Abstract
Shprintzen–Goldberg syndrome (SGS) is a rare, systemic connective tissue disorder characterized by craniofacial, skeletal, and cardiovascular manifestations that show a significant overlap with the features observed in the Marfan (MFS) and Loeys–Dietz syndrome (LDS). A distinguishing observation in SGS patients is the presence of intellectual disability, although not all patients in this series present this finding. Recently, SGS was shown to be due to mutations in the SKI gene, encoding the oncoprotein SKI, a repressor of TGFβ activity. Here, we report eight recurrent and three novel SKI mutations in eleven SGS patients. All were heterozygous missense mutations located in the R-SMAD binding domain, except for one novel in-frame deletion affecting the DHD domain. Adding our new findings to the existing data clearly reveals a mutational hotspot, with 73% (24 out of 33) of the hitherto described unrelated patients having mutations in a stretch of five SKI residues (from p.(Ser31) to p.(Pro35)). This implicates that the initial molecular testing could be focused on mutation analysis of the first half of exon 1 of SKI. As the majority of the known mutations are located in the R-SMAD binding domain of SKI, our study further emphasizes the importance of TGFβ signaling in the pathogenesis of SGS.
INTRODUCTION
Shprintzen–Goldberg syndrome (SGS; MIM 182212) is a rare, multisystemic connective tissue disorder characterized by craniosynostosis, severe skeletal muscle hypotonia, and intellectual disability. Common features observed in SGS patients include craniofacial (craniosynostosis, proptosis, dolichocephaly, hypertelorism, high arched palate, downslanting palpebral fissures, and retrognathia), skeletal (arachnodactyly, camptodactyly, scoliosis, pectus deformity, and joint hypermobility), cardiovascular (mitral valve prolaps and aortic dilatations), and neuromuscular (infantile hypotonia and intellectual disability) anomalies. As such, a considerable clinical overlap with Marfan syndrome (MFS; MIM 154700) and Loeys–Dietz syndrome (LDS; LDS1A: MIM 609192, LDS1B: MIM 610168, LDS2A: MIM 608967, LDS2B: MIM 610380, LDS3: MIM 613795, LDS4: MIM 190220) exists.1, 2 During the last decade, it has become increasingly clear that dysregulated transforming growth factor beta (TGFβ) signaling is having a major role in the pathogenesis of MFS, LDS, and related disorders involving thoracic aortic aneurysms.3, 4, 5, 6, 7, 8, 9 Because of the overlap with MFS and LDS and because several lines of evidence have confirmed a key role of TGFβ signaling in the pathogenesis of MFS and LDS, it was hypothesized that altered TGFβ signaling also underlies the SGS pathogenesis. Indeed, de novo, heterozygous mutations in SKI (Sloan-Kettering Institute), encoding a known repressor of TGFβ signaling, were identified in 10 SGS patients.10 The causal nature of SKI mutations was confirmed in two additional cohorts of SGS patients, in which two in-frame deletions and twelve missense mutations were identified.11, 12 All SKI mutations clustered in two distinct N-terminally located regions of the protein: the R-SMAD binding domain and the Dachshund-homology domain (DHD).
SKI is an avian sarcoma viral oncogene homolog that is located on chromosome 1 and consists of seven exons. Its gene product counts 728 amino acids and functions as a repressor of TGFβ signaling by inhibiting R-SMAD (SMAD2-3) phosphorylation, hereby preventing assembly of the active heteromeric R-SMAD/SMAD4 complex and translocation to the nucleus.13, 14, 15 Additionally, the SKI protein can recruit transcriptional corepressors including SNW1, N-CoR, and mSIN3 and forms a complex with histone deacetylases.16, 17 In vivo studies in Xenopus embryos, zebrafish, and mice have shown that SKI has a critical role in the development of neuronal and muscle cells or tissues.18, 19, 20 Homozygous Ski-targeted mice show a major reduction in skeletal muscle mass and defects in cranial neural tube closure, manifestations that resemble those observed in SGS patients.19 These experiments suggest a critical role for SKI in the development of muscle, craniofacial structures, and the central and peripheral nervous system, providing additional evidence for the pathogenic character of SKI mutations in SGS.
Here, we report on the identification of ten additional SKI mutations, three novel and seven previously reported, in eleven unpublished SGS patients. Except one, all mutations are located in the R-SMAD binding domain of SKI, confirming the importance of this domain. Moreover, our new data reveal a mutational hotspot, with 73% of the hitherto described patients having mutations affecting four SKI residues.
MATERIALS AND METHODS
Study participants
In total, 19 patients with clinically suspected SGS were recruited from the Howard Hughes Medical Institute (Baltimore, MD, USA), the Johns Hopkins University School of Medicine (Baltimore, MD, USA), the SW Thames Regional Genetics Service, St George's (London, UK), the King's College London School of Medicine (London, UK), the National and Kapodistrian University of Athens Medical School (Athens, Greece), the Birmingham Women's Hospital (Birmingham, UK), Gendia (Antwerp, Belgium), the Erasmus Medical Center (Rotterdam, The Netherlands), Nebraska Medical Center (Omaha, USA), Murdoch Childrens Research Institute (Parkville, Australia), Radboud University Nijmegen Medical Center (Nijmegen, The Netherlands), School of Medicine of Ribeirao Preto (Sao Paulo, Brazil), Churchill Hospital (Oxford, UK), Chapel Allerton Hospital (Leeds, UK), Institute for Pathology and Genetics (Gosselies, Belgium), Kasturba Medical College (Manipal, India), and All Childrens Hospital (Florida, USA). Informed consent was obtained from all patients or their families.
Mutation analysis and Sanger sequencing
DNA was extracted from blood using standard procedures. PCR was performed using standard conditions on a GeneAmp PCR System 2700 thermal cycler (Applied Biosystems, Foster City, CA, USA). PCR products were bidirectionally sequenced using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems) and separated on an ABI 3130XL Genetic Analyzer in accordance with the manufacturer's instructions (Applied Biosystems). Primer sequences and conditions have been described previously.10 Sequences were analyzed using the CLC Sequence Viewer (CLC bio, Aarhus, Denmark). Sequence comparison and numbering are based on Ensembl transcript ENST00000378536 (Refseq NM_003036.3).
RESULTS
Sanger sequencing of the complete coding region of SKI in nineteen patients with a clinical picture compatible with SGS revealed eight different, heterozygous missense mutations in addition to an in-frame deletion of 12 bp (Figures 1 and 2) in eleven patients. The p.(Pro35Ser) mutation occurred in two unrelated patients (pat. 4 and 6) and the p.(Gly34Asp) was found in two siblings (pat. 10 and 11). We could prove the de novo occurrence of the mutations in five patients. For patient 8 only the mother, in whom the mutation was absent, could be tested. No DNA was available of the father of patient 8, nor of the parents of the patients 1–3. We did not find any evidence of somatic mosaicism in the blood of the parents of the siblings (pat. 10 and 11), thus germline mosaicism is the most likely explanation for the occurrence of two diseased children from healthy parents. Six different mutations have been described previously,10, 11 while three were novel (p.(Ser28Thr), p.(Gly34Ala), and p.(Ser97_Arg100del)). Except the p.Ser97_Arg100 in-frame deletion, which was positioned in the DHD domain, all mutations clustered in the R-SMAD binding domain of SKI (Figure 2). Besides the de novo occurrence, we considered the following facts as additional proof of causality: (1) six out of nine different mutations had previously been described as disease causing; (2) the mutations were not present in the 1000 Genomes project, in dbSNP135, or in more than 10 000 alleles of the National Heart, Lung and Blood Institute (NHLBI) Exome Variant Server; (3) all mutations affected highly conserved SKI residues; (4) MutationTaster, SIFT, and Polyphen categorized the eight missense mutations as disease causing, not tolerated and as possibly or probably damaging, respectively. All variants described in this paper are online available at the SKI locus-specific database on the HGVS website.
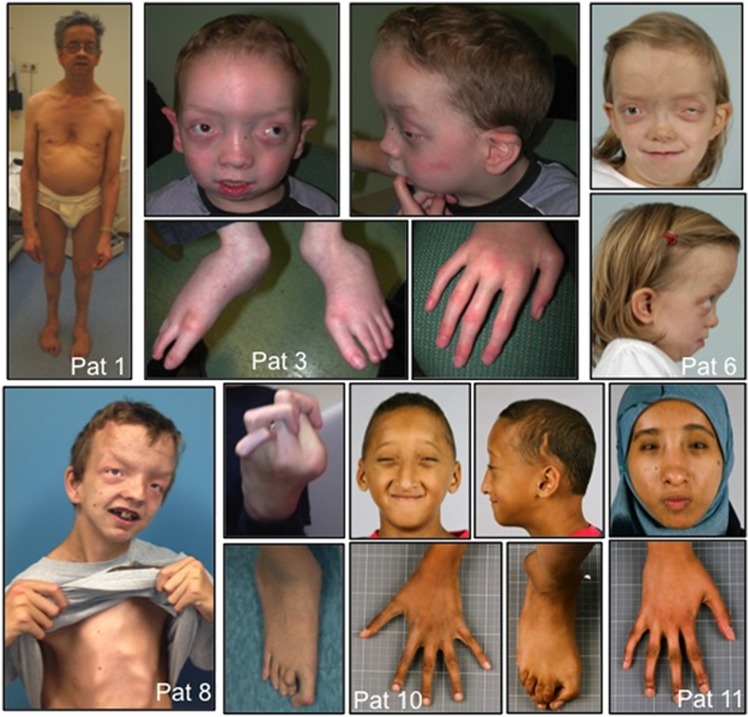
Figure 1.
Clinical pictures of SGS patients. Note typical craniofacial features including craniosynostotic skull with proptosis, dolichocephaly with retrognathia, low set ears, and hypertelorism. Skeletal findings include arachnodactyly, scoliosis, and a combination of joint hyperlaxity and contractures with camptodactyly.
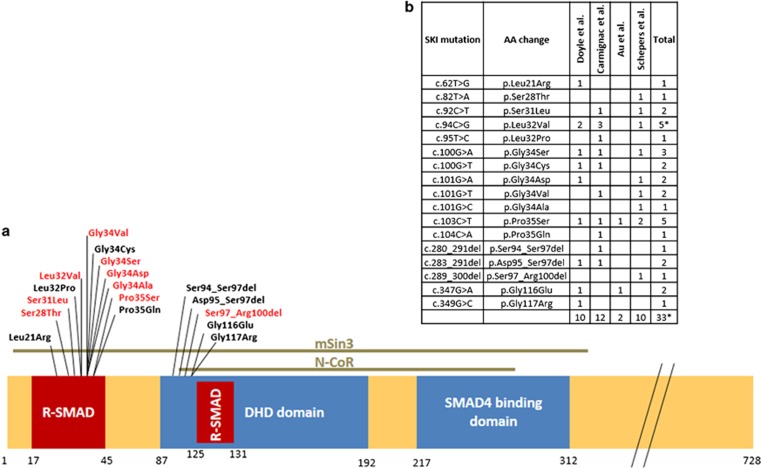
Figure 2.
(a) Schematic representation of the SKI domains in exon 1. Location of all SKI mutations known so far with respect to the binding sites of SKI-binding partners. Mutations in red are identified and discussed in this paper. (b) Overview of all published mutations identified so far in SKI. *One patient was identical in Doyle et al10 (patient 4) and Carmignac et al11 (patient 12), resulting in 33 independent SGS patients with a mutation in SKI. A full color version of this figure is available at the European Journal of Human Genetics journal online.
The clinical picture of the 11 patients having an SKI mutation is compatible with previously reported patients. Typical craniofacial features include craniosynostosis with proptosis, dolichocephaly with retrognathia, hypertelorism, low set ears, and the absence of ectopia lentis. Skeletal findings include arachnodactyly, scoliosis, and a combination of joint hyperlaxity and contractures with camptodactyly. Except one, all patients had invariable mild-to-moderate developmental delay and intellectual disability. As seen in previous studies some patients had mild aortic root dilatation. Table 1 and Figure 1 summarize the clinical picture of the 11 SGS patients. Most patients who were negative for SKI mutations had atypical clinical features (see Supplementary Table 1).
Table 1. Clinical description of the SGS patients.
| Subject ID | Pat. 1 | Pat. 2 | Pat. 3 | Pat. 4 | Pat. 5 | Pat. 6 | Pat. 7 | Pat.8 | Pat. 9 | Pat. 10a | Pat. 11a | Literature | Total (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SKI mutation | c.94C>G | c.101G>T | c.82T>A | c.103C>T | c.100G>A | c.103C>T | c.92C>T | c.289_300del | c.101G>C | c.101G>A | c.101G>A | ||
| AA substitution | p.Leu32Val | p.Gly34Val | p.Ser28Thr | p.Pro35Ser | p.Gly34Ser | p.Pro35Ser | p.Ser31Leu | p.Ser97_Arg100del | p.Gly34Ala | p.Gly34Asp | p.Gly34Asp | ||
| De novo mutation | ? | ? | ? | Y | Y | Y | Y | Not in mother | Y | Not in parents | Not in parents | ||
| Age (years) | 50 | 44 | 5 | 4 | 10 | 10 | 12 | 16 | 9 | 13 | 22 | ||
| Sex | M | F | M | F | M | F | F | M | M | M | F | ||
| Craniofacial | |||||||||||||
| Craniosynostosis | − | − | + | + | + | − | + | + | + | 23/30 | 29/39 (74) | ||
| Dolichocephaly | + | + | + | + | + | + | − | + | 28/30 | 36/38 (95) | |||
| Hypertelorism | + | + | + | + | + | + | + | + | + | + | + | 29/30 | 40/41 (98) |
| Downslanting palp. fissure | + | + | + | + | + | − | + | + | + | + | 27/30 | 36/40 (90) | |
| Proptosis | + | + | + | + | + | + | + | + | − | − | 24/30 | 32/40 (80) | |
| Malar hypoplasia | + | + | + | + | + | + | + | + | + | + | + | 12/12 | 23/23 (100) |
| High/narrow palate | + | + | + | + | + | + | + | + | + | + | 12/12 | 22/22 (100) | |
| Micrognathia | + | + | + | + | + | + | + | + | 26/30 | 34/38 (89) | |||
| Low/posteriorly rotated ears | + | + | + | + | + | − | + | + | + | + | 12/12 | 21/22 (95) | |
| Ectopia lentis | − | − | − | − | − | − | 0/12 | 0/18 (0) | |||||
| Cleft palate/bifid uvula | − | − | − | − | − | − | − | 5/12 | 5/19 (26) | ||||
| Skeletal | |||||||||||||
| Arachnodactyly | + | + | + | + | + | + | + | + | + | + | + | 29/30 | 40/41 (98) |
| Camptodactyly | −(hands)+(toes) | + | − | + | + | − | + | 18/30 | 23/37 (62) | ||||
| Scoliosis | + | + | − | − | + | − | − | 24/30 | 27/37 (73) | ||||
| Pectus | + | + | + | + | + | + | + | − | − | 25/30 | 32/39 (82) | ||
| Joint hypermobility | − | − | − | − | + | + | + | + | + | 12/12 | 17/21 (81) | ||
| Joint contracture | + | + | + | + | + | + | 25/30 | 31/36 (86) | |||||
| Dural ectasia | − | − | 0/2 (0) | ||||||||||
| Neuromuscular | |||||||||||||
| Hypotonia | − | − | + | + | + | 11/11 | 14/16 (88) | ||||||
| Developmental disability | + | + | + | + | + | + | + | + | + | + | − | 30/30 | 39/41 (95)b |
| Cardiovascular | |||||||||||||
| Mitral valve prolapse | − | − | − | − | − | + | − | − | − | 11/28 | 12/37 (32) | ||
| Aortic root dilatation | − | − | − | − | − | − | + | + | − | 11/28 | 13/39 (33) | ||
| Arterial tortuosity | − | − | − | − | 2/4 | 2/8 (25) | |||||||
| Other aneurysms | − | − | − | − | − | 2/4 | 2/9 (22) | ||||||
| Other features | (1) | (2) | (3) | (4) | (3,5) | (3,5) | |||||||
Abbreviations: F, female; M, male; Y, yes; ?, parents not available.
Empty cells, not determined; +, feature present; −, feature absent.
(1) Inguinal and umbilical hernia.
(2) Feeding difficulties requiring gastrostomy, fixed talipes.
(3) Bilateral Hallux valgus.
(4) Neonatal intracranial hemorrhage (it is unclear whether this is related to SGS or not), mitral valve regurgitation.
(5) Blepharophimosis, shallow orbits, 2/3 syndactyly.
Variant numbering based on RefSeq NM_003036.3.
Two siblings.
One of the previously reported patients (patient 2 from Doyle et al10) is now also attending regular school after initially been reported with developmental delay.
DISCUSSION
As shown in this and three previous reports,10, 11, 12 mutations in SKI cause SGS, a disorder that is usually classified among the syndromic forms of thoracic aortic aneurysms, more specifically among the MFS- and LDS-related disorders. The majority of the patients described within our first paper had dilatations of the aortic root, with z-scores of at least 2.0.10 Within the current series only two patients presented with aortic root dilatation, the hallmark of MFS and LDS. The difference between these two reports may be due to selection bias that may have occurred for the first screened set of SGS patients in addition to the relatively young age of the patients in the current series. Nevertheless, it is known that the aneurysmal phenotype is usually less severe and less penetrant in SGS cases compared with MFS and LDS cases.2, 10 Still, lifelong follow-up seems to be warranted. If baseline echocardiography is normal, then we recommend yearly follow-up in children and subsequently every 2–3 years in adults. Besides the low frequency of cardiovascular features, the patients in the current study display the typical craniofacial, skeletal, and neuromuscular features of SGS, including craniosynostosis, arachnodactyly, and developmental and intellectual disability. Importantly, not all patients have intellectual disability as patient 11 in our series is pursuing university level education and one of the previously reported patients (patient 2 from Doyle et al10) is now also attending regular school after initially been reported with some developmental delay. As such, the absence of intellectual disability does not preclude the diagnosis of SGS. In addition, severe intellectual disability seems to make a diagnosis of SGS unlikely, as we observed most often mild/moderate intellectual disability.
Dysregulated TGFβ signaling has clearly been implicated in the pathogenesis of thoracic aortic aneurysm phenotypes. Since SGS shows a major clinical overlap with both MFS and LDS, it was not unexpected to find impaired TGFβ signaling also at the core of the SGS pathogenesis. Indeed, both canonical and non-canonical TGFβ signaling were increased in primary dermal fibroblasts from SGS patients, findings that were similar to those observed in MFS and LDS patients.10 SKI is a negative regulator of TGFβ signaling, but has no catalytic activities nor can it bind DNA directly. SKI is dependent on interactions with other cellular partners, such as SMAD proteins, and transcriptional co-regulators including N-CoR, mSin3A, and histone deacetylases.14, 16 Remarkably, all SKI mutations described so far cluster into two domains: the R-SMAD binding domain, involved in TGFβ signaling, and the DHD domain, which mediates binding to SNW1 and N-Cor proteins. The latter are essential for the transforming activity of SKI and for the recruitment of transcriptional corepressors. On the one hand, the SKI mutations most likely destroy the binding capacities of these specific domains, leading to the typical MFS- and LDS-like manifestations observed in SGS patients. On the other hand, SKI also has a prominent role in neurogenesis, which may explain why SGS patients show intellectual disability.
So far 17 different SKI mutations have been identified in 33 unrelated patients (1 patient was identical in Doyle et al10 and Carmignac et al11). Adding our new findings to the existing data clearly reveals a mutational hotspot, with all mutations localized in exon 1 and with 73% (24 out of 33) of the hitherto described independent patients having mutations affecting a stretch of five SKI residues (from p.(Ser31) to p.(Pro35)). This fact has important implications for molecular testing, which most probably can be restricted to mutation analysis of exon 1 of SKI, or, as this exon is huge, to the first half of exon 1.
In conclusion, we have extended the mutational spectrum of SKI and have revealed a mutational hotspot in SKI, with 73% of the hitherto described SGS patients having mutations affecting four adjacent residues in the R-SMAD binding domain of SKI: p.(Ser31) p.(Leu32), p.(Gly34), and p.(Pro35).
Acknowledgments
DS is supported by a PhD grant from the Agency for Innovation by Science and Technology. BLL is a senior clinical investigator of the Fund for Scientific Research, Flanders (FWO, Belgium). This research was supported by funding from the Fund for Scientific Research, Flanders (FWO, Belgium) [G.0458.09; G.0221.12], the Fondation Leducq, the European Research Council (ERC), the University of Antwerp (Lanceringsproject), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Shprintzen RJ, Goldberg RB. A recurrent pattern syndrome of craniosynostosis associated with arachnodactyly and abdominal hernias. J Craniofac Genet Dev Biol. 1982;2:65–74. [PubMed] [Google Scholar]
- Robinson PN, Neumann LM, Demuth S, et al. Shprintzen-Goldberg syndrome: fourteen new patients and a clinical analysis. Am J Med Genet A. 2005;135:251–262. doi: 10.1002/ajmg.a.30431. [DOI] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucke PJ, Willaert A, Wessels MW, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar IMBH, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–U165. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AJ, Doyle JJ, Bessling SL, et al. Mutations in the TGF-beta repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44:1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignac V, Thevenon J, Ades L, et al. In-frame mutations in exon 1 of SKI cause dominant Shprintzen-Goldberg syndrome. Am J Hum Genet. 2012;91:950–957. doi: 10.1016/j.ajhg.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au PY, Racher HE, Graham JM, Jr, et al. De novo exon 1 missense mutations of SKI and Shprintzen-Goldberg syndrome: Two new cases and a clinical review. Am J Med Genet A. 2013;164:676–684. doi: 10.1002/ajmg.a.36340. [DOI] [PubMed] [Google Scholar]
- Akiyoshi S, Inoue H, Hanai J, et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J Biol Chem. 1999;274:35269–35277. doi: 10.1074/jbc.274.49.35269. [DOI] [PubMed] [Google Scholar]
- Luo K, Stroschein SL, Wang W, et al. The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev. 1999;13:2196–2206. doi: 10.1101/gad.13.17.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Angelis K, Danielpour D, et al. Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type beta transforming growth factor. Proc Natl Acad Sci USA. 2000;97:5924–5929. doi: 10.1073/pnas.090097797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Khan MM, Kaul SC, et al. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 1999;13:412–423. doi: 10.1101/gad.13.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res. 2009;19:47–57. doi: 10.1038/cr.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CD, Martinez-Rodriguez G, Hackett PB., Jr Ectopic expression of c-ski disrupts gastrulation and neural patterning in zebrafish. Mech Dev. 2000;95:147–162. doi: 10.1016/s0925-4773(00)00351-8. [DOI] [PubMed] [Google Scholar]
- Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes Dev. 1997;11:2029–2039. doi: 10.1101/gad.11.16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi LS, Neff AW, Sleeman JP, Smith RC. Autonomous neural axis formation by ectopic expression of the protooncogene c-ski. Dev Biol. 1997;192:392–404. doi: 10.1006/dbio.1997.8780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.