Abstract
Estrogens play an important role in development and function of the brain and reproductive tract. Accordingly, it is thought that developmental exposure to environmental estrogens can disrupt neural and reproductive tract development potentially resulting in long-term alterations in neurobehavior and reproductive function. Many chemicals have been shown to have estrogenic activity whereas others affect estrogen production and turnover resulting in disruption of estrogen signaling pathways. However, these mechanisms and the concentrations required to induce these effects cannot account for the myriad adverse effects of environmental toxicants on estrogen sensitive target tissues. Hence, alternative mechanisms are thought to underlie the adverse effects documented in experimental animal models and thus could be important to human health. In this review, the epigenetic regulation of gene expression is explored as a potential target of environmental toxicants including estrogenic chemicals. We suggest that toxicant-induced changes in epigenetic signatures are important mechanisms underlying disruption of ovarian follicular development. In addition, we discuss how exposure to environmental estrogens during early life can alter gene expression through effects on epigenetic control potentially leading to permanent changes in ovarian physiology.
Keywords: estrogens, methoxychlor, BPA, DEHP, ovary, epigenetic
1) Introduction
The gonadal steroid estradiol (E2) plays a central role in neurogenesis, synaptogenesis, regulation of neurotrophin expression, and sexual differentiation of the brain. Recent evidence suggests that the estrogenic activity of environmental contaminants is also associated with adverse effects on neurodevelopment and neurobehavior (1–6). The developing brain undergoes phases of cell neurogenesis, proliferation, differentiation, density, migration, and death; the brain also expresses high levels of estrogen receptors. While it has been shown that sexual dimorphism exists in brain development, it is clear that regardless of gender, the actions of estrogens on the developing brain are typically permanent and range from establishing sex differences to trophic and neuroprotective effects. Gonadal estrogens affect target organs throughout the body including the brain. Estrogens can also act as local autocrine or paracrine signals that impact only the microenvironment, including various regions of the brain. The fetal environment is replete with estrogens originating in the maternal circulation and placenta as well as from direct synthesis in fetal neurons. All of these parameters occur to varying degrees, but depending on the response to estrogenic (or androgenic) signals, the cells are either protected from naturally occurring apoptosis or induced to undergo cell death (7).
Estrogens both promote and prevent synaptogenesis in the developing brain. Estrogens are involved in establishment of synaptic patterns that endure into adulthood and subserve the neural networks of steroid-regulated physiology and behavior. Estrogens can suppress the formation of dendritic spine synapse in the arcuate nucleus. Estrogens increase levels of the amino acid transmitter GABA by up-regulating the rate-limiting enzyme glutamic acid decarboxylase. GABA acts on GABA-A receptors of astrocytes and induces process growth and branching, resulting in an increased stellate morphology (8) compared to those not exposed to estrogens. On the other hand, estrogen exposure in the preoptic and ventromedial hypothalamus promotes the development and stabilization of dendritic spine synapses which involve glutamate and ionotropic glutamate receptors (9). Because estrogens are an important aspect of neurodevelopment with multifaceted signaling mechanisms that not only influence sex differentiation but a myriad of unique and opposing effects in different regions of the brain at different times during development, there is a potential for both direct but also indirect (via ovarian function) effects of environmental estrogens.
Exposure to endocrine disruptors during fetal and early postnatal life produces different long-term alterations on various brain areas. For example, neonatal exposure to bisphenol A (BPA) decreased the inhibition of GnRH activity by RFamide-related peptide-3 (RFRP3) neurons. This effect may be related with the advanced puberty observed in these animals (10). On the other hand, the perinatal exposure to estradiol benzoate (EB) or methoxychlor (MXC) produced an early reproductive senescence in rats which is related with developmental reprogramming of hypothalamic genes. Specifically, expression of Esr1 and Kiss1 genes were altered in this animal model (11). Other brain areas not related to reproduction could be affected by early exposure to endocrine disruptors. For example, exposure to estradiol valerate (EV) at postnatal day (PND) 1 in rats produced an increase in dopamine content in striatum, substantia nigra, and ventral tegmental area when rats were adult. In addition, these rats lacked of amphetamine induced locomotor activity in the adulthood, possibly because of a decrease of dopamine transporter (DAT), the molecular target of amphetamine (12).
As in the brain, exposure to environmental estrogens produces adverse effects in other estrogen-sensitive tissues including the uterus (13) and the ovaries (14–17). Human exposure to environmental contaminants has been widely documented through large scale biomonitoring (18–24) and epidemiological studies (25–29). While several different environmental contaminants have been measured in human ovarian follicular fluid (28–31), the impact on circulating E2 concentrations and associated health consequences are largely unknown. However, evidence from studies with wildlife suggests that chemicals in the environment can adversely affect ovarian biology (32, 33) across the lifespan.
The ovary is composed of follicles at different stages of development and stroma. Follicles at their advance stages are composed of the theca externa and interna (androgen production), a basement membrane, granulosa cells (sites of E2 and anti-Müllerian hormone (AMH) synthesis) and an oocyte. Folliculogenesis (follicle recruitment and growth to ovulation) is regulated in a stage-dependent manner by oocyte derived factors (e.g. bone morphogenetic protein-15 (BMP-15), growth differentiation factor-9 (GDF-9)), gonadotropins (follicle stimulating hormone (FSH) and luteinizing hormone (LH) in early antral and Graafian follicles) and several growth regulators (e.g. transforming growth factor-β (TGF-β), AMH, inhibin-B, activin, vascular endothelial growth factor, FOXL2, and insulin like growth factors and binding proteins (reviewed in (34)). Follicles are recruited into the growing pool of follicles where their initial stages of development are gonadotropin independent. From the time follicles are formed in utero (or early postnatal in case of rodents), follicles begin to develop but in the absence of FSH stimulation they undergo atresia with much of the follicle population lost before regular menstrual/estrous cycles commence with the onset of puberty. Once regular reproductive cycles are established follicles reach the secondary stage where FSH support from the pituitary provides the trigger to begin steroidogenesis. Steroid production in the ovary involves a two-cell process in which androgens produced in theca cells are transported to the granulosa where they are converted by aromatase to E2 or estrone. Tissue culture studies demonstrate that environmental contaminants can both increase the expression of steroidogenic enzymes such as steroidogenic acute regulatory protein (StAR) and aromatase (35, 36), the rate limiting enzyme in the conversion of androgens to estrogens (estrone and E2), as well as increase the expression of enzymes involved in the metabolism of gonadal steroids (37, 38). Furthermore, animal studies have demonstrated that exposure to chemicals with hormone-like activity change functional characteristics of steroid-dependent target tissues (reviewed in (39)).
Studies performed in animal models illustrate potential effects of early exposure to estrogenic compounds on ovarian development and function. The specific processes that are most vulnerable to estrogenic compounds are (i) follicular formation (also known as follicular assembly), (ii) follicular growth, and (iii) follicular maturation and ovulation.
(i) Follicular formation/follicular assembly
In mice, primordial germ cells (PGCs) arrive in the ovary and divide by mitosis between PND10.5 and PND13.5. These cells named oogonia enter meiosis I by PND 13.5 and form the oocytes. Then oocytes progress until prophase I and arrest in the diplotene stage. During maturation, oocytes are disposed in clusters and are surrounded by somatic cells forming germ cell cysts (also named oocyte nests). From gestational day 17.5 until PND 5 this germ cell nest breakdown, many of the oocytes undergo programmed cell death and remaining oocytes are surrounded by pre-granulosa cells. This process, known as follicular formation or follicular assembly, sets the primordial pool of follicles (40, 41). A large list of factors, including neurotrophins, growth factors and transcription factors participate in the follicular assembly (40). Exposure to E2 or estrogenic compounds in newborn rodents inhibits and delays the follicular assembly (42, 43) and is associated with abnormally assembled follicles, containing more than one oocyte per follicle (multi-oocyte follicles; MOF) (44–49). Compounds with estrogenic activity have been demonstrated to decrease the number of primordial follicles (14, 15, 46, 50) potentially shortening reproductive lifespan.
(ii) Follicular growth
Once formed most primordial follicles remain quiescent to be recruited throughout the reproductive life although some immediately start growing prior to puberty. After female reach puberty, group of follicles are recruited in cohort to grow up until early antral stage, independently of central control as mentioned earlier. Neonatal exposure to estrogenic chemicals, such as BPA (51), MXC (17, 52), and EV (53) has been linked to reduced growth and development of primary, secondary and antral follicles. Since, early stages of follicular development do not depend upon gonadotropin influence; the ovary could be a direct target of the disruption by estrogenic compounds.
(iii) Follicular maturation and ovulation
Developmental exposure to estrogenic compounds could interfere with the final maturation of follicles. Thus, follicles, instead of completing their final maturation to ovulate, they form follicular cysts in the ovaries (16, 51, 54, 55). Therefore, environmental estrogens are thought to be potentially important in the pathogenesis of polycystic ovary syndrome (56, 57). This could result from a direct effect in the ovary as well as indirectly at the level of brain under the influence of estrogenic chemicals. The evidence for the disruptive effects of estrogenic compounds on the differentiation of brain in female has long been known (58–61). Evidence for direct effects of environmental estrogens in the ovary exists. For example, prepubertal rats that were exposed to estrogenic chemicals (e.g., MXC) during fetal and neonatal developmental periods ovulate fewer eggs in response to exogenous gonadotropins (52), suggesting reduced gonadotropin responsiveness in the ovary.
Although exposure to environmental estrogenic compounds and their effects on human reproductive function remains uncertain, there is a growing literature demonstrating adverse effects of environmental chemicals in animal models as well as wildlife species (reviewed in (62)). However, owing to inherent limitations of animal models and the assumption that human exposure to environmental chemicals are frequently thought to be too low to induce adverse health effects in humans, analogous to those documented in animal models, alternative mechanisms are sought to explore the links between exposure and adverse effects documented in epidemiological studies. There is a growing concern that exposure to environmental estrogens during early development can produce irreversible long-term programming of physiology including adverse effects on reproductive physiology and development (Summarized in Table 1). Recent research has advanced the knowledge of mechanisms that could explain how exposure to estrogens during fetal and neonatal development produces long-term alterations in the function of different organs. In this context, we review the epigenetic mechanisms of gene expression that regulate ovarian development and function and provide examples of epigenetic reprogramming that could explain long-term alterations in ovarian function produced by exposure to estrogenic compounds during development.
Table 1.
Endocrine Disrupting Chemicals (EDCs): Mechanism, Source and Effects on Ovarian Function.
| Compound/Potential Targets/Source | Does of exposure/species | Main Effects | References |
|---|---|---|---|
|
Bisphenol-A (BPA) ESR Plastics industry |
1, 10 and 100 μg/ml in vitro, mice cultured antral follicles |
Inhibits follicular growth, induces atresia, and inhibits steroidogenesis and expression of steroidogenic enzymes in antral follicles from adult FVB, C57BL/6, and CD-1mice. | 127 |
| 500 μg per pup PND1-10 daily rat sc injection |
Alters the hypothalamic-pituitary-gonadal axis in female Sprague-Dawley rats and induces development of follicular cysts in the ovary, leading to infertility. Increases testosterone and estradiol levels and decreases progesterone. | 51 | |
| 20 μg/kg PND7-14 daily mice hypodermic injection |
Inhibits methylation of imprinted genes during oogenesis via the ER signaling pathway, affecting the development of oocytes and folliculogenesis. | 106 | |
| 50 mg or 50 μg/kg PC14-E9.5 or PC14-E12.5 daily mice diet |
Disturbances in tissue-specific DNA methylation and gene expression in imprinted loci, leading to abnormalities in placental development. | 78 | |
| 0.5 mg/kg E30-90 daily ewe sc injection |
Decreases miRNAs targeting SOX family genes (sex determination and embryonic development), kit ligand (follicular assembly establishment of reserve pool of primordial follicles) and insulin related genes (follicular development and steroid production). | 121 | |
| 150 μg/pup PND 1-5 daily mice sc injection |
Induces polyovular follicles or multi-oocyte follicles (having more than one oocyte in a follicle). | 47 | |
| 0.05 and 20 mg/kg PND 1, 3, 5, and 7 rats sc injection |
Reduces primordial follicles and increased growing follicles. Primordial and recruited showed increased ERβ and proliferation in recruited follicles. | 15 | |
| 50 ug/kg and 50 mg/kg PND 1 rats sc injection |
Produces early pubertal onset. Large antral-like follicles and follicular cysts, lower numbers of corpora lutea and acyclicity. | 54 | |
| 2, 20, and 200 μg/kg E1-19 daily mice orally |
Dose-dependent, brain region-specific and sex-specific effects of BPA on gene expression of ERs, ERα, ERβ and estrogen-related receptor-γ that varied across brain regions, and changes DNA methyltransferase (DNMT) 1 and DNMT3A in the juvenile cortex and hypothalamus. Changes in behavior: hyperactive phenotype in males and hypoactive phenotype in females. | 104 | |
| 4.4, 44, and 440 uM in vitro cultured antral follicles PND 32 mice |
BPA (440 μM) inhibits follicle growth. BPA (44 and 440 uM) inhibit progesterone, dehydroepiandrosterone, androstenedione, estrone, testosterone, and estradiol production. | 115 | |
| 3 nM and 300 nM in vitro cultured Metaphase II oocytes mice |
Exposure to 3 nM BPA was associated with slightly accelerated follicle development, increased allele methylation errors in differentially methylated regions of maternally imprinted genes, and decreased histone H3K9 trimethylation and interkinetochore distance. | 124 | |
| 10 and 100 ug per pup PND1-5 daily mice sc injection |
Blocks cyst breakdown and rise polyovular follicles and primordial follicles. | 46 | |
|
Methoxychlor (MXC) ESR1 ESR2 AR Pesticide |
50, 100, or 500 mg/kg PND3-10 daily rats sc injection |
Inhibits early ovarian development and stimulates AMH production directly in the ovary. | 17 |
| 100 mg/kg E19 to PND7 daily rats ip or sc injection |
Induces hypermethylation of the promoter region of Esr2 in the adult ovary with a decrease in the ESR2 protein and induces Dnmt3b expression in the ovary. | 101 | |
| 0.5 or 1mg per pup PND 1-14 daily mice ip injection |
Produces ovarian atrophy, relative ovarian weight loss, depletion of corpora lutea and follicular cysts in 0.5 mg doses | 55 | |
| 20 μg/kg and 100 mg/kg E19 to PND7 daily rats ip or sc injection |
Hypermethylation and/or downregulation in: regulatory subunit p85 of phosphoinositide-3-kinase, insulin-like growth factor-1 receptor, Harvey rat sarcoma viral oncogene, insulin receptor, and FOXO3 in the ovary. | 102 | |
| 20 μg/kg and 100 mg/kg E19 to PND7 daily rats ip or sc injection |
Reduces ovulation and induces early puberty, irregular cyclicity, sub-fertility, and premature reproductive aging. | 52 | |
|
Di(2-ethylhexyl) phthalate (DEHP) ESR Androgen production Plastic Industry medical devices building products containing polyvinyl chloride |
20 and 40 μg/kg PND7-14 daily mice hypodermic injection |
Decreases number of the primordial follicles at pubertal and adult age. Decreases methylation of maternal imprinted genes Igf2 and Peg3 in oocytes, which leads to abnormalities in oocyte and embryo development. | 50 |
|
Genistein ESR Phytoestrogen (soy) |
1 nM to 100 uM in vitro cultured ovaries for 7 days PND1 mice |
Impairs formation of primordial follicular pool in the ovary. | 42 |
| 10 and 100 μg/pup PND1-5 daily mice sc injection |
Increases number of multi-oocyte follicles. | 48 | |
|
Estradiol valerate (EV) and Estradiol Benzoate (EB) ESR Synthetic estradiol |
10 mg/kg PND1, 7 and 14 rats sc injection |
Reduces follicular growth and maturation in the ovary, decreases steroidogenesis, disrupts estrous cyclicity, and impairs reproductive performance. | 53 |
| 5 mg/kg PND 1-14 daily mice ip injection |
Induces multi-oocyte follicles in adult ovaries. | 42 | |
| 10 μg/kg PN1-34 day rats sc injection |
Advances vaginal opening, and induces multi-oocyte follicles in the ovary. | 49 | |
| 0,1 mg per pup PND1 sc injection |
Increased ovarian expression of nerve growth factor (Ngfb) and p75 low-affinity neurotrophic receptor (Ngfr) mRNAs. Early vaginal opening, disrupted cyclicity, appearance of follicular cyst, absence of corpus luteum, infertility and reduced number of primordial follicles | 16 | |
| 25 ug per pup PND 1 rats sc injection |
Early pubertal onset. Ovaries undersized and absence of folliculogenesis. Absense of sexual receptivity after ovariectomy and hormone replacement. | 54 | |
|
Diethylstilbestrol (DES) ESR Synthetic estrogen |
0, 0.75, 1.25, 2.5, or 25ug per pup PND1-5 daily Rats sc injection |
Dose-dependent increase in miR-29a, miR-29b, and miR-29c expression, this resulted in a decrease in DNMT1, DNMT3a, and DNMT3b expression in testes | 105 |
| 5 μg/kg PND 1-14 daily lambs sc injection |
Decline in the stock of primordial follicles, increase in number of multi-oocyte follicles and increase in the number of antral atretic follicles | 14 | |
| 0.2 and 20 ug/kg PND 1,3,5 and 7 rats sc injection |
Produce a decrease in primordial follicles and increased growing follicles. Increase the incidences of multi-oocyte follicles. | 15 | |
| 1 ug per pup PND1-5 daily mice sc injection |
Produces a higher incidence in polyovular follicles and the oocytes from polyovular follicles have a smaller capacity for fertilization. | 44 | |
| 3 ug per pup PND1 and 2 mice sc injection |
Induces appearance of polyovular follicles. | 45 | |
| 10 and 100 ug per pup PND1-5 daily mice sc injection |
Blocks the cyst breakdown and rises polyovular follicles and primordial follicles. | 46 |
AMH, anti-Mullerian hormone; E, embryonic day; PND, postnatal day; PC, pre-coital day.
2) Epigenetic mechanisms and their roles in ovarian development and function
The term epigenetics was first coined by Conrad Waddington in 1942 to describe “the branch of biology which studies the causal interactions between genes and their products which bring the phenotype into being.” (63). Today, epigenetics is commonly defined as the study of long-lived but reversible DNA modifications that can change gene expression without changing the DNA sequence (64). There are three major epigenetic mechanisms: (i) DNA methylation, which refers to methylation of cytosine in CpG dinucleotides and is generally associated with a down regulation of gene expression; (ii) histone modifications, which refers to post-translational modifications of various amino acid moieties in histone core proteins and include acetylation and methylation of lysines. Some of these modifications (permissive histone marks) render the regulatory regions of genes more accessible to binding of transcription factors and increase gene expression while some (suppressive histone marks) renders it less accessible and decrease gene expression, and (iii) non-coding RNA (ncRNAs), refer to functional RNAs that do not encode proteins, but generally inhibit the expression of other genes. Non-coding RNAs are classified according to their sizes: microRNAs (miRNAs), which are comprised of 21–25 nucleotides; small RNAs, 100–200 nucleotides; and long ncRNAs > 200–10,000 nucleotides. All three mechanisms collectively and cooperatively modify chromatin structures and regulate gene expression (for reviews (64–69)). The present discussion will focus on the specific mechanisms that regulate ovarian development and function, including germ cell differentiation and epigenetic reprogramming (Figure 2). The role of epigenetic mechanisms in the development and function of the ovary can be viewed from two perspectives: (a) germ cell epigenetic reprogramming; and (b) follicular formation and development.
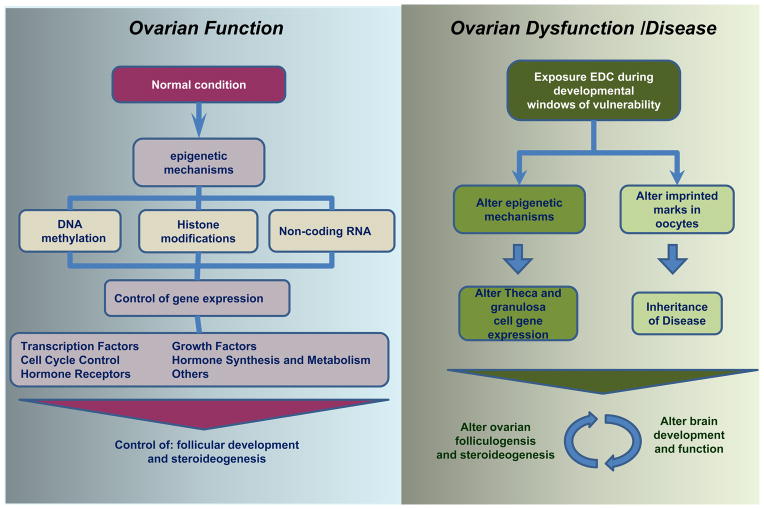
Figure 2. Developmental EDC exposure and potential epigenetic mechanisms in altered brain and ovarian function.
The normal ovarian development and function are strictly regulated by precise epigenetic mechanisms (DNA methylation, histone modifications, and miRNA) that coordinate the spatio-temporal expression of key genes. These genes are transcription factors, cell cycle proteins, growth factors, hormone synthesis enzymes and receptors, which participate in different stages of development of the follicles. Exposure to environmental estrogens alters these mechanisms of control, affecting gene expression in the ovary and leading to dysfunction and disease (See section 3).
2.1. Germ cell epigenetic reprogramming
During mammalian development, two major epigenetic reprogramming events occur: one in the preimplantation embryo and the other is in PGCs. Epigenetic programming in preimplantation embryo refers to the global erasure of DNA methylation patterns in the maternal and paternal genomes in all loci except imprinted genes and tandem repeats such as intracisternal A particles (IAPs) (70), and the reestablishment of new DNA methylation patterns according to the cell lineage fate (71). Although epigenetic modifications continue as the cells and tissues further differentiate, these modifications are limited and remain in the trajectory that is initially established.
However, PGCs is an exception, and they undergo a global epigenetic reprogramming at later stage of embryonic development. Specifically, around embryonic (E) day 7.5 in mice, PGCs begin to differentiate within the embryo proper (72), and their initial DNA methylation profile resembles that of the embryonal cells from which they differentiate (71). The PGCs gain germ cell-specific expression patterns between E8.5 and E11.5, during which they migrate and colonize the bipotential gonad, an embryonic structure that differentiates into the testis or ovary depending on the genetic sex. Gonadal sex determines the fate of PGCs, which differentiate into either female or male germ cells. Germ cells in both sexes proliferate rapidly until E13–14, when male germ cells enter mitotic arrest and female germ cells enter meiosis and arrest at meiosis I (73, 74). Between E8.5 and E12.5, the genome of PGCs, including the imprinted loci, undergoes a major demethylation process. While the timing of the demethylation process in male and female germs cells is the same, the remethylation timelines are sex-specific. Remethylation in male PGCs takes place in utero several days after erasure between E14.5 and E16.5 and is mostly complete by birth (75, 76). In contrast, female germ cell remethylation begins postnatally (PND 1 – 5) and continues throughout the growth of the oocyte. In addition, imprinting patterns are established based on the size of the oocyte rather than the age of the animal (77). Thus the evidence suggests that as each follicle is recruited into the growing follicular pool, the imprinting patterns of the associated oocyte are established. Any exposure to environmental chemicals such as BPA during imprinting establishment can disrupt the imprinting patterns and the resultant embryo suffers from altered gene expression at these loci and developmental abnormalities, especially in the placenta (78).
DNA methylation and the enzymatic machinery, namely de novo DNA methyltransferases (DNMTs) that establish DNA methylation patterns play major roles in genomic imprinting (65). Therefore, gonadal expression levels of DNMTs closely follow the timing of the establishment of the imprinting marks (79). Some imprinted loci also involve histone marks (80) and non-coding RNAs (81). During germ cell epigenetic reprogramming, besides imprinted loci, some repeat sequences (e.g., LINE1) lose their methylation marks and are de novo remethylated along with imprinted sequences (82). However, sequences with high mutagenic potential such as IAPs are protected from demethylation, which are believed to mediate transgenerational epigenetic inheritance (83).
2.2. Follicle formation and development
The main somatic cell types that are associated directly with follicles (granulosa and thecal cells) are regulated by various epigenetic mechanisms, including DNA methylation (84), histone modifications (85), and non-coding RNA (86) throughout different follicular stages. Steroidogenic factor 1 (Sf1), which is expressed in granulosa cells in the embryonic gonad (87), is regulated by promoter DNA methylation at the earliest stage of gonadal differentiation. While the Sf1 promoter in the embryonic ovary had no or minimal DNA methylation, it is highly methylated in embryonic liver or kidney, which reflects the expression patterns of the gene in these organs (88). It is likely that theca cell differentiation also involves epigenetic mechanisms, but relatively little is known regarding the initial cell specification of thecal cell precursors (89).
In postnatal and adult ovary, epigenetic mechanisms continue to play roles in differentiation of these two somatic cells. In granulosa cell, down-regulation of inhibin α during luteinization is closely regulated by histone modifications as well as DNA methylation in the promoter region of this gene (90). In contrast, up-regulation of steroidogenic acute regulatory protein (StAR) and down-regulation of aromatase (Cyp19a1) following gonadotropin treatment do not involve DNA methylation but are regulated primarily by histone modifications (91). In thecal, cells, when the levels of permissive histone marks are pharmacologically elevated at the promoter region in cytochrome P450 17A1 (CYP17A1), gene expression increases leading to an increase in androgen production (92). In contrast, in bovine thecal cells, the LH surge reduces chromatin accessibility of the CYP17A1 gene and its mRNA levels (93). In addition, patterns of epigenetic marks are determined by the cell types within the follicle as well as the follicular stage. While DNA methylation is not involved in preovulatory down-regulation of CYP19A1 in the follicles, it plays a major role in the permanent silencing of CYP19A1 in the corpus luteum (94). The roles of miRNAs in various aspects of ovarian development and function, including, gonadal differentiation (95), follicle formation (96), selection, maturation (97), ovulation (98), and luteinization (99) as well as atresia (100), have also been shown.
In summary, the main epigenetic events as relate to the ovary that are likely to be vulnerable to adverse effects of environmental factors, including endocrine disruptors are (i) the epigenetic reprogramming of female germ cells and establishment of maternal imprinting patterns, and (ii) initial differentiation and continuous adaptation of somatic components of ovarian follicles to the various stages of folliculogenesis and corpus luteum function. Experimental evidence supporting these potential epigenetic events is provided in Section 3. In addition, it is important to note that, the epigenetic reprogramming of the early embryo is also vulnerable to influence of environmental factors including endocrine disruptors. The consequence of this influence not only would be on the ovary and female germ cells, but it would also be on the entire developing organism ((78) and references within).
3) Effects of environmental estrogenic compounds on epigenetic regulation of gene expression in the ovary and its relation with ovarian function and development
Emerging evidence suggests changes in epigenetic control of gene expression as a potential mechanism underlying long-term and irreversible alterations in ovarian physiology arising from developmental exposure to endocrine disruptors (62, 101, 102). Owing to the central role of estrogen receptors (ESR) in the ovary during development (103), cells of ovarian follicles are thought to be potential targets of disruption by endogenous or environmental estrogens. Exposure to environmental chemicals with hormone-like activity in early life, such as MXC, BPA, EB and di(2-ethylhexyl) phthalate (DEHP) produces changes in the expression of DNMT in different tissues (50, 101, 104–106). DNMT can affect the patterns of methylation of promoter regions of genes usually causing a repression of gene expression. Methylation of CpG island or promoter regions decreases the accessibility of transcription factors to the binding sites in the DNA. If the binding of a stimulating transcription factor to the DNA is blocked the gene down-regulates. Conversely, if the binding of a transcription repressor is blocked the gene up-regulates. Ovarian exposure to MXC, BPA, and DEHP has been shown to alter the expression of DNMT resulting in potential alterations to the epigenome (50, 101, 106). The ovary is regulated by a myriad of transcription factors (107) which in turn regulate the expression of genes related to hormone action, extrinsic innervation, and paracrine growth factors. All these regulators act coordinately to promote follicular development, ovulation and ovarian hormone production. As may be predicted, changes in DNMT can deregulate these highly coordinated inputs and alter ovarian physiology. Despite this, the mechanism which explains how an exposure in early life produce a change in the expression of genes that can persist until adulthood, in the absence of the stimuli, remains unknown.
Long-term ovarian alterations produced by different environmental signals during fetal and early postnatal development could result from an adaptive or maladaptive response of the body to the environmental signal. In this context, it was shown that exposure to chemicals with hormone-like activity in early life leads to long-term alterations in expression of critical genes, such as ESR2, in the ovary. One such chemical is chlorinated organic pesticide MXC. Although it is currently de-registered by the EPA in the U.S., MXC is an excellent model endocrine disruptor since its major metabolites (i.e., HPTE and mono-OH-MXC) act as ESR1 agonists as well as ESR2 and androgen receptor antagonists (108). These activities represent many hormone-like chemicals in the environment. Exposure to MXC during fetal and neonatal development produces hypermethylation of the promoter region of Esr2 in the adult ovary with a decrease in the ESR2 protein (101). This decrease in ESR2 could produce a lower response to endogenous E2 in adulthood and as a consequence, an alteration in follicular development. Direct effects of E2 which favor cell proliferation and follicular development have been demonstrated (109); however, much of the paracrine regulators of folliculogenesis and its receptors have estrogen response elements (EREs). The expression of these paracrine factors can be affected by this permanently altered response to E2 through the estrous cycle. For example, AMH which is produced by granulosa cells of growing pre-antral follicles has EREs and is increased in adulthood after exposure to MXC or BPA in early life (17, 110). E2 may have a repressive effect on Amh expression and the high expression of Amh could be the result of lower response to E2 during the estrous cycle in adulthood. Alternatively, exogenous estrogens may stimulate Amh expression through the ERE leading to an increase in AMH (111). AMH has a repressive effect on early follicular development and primordial follicle recruitment as well as FSH-induced follicular maturation (112–114) which is consistent with the findings of a lower number of developing follicles in different models of estrogenic disruption (16, 17, 53, 115).
Other genes subject to epigenetic control are important in regulation of ovarian function and are permanently modified by developmental exposure to estrogenic compounds (102) including insulin growth factor-1 receptor (IGF-1R). IGF-1R agonism leads to activation of PI3K/AKT and MAPK pathways which is related to a protection of cells from Fas-L induced apoptosis and stimulation of granulosa cell proliferation (116, 117). IGF-1R is down-regulated in adult rats exposed to MXC in the neonatal period. This is consistent with the phenotype of MXC-treated animals, which showed reduced follicular development (17). Interestingly, IGF-1 secretion is influenced by FSH (118) and hence some of the effects of FSH on follicular development are mediated by IGF1. In this context, the lower expression of IGF1-R in MXC treated rats could produce a lower response of the ovary to the effects of FSH. Sympathetic innervation of the ovary is also thought to be important in ovarian regulation (119). Norepinephrine activates the β-2 adrenoreceptor and increase expression of FSHR favoring follicular development (120). β-2 adrenoreceptor gene is hypermethylated after early exposure to MXC (102). Other genes of intracellular proteins important for cell signaling pathways such as Pik3r1, Prkar2a, Pak3, and Foxo3a, are hypermethylated in the adult rat ovary after early exposure to MXC (102). On the other hand, the exposure to DEHP decreases methylation of maternal imprinted genes Igf2 and Peg3 in oocytes, which leads to abnormalities in oocyte and embryo development (50). In the same way, BPA is also known to alter methylation pattern of Igf2 and Peg3 in oocytes affecting the development of oocytes and folliculogenesis (106). In addition, daily exposure to 10 mg BPA per kg bw from 2 weeks prior mating to E9.5 or E12.5, which corresponds to germ cell and early embryonic epigenetic reprogramming, produces a tissue-specific DNA methylation and gene expression disturbances in imprinted loci (Snrpn, Ube3a, Igf2, Kcnq1ot1), leading to placental abnormalities at E12.5 in mice. Daily exposure to 10 μg BPA per kg bw, a dose considered to be safe for humans, was also effective in causing alteration at some of these loci. Importantly, effects of both doses were stage-dependent: A daily exposure between E5.5 and E12.5 had no observable effect (78).
Exposure to environmental estrogens during pregnancy can also modulate ovarian miRNA expression in the fetus (121). Exposure to BPA in sheep produces a decrease in miRNAs targeting Sry-related high-mobility-group box (SOX) family genes, kit ligand, and insulin-related genes (121). SOX genes are implicated in sex determination and embryonic development (122), while Kit ligand signaling is important for follicular assembly and the correct establishment of the reserve pool of primordial follicles (123). A silencing of Kit ligand by miRNA may result in an abnormal follicular assembly and could explain defects observed in animals exposed to environmental estrogens. On the other hand silencing of insulin related genes as IGF1 and IGF2 could explain the phenotype of reduced follicular growth in animals exposed to environmental estrogens during fetal and neonatal development. With regard to histone modifications, BPA produces a significant decrease in histone H3K9 trimethylation, a repressive histone mark, in cultured follicles (124); however, in vivo effects of developmental exposure to estrogenic compounds on histone modifications in the ovary are not known. Regardless, recent studies have shown that developmental exposure to phytoestrogen, genistein, affects histone marks in the uterus leading to alteration in gene expression and abnormal growth during adulthood (125).
In addition to the epigenetic reprogramming of ovarian somatic cells after the exposure to environmental estrogens, the PGC can also be a target of epigenetic disruption (50, 106). These epigenetic alterations could potentially damage the function of oocytes affecting both oogenesis and embryogenesis. Another consideration of epigenetic modifications of PGCs by environmental estrogens is whether these modifications of the epigenome can be hereditable to future generations. There is little information about the transgenerational inheritance of epigenetic modifications in ovarian follicular development after exposure to estrogenic compounds (126). The possibility that estrogens can produce a transgenerational inheritance of ovarian disease through the permanent alteration of germ cell epigenome needs to be considered.
4) Conclusions
Exposure to environmental estrogens has been of concern due to the potential impact in the development and function of the ovaries, reproductive tract and other estrogen sensitive tissues such as the brain. Because fetal and neonatal developmental stages are sensitive periods, exposure to estrogenic compounds could produce irreversible epigenetic reprogramming of ovarian genes that control ovarian follicular development that could, in turn, lead to abnormalities in adulthood. Studies in animal models have shown that developmental exposure to environmentally relevant levels of these chemicals can produce changes in epigenetic mechanisms and gene expression changes (50, 101, 102, 106, 121, 124). In some of these studies, these changes are also associated with ovarian dysfunction (11, 50, 106). In the future it will be important (i) to establish causal links among epigenetic alterations and gene expression and functional changes in the ovary, (ii) to determine whether these chemicals can induce additional epigenetic alterations, gene expression changes and ovarian dysfunction or whether additional environmental chemicals can cause similar changes in the ovary, and (iii) to determine whether human females with reproductive diseases/dysfunction display similar patterns of epigenetic alterations that have been demonstrated in animal models. Finally, the possibility that the effects of the estrogenic chemicals can be transmitted through epigenetic reprogramming of germ cells affecting ovarian follicular development should be assessed in the future.
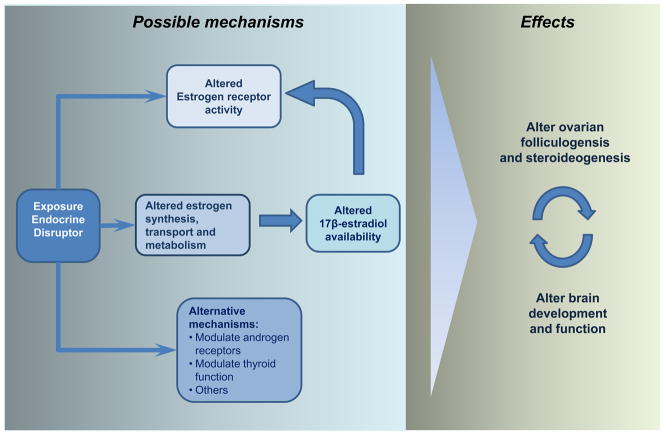
Figure 1. Developmental EDC exposure and possible mechanisms to alter brain and ovarian functions.
Endocrine-disrupting chemicals (EDC) have different mechanism of actions which mediate the final effects on ovarian function. Among them, the most recognized are through activating estrogenic receptors either by binding to them or by modifying estradiol (E2) availability [e.g., E2, bisphenol A, methoxychlor (MXC), di(2-ethylhexyl) phthalate (DEHP), diethylstilbestrol]. Other important mechanisms are the antiandrogenic proprieties of some compounds (e.g., MXC and DEHP) and the thyroid modulating effects of others (not reviewed). EDC can directly target the brain or the ovaries due the presence estrogen and androgen receptors in both tissues. However, the mechanisms of regulations between both tissues lead to the possibility that disruption of ovarian physiology affect the brain and vice versa.
Acknowledgments
This work is supported in part by National Institute of Environmental Health Sciences grants ES017847 and ES017059 and NIEHS Center grant ES005022 to MU, Fondecyt grant 1120147 to AP and Fondecyt grant 11130707 to GC.
References
- 1.Boersma ER, Lanting CI. Environmental exposure to polychlorinated biphenyls (PCBs) and dioxins. Consequences for longterm neurological and cognitive development of the child lactation. Adv Exp Med Biol. 2000:478271–87. [PubMed] [Google Scholar]
- 2.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson JL, Jacobson SW. Dose-response in perinatal exposure to polychlorinated biphenyls (PCBs): the Michigan and North Carolina cohort studies. Toxicol Ind Health. 1996;12(3–4):435–45. doi: 10.1177/074823379601200315. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18(2):415–24. [PubMed] [Google Scholar]
- 5.Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr. 1999;134(1):33–41. doi: 10.1016/s0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- 6.Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, Sparks A, Weiss B. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl. 2010;33(2):259–69. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual review of neuroscience. 2002:25507–36. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 8.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain research Developmental brain research. 2002;139(2):151–8. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58(4):584–98. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losa-Ward SM, Todd KL, McCaffrey KA, Tsutsui K, Patisaul HB. Disrupted organization of RFamide pathways in the hypothalamus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol Reprod. 2012;87(2):28. doi: 10.1095/biolreprod.112.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Molecular endocrinology. 2011;25(12):2157–68. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz G, Riquelme R, Espinosa P, Jara P, Dagnino-Subiabre A, Renard GM, Sotomayor-Zarate R. Neonatal exposure to estradiol valerate increases dopamine content in nigrostriatal pathway during adulthood in the rat. Hormone and metabolic research = Hormonund Stoffwechselforschung = Hormones et metabolisme. 2014;46(5):322–7. doi: 10.1055/s-0033-1361159. [DOI] [PubMed] [Google Scholar]
- 13.Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol. 2010;30(3):393–400. doi: 10.1016/j.reprotox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Rivera OE, Varayoud J, Rodriguez HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32(3):304–12. doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30(4):550–7. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Sotomayor-Zarate R, Dorfman M, Paredes A, Lara HE. Neonatal exposure to estradiol valerate programs ovarian sympathetic innervation and follicular development in the adult rat. Biol Reprod. 2008;78(4):673–80. doi: 10.1095/biolreprod.107.063974. [DOI] [PubMed] [Google Scholar]
- 17.Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. The Journal of endocrinology. 2006;191(3):549–58. doi: 10.1677/joe.1.06592. [DOI] [PubMed] [Google Scholar]
- 18.Axelrad DA, Goodman S, Woodruff TJ. PCB body burdens in US women of childbearing age 2001–2002: An evaluation of alternate summary metrics of NHANES data. Environ Res. 2009;109(4):368–78. doi: 10.1016/j.envres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Foster WG, Cheung AP, Davis K, Graves G, Jarrell J, Leblanc A, Liang CL, Leech T, Walker M, Weber JP, Van Oostdam J. Circulating metals and persistent organic pollutant concentrations in Canadian and non-Canadian born primiparous women from five Canadian centres: results of a pilot biomonitoring study. Sci Total Environ. 2012;435–436:326–36. doi: 10.1016/j.scitotenv.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 20.LaKind JS, Hays SM, Aylward LL, Naiman DQ. Perspective on serum dioxin levels in the United States: an evaluation of the NHANES data. J Expo Sci Environ Epidemiol. 2009;19(4):435–41. doi: 10.1038/jes.2008.63. [DOI] [PubMed] [Google Scholar]
- 21.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Needham LL, Barr DB, Calafat AM. Characterizing children’s exposures: beyond NHANES. Neurotoxicology. 2005;26(4):547–53. doi: 10.1016/j.neuro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Nichols BR, Hentz KL, Aylward L, Hays SM, Lamb JC. Age-specific reference ranges for polychlorinated biphenyls (PCB) based on the NHANES 2001–2002 survey. J Toxicol Environ Health A. 2007;70(21):1873–7. doi: 10.1080/15287390701457688. [DOI] [PubMed] [Google Scholar]
- 24.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–8. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloom MS, Buck-Louis GM, Schisterman EF, Kostyniak PJ, Vena JE. Changes in maternal serum chlorinated pesticide concentrations across critical windows of human reproduction and development. Environ Res. 2009;109(1):93–100. doi: 10.1016/j.envres.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloom MS, Kim D, Vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril. 2011;96(3):672–7. e2. doi: 10.1016/j.fertnstert.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril. 2011;95(5):1816–9. doi: 10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Jarrell JF, Villeneuve D, Franklin C, Bartlett S, Wrixon W, Kohut J, Zouves CG. Contamination of human ovarian follicular fluid and serum by chlorinated organic compounds in three Canadian cities. CMAJ. 1993;148(8):1321–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Younglai EV, Foster WG, Hughes EG, Trim K, Jarrell JF. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch Environ Contam Toxicol. 2002;43(1):121–6. doi: 10.1007/s00244-001-0048-8. [DOI] [PubMed] [Google Scholar]
- 30.Gerhard I, Monga B, Krahe J, Runnebaum B. Chlorinated hydrocarbons in infertile women. Environ Res. 1999;80(4):299–310. doi: 10.1006/enrs.1998.3890. [DOI] [PubMed] [Google Scholar]
- 31.Weiss JM, Bauer O, Bluthgen A, Ludwig AK, Vollersen E, Kaisi M, Al-Hasani S, Diedrich K, Ludwig M. Distribution of persistent organochlorine contaminants in infertile patients from Tanzania and Germany. J Assist Reprod Genet. 2006;23(9–10):393–9. doi: 10.1007/s10815-006-9069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillette LJ, Jr, Moore BC. Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Seminars in reproductive medicine. 2006;24(3):134–41. doi: 10.1055/s-2006-944419. [DOI] [PubMed] [Google Scholar]
- 33.Moore BC, Kohno S, Cook RW, Alvers AL, Hamlin HJ, Woodruff TK, Guillette LJ. Altered sex hormone concentrations and gonadal mRNA expression levels of activin signaling factors in hatchling alligators from a contaminated Florida lake. Journal of experimental zoology Part A, Ecological genetics and physiology. 2010;313(4):218–30. doi: 10.1002/jez.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oktem O, Oktay K. The ovary: anatomy and function throughout human life. Ann N Y Acad Sci. 2008:11271–9. doi: 10.1196/annals.1434.009. [DOI] [PubMed] [Google Scholar]
- 35.Heneweer M, van den Berg M, Sanderson JT. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol Lett. 2004;146(2):183–94. doi: 10.1016/j.toxlet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci. 2000;54(1):121–7. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- 37.Peters AK, van Londen K, Bergman A, Bohonowych J, Denison MS, van den Berg M, Sanderson JT. Effects of polybrominated diphenyl ethers on basal and TCDD-induced ethoxyresorufin activity and cytochrome P450-1A1 expression in MCF-7, HepG2, and H4IIE cells. Toxicol Sci. 2004;82(2):488–96. doi: 10.1093/toxsci/kfh284. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson JT, Slobbe L, Lansbergen GW, Safe S, van den Berg M. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and diindolylmethanes differentially induce cytochrome P450 1A1, 1B1, and 19 in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2001;61(1):40–8. doi: 10.1093/toxsci/61.1.40. [DOI] [PubMed] [Google Scholar]
- 39.Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142(5):633–46. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- 40.Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143(2):139–49. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 41.Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Developmental dynamics: an official publication of the American Association of Anatomists. 1992;194(3):177–92. doi: 10.1002/aja.1001940303. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148(8):3580–90. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 43.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144(8):3329–37. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 44.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43(3):478–84. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Nakajima T, Hayashi S, Chambon P, Watanabe H, Iguchi T, Sato T. Effects of diethylstilbestrol on programmed oocyte death and induction of polyovular follicles in neonatal mouse ovaries. Biol Reprod. 2009;81(5):1002–9. doi: 10.1095/biolreprod.108.070599. [DOI] [PubMed] [Google Scholar]
- 46.Karavan JR, Pepling ME. Effects of estrogenic compounds on neonatal oocyte development. Reprod Toxicol. 2012;34(1):51–6. doi: 10.1016/j.reprotox.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16(2):107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 48.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67(4):1285–96. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 49.Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol. 2011;31(3):280–9. doi: 10.1016/j.reprotox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environmental and molecular mutagenesis. 2013;54(5):354–61. doi: 10.1002/em.21776. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118(9):1217–22. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicology and applied pharmacology. 2008;233(2):286–96. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cruz G, Barra R, Gonzalez D, Sotomayor-Zarate R, Lara HE. Temporal window in which exposure to estradiol permanently modifies ovarian function causing polycystic ovary morphology in rats. Fertil Steril. 2012;98(5):1283–90. doi: 10.1016/j.fertnstert.2012.07.1060. [DOI] [PubMed] [Google Scholar]
- 54.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81(4):690–9. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eroschenko VP, Abuel-Atta AA, Grober MS. Neonatal exposures to technical methoxychlor alters ovaries in adult mice. Reprod Toxicol. 1995;9(4):379–87. doi: 10.1016/0890-6238(95)00025-6. [DOI] [PubMed] [Google Scholar]
- 56.Rutkowska A, Rachon D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS) Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2014 doi: 10.3109/09513590.2013.871517. [DOI] [PubMed] [Google Scholar]
- 57.Tsutsumi O. Assessment of human contamination of estrogenic endocrine-disrupting chemicals and their risk for human reproduction. The Journal of steroid biochemistry and molecular biology. 2005;93(2–5):325–30. doi: 10.1016/j.jsbmb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicology and applied pharmacology. 2011;252(1):36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152(2):581–94. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarro VM, Sanchez-Garrido MA, Castellano JM, Roa J, Garcia-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology. 2009;150(5):2359–67. doi: 10.1210/en.2008-0580. [DOI] [PubMed] [Google Scholar]
- 61.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 62.Zama AM, Uzumcu M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: an ovarian perspective. Frontiers in neuroendocrinology. 2010;31(4):420–39. doi: 10.1016/j.yfrne.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waddington CH. The epigenotype. 1942. Int J Epidemiol. 2012;41(1):10–3. doi: 10.1093/ije/dyr184. [DOI] [PubMed] [Google Scholar]
- 64.Lasalle JM, Powell WT, Yasui DH. Epigenetic layers and players underlying neurodevelopment. Trends Neurosci. 2013;36(8):460–70. doi: 10.1016/j.tins.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly TL, Trasler JM. Reproductive epigenetics. Clin Genet. 2004;65(4):247–60. doi: 10.1111/j.0009-9163.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 66.Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14(6):341–56. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabin LR, Delas MJ, Hannon GJ. Dogma derailed: the many influences of RNA on the genome. Mol Cell. 2013;49(5):783–94. doi: 10.1016/j.molcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes Dev. 2013;27(12):1318–38. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20(3):282–9. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- 71.Hackett JA, Surani MA. DNA methylation dynamics during the mammalian life cycle. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20110328. doi: 10.1098/rstb.2011.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110(2):521–8. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 73.McCarrey JR. Development of the germ cell. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. New York: Oxford Univesity Press; 1993. pp. 58–89. [Google Scholar]
- 74.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ueda T, Abe K, Miura A, Yuzuriha M, Zubair M, Noguchi M, Niwa K, Kawase Y, Kono T, Matsuda Y, Fujimoto H, Shibata H, Hayashizaki Y, Sasaki H. The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage during foetal testis development. Genes Cells. 2000;5(8):649–59. doi: 10.1046/j.1365-2443.2000.00351.x. [DOI] [PubMed] [Google Scholar]
- 76.Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M, Sasaki H. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet. 2007;16(19):2272–80. doi: 10.1093/hmg/ddm179. [DOI] [PubMed] [Google Scholar]
- 77.Hiura H, Obata Y, Komiyama J, Shirai M, Kono T. Oocyte growth-dependent progression of maternal imprinting in mice. Genes Cells. 2006;11(4):353–61. doi: 10.1111/j.1365-2443.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 78.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS genetics. 2013;9(4):e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lucifero D, La Salle S, Bourc’his D, Martel J, Bestor TH, Trasler JM. Coordinate regulation of DNA methyltransferase expression during oogenesis. BMC Dev Biol. 2007:736. doi: 10.1186/1471-213X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ciccone DN, Su H, Hevi S, Gay F, Lei H, Bajko J, Xu G, Li E, Chen T. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature. 2009;461(7262):415–8. doi: 10.1038/nature08315. [DOI] [PubMed] [Google Scholar]
- 81.Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21(3):416–25. doi: 10.1016/j.ceb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35(2):88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- 83.Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100(5):2538–43. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pan Z, Zhang J, Li Q, Li Y, Shi F, Xie Z, Liu H. Current advances in epigenetic modification and alteration during mammalian ovarian folliculogenesis. J Genet Genomics. 2012;39(3):111–23. doi: 10.1016/j.jgg.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 85.LaVoie HA. Epigenetic control of ovarian function: the emerging role of histone modifications. Mol Cell Endocrinol. 2005;243(1–2):12–8. doi: 10.1016/j.mce.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Christenson LK. MicroRNA control of ovarian function. Anim Reprod. 2010;7(3):129–33. [PMC free article] [PubMed] [Google Scholar]
- 87.Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15(3):417–31. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- 88.Hoivik EA, Aumo L, Aesoy R, Lillefosse H, Lewis AE, Perrett RM, Stallings NR, Hanley NA, Bakke M. Deoxyribonucleic acid methylation controls cell type-specific expression of steroidogenic factor 1. Endocrinology. 2008;149(11):5599–609. doi: 10.1210/en.2008-0104. [DOI] [PubMed] [Google Scholar]
- 89.Ungewitter EK, Yao HH. How to make a gonad: cellular mechanisms governing formation of the testes and ovaries. Sexual development: genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2013;7(1–3):7–20. doi: 10.1159/000338612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meldi KM, Gaconnet GA, Mayo KE. DNA methylation and histone modifications are associated with repression of the inhibin alpha promoter in the rat corpus luteum. Endocrinology. 2012;153(10):4905–17. doi: 10.1210/en.2012-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee L, Asada H, Kizuka F, Tamura I, Maekawa R, Taketani T, Sato S, Yamagata Y, Tamura H, Sugino N. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology. 2013;154(1):458–70. doi: 10.1210/en.2012-1610. [DOI] [PubMed] [Google Scholar]
- 92.Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, Wood JR, Legro RS, Strauss JF, 3rd, McAllister JM. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology. 2004;145(2):799–808. doi: 10.1210/en.2003-0940. [DOI] [PubMed] [Google Scholar]
- 93.Nimz M, Spitschak M, Furbass R, Vanselow J. The pre-ovulatory luteinizing hormone surge is followed by down-regulation of CYP19A1, HSD3B1, and CYP17A1 and chromatin condensation of the corresponding promoters in bovine follicles. Mol Reprod Dev. 2010;77(12):1040–8. doi: 10.1002/mrd.21257. [DOI] [PubMed] [Google Scholar]
- 94.Vanselow J, Spitschak M, Nimz M, Furbass R. DNA methylation is not involved in preovulatory down-regulation of CYP11A1, HSD3B1, and CYP19A1 in bovine follicles but may have a role in permanent silencing of CYP19A1 in large granulosa lutein cells. Biol Reprod. 2010;82(2):289–98. doi: 10.1095/biolreprod.109.079251. [DOI] [PubMed] [Google Scholar]
- 95.Real FM, Sekido R, Lupianez DG, Lovell-Badge R, Jimenez R, Burgos M. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol Reprod. 2013;89(4):78. doi: 10.1095/biolreprod.113.110957. [DOI] [PubMed] [Google Scholar]
- 96.Zhang J, Ji X, Zhou D, Li Y, Lin J, Liu J, Luo H, Cui S. miR-143 is critical for the formation of primordial follicles in mice. Front Biosci (Landmark Ed) 2013:18588–97. doi: 10.2741/4122. [DOI] [PubMed] [Google Scholar]
- 97.Schauer SN, Sontakke SD, Watson ED, Esteves CL, Donadeu FX. Involvement of miRNAs in equine follicle development. Reproduction. 2013;146(3):273–82. doi: 10.1530/REP-13-0107. [DOI] [PubMed] [Google Scholar]
- 98.Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83(2):286–95. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McBride D, Carre W, Sontakke SD, Hogg CO, Law A, Donadeu FX, Clinton M. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction. 2012;144(2):221–33. doi: 10.1530/REP-12-0025. [DOI] [PubMed] [Google Scholar]
- 100.Lin F, Li R, Pan ZX, Zhou B, Yu de B, Wang XG, Ma XS, Han J, Shen M, Liu HL. miR-26b promotes granulosa cell apoptosis by targeting ATM during follicular atresia in porcine ovary. PLoS One. 2012;7(6):e38640. doi: 10.1371/journal.pone.0038640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150(10):4681–91. doi: 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zama AM, Uzumcu M. Targeted genome-wide methylation and gene expression analyses reveal signaling pathways involved in ovarian dysfunction after developmental EDC exposure in rats. Biol Reprod. 2013;88(2):52. doi: 10.1095/biolreprod.112.104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Drummond AE, Baillie AJ, Findlay JK. Ovarian estrogen receptor alpha and beta mRNA expression: impact of development and estrogen. Mol Cell Endocrinol. 1999;149(1–2):153–61. doi: 10.1016/s0303-7207(98)00247-0. [DOI] [PubMed] [Google Scholar]
- 104.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110(24):9956–61. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meunier L, Siddeek B, Vega A, Lakhdari N, Inoubli L, Bellon RP, Lemaire G, Mauduit C, Benahmed M. Perinatal programming of adult rat germ cell death after exposure to xenoestrogens: role of microRNA miR-29 family in the down-regulation of DNA methyltransferases and Mcl-1. Endocrinology. 2012;153(4):1936–47. doi: 10.1210/en.2011-1109. [DOI] [PubMed] [Google Scholar]
- 106.Chao HH, Zhang XF, Chen B, Pan B, Zhang LJ, Li L, Sun XF, Shi QH, Shen W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochemistry and cell biology. 2012;137(2):249–59. doi: 10.1007/s00418-011-0894-z. [DOI] [PubMed] [Google Scholar]
- 107.Sirotkin AV. Transcription factors and ovarian functions. Journal of cellular physiology. 2010;225(1):20–6. doi: 10.1002/jcp.22248. [DOI] [PubMed] [Google Scholar]
- 108.Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology. 1999;140(12):5746–53. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- 109.Drummond AE, Fuller PJ. The importance of ERbeta signalling in the ovary. The Journal of endocrinology. 2010;205(1):15–23. doi: 10.1677/JOE-09-0379. [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Zhang W, Liu J, Wang W, Li H, Zhu J, Weng S, Xiao S, Wu T. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod Toxicol. 2013 doi: 10.1016/j.reprotox.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Increased expression of Mullerian-inhibiting substance correlates with inhibition of follicular growth in the developing ovary of rats treated with E2 benzoate. Endocrinology. 2002;143(1):304–12. doi: 10.1210/endo.143.1.8603. [DOI] [PubMed] [Google Scholar]
- 112.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–84. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 113.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–96. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 114.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124(5):601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 115.Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119(1):209–17. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu CL, Cowan RG, Harman RM, Quirk SM. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Molecular endocrinology. 2004;18(2):326–38. doi: 10.1210/me.2003-0178. [DOI] [PubMed] [Google Scholar]
- 117.Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. Journal of animal science. 2004;82(E-Suppl):E40–52. doi: 10.2527/2004.8213_supplE40x. [DOI] [PubMed] [Google Scholar]
- 118.Khamsi F, Roberge S, Yavas Y, Lacanna IC, Zhu X, Wong J. Recent discoveries in physiology of insulin-like growth factor-1 and its interaction with gonadotropins in folliculogenesis. Endocrine. 2001;16(3):151–65. doi: 10.1385/ENDO:16:3:151. [DOI] [PubMed] [Google Scholar]
- 119.Ricu M, Paredes A, Greiner M, Ojeda SR, Lara HE. Functional development of the ovarian noradrenergic innervation. Endocrinology. 2008;149(1):50–6. doi: 10.1210/en.2007-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mayerhofer A, Dissen GA, Costa ME, Ojeda SR. A role for neurotransmitters in early follicular development: induction of functional follicle-stimulating hormone receptors in newly formed follicles of the rat ovary. Endocrinology. 1997;138(8):3320–9. doi: 10.1210/endo.138.8.5335. [DOI] [PubMed] [Google Scholar]
- 121.Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology. 2013;154(5):1873–84. doi: 10.1210/en.2012-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kiefer JC. Back to basics: Sox genes. Developmental dynamics: an official publication of the American Association of Anatomists. 2007;236(8):2356–66. doi: 10.1002/dvdy.21218. [DOI] [PubMed] [Google Scholar]
- 123.Jones RL, Pepling ME. KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Developmental biology. 2013;382(1):186–97. doi: 10.1016/j.ydbio.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 124.Trapphoff T, Heiligentag M, El Hajj N, Haaf T, Eichenlaub-Ritter U. Chronic exposure to a low concentration of bisphenol A during follicle culture affects the epigenetic status of germinal vesicles and metaphase II oocytes. Fertil Steril. 2013;100(6):1758–67. e1. doi: 10.1016/j.fertnstert.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 125.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, Mittelstadt ML, Ho SM, Walker CL. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Molecular cancer research: MCR. 2012;10(4):546–57. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7(5):e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peretz J, Neese SL, Flaws JA. Mouse strain does not influence the overall effects of bisphenol a-induced toxicity in adult antral follicles. Biology of reproduction. 2013;89:108. doi: 10.1095/biolreprod.113.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]