Abstract
Introduction
Lipodystrophy is a term used to describe a metabolic complication of fat loss, fat gain, or a combination of fat loss and gain, which is associated with some antiretroviral (ARV) therapies given to HIV-infected individuals. There is limited research on lipodystrophy in low- and middle-income countries, despite accounting for more than 95% of the burden of HIV/AIDS. The objective of this review was to evaluate the prevalence, pathogenesis and prognosis of HIV-related lipoatrophy, lipohypertrophy and mixed syndrome, to inform clinical management in resource-limited settings.
Methods
We conducted a structured literature search using MEDLINE electronic databases. Relevant MeSH terms were used to identify published human studies on HIV and lipoatrophy, lipohypertrophy, or mixed syndrome in low-, low-middle- and upper-middle-income countries through 31 March 2014. The search resulted in 5296 articles; after 1599 studies were excluded (958 reviews, 641 non-human), 3697 studies were extracted for further review. After excluding studies conducted in high-income settings (n=2808), and studies that did not meet inclusion criteria (n=799), 90 studies were included in this review.
Results and Discussion
Of the 90 studies included in this review, only six were from low-income countries and eight were from lower middle-income economies. These studies focused on lipodystrophy prevalence, risk factors and side effects of antiretroviral therapy (ART). In most studies, lipodystrophy developed after the first six months of therapy, particularly with the use of stavudine. Lipodystrophy is associated with increased risk of cardiometabolic complications. This is disconcerting and anticipated to increase, given the rapid scale-up of ART worldwide, the increasing number and lifespan of HIV-infected patients on long-term therapy, and the emergence of obesity and non-communicable diseases in settings with extensive HIV burden.
Conclusions
Lipodystrophy is common in resource-limited settings, and has considerable implications for risk of metabolic diseases, quality of life and adherence. Comprehensive evidence-based interventions are urgently needed to reduce the burden of HIV and lipodystrophy, and inform clinical management in resource-limited settings.
Keywords: HIV, AIDS, lipodystrophy, fat redistribution, antiretroviral therapy
Introduction
Since the introduction of highly active antiretroviral therapy (HAART) as a treatment for HIV and AIDS, HIV-related mortality has been reduced by 50 to 80% [1]. However, HIV-infected individuals on long-term therapy are at increased risk for developing a variety of metabolic disturbances, including fat redistribution, dyslipidemia, insulin resistance, lactic acidemia and abnormalities in bone mineral metabolism, particularly with nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs) [2–6]. Several of these complications, particularly lipodystrophy, can predispose individuals to cardiovascular disease and impact quality of life and adherence to antiretroviral therapy (ART) [5,7–9].
Research on metabolic complications of ART has been primarily conducted in high-income countries. There is increasing evidence from low- and middle-income countries evaluating the short- and long-term effects of ART on lipodystrophy syndromes such as lipoatrophy, lipohypertrophy and mixed syndrome. Lipoatrophy is the loss of subcutaneous fat, particularly evident in the face, buttocks and limbs. Lipohypertrophy is the accumulation of visceral and central fat in the abdomen, dorsocervical region (“buffalo hump”), or breasts. Fat redistribution is an all-encompassing terms used to describe lipohypertrophy, lipoatrophy and/or mixed syndrome [10–12]. In early studies, lipodystrophy was used to describe a syndrome of subcutaneous fat loss with or without visceral fat accumulation, insulin resistance and/or dyslipidemia [13]. Although most studies to date use “lipodystrophy” as an all-encompassing term for fat redistribution, current research emphasizes the need to differentiate isolated lipoatrophy, lipohypertrophy and mixed syndrome as separate disease processes [13–15]. In this review, fat redistribution syndromes will be defined based on clinical guidelines and as defined by study authors.
In the context of rapid ART scale-up in resource-limited settings, addressing lipodystrophy is a critical component of HIV/AIDS care and treatment. The antiretroviral (ARV) medications linked to lipodystrophy, particularly lipoatrophy, are prevalent in low-income and middle-income settings [16], where HIV-infected populations face the dual burden of obesity and undernutrition [17,18]. There is a need to evaluate the prevalence, pathogenesis and prognosis of lipoatrophy, lipohypertrophy and mixed syndrome, to inform clinical management in low- and middle-income countries.
Methods
Search strategy
We conducted a structured literature search using MEDLINE electronic databases to identifiy published studies through 31 March 2014. Search terms included: (HIV [MH] OR HIV Infect*[MH] OR Human Immunodeficiency Virus [tw] OR Human Immuno-deficiency Virus [tw] OR hiv-1 [tw] OR hiv-2 [tw] OR hiv*[tw] OR AIDS [MH] OR AIDS [tw]) AND (Lipodystrophy [MH] OR Lipoatrophy [MH] OR -hypertrophy [MH] OR buffalo hump [tw] OR lipo-accumulation [tw] OR lipo*[MH] OR adiposity [tw] OR Lipodystrophy [tw] OR Lipoatrophy [tw] OR Lipohypertrophy [tw] or Fat distrib*[tw] or Fat redistrib*[tw] OR fat accumulation [tw] or lipo-*[tw]) [19]. The search strategy and results are summarized in Figure 1, and findings from these studies are summarized in detail in the supplementary tables (Tables 1–3).
Figure 1.
Search strategy.
Table 1.
Low-income countries
| Region | Population | N | Study objective | Lipodystrophy, lipoatrophy, lipohypertrophy | Other findings |
|---|---|---|---|---|---|
| AFRICA | |||||
| Prospective cohort | |||||
| Benin [33] | HIV+ initiating HAART | 79 | LD incidence and risk factors of NNRTIs | LD: 30% (11 mo) Mixed: 2.5%, LH: 24%, LA: 9% |
Significant weight gain on ART, 13% developed metabolic syndrome |
| Uganda [107] | HIV+ initiating HAART | 76 | Anthropometry, total fat, lean mass with AZT | HIV+: increased lean and fat mass; arm, waist, hip and thigh circumferences; and abdomen, subscapular, thigh SFT at 6 mo vs. controls | |
| Cross-sectional | |||||
| Ethiopia [154] | HIV+ on HAART >1 y | 356 | Prevalence and risk factors of LD | LD: 68.3% | LA/LH risk factors: smoking, d4T, >1 y ART |
| Ethiopia [152] | HIV+ on HAART | 313 | Prevalence of BFR and metabolic changes | LD: 12.1% | LD risk factors: ART >12 mo |
| Malawi [165] | HIV+ on d4T for ≥6 mo | 203 | d4T toxicity and mtDNA/nDNA ratio | LD: 18% (25 mo) | No association between LD and mtDNA/nuclear DNA ratio |
| ASIA | |||||
| Cross-sectional | |||||
| Cambodia [44] | HIV+ initiating ART | 467 | Immunovirological ART outcomes, adverse events | LD: 63% (4 y) | d4T adverse events: BFR, dyslipidemia, increased liver enzymes, peripheral neuropathy |
| Cambodia [166] | HIV+ on ART ≥2 y | 346 | Prevalence and predictors of non-adherence | Independent predictors of non-adherence: LD- related side-effects, rural residence, HIV disclosure to <2 family members | |
| Retrospective | |||||
| Cambodia [32] | HIV+ initiating d4T | 2481 | Long term toxicity (6 y) with d4T | LA: 0.8% (6 mo), 7% (1 y), 56.1% (3 y), 72.4% (6 y) | d4T Regimen change: 37.1% due to LD Risk Factors: age >40 y, female, CD4 <200 cells/μL |
WHO GDP $1,025 or less. ABC: abacavir; ART: antiretroviral therapy; AZT: zidovudine; BFR: body fat redistribution; d4T: stavudine HAART: highly active antiretroviral therapy; HIV: human immunodeficiency virus; LA: lipoatrophy; LD: lipodystrophy; LH: lipohypertrophy; mo: months; SFT: skinfold thickness; TC: total cholesterol; TG: triglycerides; WC: waist circumference; WHR: waist to height ratio; y: years. Prevalence unless otherwise noted.
The structured literature search resulted in 5296 articles; after 1599 studies were excluded (958 reviews, 641 non-human), 3697 studies were extracted for further review. Sources were retrieved, collected, indexed and assessed for HIV- and lipodystrophy-related data. The inclusion criteria for this review were availability of data on human HIV-related fat redistribution (lipodystrophy, lipoatrophy, fat redistribution, lipo-accumulation and/or lipohypertrophy), abstract availability, age ≥18 years, and low-, low-middle- and upper-middle-income countries, as defined by the World Bank classification [20].
After excluding studies conducted in high-income settings (n=2808), and studies that did not meet inclusion criteria (n=799), 90 studies were included in this review. These included 28 from Africa, 26 from Asia and 36 from Latin America.
Literature review
Prevalence of lipodystrophy
The global prevalence of lipodystrophy among HIV-infected adults on ART ranges considerably, from less than 1 to 84% [4,12,21–41]. The burden of HIV-related lipodystrophy is estimated to be high in low- and middle-income countries [4,12,24–41], with an incidence of 1.4 to 20.6 per 100 person-years [33,42,43], and 6.7 to 22.1 per 100 person-years [27,32], in studies in Asia and Africa, respectively. The wide ranges in prevalence reflect variations in study design, gender balance in studies, types of ARVs and duration of therapy. Understanding the major risks factors for lipodystrophy, lipoatrophy, lipohypertrophy and mixed syndrome may help explain the wide range in prevalence around the world and inform prevention and clinical management. Lipodystrophy may develop within four to six months of ART initiation, and increases considerably after 12 months [11,32,44]. For example, the prevalence of lipodystrophy was 0.8% in a study in Thailand after six months of ART [32], 34% in a study in Rwanda after 16 months of ART [11], and 63% in a study in Cambodia after four years of ART [44]. However, this trend has not been observed in all studies, and newer studies indicate that it is actually isolated lipoatrophy, not lipohypertrophy that progresses over time with tNRTIs (thymidine-based) [13,45]. In contrast, some cohort studies have noted that lipoatrophy persists but does not increase with time [46], while some trials have demonstrated that ARVs may be associated with increases in limb fat over time [47].
In a South African study in 2670 HIV-infected adults initiating HAART, the incidence of lipodystrophy was 1.4 per 100 person-years, but nearly doubled to 2.6 per 100 person-years when the follow-up period excluded the first six months of therapy [48]. Thus, ART type and duration need to be considered when comparing rates of lipodystrophy across studies. Some studies have reported the prevalence of lipoatrophy, lipohypertrophy and a mixed syndrome separately, and are presented in Table 1 [11,12,33,49–51].
Table 1.
Prevalence of lipohypertrophy, lipoatrophy and mixed syndrome in low and middle-income countries versus high-income countries
In a study of 180 HIV-infected adults from Brazil, sex-specific differences in the prevalence of different lipodystrophy syndromes were observed. Women were more likely to develop lipohypertrophy (43%) or mixed syndrome (40%), while men presented with a similar prevalence for all three lipodystrophy syndromes (34% lipoatrophy, 32% lipohypertrophy, 34% mixed syndrome) [39]. The propensity of women to lipohypertrophy has also been observed in high-income countries [52].
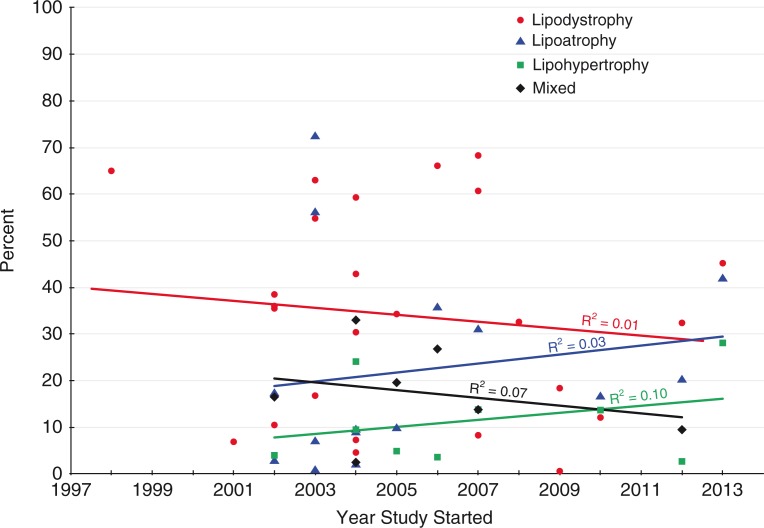
In contrast to lower-income countries, the prevalence of lipoatrophy is decreasing in high-income countries, as older drug regimens are being replaced with less toxic alternatives. For example, in the Swiss Cohort Study, there was a reduced risk of lipodystrophy in 6016 HIV-infected patients who initiated ART between 2003 and 2006 compared to the 5777 who started ART between 2000 and 2002 (p=0.02) [22,53]. Zidovudine and stavudine use declined considerably during this period, from 88.5% and 4.2% (2000–2002) to 64.2% and 0.7% (2003–2006), respectively [22]. This does not appear to be the case in the countries included in this review (Figure 2).
Figure 2.
Prevalence of lipodystrophy by year study started.
The risk factors for fat redistribution syndromes and their relative importance also vary in different settings. For example, stavudine was used as standard therapy in Thailand as part of the GPO-VIR regimen [28,29]. However it has not been used as frequently in Brazil, affecting the prevalence of lipoatrophy [54–59] as seen in Table 1.
When comparing prevalence of lipodystrophy among studies, country-specific drug regimens, gender proportions and duration of ART use should also be considered.
Diagnosing lipodystrophy, lipoatrophy and lipohypertrophy
Fat redistribution can be difficult to diagnose, particularly in settings where populations face the dual burden of obesity and undernutrition [17,18]. There is no universal definition for lipodystrophy or body fat redistribution; the term “lipodystrophy” is often used to categorize any form of fat redistribution [60]. As a result, the diagnosis, clinical management, and assessment of prevalence and aetiology are challenging. There is also a lack of concordance in the methods used to diagnose and monitor fat redistribution.
Lipodystrophy assessment methods include clinical examination, self-report, anthropometry, bioelectrical impedance analysis (BIA) and imaging techniques such as dual-energy X-ray absorptiometry (DEXA), magnetic resonance imaging (MRI) and computed tomography (CT).
Anthropometry
Anthropometric measurements are the most common method of diagnosing lipodystrophy and include height, weight, body mass index (BMI), mid-upper arm circumference, waist circumference, hip circumference, waist-to-hip ratio and skinfold thickness measurements. These methods are non-invasive, relatively low-cost and interpretable, and compare well with more advanced techniques of assessing lipodystrophy, such as DEXA or MRI [61–63]. However, accurate and precise measurements require careful training and ongoing supervision of field workers [64].
Standardized techniques are used to assess skinfold thickness measurements, including tricipital, bicipital, subscapular and suprailiac folds. The Durnin-Wormersley equation is often used to calculate body fat percentage from skinfold thickness measurements and has been validated in different populations, including African-Americans in the United States [65–67]. These tools are feasible and widely used in resource-limited settings [68,69]. However, these equations have not been validated in HIV-infected populations, or in the context of lipodystrophy [70,71].
Standardized scales
The Carr Lipodystrophy Case Definition (LDCD) and the Lichtenstein HIV Outpatient Scale (HOPS) are standardized scales that are commonly used to assess lipodystrophy. LDCD uses a combination of demographic (age) and clinical (duration of HIV infection) information, metabolic parameters (anion gap, serum HDL cholesterol), anthropometry (waist-to-hip ratio) and radiologic assessments (DEXA, CT) to define lipodystrophy with 79% sensitivity and 80% specificity [60]. The LDCD has also been expanded to include a scale for lipodystrophy severity [72]. The required use of DEXA or CT limits the applicability of LDCD in resource-limited and field settings. The Lichtenstein HOPS scale was designed to assess lipodystrophy severity using standardized clinical evaluations, laboratory values and anthropometric measurements. It uses a more specific definition, including only moderate to severe lipodystrophy [73]. However, the LDCD and HOPS do not differentiate among the three lipodystrophy syndromes.
Two studies from Africa demonstrated the importance of a standardized definition for different lipodystrophy syndromes. In Senegal, 180 HIV-infected adults on HAART for four to nine years were evaluated for lipodystrophy [12]. The prevalence of mild to severe lipodystrophy diagnosed by physicians trained to evaluate lipodystrophy, lipohypertrophy and lipoatrophy using the Carr criteria was 65% (35.6% lipohypertrophy). The prevalence was 31% (14.5% lipohypertrophy) using a stricter definition which included only moderate to severe lipodystrophy, and 50% (no separate criteria for lipohypertrophy) using objective metabolic and anthropometric criteria [12]. In a study in Cameroon, the prevalence of mild to severe lipodystrophy was 60%, compared to 18% using a stricter definition that only included moderate to severe lipodystrophy [36]. In addition, a lack of concordance between clinical diagnosis and patient self-report of lipodystrophy frequently occurs, as seen in a study of 72 HIV-infected patients on HAART from Argentina. In this study, the use of two different patient questionnaires resulted in a diagnosis of body fat redistribution of 49% and 86%. When two different clinical criteria were used, the diagnosis of body fat redistribution was considerably lower, or 24% and 48%, respectively [74].
Body composition
Assessment is particularly important in HIV-infected individuals, since both HIV and ART are associated with changes in body composition and metabolic complications [75,76]. Weight and BMI are crude measures of body composition, particularly at the individual level. However, some methods to assess body composition, such as DEXA, are prohibitively expensive and challenging to implement in resource-limited settings.
Bioelectrical impedance
BIA is a non-invasive method of body composition assessment, which is more precise than skinfold thickness [70]. The use of BIA has become widespread in body composition research due to its ease of use, portability and relatively low cost. BIA indirectly estimates different body tissues and compartments based on their conduction of a low-voltage, alternating current [77]. BIA can be used to estimate total body water, fat-free body mass and body fat. BIA is useful in monitoring body changes over time and is highly correlated with other radiologic measures of body fat [70]. However, the calculation of total fat is affected by fat distribution, making it a less accurate tool for evaluation of lipodystrophy [71]. Some data suggest that BIA may not accurately reflect body composition changes in the context of HIV infection [77], and BIA has not been extensively validated in the ART era.
Other methods
Additional methods for assessment of body composition include imaging techniques such as DEXA, MRI and CT. However, DEXA cannot differentiate between subcutaneous and visceral fat, or evaluate facial lipoatrophy, one of the more disturbing morphologic complications of ART reported by patients [78,79]. The error in measurement of body composition, particularly central fat, using DEXA may also be higher in the context of obesity [80], suggesting that its applications may be limited in the context of lipohypertrophy assessment. In contrast to DEXA, which is unable to differentiate type of fat, MRI techniques require longer imaging time and specialized equipment, and CT has improved resolution but necessitates greater ionizing radiation exposure [80]. Additional methods, such as MRI, are considered superior in assessment of body composition in the context of HIV, though are less widely available in lower-income settings. Other methods, such as ultrasound, have not been validated for lipodystrophy assessment [81], and vary considerably on the operator [82].
In a pilot study in Canada comparing BIA, skinfold measurements, and DEXA in assessment of percent body fat mass among 47 HIV-infected men receiving HAART [83], the level of error and bias in BIA and skinfold methods were relatively low, compared to DEXA. Authors concluded that BIA and skinfold techniques are acceptable methods of monitoring total body fat mass in HIV-infected men on HAART as long as training and technique are standardized.
Lipodystrophy pathogenesis in HIV
Lipodystrophy has a multi-factorial etiology, including HIV, effects of ART and host factors. Furthermore, lipoatrophy and lipohypertrophy likely have different pathogenic and genetic mechanisms, as reviewed elsewhere [84,85], which pose challenges to clinical diagnosis and management. HIV infection may contribute to fat redistribution by infecting macrophages in adipose tissue, which release pro-inflammatory cytokines and enhance local inflammation [86]. This is supported by increased tumour necrosis factor-alpha (TNF-α) expression observed in ART-naïve HIV-infected patients, a pro-inflammatory cytokine that initiates adipocyte apoptosis [86,87].
Although the risk of developing lipodystrophy was thought to be associated with the type and duration of ART, as seen in many studies reviewed, this inadequately describes the association between ARTs and different lipodystrophy syndromes. In vitro and in vivo research has demonstrated effects of ARVs on functions of various organs, including adipose, liver and muscle. Lipodystrophy may develop as a result of the effects of specific NRTIs and PIs on lipid metabolism [88,89]. PIs and NRTIs have been shown to alter regulation of genes involved in adipocyte differentiation, metabolism, cell cycle control and apoptosis. PIs are linked with adipocyte toxicity through several potential mechanisms [90]. PIs induce or up-regulate genes that inhibit adipocyte differentiation and down-regulate adipogenesis-related transcription factors [91,92], including interference with essential cellular transcription factors (e.g. SREBP-1c) [92]. PIs inhibit pre-adipocyte differentiation by up-regulating the Wingless-related integration site (wnt)/B-catenin signalling pathway. One of the functions of this pathway is to inhibit adipogenic gene expression. Specifically, activation of the wnt/B-catenin signalling pathway prevents the induction of PPAR-γ [93,94], a nuclear-receptor which regulators adipocyte differentiation and maintenance [95]. PIs are also linked to reduced lipid accumulation in adipocytes, increased adipocyte apoptosis and induction of insulin resistance [91].
NRTIs, stavudine and didanosine, are linked to inhibition of mitochondrial RNA transcription, depletion of mitochondrial DNA (mtDNA) and mitochondrial dysfunction, via inhibition of DNA polymerase γ [96]. The toxic effects of NRTIs on mitochondrial function are likely a major contributor to the development of lipoatrophy [96–99]. NRTIs inhibit mtDNA in vitro with varying affinity, as per below:
zalcitabine>didanosine>stavudine>zidovudine >lamivudine=abacavir=tenofovir [100]
Most potent mtDNA inhibition to Least potent mtDNA inhibition
Previous studies found that HIV-infected adults are less likely to develop lipoatrophy on tenofovir compared to stavudine, and tenofovir improved lipoatrophy in adults who previously developed lipoatrophy on stavudine [101,102]. These findings are of particular relevance to resource-limited settings where older NRTIs (e.g. stavudine) are still relatively common [103].
Natural history of lipodystrophy
Lipodystrophy is often measured as a dichotomous outcome at a single time point in studies, rather than as a continuum of fat redistribution assessed prospectively. HIV-infected adults initiating ART often experience an increase in weight, trunk and limb fat during the first four to six months of therapy; after this point, trunk fat stabilizes and limb fat progressively decreases [104,105]. This may be due to an initial period of immune reconstitution, followed by NRTI or PI dose-dependent adipocyte toxicity. In a study in Rwanda, participants taking stavudine-based HAART experienced a rapid increase in body weight during the first 6 to 12 months of treatment, followed by a progressive decline in body weight during the second year of therapy, with a median loss of 3.1 kg per year (IQR: −1.6 to −5.6; p<0.01) [106]. In a study from Uganda, during the first six months of primarily AZT-based ART initiated by 76 HIV-infected adults, total lean and fat mass, and arm, waist, hip and thigh circumferences significantly increased [107]. The total lean mass plateaued 6 to 12 months post-ART initiation at an increased level compared to at the initiation of AZT-based ART. Abdomen, subscapular and thigh skinfold thicknesses continued to increase significantly [107].
Lipoatrophy may be associated with overall weight loss in HIV-infected adults on ART. In a study of 705 HIV-infected patients initiating stavudine in Rwanda, lipoatrophy was associated with a 2.0 kg per year weight loss (95% CI: −0.6 to −3.4 kg; p<0.01). Weight loss had a positive predictive value for lipoatrophy of 21% and 32% and a negative predictive value of 99% and 92% for men and women, respectively [106]. In contrast, some research suggests that lipohypertrophy presents with physiologic processes similar to regaining weight after starvation, as seen in patients recovering from anorexia nervosa [108]. Thus, this may indicate a restoration to health, rather than a disease process [109].
Differentiating between HIV itself, immune reconstitution and ART toxicity in the aetiology of lipodystrophy needs to be further elucidated. Prospective studies are needed to examine changes in fat redistribution over time and progression of lipodystrophy, to inform prevention and clinical management of lipodystrophy in HIV-infected patients.
Risk factors of lipodystrophy
Risk factors for lipodystrophy syndromes identified in studies from lower-income countries are summarized in Table 2. Stavudine use, ART duration and female sex were risk factors for lipodystrophy and lipoatrophy. There were limited studies on lipohypertrophy and no risk factors were identified for mixed syndrome.
Table 2.
Risk factors for lipodystrophy identified in studies from low- and middle-income countries
| Variables | Lipodystrophya | Lipoatrophy | Lipohypertrophy |
|---|---|---|---|
| ARV | Stavudine use [12,50] | Stavudine use [11,42,62] | |
| Indinavir use [62] | |||
| Duration of ART [29,34,48] | Duration of ART [11] | ||
| Demographic | Female sex [27,29,32,48,49,51] | Female sex [11,32,42,43,49,106] | |
| Age >40 years [32] | Younger age [33] | ||
| Nutritional | Baseline BMI >25 kg/m2 [11] | ||
| Onset and rate of weight loss [11] | |||
| Immune | Undetectable HIV RNA [50] |
These studies identified risk factors for lipodystrophy and did not differentiate between types of lipodystrophy syndromes (i.e. lipoatrophy, lipohypertrophy and/or mixed syndrome). No risk factors were identified for mixed syndrome alone.
Female sex
Several studies in lower-income countries have identified female sex as a risk factor for lipodystrophy. In a study of 2190 Rwandan HIV-infected patients initiating HAART, women had a 9.7 (HR: 9.7, 95% CI: 4.5–21.0, p<0.05) times greater risk of developing lipoatrophy, compared to men [43]. In a study in South Africa, the prevalence of lipoatrophy was 57% in women, compared to 13% in men (p<0.05) [49]. In some studies, female sex was the only significant risk factor for lipodystrophy, after adjusting for other variables [42,49,106]. Women may also be less likely to receive adequate health care, education and support for HIV/AIDS [8,110] and may be more vulnerable to the social impact of stigma and metabolic consequences [8,110].
Stavudine treatment
Stavudine is associated with severe dose-related side-effects of lipoatrophy, lactic acidosis and peripheral neuropathy [32,48,99,111]. Based on these findings, the World Health Organization (WHO) recommended a lower maximum stavudine dose for all adults in 2007 (40–30 mg) [36,51,112], and discontinued use in 2009 [113]. Despite WHO recommendations, 14 (of 52) developing countries still used stavudine as a first-line HIV therapy in 2010, due to its comparatively lower cost (~18 USD/year) and widespread availability [16,114,115].
Stavudine toxicity continues to be a common cause of metabolic complications and regimen changes in resource-limited settings. HIV-infected patients taking stavudine had a 2.8 (OR: 2.8, 95% CI: 1.4–5.5, p<0.05; OR: 4.7, 95% CI: 1.3–17.1, p=0.019) [11,12] to 7.4 (OR: 7.4, 95% CI: 1.3–40.8, p=0.022) [62] times greater odds of lipoatrophy, compared to other NRTI and ARV regimens. In Rwanda, the prevalence of lipodystrophy was three times higher in HIV-infected patients on stavudine, compared to zidovudine, which also causes lipoatrophy [11]. In Cameroon, HIV-infected patients on stavudine were 5.5 times as likely to have lipoatrophy, compared to zidovudine (RR: 5.5, 95% CI: 1.3–23.5, p=0.02) [36,116]. Stavudine toxicity also increases with duration and dose [117]. In a cross-sectional study of 103 HIV-infected adults in Thailand, stavudine duration was associated with increased odds of lipodystrophy, with a 2% higher odds per month of treatment [117]. Despite WHO recommendations and scientific evidence on its severe side-effects, however, its lower price and widespread availability makes stavudine replacement challenging in lower-income countries.
Protease inhibitors
PIs were originally thought to be the main causative agent of lipodystrophy, though more recent evidence identified thymidine NRTIs as the leading contributor to ARV-related lipodystrophy. Lipohypertrophy has been attributed to PI use [14,118].
There is limited research on PIs in lower-income countries, due in part to their prohibitive cost, compared to NRTIs and non-nucleoside reverse transcriptase inhibitors (NNRTIs) [114,119]. PIs are widely used in upper-middle-income countries [30,62,120–122]. In this review, studies in only three countries – Thailand, India and Brazil – evaluated the effects of PI use on lipodystrophy. In Thailand, 247 HIV-infected adults taking PIs had a higher prevalence of facial atrophy (50% vs. 30%) and female breast hypertrophy (52% vs. 12%), compared to non-PI ART [62]. In India, patients who switched to a PI after immunologic failure on NNRTIs had a 10% incidence of lipodystrophy within the first year [30]. In a cross-sectional study of 457 HIV-infected adults and adolescents in Brazil, longer PI use was associated with self-reported lipodystrophy [122].
NNRTIs
NNRTIs, particularly nevirapine, are used frequently in lower-income countries and are not commonly linked to lipodystrophy syndromes. In one study, nevirapine was associated with a 50% lower odds of lipoatrophy (OR 0.50, 95% CI: 0.26–0.95, p<0.001) [62].
Other risk factors
Additional risk factors for lipodystrophy identified in the studies reviewed include: older age (>40 years) [32,73,123,124], greater baseline body weight at ARV initiation [29,48], undetectable HIV RNA [50], recent weight loss and faster rate of weight loss [11]. Increased baseline weight and BMI are risk factors that should be carefully examined in settings characterized by concurrent obesity and undernutrition.
Implications of lipodystrophy
The development of lipodystrophy is associated with cardiometabolic risk factors. In a study of 68 underweight (BMI <18.0 kg/m2) HIV-infected adults initiating NRTIs in India, 19 to 25% (Diabetes Federation criteria versus National Cholesterol Education Program criteria) of participants developed new onset metabolic syndrome within six months of ART initiation [125]. Patients with ART-induced lipodystrophy may also develop impaired glucose tolerance, insulin resistance, hyperlactatemia, dyslipidemia and increased inflammatory markers [126,127]. Although the interrelationship between ART use, insulin resistance and lipodystrophy has been noted in several studies [127–129], the temporal relationship and biological mechanisms involved are not well-understood. Insulin resistance often precedes lipodystrophy, so it is thought to be involved in the pathogenesis and development of lipodystrophy [127]; however, other studies have suggested that lipodystrophy contributes to the development of insulin resistance [128,129].
Lipodystrophy is associated with a variety of metabolic disturbances, though the causal mechanisms are unknown [50]. In a study in Thailand, 93% of HIV-infected patients with lipodystrophy had at least one metabolic abnormality [130]. Lipodystrophy has been associated with increased triglycerides [27], total cholesterol [34,130] and elevated glucose [130], but no associations have been found with HDL or LDL [27]. Sex differences have been observed in the presentation of distinct metabolic risk profiles. For example, in a prospective study of 332 HIV-infected adults in Brazil, BMI was higher in HIV-infected women with lipodystrophy (p=0.001) but lower in HIV-infected men with lipodystrophy (p=0.04) [37]. In contrast, HIV-infected men with lipodystrophy had lower HDL, compared to women [37].
Quality of life and ARV adherence
Lipodystrophy is also associated with psychological consequences [131,132], reduced self-reported quality of life and ARV adherence [8,59]. In a cross-sectional study in Thailand, 278 HIV-infected adults ranked the perceived severity of their lipodystrophy as mild (28%), moderate (54%) and severe (15%) [130]. Self-reported lipodystrophy correlated poorly with clinical diagnosis of lipodystrophy (60% mild, 20% moderate, 2% severe) [130]. Lipodystrophy has also been associated with lower reported quality of life. Patients have described the effects of lipodystrophy as disturbing and reported reduced satisfaction with their body image, self-esteem and social relationships, less confidence about their health and embarrassment due to body changes [8,11,133]. These negative psychosocial consequences can also be associated with a fear of disclosure of HIV status and of stigma associated with HIV/AIDS. In a study of 50 adults in South India, there was a significant decrease in quality of life regarding financial (p<0.045) and disclosure (p<0.032) concerns between patients with lipodystrophy and without lipodystrophy [134]. In a study in patients with lipodystrophy in Thailand, despite improvements seen on DEXA (after stavudine/didanosine substitution with tenofovir/lamivudine), there were no significant changes in patients’ perceptions of their own lipodystrophy [99]. In addition to the physical consequences of lipodystrophy, evaluating the impact of lipodystrophy on emotional well-being and quality of life is an important component to clinical management.
Management and treatment of lipodystrophy
The primary therapy for severe lipodystrophy, particularly lipoatrophy, is a change in ARV regimen. There are also an increasing number of clinical management and treatment options for lipoatrophy and lipohypertrophy in high-income countries. However, there is comparatively little research on clinical management and treatment of lipodystrophy in lower-income countries.
Lifestyle modifications
Diet and exercise can help reduce the progression of lipodystrophy, and mitigate its impact on health and quality of life. In a study in Rwanda, among HIV-infected individuals with lipodystrophy, a six-month exercise programme improved cardiorespiratory fitness, metabolic parameters and quality of life, compared to individuals not enrolled in exercise therapy [133,135]. Participants also experienced a reduction in total cholesterol and insulin levels and improved body image, self-esteem, psychological well-being and social relationships [133,135]. Several studies from Brazil have reported similar effects of lifestyle programmes [136,137]. Further research is needed to determine the impact of exercise programmes on the treatment of lipodystrophy, and particularly on severe lipoatrophy, as exercise may exacerbate this condition [138,139]. In contrast, in a study from Brazil where an intervention group received diet counselling every two months, there were no differences in anthropometric measurements between the intervention and control groups [140].
Dose reduction
In 2007, the WHO recommended a stavudine dose reduction from 40 to 30 mg for adults and adolescents [36]. During this time period, researchers in Cameroon and South Africa monitored changes in lipodystrophy and metabolic parameters [36,116]. In both cohort studies, the prevalence of lipoatrophy was significantly higher in patients who had taken the 40 mg dose of stavudine, compared to those who had only taken the 30-mg dose [36,116]. In the cohort study in South Africa among 249 HIV-infected adults, patients taking the 40 mg dose of stavudine had a 12 times greater odds of moderate to severe lipoatrophy (OR: 11.8, 95% CI: 3.2–43.8, p<0.05), compared to 30 mg [116]. In a meta-analysis of trials comparing these doses of stavudine in high-income countries, there was strong evidence that the lower 30 mg dose of stavudine was associated with lower rates of side-effects (e.g. peripheral neuropathy) and discontinuation, and similar efficacy in suppressing HIV viral load, compared to the 40 mg dose. These findings informed the WHO recommendations for lower dose stavudine in 2007 [141] and the subsequent discontinuation of stavudine due to severe side-effects in 2009 [113].
Regimen switches
Lipodystrophy and lipoatrophy have been the predominant reasons for stavudine substitutions. In a study in Thailand, 35 HIV-infected adults were switched from stavudine/didanosine to tenofovir/lamivudine. There was an increase in limb fat mass (+0.38 kg, p<0.006) and total fat mass (+0.69 kg, p<0.02), as measured by DEXA 48 weeks after the regimen change [99]. In addition to improving lipoatrophy, switching from stavudine to tenofovir was associated with increased mtDNA copies (386–1537, p<0.001) [142] and decreased total cholesterol, LDL and triglycerides [99,142,143].
Several studies in low-income and middle-income countries have examined the effects of stavudine substitution with zidovudine. In a study in Rwanda among HIV-infected adults with lipodystrophy who were switched to either zidovudine or tenofovir/abacavir, the zidovudine group had significantly greater weight loss three months after substitution, with a mean loss of 1.6 kg (−1.62±4.23 kg) by 12 months. The tenofovir/abacavir group had a stable body weight for the first three months and a small but significant weight increase by 12 months (+0.70±4.18 kg) [144]. No studies to date have evaluated the efficacy of switching to NRTI-sparing regimens in low-income and middle-income countries. In high-income countries, this regimen change has been effective in maintaining HIV viral load suppression and reducing the risk of side-effects, including lipodystrophy, though NRTI-sparing regimens are not commonly used in clinical practice [145,146].
Pregnant women
There is a major research gap in our understanding of lipodystrophy during pregnancy. Pregnancy is a time of many hormonal and metabolic changes that directly affect the health of the woman and foetus. During pregnancy, there is a transient increase in lipid parameters, which may be exacerbated with NRTI and PI use [147,148]. Further research is warranted to understand how lipodystrophy presents during pregnancy, and the impact of lipodystrophy on maternal and neonatal health. Further understanding of lipid alterations and naturally occurring insulin sensitivity in early trimesters and insulin resistance later in pregnancy [149] should inform ARV treatment options, particularly in resource-limited settings where perinatal HIV infection is common.
Discussion
ART is the gold standard for HIV/AIDS treatment, based on its demonstrated benefits on disease progression, immune reconstitution and mortality. However, lipodystrophy, lipoatrophy, lipohypertrophy and a mixed syndrome are common metabolic complications of ART, and increases with ART type and duration. There is limited research on ART-related lipodystrophy in low- and middle-income countries, despite accounting for more than 95% of the burden of HIV/AIDS. Of the 90 studies reviewed among adults, only eight were published from five low-income countries and nine from two lower middle-income economies. These studies focused on lipodystrophy prevalence, risk factors and ART side-effects, reflecting the continued use of older toxic ART regimes such as stavudine. Further research is needed to characterize lipodystrophy, its implications and treatment options in lower-income countries.
The prevalence of lipodystrophy is considerable in low- and middle-income countries, with lipoatrophy specifically increasing with type and duration of therapy. In most studies, lipoatrophy developed after the first six months of therapy, particularly with use of stavudine. Despite WHO recommendations, 14 out of 52 developing countries still use stavudine as a first-line HIV therapy, due to its comparatively lower cost and widespread availability [16,114,115]. Therefore, in addition to prevalent cases of lipoatrophy from past use of stavudine, there continue to be incident cases from continued stavudine use in resource-poor settings.
Lipodystrophy, lipoatrophy, lipohypertrophy and a mixed syndrome are all associated with increased risk of cardiometabolic complications, including hypertension, diabetes, insulin resistance and cardiovascular disease. Many studies reviewed highlighted the increased odds of developing metabolic syndrome with the presence of lipodystrophy [150–155]. Newer therapies, such as thiazolidinediones, target not just fat redistribution but also insulin resistance associated with lipodystrophy [156–158]. The incidence of metabolic complications from HAART and/or lipodystrophy are anticipated to increase, given the rapid sale-up of ARVs worldwide, the increasing number and lifespan of HIV-infected patients on long-term ART, and emergence of obesity and non-communicable diseases in settings with extensive HIV burden.
Improved prevention and timely resolution of lipodystrophy could impact health, psychosocial well-being, quality of life and adherence. The stigma associated with HIV in some developing countries is still severe, therefore external fat redistribution changes can significantly negatively impact an individual's quality of life and ART adherence [8,131,134,159].
Lipodystrophy management is integral to HIV care and treatment, and warrants further investigation in low- and middle-income countries.
Limitations
There are several limitations in the studies reviewed. These include a lack of standard clinical definition of lipodystrophy syndromes, varying assessment methods, limited number of prospective studies to examine the effects of ART on lipodystrophy over time, small sample sizes, different combinations of ARV regimens and limited assessment of other nutritional, immunological and metabolic factors. Some of the aforementioned studies were conducted among HIV-infected patients with failed first-line therapy or in case-control studies where patients were selected based on lipodystrophy or other clinical conditions, which limits generalizability to other HIV-infected populations and settings [30,120]. These limitations constrain the interpretability and comparability of findings across studies.
The lack of standard clinical definition and heterogeneous characterization of lipodystrophy pose challenges to examine the aetiologies and risk factors of lipoatrophy, lipohypertrophy and mixed syndrome. Most studies have examined these conditions together as a combined endpoint and had insufficient sample size to examine risk factors for each condition individually over time. Many studies cited the need for a universal standardized method to diagnose lipodystrophy syndromes, validated in both clinical and field settings, to compare findings across studies [12,36].
Prospective studies are needed to examine the effects of ART on lipodystrophy over a sufficient period of time after ART initiation. Patterns of lipodystrophy are also dynamic and may change due to HIV infection itself, and other potential factors. Some factors that were rarely adjusted for in multivariate analyses include other nutritional deficiencies, micronutrient supplement use, opportunistic infections, other co-infections and access to health care services, which are important covariates that can confound the results of the studies reviewed and limit the ability to interpret findings on the relationships between HIV, ART and lipodystrophy.
Future research
Further research is needed to better understand fat redistribution syndromes in resource-limited settings. There is inadequate research on lipohypertrophy, a condition that unlike mixed syndrome or lipoatrophy is also found in HIV-uninfected populations. Lipohypertrophy may be linked to increased risk of metabolic abnormalities, as there is strong evidence that links visceral fat accumulation to non-communicable diseases such as diabetes and cardiovascular disease [85,160]. However, there is limited data on risk factors for lipohypertrophy in HIV-infected populations on ART.
In low- and middle-income countries, HIV/AIDS, food insecurity, micronutrient deficiencies, other infectious diseases and underweight co-occur with the emergence of obesity and non-communicable diseases. Despite the extensive burden of malnutrition, few studies take malnutrition into consideration when evaluating lipodystrophy [106]. Further research is needed on the role of nutrition in the aetiology and clinical management of lipodystrophy, including the interplay between nutrition, metabolic disease, lipodystrophy and HIV infection.
Conclusions
HIV represents a serious threat to global health, and ART is the gold standard for HIV/AIDS care and treatment. However, adverse ART-related outcomes, including lipodystrophy, are common in low- and middle-income countries. This has considerable implications for risk of metabolic diseases, quality of life and adherence. As ART is rapidly scaled up worldwide and early HIV detection, ARV initiation and lifespans of infected individuals increase, the length of ARV exposure and burden of related metabolic complications are also expected to rise in low- and middle-income countries. Comprehensive evidence-based interventions are urgently needed to reduce the burden of HIV and lipodystrophy, and inform clinical management in resource-limited settings.
Footnotes
These authors contributed equally to this work.
Competing interests
The authors declare no competing interests.
Authors' contributions
JLF conducted the literature review and wrote the first draft of the manuscript. PG updated the literature review and revised the manuscript. All authors read and approved the final version.
Supplementary Tables
Table 2.
Lower middle-income countries
| Country | Population | N | Study objective | Lipodystrophy, lipoatrophy, lipohypertrophy** | Other findings |
|---|---|---|---|---|---|
| AFRICA | |||||
| Cross-sectional | |||||
| Cameroon [12,36] | HIV+ adults on d4T or AZT ≥6 mo | 249 | LD prevalence, effect of lower d4T dose on LD | Mild-severe LD: 60% Moderate-severe LD: 18% |
Odds of LD: higher in d4t vs. AZT and group on 40 mg d4T switched to 30 mg vs. 30 mg only |
| ASIA | |||||
| Prospective cohort | |||||
| India [167] | HIV+ initiating NVP ART | 190 | Effects of NVP on weight, BMI, body composition | Weight changes: At 6 mo, 22% lost mean 3.6 kg, 19% stable, 59% gained mean 6 kg. No differences in WHR ratio, BFR, or CD4 counts. | |
| India [30] | HIV+≥12 mo of RTV-boosted PI | 91 | Safety, tolerability, efficacy of PI vs. NNRTI | LD incidence: 10.4% in 1 y after NNRTI to PI regimen change | Adverse effects after switch: LD, nausea, peripheral neuropathy |
| India [125] | HIV+ initiating ART | 68 | Metabolic and BFR changes with ART | 6 mo on ART, 19.1% had new-onset metabolic syndrome; total body fat, body fat mass, lean body mass, SFT significantly increased | |
| India [31] | Malnourished HIV+ adults initiating ART | 34 | Clinical markers used to monitor BFR | LA: 26% LH: 24 |
More body weight changes diagnosed than self-reported; 28% men, 75% women |
| Cross-sectional | |||||
| India [51] | HIV+ on ART, HIV+ ART naïve, and HIV− | 363 | LD prevalence in rural patients receiving ART | LD: 60.7%, LH: 22.7%, LA: 51.1%, mixed: 22.7% | Risk factors for LD/LA: female, ART duration; BMI: HIV−>HIV+ART+>HIV+ART− |
| India [161] | HIV+ adults on 2-drug ART or HAART | 286 | Long-term metabolic and BFR changes on ART | LA: 2.8% (21 mo) | 37.5% with LA received 2 drug regimen, 50% received HAART, 12.5% both |
| India [61] | HIV+ men, on ART vs. ART naïve | 121 | Objective LD definition w/regional fat mass ratios | LD: 54.3% via trunk fat/lower limb fat mass ratio >2.28 | Clinical diagnosis of LD with many FP/FN results. Triceps SFT/Abd SFT: poor specificity |
| India [134] | HIV+ adults on HAART | 50 | QoL among HIV+ adults with and without LD | LA: 42% (3.71 y) | Significant differences in financial (p<0.045) and disclosure (p<0.023) concerns with LD |
GDP per capita: $1026 to $4,035. ABC: abacavir; ART: antiretroviral therapy; AZT: zidovudine; BFR: body fat redistribution; d4T: stavudine; FN, false negative; FP, false positive; HAART: highly active antiretroviral therapy; HIV: Human Immunodeficiency Virus; LA: lipoatrophy; LD: lipodystrophy; LH: lipohypertrophy; mo: months; NVP: nevirapine; PI: protease inhibitor; QoL: quality of life; RTV: ritonavir; SFT: skinfold thickness; TC: total cholesterol; TDF: tenofovir; TG: triglycerides; WC: waist circumference; WHR: waist to height ratio; ZDV: zidovudine; y: year. Prevalence unless otherwise noted.
Table 3.
Upper middle-income countries
| Country | Population | N | Study objective | Lipodystrophy, lipoatrophy, lipohypertrophy | Other findings |
|---|---|---|---|---|---|
| AFRICA | |||||
| Prospective cohort | |||||
| Botswana [168] | HIV+ subtype C, initiating ART | 650 | Efficacy of ZDV/ddI/3TC switched from d4T/3TC | Treatment-modifying LD: 36% (2 y) | 6.2 kg and 3 kg weight increase from baseline in HIV wasting and HIV non-wasting, respectively |
| Botswana [43] | HIV+ initiating d4T, NVP | 2190 | Incidence, risk factors for d4T and NVP toxicity | LA prevalence requiring regimen change: 7.2% (18 mo) | LA risk factors: female sex, 40 mg d4T |
| Botswana [34] | HIV+ on ART (100 LD, 50 non-LD), 50 HIV− | 571 | Prevalence of LD and HAART toxicity | LD: 34% (LA: 9%, LH: 19%, mixed: 72%), 18 mo: 69.6% | LD risk factors: urban residence, ART duration; LD associated with significantly higher TC, WHR |
| Rwanda [11] | HIV+ adults on ART for >1 y | 409 | Prevalence and risk factors of LD | LD: 34.3% (16 mo), mixed: 19.6%, LH: 4.9%, LA: 9.8% | LA risk factors: recent onset/faster rate weight loss, 3x higher LA with d4T vs. AZT, female sex |
| Rwanda [144] | HIV+ requiring d4T substitution for LA | 114 | Weight after d4T switch to ZDV or TDF/ABC | Switch to ZDV: progressive weight loss (significant at 2 and 12 mo); Switch to TDF/ABC: stable body weight, increased slightly after 3 mo | |
| Rwanda [133,135] | HIV+ with and without BFR, HIV− | 100 | Weight and QoL after 6 mo of exercise therapy | Greater decline in WC, WHR; improved emotional stress, body image, self-esteem psychological well-being, social relationships, and independence in exercise group | |
| South Africa [42] | HIV+ initiating ART | 9040 | Long term incidence of d4T toxicity, risk factors | LA incidence: 7.3%/4.6% after 23.1 mo of d4T/non-d4T ART | LA risk factors: d4t and female sex |
| South Africa [116] | HIV+ initiating ART | 3910 | Efficacy and safety of 30 mg vs. 40 mg of d4T | LA incidence: <2% (12 mo) | No evidence of impaired viral suppression with 30 mg d4T; LA: Increased odds with 40 mg d4T |
| South Africa [4] | HIV-infected on HAART | 2815 | ART toxicity and reasons for regimen change | LD prevalence: 8.3% (14.9 mo) | HAART adverse effects: LD, lactic acidosis |
| South Africa [48] | HIV+ initiating HAART | 2679 | ART toxicity and reasons for regimen change | LD prevalence requiring d4T substitution: 9% (3 y) | LD risk factors: female sex, ART ≥6 mo, d4T regimen change: 21% (9% due to LD) |
| South Africa [35] | HIV+ initiating ART | 230 | Changes in perception and anthropometry | LD prevalence: 35% (12 mo) | Significant discrepancy between BMI and patient's perception of weight |
| South Africa [49] | HIV+ initiating d4T ART | 42 | Changes in BFR and metabolic factors on d4T | LD: 42.9% (20.5 mo) mixed: 33%, LH: 9.5%, LA: 9.5% | LD risk factors: greater triceps and iliac crest SFT at baseline |
| Retrospective | |||||
| Rwanda [106] | HIV+ initiating d4T | 705 | Weight change with LA and efficacy of d4T | LA requiring regimen change: 12.2% (2 y) | Rapid weight increase in first 6–12 mo of ART, progressive decline in weight year 2 |
| Cross-sectional | |||||
| Rwanda [21] | HIV+ ART naïve and HIV− controls | 821 | BFR in HIV− and HIV+, ART naïve women | Mean body weight, BMI (19% HIV+, 26% HIV−), total body fat by BIA, WHRs similar in both groups; no associations between CD4 counts and body composition | |
| Rwanda [8,159] | HIV+BFR+ and HIV+ BFR− | 100 | Relationship between BFR and QoL | HIV+BFR+ vs. HIV+BFR−: former with larger WHR, less satisfied with self-esteem, social life, body appearance, more emotional stress, ashamed in public places | |
| Senegal [12] | HIV+ on HAART 4–9 y and HIV− controls | 180 | Prevalence, risk factors, metabolic disorders | LD: 65%, mild: 33.9%, severe: 31.1%; 2nd LD definition: 49.5% | Lower BMI, skinfolds and greater WHRs and trunk/arm skinfold ratios on HAART |
| Senegal [169] | HIV+ on HAART | 20 | Perceptions of LD | LD: 31.1% (9–16 y), LA: 60%, LH: 25%, Combined: 15% | Most unable to recognize changes as LD. LH tolerated better; excess weight sign of wealth |
| Senegal [170] | HIV+ women, ART naïve, d4T/AZT, LPV/r | 744 | Body composition (DEXA) of d4T/AZT vs. LPV/r | D4T/AZT or LPV/r: more central fat mass (p<0.01) and less leg fat mass (p<0.01) than ART naïve. D4T: WHR greater, calf skinfold lower (p<0.033) with increasing d4T time | |
| Senegal [171] | HIV+ exposed to thymidine analogue | 171 | mtDNA haplotypes, metabolic changes | No significant associations between mtDNA haplogroups and lipoatrophy | |
| South Africa [172] | HIV+ on HAART | 479 | Demand for surgical LA correction | LD: 11.7% | 5.9% considered stopping therapy due to LD, 47% consider surgery to correct LA/LD |
| South Africa [173] | HIV+, ART naïve women on ART | 83 | VL, immune activation, adipose tissue | High VL women had a lower BMI, subcutaneous abdominal fat, WC, trunk fat measurements with DEXA vs. low VL group | |
| South Africa [174] | HIV+ ART naïve, ART with LD+, ART LD− | 39 | PET glucose uptake by fat and muscle | Standardized uptake values (SUV): Higher in HAART patients with LD; SUV values for subcutaneous fat correlated with treatment duration and CD4 count | |
| ASIA | |||||
| Randomized trials | |||||
| China [175] | HIV+ on d4T vs. switch to AZT after 24 weeks | 199 | Effects of switching from d4T to AZT after 24 weeks | LD: 18.4%, d4T to AZT group; LD: 0.6%, AZT only group | |
| Prospective cohort | |||||
| China [24] | AIDS+, ART naïve adults | 103 | Efficacy/side-effects of HAART in AIDS patients | LD: 9.7% (12 mo) | HAART side-effects: peripheral neuropathy, gastrointestinal disorders, LD |
| China [25] | HIV+ adults initiating ART | 57 | Long-term ART outcomes | LD: 10.5% (72 mo) | 44% changed from d4T to ddI (24 mo); 26.3% with elevated cholesterol and TG (72 mo) |
| China [26] | HIV+, ART naïve adults initiating ART | 52 | Adipocytokine and adipose distribution in HIV/LD | LD incidence: 2% (12 mo), 9.6% (18 mo), 52% (30 mo) | Adiponectin incresed (6 mo) then decreased (18–30 mo) in LD+ group, no change in LD− group |
| Thailand [27] | HIV+ adults on HAART | 829 | Genetic polymorphisms correlated with LA/LD | LA or LD: 32.6% (4 y) | LA or LD risk factors: female sex, d4T (compared to AZT), Fas-670-AA |
| Thailand [28] | HIV+ initiating d4T/NVP | 152 | Efficacy and adverse effects of d4T/3TC/NVP | LD: 35.5% (3 y) | Median % body weight increased 8.1% (1 y) then plateaued until end of study |
| Thailand [29] | HIV+ initiating d4T/NVP | 83 | Efficacy and adverse effects of d4T/3TC/NVP | LD: 16.8% (2 y) | LD risk factors: low baseline body weight (≤50 kg), female sex, duration of treatment |
| Thailand [120] | HIV+, NRTIs to RTV boosted IDV/EFV | 61 | Metabolic disturbances and changes in BFR | VAT mass increased by 20% (96 weeks) after switch; 4.1 and 15.9% increase in TC and TG, respectively, with 1 kg reduction in total limb fat mass at baseline | |
| Thailand [121] | HIV+, NRTI to NRTI-sparing regimens | 60 | Effect of switch: NRTI to NRTI-sparing ART | All had LD | DEXA: mean total limb fat increase, mean lean limb mass loss (48 weeks) after switch |
| Thailand [99] | HIV+ 48 weeks after d4t/ddI to TDF/3TC | 35 | Effect of medication change on mtDNA | LD: 54% | After switch, mtDNA content rose significantly, lipid factors reduced; reversal of LA on DEXA |
| Cross-sectional | |||||
| Thailand [155] | 71% HIV+ on ART, 29% ART naïve | 580 | Risk of metabolic syndrome with LD | LD: 45.2%, LA: 41.9%, LH: 28.1% (2.6 y) | LD+: OR 1.8 (95% CI 1.0–3.0, p=0.032) of developing metabolic syndrome |
| Thailand [130] | HIV+ 33% treatment naïve, 77% ART | 278 | Prevalence, clinical characteristics of LD | LD: 21% in ART (43 mo) | LD associations: higher CD4 counts and lower viral loads; 93% had 1+ metabolic abnormality |
| Thailand [62] | HIV+ART naïve, on PI ART, or non-PI ART | 247 | Prevalence of BFR and metabolic changes | LA: 21% temporal, 28% facial LH: 53% PI, 12.15% non-PI |
ART treated: higher WC and WHR LA risk factors: Use of d4T and indinavir |
| Thailand [117] | HIV+ >15 y old, exposed to d4T | 103 | LD risk factors | Absent to mild LD: 47% Moderate to severe LD: 53% |
Increased odds of LD: HLA-B*4001 status, 2% increase of LD for every month of d4T |
| Thailand [50] | HIV+ initiating ART | 56 | Prevalence, clinical, risk factors of LD | LD: 66.1%; mixed 26.8%, LA 35.7%, LH 3.6% | LD risk factors: undetectable HIV RNA, d4T |
| CENTRAL/SOUTH AMERICA | |||||
| Prospective cohort | |||||
| Brazil [37] | HIV+ on HAART | 332 | Identify risk factors for LD in HIV/AIDS | LD: 54.8% (5.7 y) | LD: higher BMI (women), lower BMI (men), LD risk factors: d4T >3 y, HAART >4 y |
| Brazil [54] | HIV+LD+ and HIV− with PMMA fillers | 266 | Determine efficacy/safety of PMMA as facial filler | 90% of subjects were satisfied with treatment, significant improvements in self-esteem; Adverse effects all transient and resolved spontaneously |
|
| Brazil [176] | HIV+ LD+ on HAART>1 year | 187 | Evaluate body fat content based on ART duration | LD+ had 11 mm of additional abdominal fat after 1 y of ARVs vs. LD− Women had greater peripheral fat and men had 7.2 mm more visceral fat | |
| Brazil [177] | HIV+LD+ with/without fibric acid therapy | 53 | Compare changes in lipid metabolism in LD adults | No change in metabolic parameters at 6 mo post-nutritional counseling | |
| Brazil [58] | HIV+ LA+, ART naive | 44 | PMMA treatment on HIV disease progression | Non-significant increase in patients with undetectable viral load (p=0.67) and significant increase in CD4 count (p=0.02) after PMMA treatment | |
| Brazil [55] | AIDS+ with PMMA filling for LA | 49 | Evaluate the use of PMMA to treat LA | Most patients on d4T or ZDV; side effects infrequent; 85.7% of patients satisfied after >12 mo with PMMA | |
| Brazil [178] | HIV+ LD+ on HAART for ≥18 mo | 24 | Metformin's effects on HIV+LD+ adults | Mean weight loss: 2.2% of initial body weight (3 mo), 3.8% (6 mo), BMI reduction, WHR reduction, CT: total abdominal tissue reduced by 10.3% (6 mo) | |
| Brazil [56] | HIV+ with facial LA | 20 | Using Index for facial LA (ILA) to diagnose LA | Good to excellent response to treatment for moderate to severe facial LA; short-term side effects resolved <1 week and all within 6 mo | |
| Randomized trial | |||||
| Brazil [140] | HIV+ on HAART for ≤12 mo | 50 | Nutritional counseling on diet to prevent BFR | No differences between intervention (dietary counseling) and control; both groups showed an increase in SSF from 1–8 mo (p<0.001) | |
| Brazil [179] | HIV+ LA+: NRTI-sparing for >24 weeks | 71 | Rosiglitazone-induced fat recovery in LA | Limb fat (DEXA) increased significantly (448 g vs. 153 g) in LA group treated with rosiglitazone vs. placebo; facial LA scores changed significantly | |
| Brazil [59] | HIV+ on ART eligible for PMMA for LA | 40 | Effects and impact of facial LA on patient QoL | Slight improvement in medical outcomes, self-esteem, and Beck depression scale in PMMA group | |
| Brazil [137] | HIV+ LD+ HL+ adults on HAART >6 mo | 30 | Evaluate the effect of exercise therapy on LD | Weight, BMI,% BF decreased similarly in both exercise/diet and diet groups; peak oxygen uptake improved only in exercise/diet | |
| Cross-sectional | |||||
| Argentina [180] | HIV+ART naïve, ART LD+/LD− | 101 | Markers of endothelial function in HIV+ adults | LD group: highest TG, sVCAM-1 correlated with vWF (endothelial activation markers), % arm fat, CD4 T-cell counts, TG, ART naïve group: highest sVCAM-1 | |
| Argentina [74] | HIV+ on HAART | 72 | Congruency between methods measuring BFR | Questionnaire A vs. B: 86%/49% with BFR; Clinical criteria A vs. B: 48%/24% Best assessment: DEXA ratio between trunk fat content and the leg fat content |
|
| Brazil [181] | HIV/AIDS+ | 958 | LD and HTN prevalence | LD: 46.1% (HTN) LD: 47.7% (pre-HTN) |
Increased risk of HTN: age >40 y, male sex, BMI >25, TG >150 mg/dL |
| Brazil [182] | HIV+ | 819 | Metabolic disturbances and LD prevalence | LD: 38.5%; LA: 45%, mixed: 42.9%, LH: 12.1% | Metabolic parameters: 86% had ≥1 metabolic disturbance (low HDL, high TG) |
| Brazil [183] | HIV+ receiving HAART | 620 | ART on lipid profile pre- and post-HAART | Prevalence of DL/LD after HAART was 32.4% 30.1 mo vs. 11.3% before HAART Higher prevalence of DL/LD with PI based regimen and d4T (p=0.006) |
|
| Brazil [184] | HIV+ on HAART >1 y | 614 | Genetic SNPs in estrogen receptor-a and -b in LD | LA more common in men, LH more common in women (35.6% of all LD in women) LA risk factors: age, European ancestry, PI use, AA homozygotes for ESR2 rs3020450 |
|
| Brazil [122] | HIV/AIDS adolescents on HAART | 457 | Analysis of patient self-perception of BFR | 64.3% reported perception of body changes (15% peripheral loss, 27% central gain); Perceived body changes significantly associated with duration of PI, NRTI, not NNRTI | |
| Brazil [38] | HIV+ on HAART | 410 | Association between 4 polymorphisms and LD | Prevalence of LD: 53.4% | No difference in genotype frequencies (LD/LD−); Adiponectin higher in specific alleles in LD |
| Brazil [185] | HIV+LD+, HIV+LD−HIV−LD− | 300 | IL-18 and IFN-y gene polymorphisms (LD/LD−) | LD: increased mRNA expression of inflammatory cytokines TNF, IL-6, IL-8; Increased odds of LD: IL-18-607A, Decreased odds: IL-18-607C; IFN-y: no difference (LD/LD−) | |
| Brazil [186] | HIV+ | 256 | Estimate hyperApoB prevalence, CV risk | Self-reported LD not associated with high apoB, prevalence of hyperApoB: 32.4% Hypercholesterolemia: 32%; TG: 47%; low HDL 47.6%, high LDL 47.4% | |
| Brazil [136] | HIV/AIDS+ on HAART for ≥3 mo | 220 | Physical activity and central fat relationship | 3 physical activity questionnaires: occupation, leisure sports, locomotion; significant negative correlation for LTPA with CSF | |
| Brazil [39] | HIV or AIDS+ (96%) | 180 | Assess LD prevalence and characteristics of LD | Women: LH: 43%, mixed: 40%; men: LA: 34%, mixed: 34%, LH: 32% | Risk factors: female sex, AIDS duration >8 y |
| Brazil [82] | HIV+LD+ and HIV+LD− | 179 | U/S vs. clinical signs used to diagnose BFR | LD risk factors: age, greater duration to ART; U/S predictors of LD: facial, subq abd, visceral body compartments; ROC curves: sensitivity 69.1%, specificity 94.9% | |
| Brazil [187] | HIV+ART, HIV+ART naïve adults | 160 | Compare intra-abdominal fat thickness by U/S | No significant difference in subcutaneous adipose tissue between groups; positive correlation between intra-abdominal fat thickness and plasma TG, TC, glucose | |
| Brazil [162] | HIV+LD+; HIV+LD− controls | 139 | Different morphological alterations from LD | LA: 38.3% (25% facial); LH: 15% (8.3% abdomen); mixed: 46.7% (10% abdomen, face, UL, LL, 6.6% abdomen, face) | |
| Brazil [188] | HIV+LD+, HIV+LD− | 117 | Frequency of TNF-a-e microsatellites in HIV | TNF-308 G associated with OR: 28.40 of developing LD, protective effect of TNF-308G: 0.39, no significant association with TNF microsatellite presence polymorphisms | |
| Brazil [189] | HIV+LD+, HIV+LD− | 69 | LD+ and immune status correlation | Reduced CD4 T-cells: lower antibody production, lower anti-mLDL levels LD correlated with increased mLDL, decreased anti-mLDL antibodies |
|
| Brazil [190] | HIV+LD+, HIV+LD−healthy controls (men) | 44 | Assess HIV-LD energy expenditure | HIV+LD+ vs. LD−: REE per kg lean mass significantly greater, body fat% lower, especially in leg; all HIV+: increased carbohydrate oxidation, lower lipid oxidation | |
| Brazil [191] | AIDS+ patients on HAART | 74 | Prevalence of LD after ≥5 y on HAART | Self-reported LD: 51.4%, anthropometric: 66.2%, objective verification: 32.4%; LA: 62.5%, LH: 8.3%, mixed: 29.2% | |
| Brazil [70] | HIV+LD+, HIV+ LD−, controls (men) | 44 | Compare different methods to diagnose BFR | BIA positively and significantly correlated with DEXA for MUAC and lean mass; SFT values correlated with DEXA only in controls | |
| Brazil [40] | HIV+ on HAART | 42 | Assess physical activity in LD in HIV+ adults | LD: 42.9% | Active individuals had a 79% less chance of developing LD versus sedentary individuals |
| Retrospective | |||||
| Brazil [69] | HIV+ (PI: 47 NNRTI: 37) | 102 | Factors associated with HIV related LD | LD: 59.3% | LD associated with increase in TC, not HDL nor TG |
| Brazil [192] | HIV+ starting on HAART | 233 | Adverse effects of d4T | LD: 6.9% | |
| Chile [41] | HIV+ on HAART for ≥10 y | 121 | Clinical outcomes after 10 y of HAART | Average of 3.5 different ART regimens in 10 y due to drug toxicity (35%), allergy (9.2%) dyslipidemia (5.6%), lipodystrophy (5%) | |
GDP per capita: $4,036–12,474. 3TC: lamivudine; ABC: abacavir; AIDS: acquired autoimmune deficiency syndrome; apoB: apolipoprotein B; ART: antiretroviral therapy; AZT: zidovudine; BFR: body fat redistribution; BMI: body mass index; CSF: central subcutaneous fat; CV: cardiovascular; d4T: stavudine; ddI: didanosine; DEXA: dual energy X-ray absorptiometry; EFV: efavirenz; HAART: highly active antiretroviral therapy; HIV: human immunodeficiency virus; IDV: indinavir; LA: lipoatrophy; LD: lipodystrophy; LH: lipohypertrophy; LTPA: leisure time physical activity; mtDNA: mitochondrial DNA; mo: months; MUAC: mid upper arm circumference; NVP: nevirapine; PET: positron emission tomography; PI: protease inhibitor; PMMA: polymethylmethacrylate; QoL: quality of life; REE: resting energy expenditure; ROC: receiver operating curve; SFT: skinfold thickness; TC: total cholesterol; TDF: tenofovir; TG: triglycerides; TNF: tumor necrosis factor; U/S: ultrasound; VAT: visceral adipose tissue; VL: viral load; WC: waist circumference; WHR: waist to height ratio; ZDV: zidovudine; y: year. Prevalence unless otherwise noted.
References
- 1.Delaney M. History of HAART – the true story of how effective multi-drug therapy was developed for treatment of HIV disease. Retrovirology. 2006;3(Suppl 1):S6. [Google Scholar]
- 2.Carr A. Lactic acidemia in infection with human immunodeficiency virus. Clin Infect Dis. 2003;36(Suppl 2):S96–100. doi: 10.1086/367565. [DOI] [PubMed] [Google Scholar]
- 3.Calmy A, Hirschel B, Cooper DA, Carr A. Clinical update: adverse effects of antiretroviral therapy. Lancet. 2007;370(9581):12–4. doi: 10.1016/S0140-6736(07)61027-7. [DOI] [PubMed] [Google Scholar]
- 4.Dube NM, Summers R, Tint KS, Mayayise G. A pharmacovigilance study of adults on highly active antiretroviral therapy. South Africa: 2007–2011. Pan Afr Med J. 2012;11:39. [PMC free article] [PubMed] [Google Scholar]
- 5.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 6.Tanwani LK, Mokshagundam SL. Lipodystrophy, insulin resistance, diabetes mellitus, dyslipidemia, and cardiovascular disease in human immunodeficiency virus infection. South Med J. 2003;96(2):180–8. doi: 10.1097/01.SMJ.0000051731.69719.2E. quiz 189. [DOI] [PubMed] [Google Scholar]
- 7.Duran S, Savès M, Spire B, Cailleton V, Sobel A, Carrieri P, et al. Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS. 2001;15(18):2441–4. doi: 10.1097/00002030-200112070-00012. [DOI] [PubMed] [Google Scholar]
- 8.Mutimura E, Stewart A, Crowther NJ. Assessment of quality of life in HAART-treated HIV-positive subjects with body fat redistribution in Rwanda. AIDS Res Ther. 2007;4:19. doi: 10.1186/1742-6405-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plankey M, Bacchetti P, Jin C, Grimes B, Hyman C, Cohen M, et al. Self-perception of body fat changes and HAART adherence in the Women's Interagency HIV Study. AIDS Behav. 2009;13(1):53–9. doi: 10.1007/s10461-008-9444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baril JG, Junod P, Leblanc R, Dion H, Therrien R, Laplante F, et al. HIV-associated lipodystrophy syndrome: a review of clinical aspects. Can J Infect Dis Med Microbiol. 2005;16(4):233–43. doi: 10.1155/2005/303141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Griensven J, De Naeyer L, Mushi T, Ubarijoro S, Gashumba D, Gazille C, et al. High prevalence of lipoatrophy among patients on stavudine-containing first-line antiretroviral therapy regimens in Rwanda. Trans Roy Soc Trop Med Hyg. 2007;101(8):793–8. doi: 10.1016/j.trstmh.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Mercier S, Gueye NF, Cournil A, Fontbonne A, Copin N, Ndiaye I, et al. Lipodystrophy and metabolic disorders in HIV-1-infected adults on 4- to 9-year antiretroviral therapy in Senegal: a case-control study. J Acquir Immune Defic Syndr. 2009;51(2):224–30. doi: 10.1097/QAI.0b013e31819c16f4. [DOI] [PubMed] [Google Scholar]
- 13.Phillips DR, Hay P. Current perspectives on the management and prevention of antiretroviral-associated lipoatrophy. J Antimicrob Chemother. 2008;62(5):866–71. doi: 10.1093/jac/dkn318. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein KA. Redefining lipodystrophy syndrome: risks and impact on clinical decision making. J Acquir Immune Defic Syndr. 2005;39(4):395–400. doi: 10.1097/01.qai.0000167478.28051.3a. [DOI] [PubMed] [Google Scholar]
- 15.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40(2):121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jouquet G, Bygrave H, Kranzer K, Ford N, Gadot L, Lee J, et al. Cost and cost-effectiveness of switching from d4T or AZT to a TDF-based first-line regimen in a resource-limited setting in rural Lesotho. J Acquir Immune Defic Syndr. 2011;58(3):e68–74. doi: 10.1097/QAI.0b013e31822a9f8d. [DOI] [PubMed] [Google Scholar]
- 17.Smit E, Skolasky RL, Dobs AS, Calhoun BC, Visscher BR, Palella FJ, et al. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am J Epidemiol. 2002;156(3):211–18. doi: 10.1093/aje/kwf039. [DOI] [PubMed] [Google Scholar]
- 18.Kolčić I. Double burden of malnutrition: a silent driver of double burden of disease in low- and middle-income countries. J Glob Health. 2012;2(2):20303. doi: 10.7189/jogh.02.020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Waal R, Cohen K, Maartens G. Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction. PLoS One. 2013;8(5):e63623. doi: 10.1371/journal.pone.0063623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.W.B. Group, editor. Country and lending groups; World development indicators; Washington, DC, USA: W.B. Group; 2012. p. xxiii. [Google Scholar]
- 21.Thiébaut R, Daucourt V, Mercié P, Ekouévi DK, Malvy D, Morlat P, et al. Lipodystrophy, metabolic disorders, and human immunodeficiency virus infection: Aquitaine Cohort, France, 1999. Groupe d'Epidémiologie Clinique du Syndrome d'Immunodéficience Acquise en Aquitaine. Clin Infect Dis. 2000;31(6):1482–7. doi: 10.1086/317477. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen A, Calmy A, Schiffer V, Bernasconi E, Battegay M, Opravil M, et al. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000–2006. HIV Med. 2008;9(3):142–50. doi: 10.1111/j.1468-1293.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Misra A, Garg A. Clinical review 153: lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87(11):4845–56. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 24.Dai Y, Qiu ZF, Li TS, Han Y, Zuo LY, Xie J, et al. Clinical outcomes and immune reconstitution in 103 advanced AIDS patients undergoing 12-month highly active antiretroviral therapy. Chin Med J (Engl) 2006;119(20):1677–82. [PubMed] [Google Scholar]
- 25.Zhou HY, Zheng YH, He Y, Chen Z, Liu M, Yin W, et al. Evaluation of a 6-year highly active antiretroviral therapy in Chinese HIV-1-infected patients. Intervirology. 2010;53(4):240–6. doi: 10.1159/000302762. [DOI] [PubMed] [Google Scholar]
- 26.Luo L, Zhang L, Tao M, Qiu Z, Xie J, Han Y, et al. Adiponectin and leptin levels in Chinese patients with HIV-related lipodystrophy: a 30-month prospective study. AIDS Res Hum Retroviruses. 2009;25(12):1265–72. doi: 10.1089/aid.2009.0072. [DOI] [PubMed] [Google Scholar]
- 27.Likanonsakul S, Rattanatham T, Feangvad S, Uttayamakul S, Prasithsirikul W, Srisopha S, et al. Polymorphisms in fas gene is associated with HIV-related lipoatrophy in Thai patients. AIDS Res Hum Retroviruses. 2012;29(1):142–50. doi: 10.1089/aid.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desakorn V, Karmacharya BM, Thanachartwet V, Kyaw NL, Tansuphaswadikul S, Sahassananda D, et al. Effectiveness of fixed-dose combination stavudine, lamivudine and nevirapine (GPO-VIR) for treatment of naïve HIV patients in Thailand: a 3-year follow-up. Southeast Asian J Trop Med Public Health. 2011;42(6):1414–22. [PubMed] [Google Scholar]
- 29.Tin EE, Bowonwatanuwong C, Desakorn V, Wilairatana P, Krudsood S, Pitisuttithum P, et al. The efficacy and adverse effects of GPO-VIR (stavudine+lamivudine+nevirapine) in treatment-naïve adult HIV patients. Southeast Asian J Trop Med Public Health. 2005;36(2):362–9. [PubMed] [Google Scholar]
- 30.Kumarasamy N, Venkatesh KK, Devaleenal B, Poongulali S, Yepthomi T, Solomon S, et al. Safety, tolerability, and efficacy of second-line generic protease inhibitor containing HAART after first-line failure among South Indian HIV-infected patients. J Int Assoc Physicians AIDS Care (Chic) 2011;10(2):71–5. doi: 10.1177/1545109710382780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padmapriyadarsini C, Swaminathan S, Karthipriya MJ, Narendran G, Menon PA, Thomas BE, et al. Morphologic and body composition changes are different in men and women on generic combination antiretroviral therapy – an observational study. J Assoc Physicians India. 2010;58:375–7. [PubMed] [Google Scholar]
- 32.Phan V, Thai S, Choun K, Lynen L, van Griensven J. Incidence of treatment-limiting toxicity with stavudine-based antiretroviral therapy in Cambodia: a retrospective cohort study. PLoS One. 2012;7(1):e30647. doi: 10.1371/journal.pone.0030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zannou DM, Denoeud L, Lacombe K, Amoussou-Guenou D, Bashi J, Akakpo J, et al. Incidence of lipodystrophy and metabolic disorders in patients starting non-nucleoside reverse transcriptase inhibitors in Benin. Antivir Ther. 2009;14(3):371–80. doi: 10.1177/135965350901400307. [DOI] [PubMed] [Google Scholar]
- 34.Mutimura E, Stewart A, Rheeder P, Crowther NJ. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(4):451–5. doi: 10.1097/qai.0b013e318158c0a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurley E, Coutsoudis A, Giddy J, Knight SE, Loots E, Esterhuizen TM, et al. Weight evolution and perceptions of adults living with HIV following initiation of antiretroviral therapy in a South African urban setting. S Afr Med J. 2011;101(9):645–50. [PubMed] [Google Scholar]
- 36.Cournil A, Coudray M, Kouanfack C, Essomba CN, Tonfack CA, Biwolé-Sida M, et al. Reduced dose of stavudine and lipoatrophy in HIV-infected patients in Cameroon. Antivir Ther. 2010;15(7):1039–43. doi: 10.3851/IMP1664. [DOI] [PubMed] [Google Scholar]
- 37.Gelenske T, e Farias FA, de Alencar Ximenes RA, de Melo HR, de Albuquerque MeF, de Carvalho EH, et al. Risk factors in human immunodeficiency virus/acquired immunodeficiency syndrome patients undergoing antiretroviral therapy in the state of Pernambuco, Brazil: a case-control study. Metab Syndr Relat Disord. 2010;8(3):271–7. doi: 10.1089/met.2009.0065. [DOI] [PubMed] [Google Scholar]
- 38.Trinca JR, Sprinz E, Lazzaretti RK, Hutz MH, Kuhmmer R, de Almeida S, et al. SNPs in the APM1 gene promoter are associated with adiponectin levels in HIV-infected individuals receiving HAART. J Acquir Immune Defic Syndr. 2010;55(3):299–305. doi: 10.1097/qai.0b013e3181ecfeb7. [DOI] [PubMed] [Google Scholar]
- 39.Diehl LA, Dias JR, Paes AC, Thomazini MC, Garcia LR, Cinagawa E, et al. Prevalence of HIV-associated lipodystrophy in Brazilian outpatients: relation with metabolic syndrome and cardiovascular risk factors. Arq Bras Endocrinol Metabol. 2008;52(4):658–67. doi: 10.1590/s0004-27302008000400012. [DOI] [PubMed] [Google Scholar]
- 40.Segatto AF, Freitas Junior IF, Santos VR, Alves KC, Barbosa DA, Portelinha Filho AM, et al. Lipodystrophy in HIV/AIDS patients with different levels of physical activity while on antiretroviral therapy. Rev Soc Bras Med Trop. 2011;44(4):420–4. doi: 10.1590/s0037-86822011000400004. [DOI] [PubMed] [Google Scholar]
- 41.Wilson G, Wolff M. A decade of antiretroviral therapy: a profile of patients with 10 years of highly effective triple therapy. Rev Chilena Infectol. 2012;29(3):337–43. doi: 10.4067/S0716-10182012000300015. [DOI] [PubMed] [Google Scholar]
- 42.Menezes CN, Maskew M, Sanne I, Crowther NJ, Raal FJ. A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects. BMC Infect Dis. 2011;11:244. doi: 10.1186/1471-2334-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Griensven J, Zachariah R, Rasschaert F, Mugabo J, Atté EF, Reid T, et al. Stavudine- and nevirapine-related drug toxicity while on generic fixed-dose antiretroviral treatment: incidence, timing and risk factors in a three-year cohort in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2010;104(2):148–53. doi: 10.1016/j.trstmh.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Pujades-Rodríguez M, Schramm B, Som L, Nerrienet E, Narom P, Chanchhaya N, et al. Immunovirological outcomes and resistance patterns at 4 years of antiretroviral therapy use in HIV-infected patients in Cambodia. Trop Med Int Health. 2011;16(2):205–13. doi: 10.1111/j.1365-3156.2010.02689.x. [DOI] [PubMed] [Google Scholar]
- 45.Brown TT. Approach to the human immunodeficiency virus-infected patient with lipodystrophy. J Clin Endocrinol Metab. 2008;93(8):2937–45. doi: 10.1210/jc.2008-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grunfeld C, Saag M, Cofrancesco J, Jr, Lewis CE, Kronmal R, Heymsfield S, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24(11):1717–26. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53(2):185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boulle A, Orrel C, Kaplan R, Van Cutsem G, McNally M, Hilderbrand K, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 49.George JA, Venter WD, Van Deventer HE, Crowther NJ. A longitudinal study of the changes in body fat and metabolic parameters in a South African population of HIV-positive patients receiving an antiretroviral therapeutic regimen containing stavudine. AIDS Res Hum Retroviruses. 2009;25(8):771–81. doi: 10.1089/aid.2008.0308. [DOI] [PubMed] [Google Scholar]
- 50.Chuapai Y, Kiertiburanakul S, Malathum K, Sungkanuparph S. Lipodystrophy and dyslipidemia in human immunodeficiency virus-infected Thai patients receiving antiretroviral therapy. J Med Assoc Thai. 2007;90(3):452–8. [PubMed] [Google Scholar]
- 51.Kalyanasundaram AP, Jacob SM, Hemalatha R, Sivakumar MR. Prevalence of lipodystrophy and dyslipidemia among patients with HIV infection on generic ART in rural South India. J Int Assoc Physicians AIDS Care (Chic) 2012;11(5):329–34. doi: 10.1177/1545109711401750. [DOI] [PubMed] [Google Scholar]
- 52.Andany N, Raboud JM, Walmsley S, Diong C, Rourke SB, Rueda S, et al. Ethnicity and gender differences in lipodystrophy of HIV-positive individuals taking antiretroviral therapy in Ontario, Canada. HIV Clin Trials. 2011;12(2):89–103. doi: 10.1310/hct1202-89. [DOI] [PubMed] [Google Scholar]
- 53.Guaraldi G, Baraboutis IG. Evolving perspectives on HIV-associated lipodystrophy syndrome: moving from lipodystrophy to non-infectious HIV co-morbidities. J Antimicrob Chemother. 2009;64(3):437–40. doi: 10.1093/jac/dkp240. [DOI] [PubMed] [Google Scholar]
- 54.Carvalho Costa IM, Salaro CP, Costa MC. Polymethylmethacrylate facial implant: a successful personal experience in Brazil for more than 9 years. Dermatol Surg. 2009;35(8):1221–7. doi: 10.1111/j.1524-4725.2009.01216.x. [DOI] [PubMed] [Google Scholar]
- 55.Orsi AT, Miranda AE, Souza AC, Silva LC, Dias GR, Talhari C, et al. Lipoatrophy in patients with AIDS: treatment with polymethylmethacrylate in Amazonas, Brazil. Int J Dermatol. 2011;50(10):1255–8. doi: 10.1111/j.1365-4632.2011.04926.x. [DOI] [PubMed] [Google Scholar]
- 56.Serra MS, Oyafuso LK, Trope BM, Munhoz Leite OH, Ramos-E-Silva M. An index for staging facial lipoatrophy and evaluation of the efficacy of the treatment with polymethylmethacrylate in HIV/AIDS patients: a pilot study. J Eur Acad Dermatol Venereol. 2012;27(8):990–6. doi: 10.1111/j.1468-3083.2012.04632.x. [DOI] [PubMed] [Google Scholar]
- 57.Soares FM, Costa IM. HIV-associated facial lipoatrophy: from the advent to current knowledge. An Bras Dermatol. 2011;86(5):843–62. doi: 10.1590/s0365-05962011000500001. quiz 863–4. [DOI] [PubMed] [Google Scholar]
- 58.Soares FM, Costa IM. Treatment of HIV-associated facial lipoatrophy: impact on infection progression assessed by viral load and CD4 count. An Bras Dermatol. 2013;88(4):570–7. doi: 10.1590/abd1806-4841.2013895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warde M, Gragnani A, Gomes H, Hochman B, Ferreira LM. The impact of facial lipoatrophy treatment with polymethyl methacrylate in AIDS patients as measured by four quality-of-life questionnaires. Int J STD AIDS. 2011;22(10):596–9. doi: 10.1258/ijsa.2009.009086. [DOI] [PubMed] [Google Scholar]
- 60.Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG, et al. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet. 2003;361(9359):726–35. doi: 10.1016/s0140-6736(03)12656-6. [DOI] [PubMed] [Google Scholar]
- 61.Asha HS, Seshadri MS, Paul TV, Abraham OC, Rupali P, Thomas N. Human immunodeficiency virus-associated lipodystrophy: an objective definition based on dual-energy x-ray absorptiometry-derived regional fat ratios in a South Asian population. Endocr Pract. 2012;18(2):158–69. doi: 10.4158/EP11139.OR. [DOI] [PubMed] [Google Scholar]
- 62.Homsanit M, Nelson KE, Sonjai A, Anekthananon T, Suwanagool S, Cofrancesco J. Body shape and metabolic abnormalities in Thai HIV-infected patients. AIDS Res Hum Retroviruses. 2007;23(11):1314–21. doi: 10.1089/aid.2007.0013. [DOI] [PubMed] [Google Scholar]
- 63.Scherzer R, Shen W, Bacchetti P, Kotler D, Lewis CE, Shlipak MG, et al. Simple anthropometric measures correlate with metabolic risk indicators as strongly as magnetic resonance imaging-measured adipose tissue depots in both HIV-infected and control subjects. Am J Clin Nutr. 2008;87(6):1809–17. doi: 10.1093/ajcn/87.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sicotte M, Ledoux M, Zunzunegui MV, Ag Aboubacrine S, Nguyen VK, group A. Reliability of anthropometric measures in a longitudinal cohort of patients initiating ART in West Africa. BMC Med Res Methodol. 2010;10:102. doi: 10.1186/1471-2288-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. 1967. Br J Nutr. 2003;89(1):147–55. [PubMed] [Google Scholar]
- 66.Zillikens MC, Conway JM. Anthropometry in blacks: applicability of generalized skinfold equations and differences in fat patterning between blacks and whites. Am J Clin Nutr. 1990;52(1):45–51. doi: 10.1093/ajcn/52.1.45. [DOI] [PubMed] [Google Scholar]
- 67.Moreno S, Miralles C, Negredo E, Domingo P, Estrada V, Gutiérrez F, et al. Disorders of body fat distribution in HIV-1-infected patients. AIDS Rev. 2009;11(3):126–34. [PubMed] [Google Scholar]
- 68.Tien PC, Grunfeld C. What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis. 2004;17(1):27–32. doi: 10.1097/00001432-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Monnerat BZ, Cerutti Junior C, Caniçali SC, Motta TR. Clinical and biochemical evaluation of HIV-related lipodystrophy in an ambulatory population from the Hospital Universitário Cassiano Antonio de Morais, Vitória, ES, Brazil. Braz J Infect Dis. 2008;12(4):364–8. doi: 10.1590/s1413-86702008000400002. [DOI] [PubMed] [Google Scholar]
- 70.Siqueira Vassimon H, Jordao AA, Albuquerque de Paula FJ, Artioli Machado A, Pontes Monteiro J. Comparison of bioelectrical impedance with skinfold thickness and X-ray absorptiometry to measure body composition in HIV-infected with lipodistrophy. Nutr Hosp. 2011;26(3):458–64. doi: 10.1590/S0212-16112011000300005. [DOI] [PubMed] [Google Scholar]
- 71.Schwenk A. Methods of assessing body shape and composition in HIV-associated lipodystrophy. Curr Opin Infect Dis. 2002;15(1):9–16. doi: 10.1097/00001432-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Carr A, Law M, H.L.C.D.S. Group An objective lipodystrophy severity grading scale derived from the lipodystrophy case definition score. J Acquir Immune Defic Syndr. 2003;33(5):571–6. doi: 10.1097/00126334-200308150-00004. [DOI] [PubMed] [Google Scholar]
- 73.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15(11):1389–98. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 74.Belloso WH, Quirós RE, Ivalo SA, Perman MI, Galich AM, Stern LD, et al. Agreement analysis of variables involved in lipodystrophy syndrome definition in HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32(1):104–11. doi: 10.1097/00126334-200301010-00015. [DOI] [PubMed] [Google Scholar]
- 75.Kotler D, Wang J, Pierson R. Body composition studies in patients with the acquired immunodeficiency syndrome. Am J Clin Nutr. 1985;42(6):1255–65. doi: 10.1093/ajcn/42.6.1255. [DOI] [PubMed] [Google Scholar]
- 76.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50(3):444–7. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 77.Swanson B, Keithley JK. Bioelectrical impedance analysis (BIA) in HIV infection: principles and clinical applications. J Assoc Nurses AIDS Care. 1998;9(1):49–54. doi: 10.1016/S1055-3290(98)80076-9. [DOI] [PubMed] [Google Scholar]
- 78.Falutz J. Therapy insight: body-shape changes and metabolic complications associated with HIV and highly active antiretroviral therapy. Nat Clin Pract Endocrinol Metab. 2007;3(9):651–61. doi: 10.1038/ncpendmet0587. [DOI] [PubMed] [Google Scholar]
- 79.Arpadi SM, Bethel J, Horlick M, Sarr M, Bamji M, Abrams EJ, et al. Longitudinal changes in regional fat content in HIV-infected children and adolescents. AIDS. 2009;23(12):1501–9. doi: 10.1097/QAD.0b013e32832b7e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silver HJ, Welch EB, Avison MJ, Niswender KD. Imaging body composition in obesity and weight loss: challenges and opportunities. Diabetes Metab Syndr Obes. 2010;3:337–47. doi: 10.2147/DMSOTT.S9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carey D, Wand H, Martin A, Rothwell S, Emery S, Cooper DA, et al. Evaluation of ultrasound for assessing facial lipoatrophy in a randomized, placebo-controlled trial. AIDS. 2005;19(12):1325–7. doi: 10.1097/01.aids.0000180106.11383.cc. [DOI] [PubMed] [Google Scholar]
- 82.Signorini DJ, Netto AM, Gabbay S, Monteiro MC, Signorini DH, Andrade MeF, et al. A comparison of sonographic assessments and clinical questionnaire in the diagnosis of HIV-associated lipodystrophy. J Int Assoc Physicians AIDS Care (Chic) 2011;10(6):351–6. doi: 10.1177/1545109711399660. [DOI] [PubMed] [Google Scholar]
- 83.Aghdassi E, Arendt B, Salit IE, Allard JP. Estimation of body fat mass using dual-energy x-ray absorptiometry, bioelectric impedance analysis, and anthropometry in HIV-positive male subjects receiving highly active antiretroviral therapy. J Parenter Enteral Nutr. 2007;31(2):135–41. doi: 10.1177/0148607107031002135. [DOI] [PubMed] [Google Scholar]
- 84.Tungsiripat M, McComsey G. Pathogenesis and management of lipoatrophy. Curr HIV/AIDS Rep. 2008;5(2):55–63. doi: 10.1007/s11904-008-0010-8. [DOI] [PubMed] [Google Scholar]
- 85.Leung VL, Glesby MJ. Pathogenesis and treatment of HIV lipohypertrophy. Curr Opin Infect Dis. 2011;24(1):43–9. doi: 10.1097/QCO.0b013e3283420eef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caron-Debarle M, Lagathu C, Boccara F, Vigouroux C, Capeau J. HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol Med. 2010;16(5):218–29. doi: 10.1016/j.molmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Giralt M, Domingo P, Guallar JP, Rodriguez de la Concepción ML, Alegre M, Domingo JC, et al. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV- 1/HAART-associated lipodystrophy. Antivir Ther. 2006;11(6):729–40. [PubMed] [Google Scholar]
- 88.Minami R, Yamamoto M, Takahama S, Ando H, Miyamura T, Suematsu E, et al. Comparison of the influence of four classes of HIV antiretrovirals on adipogenic differentiation: the minimal effect of raltegravir and atazanavir. J Infect Chemother. 2011;17(2):183–8. doi: 10.1007/s10156-010-0101-5. [DOI] [PubMed] [Google Scholar]
- 89.Lagathu C, Bastard JP, Auclair M, Maachi M, Kornprobst M, Capeau J, et al. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro . Antivir Ther. 2004;9(6):911–20. [PubMed] [Google Scholar]
- 90.Flint OP, Noor MA, Hruz PW, Hylemon PB, Yarasheski K, Kotler DP, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37(1):65–77. doi: 10.1177/0192623308327119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mallewa JE, Wilkins E, Vilar J, Mallewa M, Doran D, Back D, et al. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J Antimicrob Chemother. 2008;62(4):648–60. doi: 10.1093/jac/dkn251. [DOI] [PubMed] [Google Scholar]
- 92.Mallon PW. Pathogenesis of lipodystrophy and lipid abnormalities in patients taking antiretroviral therapy. AIDS Rev. 2007;9(1):3–15. [PubMed] [Google Scholar]
- 93.Pacenti M, Barzon L, Favaretto F, Fincati K, Romano S, Milan G, et al. Microarray analysis during adipogenesis identifies new genes altered by antiretroviral drugs. AIDS. 2006;20(13):1691–705. doi: 10.1097/01.aids.0000242815.80462.5a. [DOI] [PubMed] [Google Scholar]
- 94.Harp JB. New insights into inhibitors of adipogenesis. Curr Opin Lipidol. 2004;15(3):303–7. doi: 10.1097/00041433-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 95.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 96.Côté HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med. 2002;346(11):811–20. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- 97.Villarroya F, Domingo P, Giralt M. Lipodystrophy associated with highly active anti-retroviral therapy for HIV infection: the adipocyte as a target of anti-retroviral-induced mitochondrial toxicity. Trends Pharmacol Sci. 2005;26(2):88–93. doi: 10.1016/j.tips.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Moyle G. Mechanisms of HIV and nucleoside reverse transcriptase inhibitor injury to mitochondria. Antivir Ther. 2005;10(Suppl 2):M47–52. [PubMed] [Google Scholar]
- 99.Ananworanich J, Nuesch R, Côté HC, Kerr SJ, Hill A, Jupimai T, et al. Changes in metabolic toxicity after switching from stavudine/didanosine to tenofovir/lamivudine – a Staccato trial substudy. J Antimicrob Chemother. 2008;61(6):1340–3. doi: 10.1093/jac/dkn097. [DOI] [PubMed] [Google Scholar]
- 100.Birkus G, Hitchcock MJ, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46(3):716–23. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Claas GJ, Jülg B, Goebel FD, Bogner J. Metabolic and anthropometric changes one year after switching from didanosine/stavudine to tenofovir in HIV-infected patients. Eur J Med Res. 2007;12(2):54–60. [PubMed] [Google Scholar]
- 102.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 103.van Griensven J, Lynen L, Colebunders R. Substituting tenofovir for stavudine in resource-limited settings: there are challenges ahead. AIDS. 2009;23(8):1027. doi: 10.1097/QAD.0b013e328329d04b. [DOI] [PubMed] [Google Scholar]
- 104.Brown TT, Chu H, Wang Z, Palella FJ, Kingsley L, Witt MD, et al. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS. 2007;21(13):1731–8. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 105.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17(7):971–9. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 106.van Griensven J, Zachariah R, Mugabo J, Reid T. Weight loss after the first year of stavudine-containing antiretroviral therapy and its association with lipoatrophy, virological failure, adherence and CD4 counts at primary health care level in Kigali, Rwanda. Trans Roy Soc Trop Med Hyg. 2010;104(12):751–7. doi: 10.1016/j.trstmh.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 107.Thompson V, Medard B, Taseera K, Chakera AJ, Andia I, Emenyonu N, et al. Regional anthropometry changes in antiretroviral-naïve persons initiating a Zidovudine-containing regimen in Mbarara, Uganda. AIDS Res Hum Retroviruses. 2011;27(7):785–91. doi: 10.1089/aid.2010.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mayer L, Walsh BT, Pierson RN, Heymsfield SB, Gallagher D, Wang J, et al. Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr. 2005;81(6):1286–91. doi: 10.1093/ajcn/81.6.1286. [DOI] [PubMed] [Google Scholar]
- 109.Martínez E. Disorders of fat partitioning in treated HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25(3):415–27. doi: 10.1016/j.beem.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 110.Nyblade L, Pande RP, Mathur S, MacQuarrie K, Kidd R, Banteyerga H, et al. Disentangling HIV and AIDS stigma in Ethiopia, Tanzania and Zambia; DC; International Center for Research on Women: Washington; 2003. [Google Scholar]
- 111.Palmer M, Chersich M, Moultrie H, Kuhn L, Fairlie L, Meyers T. Frequency of stavudine substitution due to toxicity in children receiving antiretroviral treatment in Soweto, South Africa. AIDS. 2013;27(5):781–5. doi: 10.1097/QAD.0b013e32835c54b8. [DOI] [PubMed] [Google Scholar]
- 112.Pujades-Rodríguez M, Dantony E, Pinoges L, Ecochard R, Etard JF, Carrillo-Casas E, et al. Toxicity associated with stavudine dose reduction from 40 to 30 mg in first-line antiretroviral therapy. PLoS One. 2011;6(11):e28112. doi: 10.1371/journal.pone.0028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.WHO, UNAIDS, UNICEF. Geneva: WHO, UNAIDS, UNICEF; 2011. Global HIV/AIDS response: epidemic update and health sector progress towards Universal Access: Progress Report. [Google Scholar]
- 114.World Health Organization. Geneva: World Health Organization; 2012. Global Price Reporting Mechanism (GPRM): AIDS medicines and diagnostic service (AMDS) [Google Scholar]
- 115.Ford N, Calmy A. Improving first-line antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5(1):38–47. doi: 10.1097/COH.0b013e3283339b41. [DOI] [PubMed] [Google Scholar]
- 116.Maskew M, Westreich D, Fox MP, Maotoe T, Sanne IM. Effectiveness and safety of 30 mg versus 40 mg stavudine regimens: a cohort study among HIV-infected adults initiating HAART in South Africa. J Int AIDS Soc. 2012;15(1):13. doi: 10.1186/1758-2652-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wangsomboonsiri W, Mahasirimongkol S, Chantarangsu S, Kiertiburanakul S, Charoenyingwattana A, Komindr S, et al. Association between HLA-B*4001 and lipodystrophy among HIV-infected patients from Thailand who received a stavudine-containing antiretroviral regimen. Clin Infect Dis. 2010;50(4):597–604. doi: 10.1086/650003. [DOI] [PubMed] [Google Scholar]
- 118.Bogner JR, Vielhauer V, Beckmann RA, Michl G, Wille L, Salzberger B, et al. Stavudine versus zidovudine and the development of lipodystrophy. J Acquir Immune Defic Syndr. 2001;27(3):237–44. doi: 10.1097/00126334-200107010-00004. [DOI] [PubMed] [Google Scholar]
- 119.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L, et al. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20(8):1163–9. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 120.Autar RS, Boyd MA, Wit FW, Ruxrungthams K, Sankote J, Lange J, et al. Relationships between drug exposure, changes in metabolic parameters and body fat in HIV-infected patients switched to a nucleoside sparing regimen. Antivir Ther. 2007;12(8):1265–71. [PubMed] [Google Scholar]
- 121.Boyd MA, Carr A, Ruxrungtham K, Srasuebkul P, Bien D, Law M, et al. Changes in body composition and mitochondrial nucleic acid content in patients switched from failed nucleoside analogue therapy to ritonavir-boosted indinavir and efavirenz. J Infect Dis. 2006;194(5):642–50. doi: 10.1086/505709. [DOI] [PubMed] [Google Scholar]
- 122.Santos CP, Felipe YX, Braga PE, Ramos D, Lima RO, Segurado AC, et al. Self-perception of body changes in persons living with HIV/AIDS: prevalence and associated factors. AIDS. 2005;19(Suppl 4):S14–21. doi: 10.1097/01.aids.0000191485.92285.c7. [DOI] [PubMed] [Google Scholar]
- 123.Savès M, Raffi F, Capeau J, Rozenbaum W, Ragnaud JM, Perronne C, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34(10):1396–405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 124.Miller J, Carr A, Emery S, Law M, Mallal S, Baker D, et al. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003;4(3):293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 125.Gupta V, Biswas A, Sharma SK. Metabolic and body composition changes after six months of highly active antiretroviral therapy in northern Indian patients. Int J STD AIDS. 2011;22(1):46–9. doi: 10.1258/ijsa.2010.010193. [DOI] [PubMed] [Google Scholar]
- 126.Haugaard SB, Andersen O, Halsall I, Iversen J, Hales CN, Madsbad S, et al. Impaired proinsulin secretion before and during oral glucose stimulation in HIV-infected patients who display fat redistribution. Metabolism. 2007;56(7):939–46. doi: 10.1016/j.metabol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 127.Grinspoon SK. Metabolic syndrome and cardiovascular disease in patients with human immunodeficiency virus. Am J Med. 2005;118(Suppl 2):23S–8S. doi: 10.1016/j.amjmed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 128.Kosmiski LA, Kuritzkes DR, Lichtenstein KA, Glueck DH, Gourley PJ, Stamm ER, et al. Fat distribution and metabolic changes are strongly correlated and energy expenditure is increased in the HIV lipodystrophy syndrome. AIDS. 2001;15(15):1993–2000. doi: 10.1097/00002030-200110190-00012. [DOI] [PubMed] [Google Scholar]
- 129.Meininger G, Hadigan C, Laposata M, Brown J, Rabe J, Louca J, et al. Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. Metabolism. 2002;51(2):260–6. doi: 10.1053/meta.2002.29999. [DOI] [PubMed] [Google Scholar]
- 130.Puttawong S, Prasithsirikul W, Vadcharavivad S. Prevalence of lipodystrophy in Thai-HIV infected patients. J Med Assoc Thai. 2004;87(6):605–11. [PubMed] [Google Scholar]
- 131.Ammassari A, Antinori A, Cozzi-Lepri A, Trotta MP, Nasti G, Ridolfo AL, et al. Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S140–4. doi: 10.1097/00126334-200212153-00011. [DOI] [PubMed] [Google Scholar]
- 132.Nachega JB, Trotta MP, Nelson M, Ammassari A. Impact of metabolic complications on antiretroviral treatment adherence: clinical and public health implications. Curr HIV/AIDS Rep. 2009;6(3):121–9. doi: 10.1007/s11904-009-0017-9. [DOI] [PubMed] [Google Scholar]
- 133.Mutimura E, Stewart A, Crowther NJ, Yarasheski KE, Cade WT. The effects of exercise training on quality of life in HAART-treated HIV-positive Rwandan subjects with body fat redistribution. Qual Life Res. 2008;17(3):377–85. doi: 10.1007/s11136-008-9319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shenoy A, Ramapuram JT, Unnikrishan B, Achappa B, Madi D, Rao S, et al. Effect of lipodystrophy on the quality of life among people living with HIV/AIDS (PLHIV) on highly active antiretroviral therapy. J Int Assoc Provid AIDS Care. 2013;13(5):471–5. doi: 10.1177/2325957413488205. [DOI] [PubMed] [Google Scholar]
- 135.Mutimura E, Crowther NJ, Cade TW, Yarasheski KE, Stewart A. Exercise training reduces central adiposity and improves metabolic indices in HAART-treated HIV-positive subjects in Rwanda: a randomized controlled trial. AIDS Res Hum Retroviruses. 2008;24(1):15–23. doi: 10.1089/aid.2007.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Florindo AA, de Oliveira Latorre MoR, Jaime PC, Segurado AA. Leisure time physical activity prevents accumulation of central fat in HIV/AIDS subjects on highly active antiretroviral therapy. Int J STD AIDS. 2007;18(10):692–6. doi: 10.1258/095646207782193795. [DOI] [PubMed] [Google Scholar]
- 137.Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP, et al. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med Sci Sports Exerc. 2006;38(3):411–17. doi: 10.1249/01.mss.0000191347.73848.80. [DOI] [PubMed] [Google Scholar]
- 138.Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7(3):137–50. doi: 10.1038/nrendo.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lindegaard B, Hansen T, Hvid T, van Hall G, Plomgaard P, Ditlevsen S, et al. The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. J Clin Endocrinol Metab. 2008;93(10):3860–9. doi: 10.1210/jc.2007-2733. [DOI] [PubMed] [Google Scholar]
- 140.Almeida LB, Segurado AC, Duran AC, Jaime PC. Impact of a nutritional counseling program on prevention of HAART-related metabolic and morphologic abnormalities. AIDS Care. 2011;23(6):755–63. doi: 10.1080/09540121.2010.525789. [DOI] [PubMed] [Google Scholar]
- 141.Hill A, Ruxrungtham K, Hanvanich M, Katlama C, Wolf E, Soriano V, et al. Systematic review of clinical trials evaluating low doses of stavudine as part of antiretroviral treatment. Expert Opin Pharmacother. 2007;8(5):679–88. doi: 10.1517/14656566.8.5.679. [DOI] [PubMed] [Google Scholar]
- 142.Gerschenson M, Kim C, Berzins B, Taiwo B, Libutti DE, Choi J, et al. Mitochondrial function, morphology and metabolic parameters improve after switching from stavudine to a tenofovir-containing regimen. J Antimicrob Chemother. 2009;63(6):1244–50. doi: 10.1093/jac/dkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boufassa F, Dulioust A, Lascaux AS, Meyer L, Boué F, Delfraissy JF, et al. Lipodystrophy in 685 HIV-1-treated patients: influence of antiretroviral treatment and immunovirological response. HIV Clin Trials. 2001;2(4):339–45. doi: 10.1310/BRE5-448N-WUPU-JWVL. [DOI] [PubMed] [Google Scholar]
- 144.van Griensven J, Zachariah R, Rasschaert F, Atté EF, Reid T. Weight evolution in HIV-1 infected women in Rwanda after stavudine substitution due to lipoatrophy: comparison of zidovudine with tenofovir/abacavir. Trans R Soc Trop Med Hyg. 2009;103(6):613–19. doi: 10.1016/j.trstmh.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 145.Allavena C, Ferré V, Brunet-François C, Delfraissy JF, Lafeuillade A, Valantin MA, et al. Efficacy and tolerability of a nucleoside reverse transcriptase inhibitor-sparing combination of lopinavir/ritonavir and efavirenz in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39(3):300–6. doi: 10.1097/01.qai.0000165914.42827.bb. [DOI] [PubMed] [Google Scholar]
- 146.Valantin MA, Lanoy E, Bentata M, Kalmykova O, Boutekadjirt A, Allavena C, et al. Recovery of fat following a switch to nucleoside reverse transcriptase inhibitor-sparing therapy in patients with lipoatrophy: results from the 96-week randomized ANRS 108 NoNuke Trial. HIV Med. 2008;9(8):625–35. doi: 10.1111/j.1468-1293.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 147.Floridia M, Tamburrini E, Anzidei G, Tibaldi C, Guaraldi G, Guerra B, et al. Plasma lipid profile in pregnant women with HIV receiving nevirapine. AIDS Patient Care STDS. 2009;23(3):147–52. doi: 10.1089/apc.2008.0148. [DOI] [PubMed] [Google Scholar]
- 148.Sturt AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD008440. CD008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–48. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 150.Barbaro G. Highly active antiretroviral therapy-associated metabolic syndrome: pathogenesis and cardiovascular risk. Am J Ther. 2006;13(3):248–60. doi: 10.1097/01.mjt.0000162013.66614.16. [DOI] [PubMed] [Google Scholar]
- 151.Barbaro G. Vascular injury, hypertension and coronary artery disease in human immunodeficiency virus infection. Clin Ter. 2008;159(1):51–5. [PubMed] [Google Scholar]
- 152.Berhane T, Yami A, Alemseged F, Yemane T, Hamza L, Kassim M, et al. Prevalence of lipodystrophy and metabolic syndrome among HIV positive individuals on highly active anti-retroviral treatment in Jimma, South West Ethiopia. Pan Afr Med J. 2012;13:43. [PMC free article] [PubMed] [Google Scholar]
- 153.Dubé MP, Parker RA, Tebas P, Grinspoon SK, Zackin RA, Robbins GK, et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19(16):1807–18. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 154.Feleke Y, Fekade D, Mezegebu Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J. 2012;50(3):221–30. [PubMed] [Google Scholar]
- 155.Jantarapakde J, Phanuphak N, Chaturawit C, Pengnonyang S, Mathajittiphan P, Takamtha P, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDS. 2014;28(7):331–40. doi: 10.1089/apc.2013.0294. [DOI] [PubMed] [Google Scholar]
- 156.Edgeworth A, Treacy MP, Hurst TP. Thiazolidinediones in the treatment of HIV/HAART-associated lipodystrophy syndrome. AIDS Rev. 2013;15(3):171–80. [PubMed] [Google Scholar]
- 157.Raboud JM, Diong C, Carr A, Grinspoon S, Mulligan K, Sutinen J, et al. A meta-analysis of six placebo-controlled trials of thiazolidinedione therapy for HIV lipoatrophy. HIV Clin Trials. 2010;11(1):39–50. doi: 10.1310/hct1101-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Slama L, Lanoy E, Valantin MA, Bastard JP, Chermak A, Boutekatjirt A, et al. Effect of pioglitazone on HIV-1-related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113) Antivir Ther. 2008;13(1):67–76. [PubMed] [Google Scholar]
- 159.Mutimura E, Anastos K, Zheng Lin, Cohen M, Binagwaho A, Kotler DP, et al. Effect of HIV infection on body composition and fat distribution in Rwandan women. J Int Assoc Physicians AIDS Care (Chic) 2010;9(3):173–8. doi: 10.1177/1545109710366472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 161.Saghayam S, Chaguturu SK, Kumarasamy N, Solomon S, Mayer KH, Wanke C, et al. Lipoatrophy is the predominant presentation of HIV-associated lipodystrophy in southern India. Clin Infect Dis. 2004;38(11):1646–7. doi: 10.1086/392516. [DOI] [PubMed] [Google Scholar]
- 162.Tsuda LC, Silva MM, Machado AA, Fernandes AP. Body changes: antiretroviral therapy and lipodystrophy syndrome in people living with HIV/aids. Rev Lat Am Enfermagem. 2012;20(5):847–53. doi: 10.1590/s0104-11692012000500005. [DOI] [PubMed] [Google Scholar]
- 163.Heath KV, Hogg RS, Singer J, Chan KJ, O'Shaughnessy MV, Montaner JS, et al. Antiretroviral treatment patterns and incident HIV-associated morphologic and lipid abnormalities in a population-based chort. J Acquir Immune Defic Syndr. 2002;30(4):440–7. doi: 10.1097/00042560-200208010-00010. [DOI] [PubMed] [Google Scholar]
- 164.Joly V, Flandre P, Meiffredy V, Leturque N, Harel M, Aboulker JP, et al. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2002;16(18):2447–54. doi: 10.1097/00002030-200212060-00010. [DOI] [PubMed] [Google Scholar]
- 165.Kampira E, Kumwenda J, Van Oosterhout JJ, Dandara C. Mitochondrial subhaplogroups and differential risk of stavudine-induced lipodystrophy in Malawian HIV/AIDS patients. Pharmacogenomics. 2013;14(16):1999–2004. doi: 10.2217/pgs.13.188. [DOI] [PubMed] [Google Scholar]
- 166.Spire B, Carrieri P, Sopha P, Protopopescu C, Prak N, Quillet C, et al. Adherence to antiretroviral therapy in patients enrolled in a comprehensive care program in Cambodia: a 24-month follow-up assessment. Antivir Ther. 2008;13(5):697–703. [PubMed] [Google Scholar]
- 167.Saghayam S, Kumarasamy N, Cecelia AJ, Solomon S, Mayer K, Wanke C, et al. Weight and body shape changes in a treatment-naive population after 6 months of nevirapine-based generic highly active antiretroviral therapy in South India. Clin Infect Dis. 2007;44(2):295–300. doi: 10.1086/510491. [DOI] [PubMed] [Google Scholar]
- 168.Bussmann H, Wester CW, Thomas A, Novitsky V, Okezie R, Muzenda T, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C-infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009;51(1):37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Desclaux A, Boye S. Perceptions of lipodystrophy among PLHIV after 10 years of antiretroviral therapy in Senegal. Bull Soc Pathol Exot. 2014;107(4):246–51. doi: 10.1007/s13149-014-0357-6. [DOI] [PubMed] [Google Scholar]
- 170.Goedecke JH, Micklesfield LK, Levitt NS, Lambert EV, West S, Maartens G, et al. Effect of different antiretroviral drug regimens on body fat distribution of HIV-infected South African women. AIDS Res Hum Retroviruses. 2013;29(3):557–63. doi: 10.1089/aid.2012.0252. [DOI] [PubMed] [Google Scholar]
- 171.Sinxadi PZ, Dave JA, Samuels DC, Heckmann JM, Maartens G, Levitt NS, et al. Mitochondrial genomics and antiretroviral therapy-associated metabolic complications in HIV-infected Black South Africans: a pilot study. AIDS Res Hum Retroviruses. 2013;29(7):1031–9. doi: 10.1089/aid.2012.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zinn RJ, Serrurier C, Takuva S, Sanne I, Menezes CN. HIV-associated lipodystrophy in South Africa: the impact on the patient and the impact on the plastic surgeon. J Plast Reconstr Aesthet Surg. 2013;66(6):839–44. doi: 10.1016/j.bjps.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 173.Azzoni L, Crowther NJ, Firnhaber C, Foulkes AS, Yin X, Glencross D, et al. Association between HIV replication and serum leptin levels: an observational study of a cohort of HIV-1-infected South African women. J Int AIDS Soc. 2010;13:33. doi: 10.1186/1758-2652-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sathekge M, Maes A, Kgomo M, Stolz A, Ankrah A, Van de Wiele C, et al. Evaluation of glucose uptake by skeletal muscle tissue and subcutaneous fat in HIV-infected patients with and without lipodystrophy using FDG-PET. Nucl Med Commun. 2010;31(4):311–4. doi: 10.1097/MNM.0b013e3283359058. [DOI] [PubMed] [Google Scholar]
- 175.Li T, Guo F, Li Y, Zhang C, Han Y, Lye W, et al. An antiretroviral regimen containing 6 months of stavudine followed by long-term zidovudine for first-line HIV therapy is optimal in resource-limited settings: a prospective, multicenter study in China. Chin Med J (Engl) 2014;127(1):59–65. [PubMed] [Google Scholar]
- 176.Signorini DJ, Oliveira Netto AM, Monteiro MC, Signorini DH, Codeço CT, Bastos FI, et al. Differences in body fat distribution assessed by ultrasonography in patients receiving antiretroviral drugs. Rev Assoc Med Bras. 2012;58(2):197–203. [PubMed] [Google Scholar]
- 177.Anjos EM, Pfrimer K, Machado AA, Cunha SF, Salomão RG, Monteiro JP, et al. Nutritional and metabolic status of HIV-positive patients with lipodystrophy during one year of follow-up. Clinics (Sao Paulo) 2011;66(3):407–10. doi: 10.1590/S1807-59322011000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Diehl LA, Fabris BA, Barbosa DS, De Faria EC, Wiechmann SL, Carrilho AJ. Metformin increases HDL3-cholesterol and decreases subcutaneous truncal fat in nondiabetic patients with HIV-associated lipodystrophy. AIDS Patient Care STDS. 2008;22(10):779–86. doi: 10.1089/apc.2008.0012. [DOI] [PubMed] [Google Scholar]
- 179.Tungsiripat M, El-Bejjani D, Rizk N, Hu B, Ross AC, Walker UA, et al. Changes in inflammation, oxidative stress, mitochondrial DNA content after Rosiglitazone in HIV Lipoatrophy. J AIDS Clin Res. 2012;3(8):174. doi: 10.4172/2155-6113.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Larrañaga GF, Bocassi AR, Puga LM, Alonso BS, Benetucci JA. Endothelial markers and HIV infection in the era of highly active antiretroviral treatment. Thromb Res. 2003;110(2–3):93–8. doi: 10.1016/s0049-3848(03)00291-3. [DOI] [PubMed] [Google Scholar]
- 181.Arruda Júnior ER, Lacerda HR, Moura LC, Albuquerque MeF, Miranda Filho DeB, Diniz GT, et al. Profile of patients with hypertension included in a cohort with HIV/AIDS in the state of Pernambuco, Brazil. Arq Bras Cardiol. 2010;95(5):640–7. doi: 10.1590/s0066-782x2010005000138. [DOI] [PubMed] [Google Scholar]
- 182.Signorini DJ, Monteiro MC, Andrade MeF, Signorini DH, Eyer-Silva WeA. What should we know about metabolic syndrome and lipodystrophy in AIDS? Rev Assoc Med Bras. 2012;58(1):70–5. [PubMed] [Google Scholar]
- 183.Ceccato MG, Bonolo PF, Souza Neto AI, Araújo FS, Freitas MI. Antiretroviral therapy-associated dyslipidemia in patients from a reference center in Brazil. Braz J Med Biol Res. 2011;44(11):1177–83. doi: 10.1590/s0100-879x2011007500129. [DOI] [PubMed] [Google Scholar]
- 184.Gasparotto AS, Sprinz E, Lazzaretti RK, Kuhmmer R, Silveira JM, Basso RP, et al. Genetic polymorphisms in estrogen receptors and sexual dimorphism in fat redistribution in HIV-infected patients on HAART. AIDS. 2012;26(1):19–26. doi: 10.1097/QAD.0b013e32834db3ac. [DOI] [PubMed] [Google Scholar]
- 185.Castelar L, Silva MM, Castelli EC, Deghaide NH, Mendes-Junior CT, Machado AA, et al. Interleukin-18 and interferon-gamma polymorphisms in Brazilian human immunodeficiency virus-1-infected patients presenting with lipodystrophy syndrome. Tissue Antigens. 2010;76(2):126–30. doi: 10.1111/j.1399-0039.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- 186.de Carvalho EH, Miranda Filho DeB, Ximenes RA, de Albuquerque MeF, de Melo HR, Gelenske T, et al. Prevalence of hyperapolipoprotein B and associations with other cardiovascular risk factors among human immunodeficiency virus-infected patients in Pernambuco, Brazil. Metab Syndr Relat Disord. 2010;8(5):403–10. doi: 10.1089/met.2009.0092. [DOI] [PubMed] [Google Scholar]
- 187.Guimarães MM, de Oliveira AR, Penido MG, Queiroz LC, Goulart EM, Greco DB, et al. Ultrasonographic measurement of intra-abdominal fat thickness in HIV-infected patients treated or not with antiretroviral drugs and its correlation to lipid and glycemic profiles. Ann Nutr Metab. 2007;51(1):35–41. doi: 10.1159/000100819. [DOI] [PubMed] [Google Scholar]
- 188.Silva MM, Sim[otilde]es RT, Castelli EC, Mendes-Junior CT, Deghaide NH, Tsuda LC, et al. TNF microsatellite alleles may confer protection against the development of lipodystrophy syndrome in Brazilian HIV patients. Int J Immunogenet. 2010;37(5):379–85. doi: 10.1111/j.1744-313X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 189.Ronchini KR, Duarte AJ, Casseb JS, Gidlund M. Cardiovascular complications and increased levels of circulating modified low density lipoprotein in HIV patients and patients with lipodystrophy. Braz J Med Biol Res. 2004;37(1):119–22. doi: 10.1590/s0100-879x2004000100016. [DOI] [PubMed] [Google Scholar]
- 190.Vassimon HS, de Paula FJ, Machado AA, Monteiro JP, Jordão AA. Hypermetabolism and altered substrate oxidation in HIV-infected patients with lipodystrophy. Nutrition. 2012;28(9):912–6. doi: 10.1016/j.nut.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 191.Justina LB, Luiz MC, Maurici R, Schuelter-Trevisol F. Prevalence and factors associated with lipodystrophy in AIDS patients. Rev Soc Bras Med Trop. 2014;47(1):30–7. doi: 10.1590/0037-8682-0240-2013. [DOI] [PubMed] [Google Scholar]
- 192.Menezes de Pádua CA, Moura CS. Availability of data on adverse reactions to antiretroviral drugs in medical charts according to the naranjo algorithm: an example of a Brazilian historical cohort. Clin Drug Investig. 2014;34(6):395–402. doi: 10.1007/s40261-014-0187-0. [DOI] [PubMed] [Google Scholar]