg2GIMA (ordinate) for the 834 group B sera that were classified as TgAb-positive according to the FS cutoff of both methods K and R. The table below shows the median TgAb for methods K and R, and the percentage of severe interferences (Tg2GIMA of <0.10 μg/L) seen when TgAb-positive group B sera were analyzed according to TgRIA values (1.0–2.5, 2.6–5.0, 5.1–10, or >10 μg/L).
Figure 3 (top panels) shows TgAb method K vs R subgroup analyses for the 607 sequential sera (group C). With use of the FS cutoffs, the percentage classified as TgAb positive was comparable (42.6% vs 46.7%, K vs R, respectively, NS), but higher than previously reported for DTC (20%–30%) (2, 3). This reflected the use of FS cutoffs as opposed to the MCOs employed by earlier studies (2) as well as preferential ordering for the TgRIA methodology used by this laboratory. Of the group C sera, 66% had low (<1.0 μg/L) Tg (both TgRIA and Tg2GIMA), 30% had TgRIA between 1 and 40 μg/L (median, 3.0 μg/L), and only 4% had TgRIA of >40 μg/L. Four specimens with Tg of >1000 μg/L (both Tg2GIMA and TgRIA) were classified as TgAb positive by method R (23, 26, 33, and 148 kIU/L) but TgAb negative by method K, confirming other reports that high Tg concentrations interfere, causing false-positive method R values (33).
Figure 3.
Top panels: distribution of TgAb concentrations found for the 607 sequential DTC sera (group C) received for routine Tg + TgAb testing. Method K (left panel) and method R (right panel) data are analyzed as subgroups with cutoff values covering the range from low to very high values, with focus on the LOD, FS, MCO, and ROC curve cutoffs. Bottom panels: method K (left panel) and method R (right panel) data for the 1426 group B DTC sera with TgRIA of ≥1.0 μg/L (n = 1426). The black bars show the group frequencies for false-negative misclassifications, expressed as a percentage of the total number of sera classified as “negative” by that cutoff. A false-negative TgAb classification was defined as a serum with a TgAb below the cutoff that had a Tg2GIMA/TgRIA ratio of <75%, suggesting the presence of interfering TgAb. The yellow bars show the group frequencies for false-positive misclassifications, expressed as a percentage of the total number of sera classified as “positive” by that cutoff. A false-positive TgAb misclassification was defined as a serum with a TgAb value more than or equal to the cutoff that had a Tg2GIMA/TgRIA ratio of ≥75%, suggesting the absence of interfering TgAb. The red bars show the frequencies for severe interference, defined as an undetectable Tg2GIMA (<0.10 μg/L) associated with an unequivocally detectable TgRIA (≥1.0 μg/L). Blue bars show the frequencies for sera with inappropriately low Tg2GIMA (<0.30 μg/L).
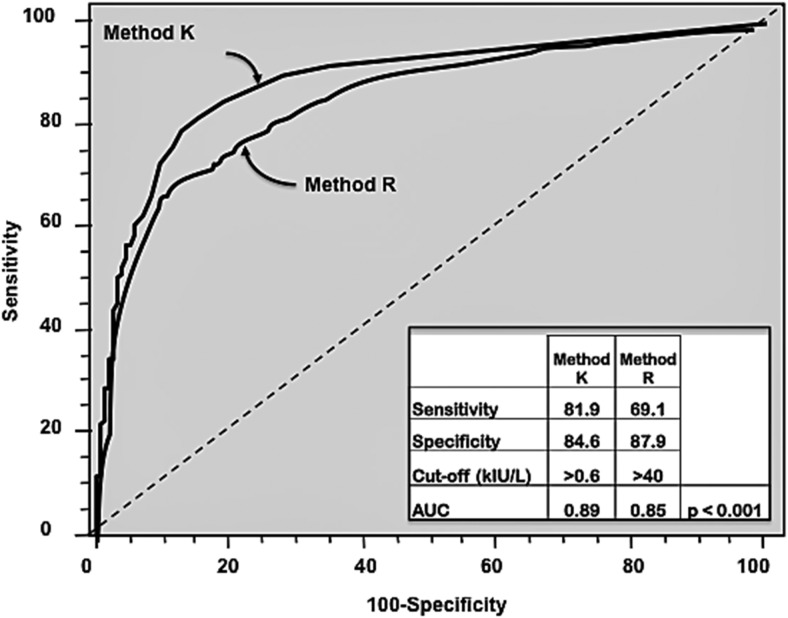
Figure 3 (bottom panels) shows Group B subgroup frequencies for false-positive and false-negative TgAb misclassifications using different method K vs R cutoffs ranging from the LOD to high TgAb concentrations and using low Tg2GIMA/TgRIA ratios (<75%) to indicate interfering TgAb. Table 1 summarizes subgroup characteristics, showing the performance at the most commonly used cutoffs (LOD, FS, ROC curve, and MCO) by bold type. Table 1 and Figure 3 show that as the TgAb cutoff increased, the number of false-negative misclassifications rose and the number of false-positive misclassifications declined. Comparable median TgRIA and Tg2GIMA were seen for the cutoffs close to the FS, but as the cutoff increased median Tg2GIMA progressively decreased to 0.10 μg/L at the highest cutoff, whereas median TgRIA remained relatively stable across the TgAb range. The preferential influence of rising TgAb on Tg2GIMA but not TgRIA produced a progressive decline in median Tg2GIMA/TgRIA ratios. The frequency of severe interference steadily rose with the increasing cutoff to peak at ∼15% at high TgAb concentrations. ROC curve analysis (Figure 4) reported optimal cutoffs of >0.6 kIU/L for method K vs >40 kIU/L for method R and showed that method K had higher sensitivity for TgAb detection (81.9% vs 69.1%, K vs R, respectively, P < .001) and more area under the curve (0.89 vs 0.85, respectively, P < .001). With ROC curve cutoffs of 0.7 kIU/L (K) vs 41 kIU/L (R), method K classified fewer sera as TgAb negative (46.3% vs 55.1%, K vs R, respectively, P < .001) of which fewer were false negatives (21.7% vs 31.6%, K vs R, respectively, P < .001). Furthermore, fewer false-negative sera displayed severe interference using method K (2.1% vs 6.2%, K vs R, respectively, P < .001). When the FS cutoff recommended by guidelines (30) was used, method K also displayed superior sensitivity. Specifically, although the percentage of TgAb negatives was comparable using FS cutoffs (32.1% vs 34.9%, K vs R, respectively, NS), fewer method K TgAb negatives were false negatives (13.5% vs 21.3%, K vs R, respectively, P < .01) and fewer displayed severe interference (0.7% vs 2.4%, K vs R, respectively, P < .05). In addition, using method K, fewer false-negative sera had inappropriately low Tg2GIMA (<0.30 μg/L) (0.7% vs 4.4%, K vs R, respectively, P < .001). Consistent with superior method K sensitivity, fewer method K false negatives were judged positive by method R compared with method R false negatives judged positive by method K (16.1% vs 50.9% K vs R, respectively, P < .001). A chart review revealed that a significant number of false-negative sera were from patients with DTC with a prior (method K) history of TgAb positivity (32.2% vs 20.8%, K vs R, respectively). Taken together, these data suggested that a low Tg2GIMA/Tg RIA ratio detected interfering TgAb more sensitively than direct TgAb measurement using either method, as reported previously (37).
Table 1.
Group B Sera Subgroup Analysis
| Cutoff, kIU/L | Sera in Group, n (%) | TgAb, Median, kIU/L |
Tg RIA, Median, μg/L | 2GTgIMA, Median, μg/L | 2GTgIMA/Tg RIA Ratio, Median, % | False Positive, % | False Negative, % | Tg2GIMA, % |
||
|---|---|---|---|---|---|---|---|---|---|---|
| K | R | <0.30 μg/L | <0.10 μg/L | |||||||
| TgAb method K | ||||||||||
| 0.3 (LOD) | 458 (32.1) | 0.3 | 15 | 6.6 | 5.5 | 93.8 | 22.4 | 13.5 | 0.7 | 0.7 |
| 0.4 (FS) | 62 (4.3) | 0.4 | 23 | 3.2 | 3.2 | 88.2 | 22.4 | 13.5 | 0.7 | 0.7 |
| 0.5 | 93 (6.5) | 0.5 | 21 | 3.5 | 3.5 | 79.3 | 19.1 | 15.4 | 1.5 | 1.2 |
| 0.6 | 47 (3.3) | 0.6 | 28 | 3.2 | 2.7 | 74.3 | 14.6 | 19.4 | 3.8 | 1.8 |
| 0.7 (ROC curve) | 39 (2.7) | 0.7 | 30 | 2.4 | 2.0 | 68.4 | 12.5 | 21.7 | 5.0 | 2.1 |
| 0.8 | 35 (2.5) | 0.8 | 34 | 1.9 | 0.8 | 43.0 | 11.0 | 23.7 | 7.7 | 2.4 |
| 0.9 | 39 (2.7) | 0.9 | 39 | 1.8 | 0.6 | 49.9 | 10.3 | 26.2 | 8.6 | 3.7 |
| 1.0 (MCO) | 58 (4.1) | 1.0 | 40 | 2.1 | 1.3 | 47.0 | 9.3 | 28.6 | 10.3 | 3.8 |
| 1.2 | 64 (4.5) | 1.3 | 45 | 2.4 | 1.5 | 55.0 | 8.7 | 32.5 | 11.4 | 4.7 |
| 1.5 | 75 (5.3) | 1.7 | 55 | 2.1 | 0.45 | 24.7 | 7.0 | 35.6 | 14.3 | 5.0 |
| 2 | 78 (5.5) | 2.5 | 75 | 1.9 | 0.34 | 16.1 | 5.3 | 39.3 | 16.8 | 6.7 |
| 3 | 97 (6.8) | 3.8 | 134 | 2.1 | 0.17 | 9.1 | 4.8 | 43.1 | 20.4 | 8.7 |
| 5 | 91 (6.4) | 6.8 | 289 | 2.4 | 0.52 | 6.8 | 4.6 | 47.6 | 22.5 | 11.4 |
| 10 | 70 (4.9) | 15 | 447 | 3.4 | 0.10 | 3.7 | 3.2 | 50.9 | 24.2 | 12.7 |
| 25 | 29 (2.0) | 33 | 661 | 3.5 | 0.03 | 1.3 | 1.7 | 53.2 | 24.7 | 14.2 |
| 50 | 29 (2.0) | 71 | 1312 | 4.7 | 0.10 | 2.6 | 1.1 | 54.2 | 25.9 | 15.2 |
| 100 | 30 (2.1) | 157 | 1347 | 4.7 | 0.10 | 2.0 | 0.0 | 55.1 | 27.2 | 15.4 |
| ≥250 | 32 (2.2) | 738 | 3621 | 7.7 | 0.10 | 2.4 | 0.0 | 59.5 | 26.6 | 15.8 |
| TgAb method R | ||||||||||
| 10 (LOD) | 229 (16.1) | 0.3 | 11 | 5.4 | 5.0 | 95.0 | 40.5 | 17.9 | 1.2 | 1.2 |
| 15 | 129 (9.0) | 0.3 | 16 | 4.5 | 3.7 | 91.7 | 34.9 | 14.8 | 1.7 | 0.9 |
| 18 | 71 (5.0) | 0.3 | 19 | 8.8 | 7.6 | 94.1 | 30.1 | 18.7 | 2.2 | 1.1 |
| 20 | 69 (4.8) | 0.4 | 21 | 3.5 | 3.6 | 84.2 | 26.5 | 18.6 | 2.3 | 0.9 |
| 22 (FS) | 71 (5.0) | 0.4 | 23 | 2.5 | 2.6 | 77.3 | 23.8 | 21.3 | 4.4 | 2.4 |
| 25 | 99 (6.9) | 0.5 | 27 | 2.4 | 2.0 | 73.5 | 21.4 | 24.4 | 6.2 | 3.2 |
| 30 | 62 (4.3) | 0.7 | 32 | 3.0 | 2.5 | 75.2 | 17.7 | 28.3 | 9.3 | 4.5 |
| 35 | 56 (3.9) | 0.9 | 37 | 2.1 | 1.5 | 68.6 | 14.7 | 30.0 | 10.1 | 5.2 |
| 41 (ROC curve) | 79 (5.5) | 0.9 | 45 | 1.8 | 0.34 | 32.0 | 11.7 | 31.6 | 11.7 | 6.2 |
| 50 | 54 (3.8) | 1.3 | 53 | 2.1 | 0.63 | 32.4 | 9.6 | 35.4 | 17.5 | 8.1 |
| 60 | 69 (4.8) | 1.8 | 69 | 2.1 | 0.83 | 40.0 | 8.3 | 37.9 | 18.8 | 9.1 |
| 80 | 68 (4.8) | 2.0 | 93 | 2.6 | 0.85 | 42.9 | 7.3 | 41.2 | 20.0 | 10.3 |
| 115 (MCO) | 91 (6.4) | 3.6 | 145 | 4.1 | 1.10 | 30.0 | 5.7 | 43.9 | 22.2 | 11.3 |
| 200 | 100 (7.0) | 6.0 | 287 | 3.5 | 0.86 | 26.8 | 5.0 | 47.8 | 24.0 | 12.8 |
| 400 | 68 (4.8) | 10.2 | 476 | 3.4 | 0.47 | 28.8 | 3.9 | 51.4 | 24.7 | 13.8 |
| 600 | 43 (3.0) | 30 | 775 | 3.3 | 0.14 | 5.0 | 2.7 | 53.6 | 25.2 | 14.5 |
| ≥1000 | 68 (4.8) | 129 | 2871 | 6.8 | 0.10 | 1.3 | 0.0 | 54.9 | 24.4 | 15.2 |
TgAb cutoffs frequently used by laboratories to define a “positive” TgAb (LOD, FS, ROC curve, and MCO) are shown in bold.
Figure 4.
Performances of TgAb methods K and R analyzed by ROC curve analyses of group B sera data for cutoffs covering the entire range of concentrations of TgAb methods K and R. AUC, area under the curve.
Methods K and R had comparable false-positive frequencies using FS cutoffs (22.4% vs 23.8%, K vs R, respectively, NS). However, method R had a wider subfunctional sensitivity range (10–21 kIU/L) vs that for method K (0.1–0.3 kIU/L), suggesting a higher method R potential for reporting false positives using cutoffs of <22 kIU/L.
Discussion
Reliable TgAb detection before Tg testing is critical when serum Tg2GIMA measurements are used as a DTC tumor marker. A “negative” TgAb test is used to authenticate the absence of TgAb interference, whereas a “positive” test suggests that the Tg2GIMA may be unreliable and report falsely low/undetectable serum Tg values that could mask disease—the most serious problem that compromises the clinical utility of Tg2GIMA testing. Laboratories often maximize the clinical sensitivity of Tg2GIMA measurement while minimizing the TgAb interference problem by reflexing TgAb-positive sera to a TgAb-resistant, but less sensitive, class of Tg method (RIA or LC-MS/MS) (5, 17). This study has clinical implications for laboratories that perform reflex Tg testing. The study confirmed that false-negative TgAb misclassifications were unacceptably high (30%–40%) using the MCOs for TgAb (10), whereas the FS cutoff minimized both false negatives and severe interferences associated with falsely low/undetectable Tg2GIMA. However, the FS cutoffs were associated with an approximate 20% false-positive frequency that could prompt unnecessarily reflexing of many sera to less sensitive RIA or LC-MS/MS methodology, which could fail to detect disease associated with low Tg concentrations (21–27). The FS cutoff also has an inherent 20% between-run imprecision (30) that could lead to false fluctuations in TgAb status (positive to negative or vice versa) while monitoring patients with low TgAb concentrations and, as a result, prompt unnecessary changes in the Tg method used. Guidelines caution about the need for Tg method continuity, because different numeric Tg values are reported when the same serum sample is measured by different methods (4, 9, 12, 17, 28–30). Although there were correlations between the methods in the absence of TgAb overall, the discordance between the Tg2GIMA, TgRIA, and TgLC-MS/MS measurements made for the individual TgAb-negative patients with disease was higher (mean 24% CV, range 0%–100%) than would be typical when Tg2GIMA is used consistently to monitor an individual patient at 6- to 12-month intervals (9). The 5 patients (14%) who displayed severe (>30% CV) between-method discordances (red circles in Figure 1A) emphasize how serum Tg monitoring could be disrupted by unnecessarily Tg method changes. Laboratories that reflex Tg testing to different methods based on the TgAb FS cutoff could guard against unnecessarily changing the Tg method by considering the patient's Tg and TgAb testing history, in addition to the TgAb status of the current specimen, before selecting an appropriate Tg method.
This study evaluated how different Kronus/RSR vs Roche TgAb method cutoffs for positivity influenced TgAb false-negative and false-positive frequencies, using a low Tg2GIMA/TgRIA ratio (<75%) to indicate the presence of interfering TgAb (2, 10, 36, 37). Table 1 and Figure 3 show that false positives declined whereas false negatives rose with a rising cutoff, but that no cutoff used for either method eliminated all false-negative and false-positive TgAb misclassifications. ROC curve analysis reported higher Kronus sensitivity vs Roche sensitivity, although both the ROC curve–determined and manufacturer-recommended cutoffs of both methods had unacceptably high false-negative frequencies (20%–40%) that included many cases of severe interference (Tg2GIMA of <0.10 μg/L) (10, 33, 34). This is the first study of TgLC-MS/MS measurement of clinically defined sera, contrasting with previous studies that were limited to methodologic correlations of TgLC-MS/MS with Tg2GIMA or TgRIA (11, 19). Severe TgAb interference causing Tg2GIMA underestimation was evident from undetectable Tg2GIMA seen for 38% of the TgAb-positive patients with disease (Figure 1B) and lower Tg2GIMA vs either TgLC-MS/MS or TgRIA seen for an additional 60% of these patients. The paradoxically undetectable TgLC-MS/MS seen for 12 of 52 (23%) TgAb-positive patients despite the presence of disease warrants further study. Two of these patients had no Tg detected by any method, and 8 had undetectable Tg2GIMA and TgLC-MS/MS but unequivocally detectable (≥1.0 μg/L) TgRIA. It was more common to see TgAb-positive sera with TgRIA higher (>50%) than TgLC-MS/MS than sera with higher TgLC-MS/MS than TgRIA (56% vs 11%, respectively, P < .01), consistent with either TgAb interference causing TgRIA overestimation or false-positive TgRIA (42, 43) or, alternatively, the presence of a polymorphic tumor Tg that failed to generate the target Tg peptide necessary for LC-MS/MS detection (20). It was striking that both TgLC-MS/MS and TgRIA values were significantly lower (P < .001) when patients with disease had TgAb detected (Figure 1, B vs A). This observation lends support to past studies, suggesting that increased metabolic clearance of Tg-TgAb complexes may be responsible (5, 44–47). If the metabolic clearance of Tg complexed with TgAb is faster than the clearance of free Tg, high Tg assay FS would be especially critical for detecting disease in TgAb-positive patients (22, 26).
Guidelines mandate that each DTC specimen have a TgAb status determined directly by immunoassay and not a Tg recovery test (1, 5, 6, 17, 28, 30). Current TgAb methods vary in sensitivity, specificity, and the numeric values they report, despite claiming to use the same primary calibrator (Medical Research Council [MRC] 65/93) (4, 10, 12, 31–35). This study of 2 well-established TgAb methods (Kronus/RSR vs Roche) found that both the intrinsic sensitivity of the method and the cutoff selected for TgAb positivity influenced the reliability of TgAb detection. In addition, Tg concentrations of >1000 μg/L interfered with the Roche method, as described previously (33). Some sera were classified as TgAb positive by one method but TgAb negative by the other, and some low TgAb concentrations caused profound interference whereas high TgAb in other sera appeared nonreactive (2, 5, 10, 17, 48). These qualitative TgAb differences reflect a complex matrix of factors that include the numeric cutoff for positivity (10, 33, 34) as well as the epitope specificity of the individual patient's serum Tg antibodies for binding the thyroglobulin reagent used in the TgAb test (10, 33, 35, 48–50). Comparative TgAb method studies fail to assess how Tg antibodies in individual sera affect Tg measurement (10, 31–35). Either a positive TgAb test and/or discordant Tg results between different analytical methods (eg, Tg2GIMA vs TgRIA) are recognized indicators for possible Tg interference (18). The current study used an abnormally low Tg2GIMA/TgRIA ratio (<75%) to assess the influence of interfering TgAb on Tg measurement, as reported previously (2, 5, 10, 18, 36, 37, 51). This Tg2GIMA/TgRIA ratio parameter had the advantages of being independent of the measured TgAb concentration as well as the potential to amplify TgAb influences by distorting Tg2GIMA and TgRIA values in opposite directions. Thus, the unidirection (underestimation) typical of TgAb interference with IMA, coupled with either an unaffected or overestimated TgRIA, would produce a lower Tg2GIMA/TgRIA ratio (2, 4, 9, 10, 36, 37). Although specimen availability and cost constraints prevented the use of Tg2GIMA/TgLC-MS/MS ratios for the entire study, the utility of the Tg2GIMA/TgRIA ratio parameter is supported by the concordance found between ratios calculated for patients with disease (Figure 1) using either TgRIA or TgLC-MS/MS as the denominator. Specifically, when Tg was detectable, making ratio calculations possible, the Tg2GIMA/TgRIA vs Tg2GIMA/TgLC-MS/MS ratios were comparable both in the presence and absence of TgAb. It should be noted that the group A TgAb-negative patients with disease displayed a wider range of Tg2GIMA/TgRIA ratios than that seen for group B TgAb-negative results that comprised a larger number of sera but probably included fewer patients with disease (with no clinical information available). The wider range of Tg2GIMA/TgRIA ratios seen for the group A TgAb-negative patients reflected the discordances seen between the 3 methods that characterized these patients (Figure 1A) and probably reflected different method specificities for detecting heterogeneous, tumor-derived, Tg molecules (51).
When FS cutoffs were used, the Roche method reported significantly more false negatives with severe interferences than the Kronus method (2.4% vs 0.7%, R vs K, respectively, P < .05), and more false negatives with inappropriately low (<0.30 μg/L) Tg2GIMA values (4.4% vs 0.7%, R vs K, respectively, P < .001). Underestimation causing falsely low (but detectable) Tg2GIMA values has as much potential to disrupt clinical management as severe interference, because a Tg2GIMA of <0.30 μg/L predicts the absence of disease (14–16, 18, 24, 25, 27, 29). Now that radioiodine remnant ablation is no longer considered a necessary treatment for low-risk DTC (52–56), an increasing number of disease-free patients will undergo lifelong monitoring of low basal (non-TSH–stimulated) Tg2GIMA concentrations (0.10–1.0 μg/L range) arising from normal remnant tissue (9, 24, 25, 27, 57, 58). TgAb positivity is associated with a higher DTC recurrence risk (59–62) and changes in a patient's TgAb status have clinical implications: a TgAb decline or disappearance is considered a good prognostic sign, whereas a rise in or de novo appearance of TgAb suggests active disease (2–7, 59, 60, 63–68). When sera are misclassified as TgAb negative and have inappropriately low Tg2GIMA values due to interference, any recurrence that causes a rising TgAb could further suppress Tg2GIMA and be misinterpreted as a good prognostic sign.
This study has a number of clinical implications. It is the first to show that a detectable or higher TgLC-MS/MS is frequently seen for TgAb-positive patients with persistent/recurrent DTC and undetectable/low Tg2GIMA (11, 19, 20), confirming that TgAb interference causing Tg2GIMA underestimation can mask disease (Figure 1B). The study emphasizes how concordance between Tg2GIMA and Tg measured by a different class of method (RIA or LC-MS/MS) may help prove that a Tg2GIMA result is free from TgAb interference (17, 18). The primary focus of the study was the influence of TgAb method cutoffs on the reliability of TgAb detection. No cutoff could be identified for either the Kronus or Roche TgAb method that eliminated both false-negative and false-positive serum TgAb misclassifications. Because TgAb interference causing Tg2GIMA underestimation is considered the most serious clinical problem, it is optimal to use the FS cutoff to define TgAb positivity because this cutoff minimizes false negatives. However, a significant percentage of sera (∼20%) would be misclassified as TgAb-positive using FS and could be unnecessarily reflexed to the less sensitive TgRIA or TgLC-MS/MS methodologies that may fail to detect disease associated with low Tg concentrations (21–27). In addition, use of the FS cutoff can cause the TgAb status of patients with low TgAb concentrations to fluctuate between “negative” and “positive” over time, as a result of assay imprecision (15%–20%) exacerbated by the TgAb biologic variability (∼15%) expected during DTC monitoring (69). Changes in a patient's TgAb status that were solely methodologic could prompt unnecessary switching between Tg methodologies (Tg2GIMA to TgRIA or TgLC-MS/MS, or vice versa) and potentially disrupt serial Tg monitoring, thereby negatively affecting clinical management. The potential for disrupting Tg monitoring is clearly evident from the significant serum Tg2GIMA, TgRIA, and TgLC-MS/MS differences seen in Figure 1A that appear unrelated to TgAb and possibly reflect method specificity differences for tumor Tg detection. Laboratories that reflex Tg testing to different methods should minimize unnecessary Tg method changes by considering the individual patient's Tg and TgAb measurement history in addition to the TgAb status of the current specimen before selecting an appropriate Tg method and establishing a new baseline for patients when a change in the Tg or TgAb method becomes necessary. Physicians should recognize the technical limitations of the current Tg and TgAb measurements illustrated by this study and interpret serum Tg and TgAb values relative to the clinical status of the patient.
Acknowledgments
We thank Dr Stefan Grebe and Brian Netzel for collaborating to provide the TgLC-MS/MS analyses at the Mayo Medical Laboratories.
Results from this work were presented in part at the 82nd Annual Meeting of the American Thyroid Association, Quebec City, Canada, 2012.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- coefficient of variation
- DTC
- differentiated thyroid cancer
- FS
- functional sensitivity
- 2GIMA
- second-generation immunometric assay
- K
- Kronus
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LOQ
- limit of quantitation
- MCO
- manufacturer-recommended cutoff
- NS
- not significant
- R
- Roche
- ROC
- receiver operating characteristic
- RIA
- radioimmunoassay
- Tg
- thyroglobulin
- TgAb
- thyroglobulin autoantibody.
References
- 1. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 2. Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127. [DOI] [PubMed] [Google Scholar]
- 3. Görges R, Maniecki M, Jentzen W, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153:49–55. [DOI] [PubMed] [Google Scholar]
- 4. Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods—strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013;27:701–712. [DOI] [PubMed] [Google Scholar]
- 5. Verburg FA, Luster M, Cupini C, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23:1211–1225. [DOI] [PubMed] [Google Scholar]
- 6. Spencer C. Commentary on: Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23:1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ringel MD, Nabhan F. Approach to follow-up of the patient with differentiated thyroid cancer and positive anti-thyroglobulin antibodies. J Clin Endocrinol Metab. 2013;98:3104–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLeod DS, Cooper DS, Ladenson PW, et al. Prognosis of differentiated thyroid cancer in relation to serum thyrotropin and thyroglobulin antibody status at time of diagnosis. Thyroid. 2014;24:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer C, LoPresti JS, Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancers, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes. 2014;21:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spencer C, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:1283–1291. [DOI] [PubMed] [Google Scholar]
- 11. Clarke NJ, Zhang Y, Reitz RE. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J Investig Med. 2012;60:1157–1163. [DOI] [PubMed] [Google Scholar]
- 12. Grebe SKG. Diagnosis and management of thyroid carcinoma: a focus on serum thyroglobulin. Expert Rev Endocrinol Metab. 2009;4:25–43. [Google Scholar]
- 13. Spencer C, Fatemi S, Singer P, Nicoloff J, LoPresti J. Serum basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid. 2010;20:587–595. [DOI] [PubMed] [Google Scholar]
- 14. Malandrino P, Latina A, Marescalco S, et al. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab. 2011;96:1703–1709. [DOI] [PubMed] [Google Scholar]
- 15. Chindris AM, Diehl NN, Crook JE, Fatourechi V, Smallridge RC. Undetectable sensitive serum thyroglobulin (<0.1 ng/mL) in 163 patients with follicular cell-derived thyroid cancer: results of rhTSH stimulation and neck ultrasonography and long-term biochemical and clinical follow-up. J Clin Endocrinol Metab. 2012;97:2714–2723. [DOI] [PubMed] [Google Scholar]
- 16. Trimboli P, La Torre D, Ceriani L, et al. High sensitive thyroglobulin assay on thyroxine therapy: can it avoid stimulation test in low and high risk differentiated thyroid carcinoma patients? Horm Metab Res. 2013;45:664–668. [DOI] [PubMed] [Google Scholar]
- 17. Giovanella L, Clark PM, Chiovato L, et al. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol. 2014;171:R33–R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giovanella L, Treglia G, Sadeghi R, Trimboli P, Ceriani L, Verburg FA. Unstimulated highly sensitive thyroglobulin in follow-up of differentiated thyroid cancer patients: a meta-analysis. J Clin Endocrinol Metab. 2014;99:440–447. [DOI] [PubMed] [Google Scholar]
- 19. Kushnir MM, Rockwood AL, Roberts WL, Abraham D, Hoofnagle AN, Meikle AW. Measurement of thyroglobulin by liquid chromatography-tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin Chem. 2013;59:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoofnagle AN, Roth MY. Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab. 2013;98:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zöphel K, Wunderlich G, Smith BR. Serum thyroglobulin measurements with a high sensitivity enzyme-linked immunosorbent assay: is there a clinical benefit in patients with differentiated thyroid carcinoma? Thyroid. 2003;13:861–865. [DOI] [PubMed] [Google Scholar]
- 22. Heilo A, Sigstad E, Fagerlid KH, et al. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2750–2755. [DOI] [PubMed] [Google Scholar]
- 23. Iervasi A, Iervasi G, Ferdeghini M, et al. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf). 2007;67:434–441. [DOI] [PubMed] [Google Scholar]
- 24. Miyauchi A, Kudo T, Miya A, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21:707–716. [DOI] [PubMed] [Google Scholar]
- 25. Wong H, Wong KP, Yau T, et al. Is there a role for unstimulated thyroglobulin velocity in predicting recurrence in papillary thyroid carcinoma patients with detectable thyroglobulin after radioiodine ablation? Ann Surg Oncol. 2012;19:3479–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hay ID, Lee RA, Davidge-Pitts C, Reading CC, Charboneau JW. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. 2013;154:1448–1454; discussion 1454–1455. [DOI] [PubMed] [Google Scholar]
- 27. Kelders A, Kennes LN, Krohn T, Behrendt FF, Mottaghy FM, Verburg FA. Relationship between positive thyroglobulin doubling time and 18F-FDG PET/CT-positive, 131I-negative lesions. Nucl Med Commun. 2014;35:176–181. [DOI] [PubMed] [Google Scholar]
- 28. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:5566–5575. [DOI] [PubMed] [Google Scholar]
- 29. Schlumberger M, Hitzel A, Toubert ME, et al. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:2487–2495. [DOI] [PubMed] [Google Scholar]
- 30. Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. [DOI] [PubMed] [Google Scholar]
- 31. Krahn J, Dembinski T. Thyroglobulin and anti-thyroglobulin assays in thyroid cancer monitoring. Clin Biochem. 2009;42:416–419. [DOI] [PubMed] [Google Scholar]
- 32. Taylor KP, Parkington D, Bradbury S, Simpson HL, Jefferies SJ, Halsall DJ. Concordance between thyroglobulin antibody assays. Ann Clin Biochem. 2011;48:367–369. [DOI] [PubMed] [Google Scholar]
- 33. Pickett AJ, Jones M, Evans C. Causes of discordance between thyroglobulin antibody assays. Ann Clin Biochem. 2012;49:463–467. [DOI] [PubMed] [Google Scholar]
- 34. Nygaard B, Bentzen J, Laurberg P, et al. Large discrepancy in the results of sensitive measurements of thyroglobulin antibodies in the follow-up on thyroid cancer: a diagnostic dilemma. Eur Thyroid J. 2012;1:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Latrofa F, Ricci D, Montanelli L, et al. Thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: comparison of different assays and evaluation of causes of discrepancies. J Clin Endocrinol Metab. 2012;97:3974–3982. [DOI] [PubMed] [Google Scholar]
- 36. Clark P, Franklyn J. Can we interpret serum thyroglobulin results? Ann Clin Biochem. 2012;49:313–322. [DOI] [PubMed] [Google Scholar]
- 37. Crane MS, Strachan MW, Toft AD, Beckett GJ. Discordance in thyroglobulin measurements by radioimmunoassay and immunometric assay: a useful means of identifying thyroglobulin assay interference. Ann Clin Biochem. 2013;50:421–432. [DOI] [PubMed] [Google Scholar]
- 38. Algeciras-Schimnich A, Lasho MA, Ness KM, Cheryk LA, Grebe SK. The Roche Elecsys and Siemens-Centaur thyroglobulin autoantibody assays show comparable clinical performance to the recently unavailable Beckman-Coulter access thyroglobulin autoantibody assay in identifying samples with potentially false-low thyroglobulin measurements due to thyroglobulin autoantibody interference. Thyroid. 2011;21:813–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spencer CA, Platler BW, Guttler RB, Nicoloff JT. Heterogeneity of 125I-labeled human thyroglobulin preparations. Clin Chim Acta. 1985;151:121–132. [DOI] [PubMed] [Google Scholar]
- 40. Netzel BC, Grebe SK, Algeciras-Schimnich A. Usefulness of a thyroglobulin liquid chromatography-tandem mass spectrometry assay for evaluation of suspected heterophile interference. Clin Chem. 2014;60:1016–1018. [DOI] [PubMed] [Google Scholar]
- 41. Rodbard D. Statistical estimation of the minimal detectable concentration (“sensitivity”) for radioligand assays. Anal Biochem. 1978;90:1–12. [DOI] [PubMed] [Google Scholar]
- 42. Schneider AB, Pervos R. Radioimmunoassay of human thyroglobulin: effect of antithyroglobulin autoantibodies. J Clin Endocrinol Metab. 1978;47:126–137. [DOI] [PubMed] [Google Scholar]
- 43. Feldt-Rasmussen U, Rasmussen AK. Serum thyroglobulin (Tg) in presence of thyroglobulin autoantibodies (TgAb). Clinical and methodological relevance of the interaction between Tg and TgAb in vitro and in vivo. J Endocrinol Invest. 1985;8:571–576. [DOI] [PubMed] [Google Scholar]
- 44. Weigle WO, High GJ. The behavior of autologous thyroglobulin in the circulation of rabbits immunized with either heterologous or altered homologous thyroglobulin. J Immunol. 1967;98:1105–1114. [PubMed] [Google Scholar]
- 45. Feldt-Rasmussen U, Petersen PH, Date J, Madsen CM. Sequential changes in serum thyroglobulin (Tg) and its autoantibodies (TgAb) following subtotal thyroidectomy of patients with preoperatively detectable TgAb. Clin Endocrinol (Oxf). 1980;12:29–38. [DOI] [PubMed] [Google Scholar]
- 46. Feldt-Rasmussen U. Serum thyroglobulin and thyroglobulin autoantibodies in thyroid diseases. Pathogenic and diagnostic aspects. Allergy. 1983;38:369–387. [DOI] [PubMed] [Google Scholar]
- 47. van der Laken CJ, Voskuyl AE, Roos JC, et al. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. Ann Rheum Dis. 2007;66:253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Latrofa F, Ricci D, Grasso L, et al. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab. 2008;93:591–596. [DOI] [PubMed] [Google Scholar]
- 49. Spencer CA. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011;96:3615–3627. [DOI] [PubMed] [Google Scholar]
- 50. Latrofa F, Ricci D, Montanelli L, et al. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab. 2012;97:2380–2387. [DOI] [PubMed] [Google Scholar]
- 51. Heilig B, Hüfner M, Dörken B, Schmidt-Gayk H. Increased heterogeneity of serum thyroglobulin in thyroid cancer patients as determined by monoclonal antibodies. Klin Wochenschr. 1986;64:776–780. [DOI] [PubMed] [Google Scholar]
- 52. Sabra MM, Grewal RK, Ghossein RA, Tuttle RM. Higher administered activities of radioactive iodine are associated with less structural persistent response in older, but not younger, papillary thyroid cancer patients with lateral neck lymph node metastases. Thyroid. 2014;24:1088–1095. [DOI] [PubMed] [Google Scholar]
- 53. Nascimento C, Borget I, Troalen F, et al. Ultrasensitive serum thyroglobulin measurement is useful for the follow-up of patients treated with total thyroidectomy without radioactive iodine ablation. Eur J Endocrinol. 2013;169:689–693. [DOI] [PubMed] [Google Scholar]
- 54. Ibrahimpasic T, Nixon IJ, Palmer FL, et al. Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer—is there a need for radioactive iodine therapy? Surgery. 2012;152:1096–1105. [DOI] [PubMed] [Google Scholar]
- 55. Nixon IJ, Ganly I, Patel SG, et al. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid. 2013;23:683–694. [DOI] [PubMed] [Google Scholar]
- 56. Tuttle RM, Sabra MM. Selective use of RAI for ablation and adjuvant therapy after total thyroidectomy for differentiated thyroid cancer: a practical approach to clinical decision making. Oral Oncol. 2013;49:676–683. [DOI] [PubMed] [Google Scholar]
- 57. Giovanella L, Trimboli P, Verburg FA, et al. Thyroglobulin levels and thyroglobulin doubling time independently predict a positive 18F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:874–880. [DOI] [PubMed] [Google Scholar]
- 58. Angell TE, Spencer CA, Rubino BD, Nicoloff JT, LoPresti JS. In search of an unstimulated thyroglobulin baseline value in low-risk papillary thyroid carcinoma patients not receiving radioactive iodine ablation. Thyroid. 2014;24:1127–1133. [DOI] [PubMed] [Google Scholar]
- 59. Chung JK, Park YJ, Kim TY, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf). 2002;57:215–221. [DOI] [PubMed] [Google Scholar]
- 60. Durante C, Tognini S, Montesano T, et al. Clinical aggressiveness and long-term outcome in patients with papillary thyroid cancer and circulating anti-thyroglobulin autoantibodies. Thyroid. 2014;24:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Adil A, Jafri RA, Waqar A, et al. Frequency and clinical importance of anti-Tg auto-antibodies (ATG). J Coll Physicians Surg Pak. 2003;13:504–506. [PubMed] [Google Scholar]
- 62. Soyluk O, Boztepe H, Aral F, Alagol F, Özbey NC. Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment. Thyroid. 2011;21:1301–1308. [DOI] [PubMed] [Google Scholar]
- 63. Tumino S, Belfiore A. Appearance of antithyroglobulin antibodies as the sole sign of metastatic lymph nodes in a patient operated on for papillary thyroid cancer: a case report. Thyroid. 2000;10:431–433. [DOI] [PubMed] [Google Scholar]
- 64. Chiovato L, Latrofa F, Braverman LE, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351. [DOI] [PubMed] [Google Scholar]
- 65. Kim WG, Yoon JH, Kim WB, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:4683–4689. [DOI] [PubMed] [Google Scholar]
- 66. Tsushima Y, Miyauchi A, Ito Y, et al. Prognostic significance of changes in serum thyroglobulin antibody levels of pre- and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients. Endocr J. 2013;60:871–876. [DOI] [PubMed] [Google Scholar]
- 67. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2013;98:2409–2414. [DOI] [PubMed] [Google Scholar]
- 68. Hsieh CJ, Wang PW. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid. 2014;24:488–493. [DOI] [PubMed] [Google Scholar]
- 69. Jensen E, Petersen PH, Blaabjerg O, Hegedüs L. Biological variation of thyroid autoantibodies and thyroglobulin. Clin Chem Lab Med. 2007;45:1058–1064. [DOI] [PubMed] [Google Scholar]