Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) based strategy is a promising targeted therapeutic approach for the treatment of a variety of cancers including ovarian cancer. However, the inherent or acquired resistance of tumor cells to TRAIL limits the potential application of TRAIL-mediated therapy. In this study, we identified that mitochondrial division inhibitor-1 (mdivi-1) is able to enhance the sensitivity of human ovarian cancer cells to death receptor ligands including TRAIL, FAS ligands, and TNF-α. Importantly, the combination of TRAIL and mdivi-1 has no apparent cytotoxic effect on non-transformed human cells, indicating a significant therapeutic window. We identified that caspase-8 and not the modulation of TRAIL receptors is required for the combination effect of TRAIL and mdivi-1. We further demonstrated that the enhanced efficacy of combination of mdivi-1 and death ligands is not dependent on the originally reported target of mdivi-1, Drp1, and is also not dependent on the two important pro-apoptotic Bcl-2 family proteins Bax and Bak. Thus, our study presents a novel strategy in enhancing the apoptotic effect of death receptor ligands and provides a new effective TRAIL-based combination approach for treating human ovarian cancer.
Keywords: Ovarian cancer, mdivi-1, death ligands, TRAIL, Drp1, Bax/Bak
Introduction
Ovarian cancer is the leading cause of death from gynecological malignancies [1]. The current standard for management of ovarian cancer is cytoreductive surgery and platinum drugs-based combination chemotherapy with taxanes [2]. Despite the high initial response rate, up to 80% of patients will recur and eventually develop resistance to therapy, which leads to incurable disease [3]. Addition of doxorubicin, topotecan, gemcitabine [4], or bevacizumab (anti-VEGF anti-angiogenic antibody) [5] to standard chemotherapy unfortunately has no advantages in improving patient overall survival, further indicating the significant therapeutic challenges in the treatment of ovarian cancer.
During the past decade, tumor necrosis factor-related apoptosis inducing ligand (TRAIL) has been recognized as one of the promising anticancer agents due to its ability to selectively induce apoptosis in many tumor cells without affecting normal cells [6]. Ovarian cancer has been shown to be one of the potential therapeutic targets of TRAIL [7,8]. TRAIL induces apoptosis by binding to death receptors 4 (DR4) and 5 (DR5), which leads to the recruitment of the adaptor protein FADD. Binding of FADD with Pro-caspase-8 leads to the formation of the death-inducing signaling complex (DISC) and resulting in the activation of caspase-8. Activated caspase-8 is able to directly activate executioner caspases (caspase-3, -6, -7), committing the cell to apoptotic death. In addition, the intrinsic apoptotic pathway can also be triggered by activated caspase-8 through cleavage of Bid. Translocation of the truncated Bid (tBid) to the mitochondria promotes cytochrome c release and activates caspase-9 and downstream caspases. However, TRAIL resistance has been reported in approximately 50% of tumor cells, including ovarian cancer cells [7]. The mechanisms underlying TRAIL resistance have been shown to occur at several levels in ovarian cancer cells. The coding sequence of TRAIL receptor is commonly deleted in ovarian cancer. For instance, lack of expression of death receptor 4 could account for the resistance of A2780 ovarian cancer cells to TRAIL [9]. Alterations in the signaling events downstream of TRAIL receptors also contribute to TRAIL resistance, such as the increased level of c-FLIP [10]. Thus, overcoming TRAIL resistance is of great significance in ovarian cancer therapy.
We have shown previously that mdivi-1 induces tumor-specific mitotic defects [11] and enhances the efficacy of platinum agents in cancer cells from various tumor types, including ovarian cancer [12]. In this study, we further identified that mdivi-1 is able to enhance the death ligands including TRAIL-induced apoptosis in both ovarian cancer cell lines and patient-derived primary ovarian cancer cells. Importantly, the combination of mdivi-1 and TRAIL has no apparent cytotoxic effect on non-transformed human fibroblasts. Thus, our study presents a novel TRAIL-based combination strategy for the treatment of ovarian cancer.
Materials and Methods
Cell Culture
The human ovarian carcinoma cell lines A2780 and the cisplatin-resistant A2780cis were obtained from Sigma-Aldrich (St. Louis, MO). Human dermal fibroblasts NHDF were obtained from Lonza (Walkersville, MD). Ovarian cancer patient ascites were obtained under an IRB protocol, Heath Sciences Tissue Bank IRB 0506140, approved by the University of Pittsburgh Cancer Institute. Primary epithelial ovarian cancer cells (EOC) presented in those ascites were isolated and cultured as described previously [13]. Drp1 wild-type and knockout MEF cells were established by Katsuyoshi Mihara [14], and kindly provided by Kasturi Mitra (University of Alabama). Bax/Bak wild-type and double knockout MEF cells were established by Dr. Stanley J. Korsmeyer [15], and kindly provided by Dr. Shivendra Singh (University of Pittsburgh Cancer Institute). Cells were cultured in their corresponding media including RPMI-1640 and DMEM, in 5% CO2 at 37°C.
Reagents
Mdivi-1 was obtained from Sigma-Aldrich (St. Louis, MO). Human TRAIL was obtained from PeproTech (Rocky Hill, NJ). Recombinant TNF-α was obtained from Invitrogen (Grand Island, NY) and recombinant Super Fas Ligand was obtained from Enzo Life Sciences (Farmingdale, NY).
Apoptosis and Viability Assays
The activity of caspase-3/7 was measured using a Caspase-Glo® 3/7 Assay Systems (Promega, Madison, WI), according to the manufacturer's instruction. Apoptosis was also determined by staining cells with Annexin V-FITC and propidium iodide (PI) using an FITC Annexin V Apoptosis Detection Kit (BD PharMingen, San Diego, CA) followed by flow cytometric analysis using Accuri 6 flow cytometer (BD bioscience). Cell survival was determined using a CellTiter-Blue Cell Viability Assay (Promega, Madison, WI).
Western Blot Analysis
Western blot was performed as we previously described [12]. Primary antibodies used were: Capase-8, Caspase-9, cleaved Caspase-3, BID, FADD, DR5 were from Cell Signaling Technology (Beverly, MA); Cytochrome c was from BD Biosciences (San Jose, CA); an Jose, CAc was from BD BioscieSt. Louis, MO); and DcR2 was from Santa Cruz Biotechnology (Dallas, TX).
Lentiviral Transduction
Caspase-8 shRNA lenti-viruses were obtained from Vector Facility at University of Pittsburgh Cancer Institute. Cells with stable knockdown were selected by 2 µg/ml of puromycin.
Statistical Analysis
Data were expressed as mean ± standard deviation. A Student's t test was used for the comparisons between the treatment with TRAIL alone and combination with mdivi-1. P < 0.05 was considered statistically significant.
Results
Mdivi-1 enhances TRAIL sensitivity in human ovarian cancer cells but not in non-transformed normal cells
We employed a caspase-3/7 activity assay to determine the apoptotic effect of TRAIL alone, mdivi-1 alone or the combination of TRAIL and mdivi-1 on A2780 ovarian cancer cells. With increasing doses of TRAIL and mdivi-1, the combination dramatically enhanced the activity of caspase-3/7 compared to either TRAIL or mdivi-1 alone (Fig 1A). Platinum drugs are highly effective at initial treatment and are thus used as standard first-line therapy in ovarian cancer. However, the development of platinum resistance leads to incurable disease. Therefore, we examined the combination effect of TRAIL and mdivi-1 on platinum-resistant A2780cis cells, which are the derivatives of cisplatin-sensitive A2780 cells and are also cross-resistant to melphalan, adriamycin and irradiation. Similar to our results in A2780 cells, mdivi-1 was able to enhance the sensitivity to TRAIL in A2780cis cells (Fig 1A), indicating the potential of the combination of TRAIL and mdivi-1 in treating drug-resistant tumor cells. We then performed a CellTiter-blue cell viability assay to confirm the combination effect and found that the combination treatment led to a stronger reduction in the viability of ovarian cancer cells compared to single agent treatment (Fig 1B). Annexin V apoptosis assay further revealed that the number of Annexin V-positive apoptotic cells after combination treatment was increased compared to treatment with TRAIL or mdivi-1 alone (Fig 1C). Importantly, we did not observe a significant cytotoxic effect in non-transformed normal human fibroblast NHDF cells following single agent or combination treatment (Fig 1D), indicating a tumor cell-selective effect.
Figure 1. Mdivi-1 enhances TRAIL sensitivity in human ovarian cancer cells but not in non-transformed normal cells.
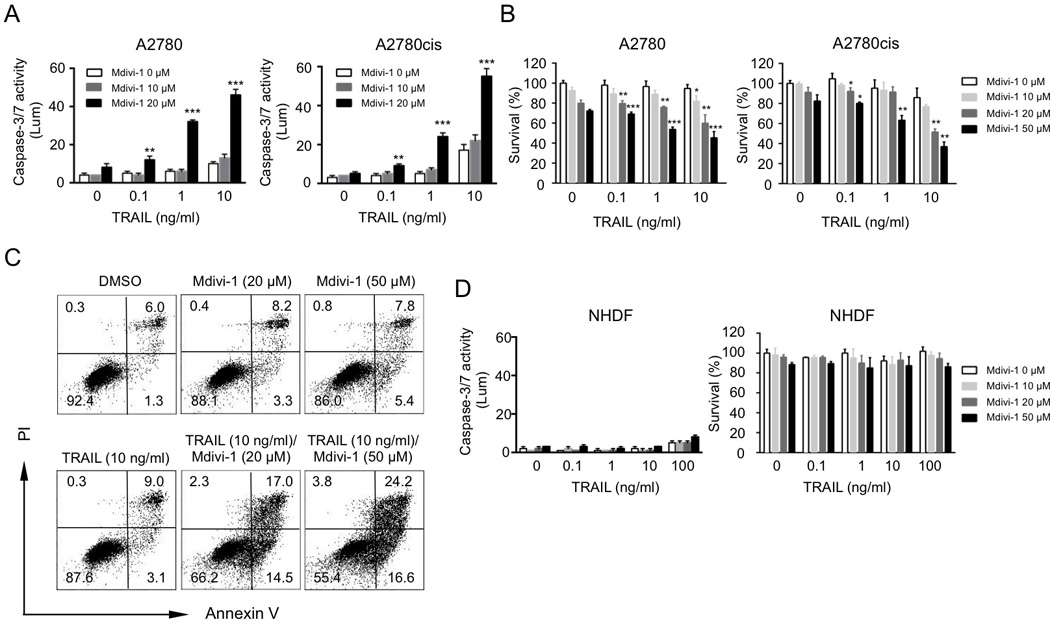
(A) Human ovarian cancer cells A2780 and cisplatin-resistant A2780cis were treated with increasing doses of TRAIL alone, mdivi-1 alone, or the combination of TRAIL and mdivi-1 for 16 h. Apoptosis was determined by the activity of caspase-3/7. (B) A2780 and A2780cis cells were treated with TRAIL alone, mdivi-1 alone, or the combination for 16 h. Cell survival was measured by CellTiter-Blue. (C) A2780 cells were treated with TRAIL alone, mdivi-1 alone, or the combination at indicated concentrations for 16 h. Apoptosis was determined by staining cells with Annexin V and PI followed by flow cytometry. (D) Normal human dermal fibroblast (NHDF) cells were treated with TRAIL alone, mdivi-1 alone, or the combination for 16 h. Apoptosis and cell viability were determined by the activity of caspase-3/7 and CellTiter-Blue, respectively. Data represent the mean ± S.D. * P<0.05, ** P<0.01, *** P<0.001.
The combination of mdivi-1 and TRAIL is highly effective against primary tumor cells from ovarian cancer patients
We then evaluated if mdivi-1 is able to enhance the effect of TRAIL on patient-derived primary epithelial ovarian cancer (EOC) cells, by ex vivo drug sensitivity assay using CellTiter-blue. High-grade serous carcinoma (HGSC) is the most frequent and lethal type of epithelial ovarian cancer, with a high capacity to develop drug resistance [16]. We isolated the primary EOC cells from the ascites fluids of three HGSC ovarian cancer patients (Fig 2A). We found that mdivi-1 was highly effective at enhancing the efficacy of TRAIL in primary EOC cells in a dose dependent manner (around 40%–50% decrease in cell survival compared to the treatment with TRAIL alone, when 50 µM mdivi-1 was combined), while TRAIL alone at 100 ng/ml only had limited effect (Fig 2B).
Figure 2. Mdivi-1 enhances TRAIL sensitivity in patient-derived primary epithelial ovarian cancer cells.
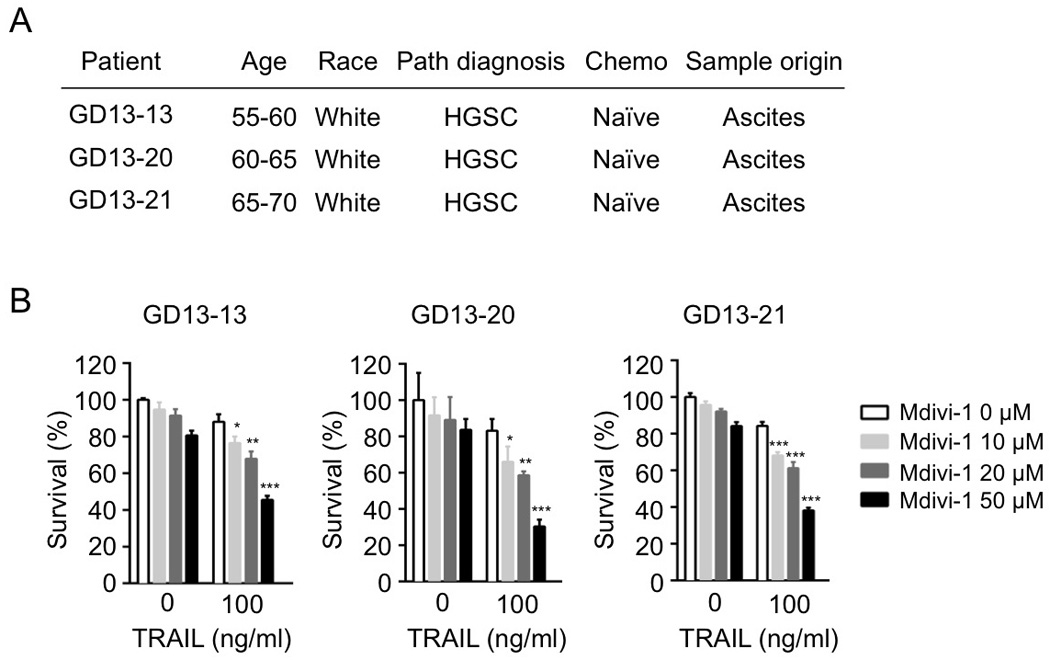
(A) Characteristics of the ovarian cancer patients. (B) Primary epithelial ovarian cancer cells isolated from the three patients were treated with TRAIL alone, mdivi-1 alone, or with the combination at indicated concentrations for 16 h. Cell survival was measured by CellTiter-Blue. Data represent the mean ± S.D. * P<0.05, ** P<00.1, *** P<0.001.
The combination of mdivi-1 and TRAIL enhances extrinsic apoptotic signaling without modulation of the expression of TRAIL receptors
We sought to understand the signaling pathway leading to the enhanced apoptosis in ovarian cancer cells following the treatment with the combination of mdivi-1 and TRAIL. The cleavage of caspase-8 was enhanced after combination treatment in A2780 cells (Fig 3A). FADD, which is an important binding protein of pro-caspase-8 in DISC, had no change with either single agent alone or combination treatment in A2780 cells. The levels of Bid, which is a substrate of caspase-8, significantly decreased following combination treatment, indicating the occurrence of Bid cleavage. A time course study of the activation of caspases revealed a time-dependent sequential cleavage of caspase-8 and then caspase-9 and caspase-3 (Fig 3B). Truncated Bid (tBid) is known to translocate onto mitochondria and activate Bax or Bak-mediated mitochondrial outer membrane permeabilization (MOMP). Consistent with this notion, we observed a time-dependent release of cytochrome c from mitochondria into cytosol (Fig 3C), indicating the mitochondrial pathway was involved in the combination-induced apoptotic signaling. We then investigated whether mdivi-1 enhances TRAIL activity through the modulation of TRAIL receptors. Since the expression of DR4 and DcR1 is undetectable in A2780 cells [17], we examined the expression of DcR2 and DR5 after the combination treatment. No significant changes were observed for the expression of both DcR2 and DR5 (Fig 3D). As caspase-8 is the essential component in DISC formation, we then examined the importance of caspase-8 in apoptosis induced by the combination treatment of TRAIL and mdivi-1. Knocking down caspase-8 through shRNA led to a reduced cleavage of caspase-8, 9 and 3, following combination treatment (Fig 3E), indicating caspase-8 is crucial for the enhanced apoptosis by the combination of TRAIL and mdivi-1.
Figure 3. Mdivi-1 enhances death receptor-mediated apoptotic signaling.
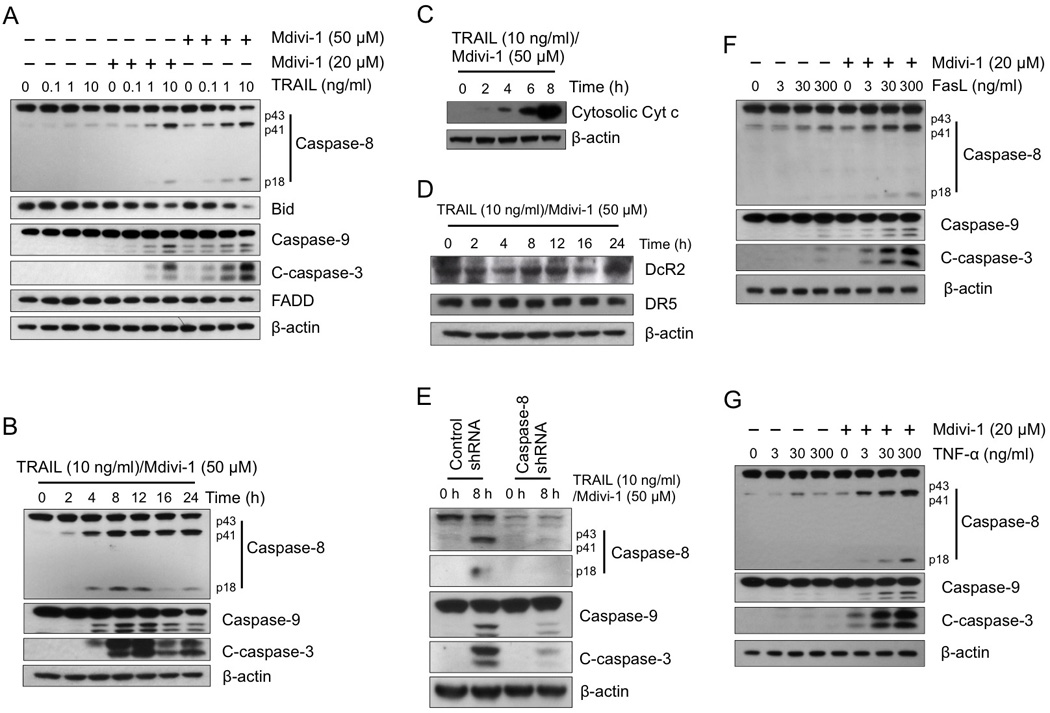
(A) A2780 cells were treated with TRAIL alone, mdivi-1 alone, or the combination at the indicated concentrations for 16 h. The activation of apoptotic signaling was examined by western blot using antibodies against caspase-8, bid, caspase-9, cleaved caspase-3 (C-caspase-3), and FADD. β-actin was used as a loading control. (B) A2780 cells were treated with the combination of TRAIL and mdivi-1 as indicated. Cleavage of caspases was detected by western blot. (C) A2780 cells were treated with the combination of TRAIL and mdivi-1 as indicated with the presence of 20 µM caspase inhibitor Q-VD-OPH. The cytosolic fraction was isolated using digitonin permeabilization followed by centrifugation. The amount of cytochrome c presented in cytosolic fraction was detected by western blot. (D) A2780 cells were treated as indicated and the expression of Decoy receptor DcR2 and death receptor DR5 were detected by western blot. (E) A2780 cells were transduced with control or caspase-8 shRNA and selected by puromycin. Cells were treated with the combination of TRAIL and mdivi-1 at indicated concentration for 8 h. The cleavage of caspases was detected by western blot. (F, G) A2780 cells were treated with increasing doses of Fas ligand (FasL) (F) or TNF-α (G) alone or the combination with mdivi-1 (20 µM) for 16 h. Cleavage of caspases was examined by western blot. These data represent three independent experiments.
Mdivi-1 enhances cellular sensitivity of ovarian cancer cells to TNF superfamily ligands Fas ligand and TNF-α
TRAIL is a member of the TNF family. Upon receptor ligation stimulated by ligand binding, TNF superfamily ligands share similar apoptotic inducing pathways. We therefore investigated whether other TNF superfamily ligands such as Fas ligand and TNF-α also have a combination effect with mdivi-1. The cleavage of caspase-8, 9 and 3 was enhanced following the combination treatment of mdivi-1 with both Fas ligand and TNF-α, compared to the agents-alone treatment (Fig 3F and G), indicating mdivi-1 is able to enhance extrinsic death receptor-mediated apoptotic signaling, irrespective of the particular type of the death ligands and their particular receptors in ovarian cancer cells.
Mdivi-1 enhances death receptor-mediated apoptosis independent of Drp1, Bax and Bak
Mdivi-1 was first discovered as an inhibitor of a mitochondrial fission protein Drp1 to prevent apoptosis [18]. Bax and Bak, which promote stable association of Drp1 with mitochondria during apoptosis [19], are important for the release of mitochondrial apoptotic factors. We thus investigated whether the enhanced apoptosis by the combination of mdivi-1 and the death ligands is dependent on the Drp1 and Bax/Bak, by using mouse embryonic fibroblasts (MEFs) depleted of Drp1 or Bax/Bak. Since the combination of TRAIL and mdivi-1 lacks effect on fibroblasts, we evaluated the combination effect of Fas ligand and mdivi-1. The cleavage of caspase-8 and 3 was enhanced in a dose dependent manner in both Drp1 wild-type and knockout cells following the treatment with the combination of mdivi-1 and Fas ligand (Fig 4A). In addition, both Drp1 wild-type and knockout cells demonstrated a similar pattern in the increase of the Annexin V-positive apoptotic cells following combination treatment (Fig 4B). We also observed the similar effects with Bax/Bak wild-type and double knockout MEF cells (Fig 4C and D). These results indicate that mdivi-1 enhances death receptor-mediated apoptosis independent of Drp1 and Bax/Bak. However, it is worth noting that after combination treatment the number of Annexin V-positive apoptotic cells in Drp1 knockout MEF cells and Bax/Bak double knockout MEF cells is less than the number in wild-type cells (Fig 4B and D), indicating that the reduced activation on mitochondrial apoptotic pathway due to the lack of Drp1 or Bax/Bak has a certain degree of protective role in the apoptotic effect of the combination of mdivi-1 and Fas ligand.
Figure 4. Mdivi-1 enhances death receptor-mediated apoptosis independent of Drp1 and Bax/Bak.
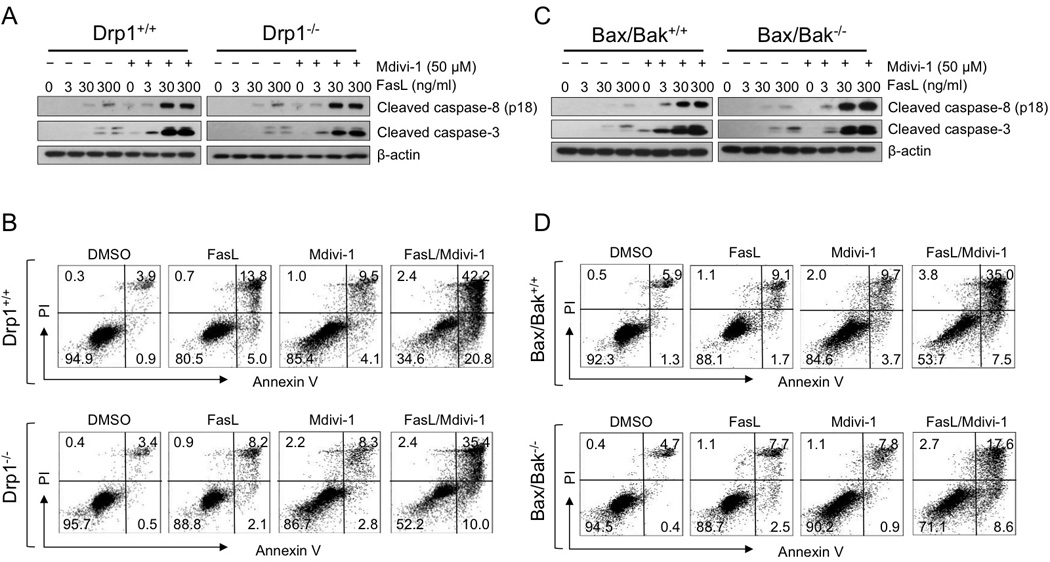
(A) Drp1 wild-type (Drp1+/+) and knockout (Drp1−/−) MEF cells and (C) Bax/Bak wild-type (Bax/Bak+/+) and double knockout (Bax/Bak−/−) MEF cells were treated with increasing doses of Fas ligand alone or with the combination of mdivi-1 (50 µM) for 16 h. Cleavage of caspase-8 and caspase-3 were detected by western blot. The mouse specific anti-caspase-8 antibody recognizes only the cleaved fragment as compared to human specific antibody. (B) Drp1 wild-type (Drp1+/+) and knockout (Drp1−/−) MEF cells and (D) Bax/Bak wild-type (Bax/Bak+/+) and double knockout (Bax/Bak−/−) MEF cells were treated with Fas ligand (300 ng/ml), mdivi-1 (50 µM), or the combination for 16 h. Apoptosis was determined by staining cells with Annexin V and PI. These data represent three independent experiments.
Discussion
Despite the fact that more than 80% of the ovarian cancer patients initially respond to first-line chemotherapy [20], most patients progress to advanced disease with the development of acquired drug resistance leading to treatment failure. In addition, the toxicities accompanied with the conventional chemotherapeutic drugs often limit the administration of the treatment. Clearly, the development of more effective strategies that selectively target ovarian cancer cells while sparing normal tissue is highly desirable. In this study, we have identified that the combination of mdivi-1 and TRAIL enhances apoptosis in ovarian cancer cells, including the cells that are resistant to standard chemotherapeutic agents, but not in normal cells. We thus provide a potential effective combination strategy for the treatment of ovarian cancer.
TRAIL, which shows great broad-spectrum antitumor effects towards numerous cancer cell lines without affecting normal cells, has been considered as a promising candidate in cancer therapy [21]. However, the clinical activity of TRAIL is limited due to the inherent or acquired resistance of tumors. Combining TRAIL with several different classes of anticancer drugs has been reported able to overcome TRAIL resistance [22,23]. Genotoxic drugs such as 5-fluorouracil and cisplatin have been shown to enhance TRAIL efficacy by upregulating the transcription of BH3-only pro-apoptotic proteins and DR5 expression, and by downregulating the expression of inactive caspase-8 homolog cFLIP and anti-apoptotic Bcl-2 family proteins [24]. Cytokines such as IFN-γ has been reported to enhance TRAIL-induced apoptosis through enhanced DR5 expression and inactivation of NF-κB in human hepatocellular carcinoma cells [25]. Mdivi-1 is known as an inhibitor of Drp1 [18], and has therapeutic value in ischemia/reperfusion injury, myocardial infarction, and neurodegenerative diseases [26]. Although, we have demonstrated that mdivi-1 enhances death ligand-mediated apoptosis independent of Drp1, the highly synergistic antitumor effect of the combination of TRAIL and mdivi-1 clearly suggests a potential new therapeutic option for TRAIL-based combination therapy for ovarian cancer.
Apoptosis is triggered through two principal signaling pathways, death receptor-mediated extrinsic and mitochondria-mediated intrinsic pathways. The crosstalk between these two pathways is mediated by the cleavage of Bid through the activation of caspase-8. Activation of Bid then triggers the oligomerization of Bax or Bak, resulting in the release of mitochondrial intermembrane apoptotic proteins such as cytochrome c. Mitochondrial pathway has been shown involved in TRAIL-activated apoptosis [27]. We found that mdivi-1 enhances the extrinsic apoptotic pathway irrespective of the death ligands and their respective receptors. In agreement with this finding, we demonstrated that the combination of mdivi-1 and TRAIL does not alter the expression of TRAIL receptors. Furthermore, we have observed enhanced Bid-cleavage and cytochrome c release after ovarian cancer cells were treated with the combination of TRAIL and mdivi-1, indicating mitochondria play a role in the combination-induced apoptosis. Drp1 has been shown to be involved in tBid-induced and Bax/Bak-dependent cytochrome c release [18]. Using Drp1 deficient cells, we found that Drp1 is not essential for the enhanced apoptosis induced by the combination of mdivi-1 with Fas ligand. However, the lack of Drp1 did confer certain degree of resistance to the combination treatment. We also observed similar phenomenon for Bax and Bak. These results indicate that the mitochondrial apoptotic pathway partially contributes to the combination-induced apoptosis, and the release of cytochrome c triggered by the combination in ovarian cancer cells may also involve the mechanism that is independent of Drp1 and Bax/Bak. Since mdivi-1 has been shown to inhibit the mitochondrial apoptotic pathway [18], the possible action site(s) of mdivi-1 responsible for the enhanced apoptosis by the combination is thus probably upstream of mitochondria and not exclusively associated with the mitochondria-related apoptotic signaling. Direct activation of caspase-3 by enhanced caspase-8 activity conceivably plays an important role in the apoptosis induced by the combination of TRAIL and mdivi-1. Further elucidation of the mechanism of action of mdivi-1 in enhancing TRAIL-mediated apoptotic cell death may lead to a better understanding of TRAIL resistance in ovarian cancer cells and a better design of effective TRAIL-based treatment strategies.
In summary, we have identified that the combination of TRAIL and mdivi-1 is highly effective in inducing apoptosis in ovarian cancer cells in a cancer cell-specific manner. The combination showed high efficacy in patient tumor samples, and also in ovarian cancer cells that are resistant to conventional chemotherapeutic drugs, which may be of particular interest from a clinical standpoint.
Highlights.
Mdivi-1 enhances death receptor-mediated apoptosis in ovarian cancer cells
Combining mdivi-1 and TRAIL induces efficient apoptosis in patient tumor samples
Combination of mdivi-1 and TRAIL induces cancer cell specific apoptosis
Enhanced apoptosis by the combination is independent of Drp1, Bax, and Bak
Acknowledgements
This work was funded by a grant with the Pennsylvania Department of Health PA CURE program, Magee-Womens Foundation, P30CA047904, P50CA097190, and P50CA121973. Jingnan Wang received funding from China Scholarship Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare that authors have no conflict of interest.
References
- 1.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, Xu X, Hamilton TC. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Mullany LK, Richards JS. Minireview: animal models and mechanisms of ovarian cancer development. Endocrinology. 2012;153:1585–1592. doi: 10.1210/en.2011-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog TJ. Recurrent ovarian cancer: how important is it to treat to disease progression? Clin Cancer Res. 2004;10:7439–7449. doi: 10.1158/1078-0432.CCR-04-0683. [DOI] [PubMed] [Google Scholar]
- 4.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K, Mutch DG, Burger RA, Swart AM, Trimble EL, Accario-Winslow C, Roth LM. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 7.Khaider NG, Lane D, Matte I, Rancourt C, Piche A. Targeted ovarian cancer treatment: the TRAILs of resistance. Am J Cancer Res. 2012;2:75–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Duiker EW, de Vries EG, Mahalingam D, Meersma GJ, Boersma-van Ek W, Hollema H, Lub-de Hooge MN, van Dam GM, Cool RH, Quax WJ, Samali A, van der Zee AG, de Jong S. Enhanced antitumor efficacy of a DR5-specific TRAIL variant over recombinant human TRAIL in a bioluminescent ovarian cancer xenograft model. Clin Cancer Res. 2009;15:2048–2057. doi: 10.1158/1078-0432.CCR-08-1535. [DOI] [PubMed] [Google Scholar]
- 9.Tomek S, Horak P, Pribill I, Haller G, Rossler M, Zielinski CC, Pils D, Krainer M. Resistance to TRAIL-induced apoptosis in ovarian cancer cell lines is overcome by co-treatment with cytotoxic drugs. Gynecol Oncol. 2004;94:107–114. doi: 10.1016/j.ygyno.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Park SJ, Kim MJ, Kim HB, Sohn HY, Bae JH, Kang CD, Kim SH. Trichostatin A sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by down-regulation of c-FLIPL via inhibition of EGFR pathway. Biochem Pharmacol. 2009;77:1328–1336. doi: 10.1016/j.bcp.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Li J, Santos L, Shuda M, Sobol R, Van Houten B, Qian W. A novel strategy for targeted killing of tumor cells: induction of multipolar acentrosomal mitotic spindles with a quinazolinone derivative mdivi-1. Mol Oncol. 2014 doi: 10.1016/j.molonc.2014.10.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian W, Wang J, Roginskaya V, McDermott LA, Edwards RP, Stolz DB, Llambi F, Green DR, Van Houten B. Novel combination of mitochondrial division inhibitor 1 (mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells. Oncotarget. 2014;5:4180–4194. doi: 10.18632/oncotarget.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd TG, Theriault BL, Campbell EJ, Nachtigal MW. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat Protoc. 2006;1:2643–2649. doi: 10.1038/nprot.2006.328. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 15.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler M, Fotopoulou C, Meyer T. The molecular fingerprint of high grade serous ovarian cancer reflects its fallopian tube origin. Int J Mol Sci. 2013;14:6571–6596. doi: 10.3390/ijms14046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duiker EW, Meijer A, van der Bilt AR, Meersma GJ, Kooi N, van der Zee AG, de Vries EG, de Jong S. Drug-induced caspase 8 upregulation sensitises cisplatin-resistant ovarian carcinoma cells to rhTRAIL-induced apoptosis. Br J Cancer. 2011;104:1278–1287. doi: 10.1038/bjc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 21.Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285:1–5. doi: 10.1016/j.canlet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Maksimovic-Ivanic D, Stosic-Grujicic S, Nicoletti F, Mijatovic S. Resistance to TRAIL and how to surmount it. Immunol Res. 2012;52:157–168. doi: 10.1007/s12026-012-8284-8. [DOI] [PubMed] [Google Scholar]
- 23.Hellwig CT, Rehm M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Mol Cancer Ther. 2012;11:3–13. doi: 10.1158/1535-7163.MCT-11-0434. [DOI] [PubMed] [Google Scholar]
- 24.Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, Krueger A, Weigand MA, Grosse-Wilde A, Stremmel W, Krammer PH, Walczak H. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11(Suppl 1):S86–S96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- 25.Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, Miyazoe S, Nakagawa Y, Ishikawa H, Hamasaki K, Nakata K, Ishii N, Eguchi K. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-kappa B inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]
- 26.Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem Biol. 2010;17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandasamy K, Srinivasula SM, Alnemri ES, Thompson CB, Korsmeyer SJ, Bryant JL, Srivastava RK. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 2003;63:1712–1721. [PubMed] [Google Scholar]


