Abstract
Chronic alcohol use can result in many pathological effects including alcoholic liver disease (ALD). While alcohol is necessary for the development of ALD, only 20–30% of alcoholics develop alcoholic steatohepatitis (ASH) with progressive liver disease leading to cirrhosis and liver failure (ALD). This suggests that while chronic alcohol consumption is necessary it is not sufficient to induce clinically relevant liver damage in the absence of a secondary risk factor. Studies in rodent models and alcoholic patients show that increased intestinal permeability to microbial products like endotoxin play a critical role in promoting liver inflammation in ALD pathogenesis. Therefore identifying mechanisms of alcohol-induced intestinal permeability is important in identifying mechanisms of ALD and for designing new avenues for therapy. Cyp2e1 is a cytochrome P450 enzyme that metabolizes alcohol has been shown to be upregulated by chronic alcohol use and to be a major source of oxidative stress and liver injury in alcoholics and in animal and in vitro models of chronic alcohol use. Because Cyp2e1 is also expressed in the intestine and is upregulated by chronic alcohol use, we hypothesized it could play a role in alcohol-induced intestinal hyperpermeability. Our in vitro studies with intestinal Caco-2 cells and in mice fed alcohol showed that circadian clock proteins CLOCK and PER2 are required for alcohol-induced permeability. We also showed that alcohol increases Cyp2e1 protein and activity but not mRNA in Caco-2 cells and that an inhibitor of oxidative stress or siRNA knockdown of Cyp2e1 prevents the increase in CLOCK or PER2 proteins and prevents alcohol-induced hyperpermeability. With our collaborators we have also shown that Cyp2e1 knockout mice are resistant to alcohol-induced gut leakiness and liver inflammation. Taken together our data support a novel Cyp2e1-circadian clock protein mechanism for alcohol-induced gut leakiness that could provide new avenues for therapy of ALD.
Keywords: Cyp2e1, Alcohol, Circadian rhythms, Intestinal permeability, Alcoholic steatohepatitis
Highlights
-
•
Mechanisms for alcohol-induced gut leakiness are not fully established.
-
•
Cyp2e1 is a P450 enzyme that causes oxidative stress and liver injury due to alcohol.
-
•
Intestinal Cyp2e1 stimulates alcohol-induced gut leakiness and liver inflammation in vitro and in Cyp2e1 knockout mice.
-
•
Alcohol-Cyp2e1 oxidative stress upregulation of intestinal clock proteins Clock and Per2 promotes gut leakiness.
-
•
These studies may provide new avenues for therapy of alcohol-induced gut leakiness and alcoholic liver disease.
Alcohol and health
Alcohol consumption worldwide is increasing and the negative consequences of chronic alcohol misuse are a healthcare burden in the United States and worldwide[1,2]. Chronic alcohol use can result in many pathological effects including liver disease (Mandayam et al., 2004), pancreatic disease [3], neurological problems [4,5], promotion of several forms of cancer [6,7], and negative effects on immune function [8]. Perhaps the best known effect of chronic alcohol use is liver inflammation known as alcoholic steatohepatitis (ASH) which can lead to progressive alcoholic liver disease (ALD) [2,9–11]. While alcohol is necessary for the development of ALD, only 20–30% of alcoholics develop alcoholic steatohepatitis (ASH) with progressive liver disease leading to cirrhosis and liver failure (ALD) [11–13] suggesting that while chronic alcohol consumption is necessary it is not sufficient to induce clinically relevant liver damage in the absence of a secondary risk factor. Studies in alcoholic patients and animal models provide compelling evidence that gut-derived microbial factors and especially bacterial endotoxin (Lipopolysaccharide, LPS) are necessary to initiate the hepatic inflammatory cascades that are required for the development of alcohol-induced liver injury [14–16]. The primary source of endotoxin is the intestine, thus disruption in intestinal barrier integrity can permit the passage of LPS into the portal circulation resulting in endotoxemia and promoting inflammatory cascades necessary to develop liver pathology. Alcohol-induced intestinal hyperpermeability (i.e., leaky gut) and/or dysbiosis of intestinal bacteria [17,18] may both contribute to the pathological effects of alcohol [19–22]. Alcohol-induced gut leakiness is particularly relevant in the etiology of ASH and progressive ALD. Alcohol universally promotes increased permeability in intestinal epithelial cells in vitro; however, alcohol consumption induces intestinal hyperpermeability and endotoxemia in only a subset of human alcoholics [21,22]. Many studies have investigated alcohol-induced effects on the intestinal barrier using in vitro, in vivo animal models, and clinical data [23]; however, these findings suggest that, especially in vivo, the mechanisms that cause alcohol-induced intestinal hyperpermeability are not fully established.
Alcohol metabolism, intestinal permeability, and Cyp2e1
Many of the deleterious effects of alcohol are thought to be the consequence of alcohol metabolism by-products[24], thus it is possible that individual variability in alcohol metabolism pathways might account for individual susceptibility to alcohol-induced gut leakiness. Several recent studies have shown that alcohol and/or alcohol metabolism products such as acetaldehyde [25] (produced as a by-product of alcohol dehydrogenase, ADH) or stimulation of inducible nitric oxide synthase (iNOS) [26–28] disrupt intestinal barrier integrity via several mechanisms especially alterations in tight junction proteins [29–31]. Intestinal epithelial tight junctions are the major regulators of intestinal permeability [32,33] thus altering tight junction protein expression and/or function will certainly impact intestinal barrier function. However, ADH-induced effects on tight junction proteins are unlikely to fully explain alcohol-induced intestinal hyperpermeability since Caco-2 intestinal epithelial cells, a widely used cell line for alcohol studies [23], do not express ADH [34] and yet low dose (0.2%) alcohol exposure results in marked disruption of Caco-2 cell monolayer barrier function [26,35].
Alcohol metabolism by Cyp2e1 may be contributing to alcohol-induced effects on the intestine. It is well-established that: (1) Cyp2e1 is expressed in Caco-2 cells as well as in both the small intestine and colon tissue in rodents and humans [36–38]; (2) Cyp2e1 protein is induced in intestinal tissue by chronic alcohol feeding in rodents and humans[36,37,39,40] mainly through post-translational mechanisms rather than mRNA expression [41]; (3) Cyp2e1 is one of the most highly expressed of the CYP450 isoforms in the human intestine [38,42]; and (4) Cyp2e1 metabolism of alcohol produces oxidative stress products [24,36,43] that can contribute to alcohol-induced tissue damage including reactive oxygen species/reactive nitrogen species (ROS/RNS) that could mediate disruption of intestinal epithelial permeability directly [23,26–28]. Oxidative stress also promotes cell signaling, cellular damage, and changes to proteins regulating permeability including tight junction proteins [23,27,28]. Cyp2e1-mediated alcohol metabolism results in the production of reactive oxygen species (oxidative stress) and other damaging products [43–45]. Thus, Cyp2e1-mediated products of alcohol could be the key mechanism of alcohol-induced gut leakiness and liver injury. Indeed, we have recently shown that Cyp2e1 protein and activity are increased in alcohol-treated Caco-2 cell monolayers and alcohol-fed rodents with gut leakiness [46]. Moreover, knocking down Cyp2e1 prevented alcohol-induced disruption of monolayer barrier integrity [46]. Also, alcohol-fed Cyp2e1 knockout mice did not develop endotoxemia and liver inflammation (discussed below) [47]. Recent findings by Seitz and Wang provide an intriguing possibility for a role of intestinal Cyp2e1 as a risk factor for developing ALD. They showed that small intestine and colonic Cyp2e1 is induced by alcohol with individual variability in humans which may be contributing to the subset of individuals that demonstrate alcohol-induced intestinal hyperpermeability and liver pathology [36].
Alcohol, gut leakiness, alcoholic steatohepatitis, circadian rhythms and oxidative stress
Circadian rhythms are 24 h biological patterns of function that synchronize humans and other organisms with the daily environmental patterns of light and dark and feeding [48–50] and are essential for the regulation of a wide range of metabolic and biological pathways. These rhythms are controlled by an approximately 24 h cyclic pattern of circadian clock gene expression (including Per1-3, Clock, etc.) that constitutes the molecular circadian clock and that this molecular clock is expressed in nearly every cell in the body [51,52]. In addition, each tissue has 5–20% of the genome directly controlled by circadian mechanisms and specific genes that are under this circadian regulation are unique for each tissue [53,54]. Recent studies have shown that circadian mechanisms regulate a significant number of intestinal genes and GI functions [55–58]. Disruption of circadian rhythms (e.g., shift work) has been implicated as a mechanism for a variety of inflammation-mediated disorders such as metabolic syndrome, obesity, cardiovascular disease, cancer, and intestinal disorders [57,59–62]. Our laboratory has demonstrated that disruption of circadian rhythms by altering the light:dark cycle in mice makes the intestine susceptible to damage by injurious agents [63,64]. We have shown that mice subjected to either environmental (repeated light/dark phase shifting) or genetic (Clock∆19 mutation) circadian rhythm disruption exhibit increased intestinal permeability and that circadian rhythm disruption markedly exacerbates alcohol-induced gut leakiness and alcoholic liver disease [65]. Therefore, circadian rhythm disruption may be one mechanism to promote alcohol-induced intestinal barrier dysfunction and liver pathology accounting for a subset of alcoholics demonstrating these features.
Several studies have demonstrated that alcohol disrupts circadian rhythms at the behavioral level [66–68] as well as at the level of gene transcription in the brain central clock in the SCN [69,70]. Other studies have shown that the liver demonstrates circadian rhythmicity on functional and molecular levels and alcohol-induced steatohepatitis (ASH) occurs concurrently with disruption of the circadian rhythms in the liver [71,72]. Thus, alcohol-induced circadian rhythm disruption in the intestine and/or liver may account for alcohol effects on intestinal barrier dysfunction and liver pathology. One mechanism accounting for alcohol-induced effects on the molecular circadian clock may be oxidative stress [73,74] as the molecular circadian clock has several elements that are redox sensitive. One recent review states that the circadian system probably evolved in response to the “Great Oxidation Event” in response to oxidative stress 2.5 billion years ago [75].
One mechanism for alcohol-induced gut leakiness may involve a circadian mechanism. Using Caco-2 intestinal epithelial cells [76] we determined that transepithelial resistance (TER) and flux of the fluorescent dye FSA [26,77,78] are decreased and increased, respectively, in response to Caco-2 cell alcohol exposure (0.1–0.5%) with alcohol causing dose- and time-dependent increases in barrier dysfunction [35]. When protein levels of components of the molecular circadian clock were measured, both CLOCK and PER2 were increased as early as 30 min after alcohol exposure and further increased until 240 min (237% and 315%, respectively) [35]. Thus, at a time when monolayers are exhibiting alcohol-induced hyperpermeability, the levels of these molecular circadian clock proteins are abnormally elevated. To further investigate the causal impact of these changes in the circadian proteins, siRNA was used to knock down gene expression of Clock and Per2 in Caco-2 cells and this knockdown prevented alcohol-induced effects on barrier integrity [35]. These data support that alcohol-induced increases in CLOCK and PER2 proteins is required for alcohol stimulation of intestinal hyperpermeability in this model. To determine if these effects were also observed in vivo intestinal tissue from rats chronically administered alcohol (6 g/kg/day, 10 weeks) and that exhibited intestinal dysbiosis, intestinal hyperpermeability, endotoxemia, and alcoholic steatohepatitis [18,79] were evaluated for levels of circadian proteins. Evaluation of PER2 protein levels in the duodenum and colon intestinal tissue from these alcohol-fed and control rats show a significant alcohol-induced elevation in intestinal PER2 protein [35]. Thus, both the Caco-2 and in vivo data support that alcohol stimulation of the circadian clock protein PER2 occurs at a time when intestinal permeability is elevated and may be an important factor in alcohol-induced gut leakiness.
Alcohol, Cyp2e1, oxidative stress, clock genes and intestinal hyperpermeability and ASH
Considerable evidence supports a role for liver Cyp2e1-mediated alcohol metabolism in the pathogenesis of alcoholic liver disease, specifically via the production of oxidative stress [43,80–85]. Oxidative stress can impact the intestinal barrier function in a variety of ways but one potentially important pathway is via influencing redox sensitive cellular signaling mechanisms [23]. A number of studies have shown that oxidative stress stimulates the transcription of clock genes that contain a redox sensitive PAS domain, including Clock and Per2 [73,75,86–89]. Oxidative stress generated as a consequence of alcohol metabolism by intestinal Cyp2e1 may be the driving force increasing CLOCK and PER2 resulting in intestinal hyperpermeability. Using Caco-2 cells exposed to alcohol (0.2%, 4 h) we determined that Cyp2e1 mRNA was not increased by alcohol but that both protein expression (93%) as well as enzymatic activity (69%) [90] were significantly increased [46]. We also measured Cyp2e1 protein and mRNA in colon tissue from BL/6 mice fed a chronic alcohol diet and again found no increase in Cyp2e1 mRNA but a 73% increase in protein. These data are consistent with previous reports of Cyp2e1 following alcohol exposure in liver cells [41] and chronically alcohol-fed rodents [37]. These results, while intriguing, do not support a causal role of Cyp2e1 in mediating alcohol-induced intestinal hyperpermeability. To establish a causal role, we showed that knocking down Cyp2e1 in Caco-2 cells via siRNA blocked alcohol-induced increases in CLOCK and PER2 proteins and prevented alcohol stimulated hyperpermeability [46]. To determine the relative contribution of oxidative stress in these effects, Caco-2 cells were pretreated with the anti-oxidantN-acetylcysteine (NAC), which blocked the alcohol-induced increase in CLOCK and PER2 proteins as well as intestinal hyperpermeability. Similarly, the oxidant H2O2 mimicked the effects of alcohol on CLOCK and PER2 proteins and this effect could also be blocked with NAC [46]. These data are in agreement with data from other labs showing H2O2 stimulates expression of PER2 protein [87,88]. Several studies have shown that changes in oxidative stress can regulate circadian clock gene proteins. Those studies have shown that the function of several circadian genes, including Clock and Per2, are sensitive to changes in the cellular redox state resulting from cellular metabolism [73,89]. Experiments with red blood cells show that redox state alone in the absence of transcription can regulate circadian rhythms [91] and cyanobacteria [92].
Together, our data support a novel model in which oxidative stress resulting from alcohol metabolism by intestinal Cyp2e1 upregulates intestinal CLOCK and PER2 circadian proteins promoting alcohol-induced intestinal hyperpermeability [46] (Fig. 1). Indeed, Cyp2e1 KO mice fed a binge alcohol diet [47] show blunted intestinal hyperpermeability, endotoxemia, and liver steatosis and inflammation similar to when wild type mice are treated with the Cyp2e1 inhibitor chlormethiazole [47]. Taken together, these studies support that Cyp2e1 not only plays a key role in alcohol-induced liver injury but also promotes intestinal leakiness to gut microbial contents necessary for alcohol-induced liver injury. This intestinal mechanism for Cyp2e1-mediated alcohol-induced hyperpermeability may be driven by a circadian based mechanism involving increased oxidative stress burden and is summarized in Fig. 1.
Fig. 1.
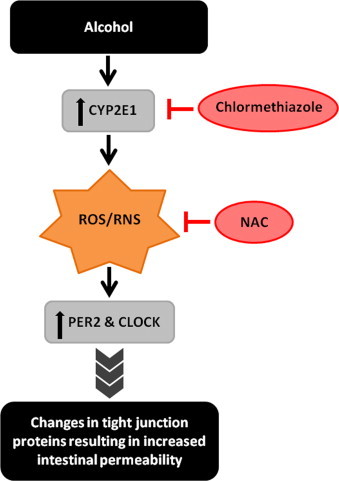
Proposed model of alcohol Cyp2e1 clock gene protein regulation of gut permeability.
Implications of these studies and future directions
Our data support a model in which alcohol metabolism by intestinal Cyp2e1 promotes intestinal hyperpermeability through a circadian clock protein-mediated mechanism. One question that arises from our studies is where does this mechanism of alcohol-induced intestinal hyperpermeability fit in with other described mechanisms for alcohol-induced gut leakiness? The simple answer is that it is too soon to tell, but we believe it is a significant contributor. Our data demonstrate significant alcohol-induced elevation of intestinal Cyp2e1 protein and function [46] as well as intestinal PER2 protein [65] in chronically alcohol fed mice. Importantly, together with our collaborators we have shown that Cyp2e1 knockout mice exhibit blunted intestinal leakiness after binge alcohol [47]. However, intestinal tissue expression of CLOCK or PER2 protein were not measured in these mice. Future studies are planned to determine the intestinal expression of these key proteins in this Cyp2e1 knockout mouse model at baseline and in response to alcohol feeding.
Our lab and others have published alternative pathways for alcohol-induced intestinal hyperpermeability and understanding how these alternative pathways may dovetail into our current model is an important next focus of our laboratory. For example, alcohol-induced oxidative stress, especially via upregulation of inducible nitric oxide synthase (iNOS) [26,28,93]. Therefore one current focus of our laboratory is in determining the relationship between Cyp2e1 and iNOS activation by alcohol. Characterizing this relationship will be a key next step to understanding alcohol effects on intestinal barrier function and gut derived endotoxin-mediated inflammation in alcohol associated diseases and how this circadian-Cyp2e1 mechanism fits together with other mechanisms of alcohol-induced gut leakiness [23].
Another key question in light of our data are how might our data explain the increased leakiness in a subset of alcoholics that develop ALD? The essential point here is that taken together our data clearly link disruption of circadian rhythms to increased gut leakiness with and without alcohol [65]. Therefore we propose that the degree of circadian disruption in an individual patient may be one critical difference that makes that subject more susceptible to alcohol-induced gut leakiness. Thus, subjects with greater circadian disruption could be more susceptible to negative alcohol-induced effects on gut leakiness. Alternatively, alcohol is known to disrupt circadian rhythms [67,69,70,94]. Our data also link alcohol stimulation itself to disruption of clock gene expression (circadian disruption) which in turn promotes gut leakiness [46]. Thus, subject to subject variability in the response of the circadian clock mechanism to alcohol could also explain differences in a subset of individuals to alcohol-induced gut leakiness. One reason for this variability for example could be individual variability in the ‘anti-oxidant capacity’ (redox state) of intestinal cells/tissue from a given individual due to diet, stress, or other factors. Another possibility very relevant to this review could be differences in individual expression of intestinal and/or liver Cyp2e1. Finally, there is the element of the effects of microbiota dysbiosis on gut leakiness. We and others have shown that chronic alcohol use is associated with dysbiosis of the intestinal microbiota and gut leakiness in rodents and humans [17,18,95]. We have also recently shown that circadian disruption promotes dysbiosis of the intestinal microbiota [96]. Therefore, differences in a subject's intestinal microbiota as a result of circadian disruption could synergize to promote increased gut leakiness in a subset of alcohol users. Identifying the contribution of each of these above possible mechanisms to result in gut leakiness and ALD in a subset of individuals will be a challenge for our studies going forward.
There are several potential therapeutic (translational) implications of the data described in this review. First, several studies suggest the use of anti-oxidants to prevent/treat alcohol-induced gut leakiness. Our studies show that the anti-oxidant NAC prevents the alcohol–Cyp2e1 mediated increase in CLOCK and PER2 that in turn promotes intestinal hyperpermeability [46,47]. Similarly, stimulation of zebrafish cells with H2O2 increases Per2 mRNA expression, an effect that is inhibited with the antioxidant catalase[87]. In addition, our data demonstrate that the Cyp2e1 inhibitor chlormethiazole prevents alcohol-induced gut leakiness in mice [46,47]. Thus, antioxidants or Cyp2e1 inhibitors might therefore be used to reduce alcohol induced gut leakiness therapeutically. A second possible therapy is the use of prebiotics such as oats or probiotics such as Lactobacillus GG because we have shown that both pre- and probiotics ameliorate intestinal oxidative stress and gut leakiness in alcohol-fed rodents [79,97,98]. One intriguing possibility is that these effects may be due in part to lowering the expression of intestinal Cyp2e1 as one study with probiotics has shown [99]. This may, in part, be the result of correcting alcohol-induced intestinal dysbiosis (and oxidative stress) as we have shown for prebiotics and Lactobacillus GG [18]. A third possible therapeutic avenue suggested by our data are greater attention to normalizing circadian disruption in the lifestyle of chronic alcohol users. Our data demonstrate that circadian mechanisms contribute to the regulation of intestinal permeability in response to alcohol whereby environmental or genetic disruption of circadian rhythms augments alcohol-induced effects on gut leakiness and liver injury [65]. Thus, perhaps normalization of sleep/wake behavior and daily routines of chronic alcohol abusers (chronotherapy) could be beneficial by minimizing the negative effects of alcohol on the gut. Such approaches have been successful with other diseases such as obesity and metabolic syndrome as well as psychiatric illness [59,62].
Studies are currently underway in our laboratory investigating mechanisms of our observed alcohol-induced effects as well as the mechanisms of potential therapeutic approaches to mitigate some of the detrimental effects of alcohol that are mediated by Cyp2e1 byproducts. These studies will help us further define the novel mechanisms by which Cyp2e1-mediated by products of alcohol metabolism promote gut leakiness and liver disease that could form the basis for new avenues of therapy.
Acknowledgments
This research was supported in part by grants AA013745 (AK), RC2AA019405 (AK), AA023417 (AK), AA020216 (AK, CBF, and RMV) and gifts from Mr. and Mrs. Larry Field (AK) and Mr. and Mrs. Silas Keehn (AK). These funding sources had no involvement in the content of this review.
References
- 1.Rehm J., Mathers C., Popova S., Thavorncharoensap M., Teerawattananon Y., Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi: 10.1016/S0140-6736(09)60746-7. 19560604 [DOI] [PubMed] [Google Scholar]
- 2.Mandayam S., Jamal M.M., Morgan T.R. Epidemiology of alcoholic liver disease. Seminars in Liver Disease. 2004;24(3):217–232. doi: 10.1055/s-2004-832936. 15349801 [DOI] [PubMed] [Google Scholar]
- 3.Vonlaufen A., Spahr L., Apte M.V., Frossard J.L. Alcoholic pancreatitis: a tale of spirits and bacteria. World Journal of Gastrointestinal Pathophysiology. 2014;5(2):82–90. doi: 10.4291/wjgp.v5.i2.82. 24891979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee S. Alcoholism and its effects on the central nervous system. Current Neurovascular Research. 2013;10(3):256–262. doi: 10.2174/15672026113109990004. 23713737 [DOI] [PubMed] [Google Scholar]
- 5.Tabakoff B., Hoffman P.L. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacology, Biochemistry, and Behavior. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. 24141171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seitz H.K., Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Reviews. Cancer. 2007;7(8):599–612. doi: 10.1038/nrc2191. 17646865 [DOI] [PubMed] [Google Scholar]
- 7.Homann N., Seitz H.K., Wang X.D., Yokoyama A., Singletary K.W., Ishii H. Mechanisms in alcohol-associated carcinogenesis. Alcoholism, Clinical and Experimental Research. 2005;29(7):1317–1320. doi: 10.1097/01.alc.0000171892.09367.6f. 16088994 [DOI] [PubMed] [Google Scholar]
- 8.Szabo G., Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcoholism, Clinical and Experimental Research. 2009;33(2):220–232. doi: 10.1111/j.1530-0277.2008.00842.x. 19053973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieber C.S. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. New England Journal of Medicine. 1988;319(25):1639–1650. doi: 10.1056/NEJM198812223192505. 3059192 [DOI] [PubMed] [Google Scholar]
- 10.Lieber C.S. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol (Fayetteville, N.Y.) 2004;34(1):9–19. doi: 10.1016/j.alcohol.2004.07.008. 15670660 [DOI] [PubMed] [Google Scholar]
- 11.Grant B.F., Dufour M.C., Harford T.C. Epidemiology of alcoholic liver disease. Seminars in Liver Disease. 1988;8(1):12–25. doi: 10.1055/s-2008-1040525. 3283941 [DOI] [PubMed] [Google Scholar]
- 12.O’Shea R.S., Dasarathy S., McCullough A.J. Alcoholic liver disease. Hepatology (Baltimore, Md.) 2010;51(1):307–328. doi: 10.1002/hep.23258. 20034030 [DOI] [PubMed] [Google Scholar]
- 13.Rao R.K., Seth A., Sheth P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;286(6):G881–G884. doi: 10.1152/ajpgi.00006.2004. 15132946 [DOI] [PubMed] [Google Scholar]
- 14.Keshavarzian A., Farhadi A., Forsyth C.B., Rangan J., Jakate S., Shaikh M., Banan A., Fields J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. Journal of Hepatology. 2009;50(3):538–547. doi: 10.1016/j.jhep.2008.10.028. 19155080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H.J., Zakhari S., Jung M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World Journal of Gastroenterology: WJG. 2010;16(11):1304–1313. doi: 10.3748/wjg.v16.i11.1304. 20238396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo G., Bala S. Alcoholic liver disease and the gut-liver axis. World Journal of Gastroenterology: WJG. 2010;16(11):1321–1329. doi: 10.3748/wjg.v16.i11.1321. 20238398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutlu E.A., Gillevet P.M., Rangwala H., Sikaroodi M., Naqvi A., Engen P.A., Kwasny M., Lau C.K., Keshavarzian A. Colonic microbiome is altered in alcoholism. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;302(9):G966–G978. doi: 10.1152/ajpgi.00380.2011. 22241860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutlu E., Keshavarzian A., Engen P., Forsyth C.B., Sikaroodi M., Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcoholism, Clinical and Experimental Research. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. 19645728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purohit V., Bode J.C., Bode C., Brenner D.A., Choudhry M.A., Hamilton F., Kang Y.J., Keshavarzian A., Rao R., Sartor R.B., Swanson C., Turner J.R. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol (Fayetteville, N.Y.) 2008;42(5):349–361. doi: 10.1016/j.alcohol.2008.03.131. 18504085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjarnason I., Peters T.J., Wise R.J. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1(8370):179–182. doi: 10.1016/s0140-6736(84)92109-3. 6141332 [DOI] [PubMed] [Google Scholar]
- 21.Bode C., Kugler V., Bode J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. Journal of Hepatology. 1987;4(1):8–14. doi: 10.1016/s0168-8278(87)80003-x. 3571935 [DOI] [PubMed] [Google Scholar]
- 22.Keshavarzian A., Holmes E.W., Patel M., Iber F., Fields J.Z., Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. American Journal of Gastroenterology. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. 9934756 [DOI] [PubMed] [Google Scholar]
- 23.Elamin E.E., Masclee A.A., Dekker J., Jonkers D.M. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutrition Reviews. 2013;71(7):483–499. doi: 10.1111/nure.12027. 23815146 [DOI] [PubMed] [Google Scholar]
- 24.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Research and Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2006;29(4):245–254. 17718403 [PMC free article] [PubMed] [Google Scholar]
- 25.Rao R.K. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcoholism, Clinical and Experimental Research. 1998;22(8):1724–1730. 9835287 [PubMed] [Google Scholar]
- 26.Forsyth C.B., Tang Y., Shaikh M., Zhang L., Keshavarzian A. Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcoholism, Clinical and Experimental Research. 2011;35(9):1635–1643. doi: 10.1111/j.1530-0277.2011.01510.x. 21535025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banan A., Choudhary S., Zhang Y., Fields J.Z., Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. Journal of Pharmacology and Experimental Therapeutics. 1999;291(3):1075–1085. 10565827 [PubMed] [Google Scholar]
- 28.Banan A., Fields J.Z., Decker H., Zhang Y., Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):997–1008. 10945852 [PubMed] [Google Scholar]
- 29.Rao R.K. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods in Molecular Biology (Clifton, N.J.) 2008;447:171–183. doi: 10.1007/978-1-59745-242-7_13. 18369919 [DOI] [PubMed] [Google Scholar]
- 30.Seth A., Basuroy S., Sheth P., Rao R.K. l-glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;287(3):G510–G517. doi: 10.1152/ajpgi.00058.2004. 15331350 [DOI] [PubMed] [Google Scholar]
- 31.Ferrier L., Bérard F., Debrauwer L., Chabo C., Langella P., Buéno L., Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. American Journal of Pathology. 2006;168(4):1148–1154. doi: 10.2353/ajpath.2006.050617. 16565490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farhadi A., Banan A., Fields J., Keshavarzian A. Intestinal barrier: an interface between health and disease. Journal of Gastroenterology and Hepatology. 2003;18(5):479–497. doi: 10.1046/j.1440-1746.2003.03032.x. 12702039 [DOI] [PubMed] [Google Scholar]
- 33.Turner J.R. Intestinal mucosal barrier function in health and disease. Nature Reviews. Immunology. 2009;9(11):799–809. doi: 10.1038/nri2653. 19855405 [DOI] [PubMed] [Google Scholar]
- 34.Koivisto T., Salaspuro M. Effects of acetaldehyde on brush border enzyme activities in human colon adenocarcinoma cell line Caco-2. Alcoholism, Clinical and Experimental Research. 1997;21(9):1599–1605. 9438518 [PubMed] [Google Scholar]
- 35.Swanson G., Forsyth C.B., Tang Y., Shaikh M., Zhang L., Turek F.W., Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcoholism, Clinical and Experimental Research. 2011;35(7):1305–1314. doi: 10.1111/j.1530-0277.2011.01466.x. 21463335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seitz H.K., Wang X.D. The role of cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Sub-Cellular Biochemistry. 2013;67:131–143. doi: 10.1007/978-94-007-5881-0_3. 23400919 [DOI] [PubMed] [Google Scholar]
- 37.Roberts B.J., Shoaf S.E., Jeong K.S., Song B.J. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochemical and Biophysical Research Communicators. 1994;205:1064–1071. doi: 10.1006/bbrc.1994.2774. 7802633 [DOI] [PubMed] [Google Scholar]
- 38.Bergheim I., Bode C., Parlesak A. Distribution of cytochrome P450 2C, 2E1, 3A4, and 3A5 in human colon mucosa. BMC Clinical Pharmacology. 2005;5:4. doi: 10.1186/1472-6904-5-4. 16253141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hakkak R., Korourian S., Ronis M.J., Ingelman-Sundberg M., Badger T.M. Effects of diet and ethanol on the expression and localization of cytochromes P450 2E1 and P450 2C7 in the colon of male rats. Biochemical Pharmacologist. 1996;51:61–69. doi: 10.1016/0006-2952(95)02154-x. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu M., Lasker J.M., Tsutsumi M., Lieber C.S. Immunohistochemical localization of ethanol-inducible P450IIE1 in the rat alimentary tract. Gastroenterology. 1990;99(4):1044–1053. doi: 10.1016/0016-5085(90)90625-b. 2203661 [DOI] [PubMed] [Google Scholar]
- 41.Roberts B.J., Song B.J., Soh Y., Park S.S., Shoaf S.E. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. Journal of Biological Chemistry. 1995;270(50):29632–29635. doi: 10.1074/jbc.270.50.29632. 8530344 [DOI] [PubMed] [Google Scholar]
- 42.Thörn M., Finnström N., Lundgren S., Rane A., Lööf L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. British Journal of Clinical Pharmacology. 2005;60(1):54–60. doi: 10.1111/j.1365-2125.2005.02389.x. 15963094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Y., Cederbaum A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radical Biology and Medicine. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. 18078827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Y., Cederbaum A.I. CYP2E1 potentiation of LPS and TNFalpha-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes and Nutrition. 2010;5:149–167. doi: 10.1007/s12263-009-0150-5. 19798529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieber C.S. Cytochrome P-4502E1: its physiological and pathological role. Physiological Reviews. 1997;77(2):517–544. doi: 10.1152/physrev.1997.77.2.517. 9114822 [DOI] [PubMed] [Google Scholar]
- 46.Forsyth C.B., Voigt R.M., Shaikh M., Tang Y., Cederbaum A.I., Turek F.W., Keshavarzian A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2013;305(2):G185–G195. doi: 10.1152/ajpgi.00354.2012. 23660503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelmegeed M.A., Banerjee A., Jang S., Yoo S.H., Yun J.W., Gonzalez F.J., Keshavarzian A., Song B.J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radical Biology and Medicine. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. 24064383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hastings M.H., Reddy A.B., Maywood E.S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature Reviews. Neuroscience. 2003;4(8):649–661. doi: 10.1038/nrn1177. 12894240 [DOI] [PubMed] [Google Scholar]
- 49.Reppert S.M., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. 11181971 [DOI] [PubMed] [Google Scholar]
- 50.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. 12198538 [DOI] [PubMed] [Google Scholar]
- 51.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annual Review of Neuroscience. 2012;35(1):445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunlap J.C. Molecular bases for circadian clocks. Cell. 1999;96(2):271–290. doi: 10.1016/s0092-8674(00)80566-8. 9988221 [DOI] [PubMed] [Google Scholar]
- 53.Bozek K., Relógio A., Kielbasa S.M., Heine M., Dame C., Kramer A., Herzel H. Regulation of clock-controlled genes in mammals. PloS One. 2009;4(3):e4882. doi: 10.1371/journal.pone.0004882. 19287494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M., Schultz P.G., Kay S.A., Takahashi J.S., Hogenesch J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. 12015981 [DOI] [PubMed] [Google Scholar]
- 55.Scheving L.A. Biological clocks and the digestive system. Gastroenterology. 2000;119(2):536–549. doi: 10.1053/gast.2000.9305. 10930389 [DOI] [PubMed] [Google Scholar]
- 56.Polidarová L., Sládek M., Soták M., Pácha J., Sumová A. Hepatic, duodenal, and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chronobiology International. 2011;28(3):204–215. doi: 10.3109/07420528.2010.548615. 21452916 [DOI] [PubMed] [Google Scholar]
- 57.Hoogerwerf W.A. Role of biological rhythms in gastrointestinal health and disease. Reviews in Endocrine and Metabolic Disorders. 2009;10(4):293–300. doi: 10.1007/s11154-009-9119-3. 19798581 [DOI] [PubMed] [Google Scholar]
- 58.Hoogerwerf W.A., Hellmich H.L., Cornélissen G., Halberg F., Shahinian V.B., Bostwick J., Savidge T.C., Cassone V.M. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133(4):1250–1260. doi: 10.1053/j.gastro.2007.07.009. 17919497 [DOI] [PubMed] [Google Scholar]
- 59.Golombek D.A., Casiraghi L.P., Agostino P.V., Paladino N., Duhart J.M., Plano S.A., Chiesa J.J. The times they're a-changing: effects of circadian desynchronization on physiology and disease. Journal of Physiology, Paris. 2013;107:310–322. doi: 10.1016/j.jphysparis.2013.03.007. 23545147 [DOI] [PubMed] [Google Scholar]
- 60.Penev P.D., Kolker D.E., Zee P.C., Turek F.W. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. American Journal of Physiology. 1998;275(6 Pt 2):H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. 9843836 [DOI] [PubMed] [Google Scholar]
- 61.Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D.R., Eckel R.H., Takahashi J.S., Bass J. Obesity and metabolic syndrome in circadian clock mutant mice. Science (New York, N.Y.) 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. 15845877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy A.B., O’Neill J.S. Healthy clocks, healthy body, healthy mind. Trends in Cell Biology. 2010;20(1):36–44. doi: 10.1016/j.tcb.2009.10.005. 19926479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang Y., Preuss F., Turek F.W., Jakate S., Keshavarzian A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Medicine. 2009;10(6):597–603. doi: 10.1016/j.sleep.2008.12.009. 19403332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Preuss F., Tang Y., Laposky A.D., Arble D., Keshavarzian A., Turek F.W. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;295(6):R2034–R2040. doi: 10.1152/ajpregu.00118.2008. 18843092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Summa K.C., Voigt R.M., Forsyth C.B., Shaikh M., Cavanaugh K., Tang Y., Vitaterna M.H., Song S., Turek F.W., Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and Promotes alcohol-induced hepatic pathology and inflammation. PloS One. 2013;8(6):e67102. doi: 10.1371/journal.pone.0067102. 23825629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruby C.L., Brager A.J., DePaul M.A., Prosser R.A., Glass J.D. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2009;297(3):R729–R737. doi: 10.1152/ajpregu.00268.2009. 19553498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenwasser A.M., Fecteau M.E., Logan R.W. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiology and Behavior. 2005;84(4):537–542. doi: 10.1016/j.physbeh.2005.01.016. 15811388 [DOI] [PubMed] [Google Scholar]
- 68.Seggio J.A., Fixaris M.C., Reed J.D., Logan R.W., Rosenwasser A.M. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. Journal of Biological Rhythms. 2009;24(4):304–312. doi: 10.1177/0748730409338449. 19625732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spanagel R., Rosenwasser A.M., Schumann G., Sarkar D.K. Alcohol consumption and the body's biological clock. Alcoholism, Clinical and Experimental Research. 2005;29(8):1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. 16156052 [DOI] [PubMed] [Google Scholar]
- 70.Chen C.P., Kuhn P., Advis J.P., Sarkar D.K. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. Journal of Neurochemistry. 2004;88(6):1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. 15009656 [DOI] [PubMed] [Google Scholar]
- 71.Filiano A.N., Millender-Swain T., Johnson R., Jr., Young M.E., Gamble K.L., Bailey S.M. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PloS One. 2013;8(8):e71684. doi: 10.1371/journal.pone.0071684. 23951220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou P., Ross R.A., Pywell C.M., Liangpunsakul S., Duffield G.E. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Scientific Reports. 2014;4:3725. doi: 10.1038/srep03725. 24430730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rutter J., Reick M., McKnight S.L. Metabolism and the control of circadian rhythms. Annual Review of Biochemistry. 2002;71(1):307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 74.Wilking M., Ndiaye M., Mukhtar H., Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxidants and Redox Signaling. 2013;19:192–208. doi: 10.1089/ars.2012.4889. 23198849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loudon A.S. Circadian biology: a 2.5 billion year old clock. Current Biology. 2012;22(14):R570–R571. doi: 10.1016/j.cub.2012.06.023. 22835791 [DOI] [PubMed] [Google Scholar]
- 76.Hidalgo I.J., Raub T.J., Borchardt R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96(3):736–749. 2914637 [PubMed] [Google Scholar]
- 77.Forsyth C.B., Banan A., Farhadi A., Fields J.Z., Tang Y., Shaikh M., Zhang L.J., Engen P.A., Keshavarzian A. Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. Journal of Pharmacology and Experimental Therapeutics. 2007;321(1):84–97. doi: 10.1124/jpet.106.113019. 17220428 [DOI] [PubMed] [Google Scholar]
- 78.Sanders S.E., Madara J.L., McGuirk D.K., Gelman D.S., Colgan S.P. Assessment of inflammatory events in epithelial permeability: a rapid screening method using fluorescein dextrans. Epithelial Cell Biology. 1995;4(1):25–34. 8563793 [PubMed] [Google Scholar]
- 79.Keshavarzian A., Choudhary S., Holmes E.W., Yong S., Banan A., Jakate S., Fields J.Z. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. Journal of Pharmacology and Experimental Therapeutics. 2001;299(2):442–448. 11602653 [PubMed] [Google Scholar]
- 80.Lu Y., Wu D., Wang X., Ward S.C., Cederbaum A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radical Biology and Medicine. 2010;49(9):1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. 20692331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gouillon Z., Lucas D., Li J., Hagbjork A.L., French B.A., Fu P., Fang C., Ingelman-Sundberg M., Donohue T.M., Jr., French S.W. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 2000;224(4):302–308. doi: 10.1046/j.1525-1373.2000.22435.x. 10964266 [DOI] [PubMed] [Google Scholar]
- 82.Morimoto M., Hagbjörk A.L., Wan Y.J., Fu P.C., Clot P., Albano E., Ingelman-Sundberg M., French S.W. Modulation of experimental alcohol-induced liver disease by cytochrome P450 2E1 inhibitors. Hepatology (Baltimore, Md.) 1995;21(6):1610–1617. 7768506 [PubMed] [Google Scholar]
- 83.Morimoto M., Hagbjörk A.L., Nanji A.A., Ingelman-Sundberg M., Lindros K.O., Fu P.C., Albano E., French S.W. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol (Fayetteville, N.Y.) 1993;10(6):459–464. doi: 10.1016/0741-8329(93)90065-v. 8123200 [DOI] [PubMed] [Google Scholar]
- 84.Lieber C.S. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998)—a review. Alcoholism, Clinical and Experimental Research. 1999;23(6):991–1007. 10397283 [PubMed] [Google Scholar]
- 85.Lieber C.S., Rubin E., DeCarli L.M. Hepatic microsomal ethanol oxidizing system (MEOS): differentiation from alcohol dehydrogenase and NADPH oxidase. Biochemical and Biophysical Research Communications. 1970;40(4):858–865. doi: 10.1016/0006-291x(70)90982-4. 4395603 [DOI] [PubMed] [Google Scholar]
- 86.Taylor B.L., Zhulin I.B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiology and Molecular Biology Reviews. 1999;63(2):479–506. doi: 10.1128/mmbr.63.2.479-506.1999. 10357859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirayama J., Cho S., Sassone-Corsi P. Circadian control by the reduction/oxidation pathway: catalase represses light-dependent clock gene expression in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(40):15747–15752. doi: 10.1073/pnas.0705614104. 17898172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamaru T., Hattori M., Ninomiya Y., Kawamura G., Varès G., Honda K., Mishra D.P., Wang B., Benjamin I., Sassone-Corsi P., Ozawa T., Takamatsu K. ROS stress resets circadian clocks to coordinate pro-survival signals. PloS One. 2013;8(12):e82006. doi: 10.1371/journal.pone.0082006. 24312621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rutter J., Reick M., Wu L.C., McKnight S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science (New York, N.Y.) 2001;293(5529):510–514. doi: 10.1126/science.1060698. 11441146 [DOI] [PubMed] [Google Scholar]
- 90.Wang X., Cederbaum A.I. S-adenosyl-l-methionine attenuates hepatotoxicity induced by agonistic Jo2 Fas antibody following CYP2E1 induction in mice. Journal of Pharmacology and Experimental Therapeutics. 2006;317(1):44–52. doi: 10.1124/jpet.105.098004. 16373529 [DOI] [PubMed] [Google Scholar]
- 91.O’Neill J.S., Reddy A.B. Circadian clocks in human red blood cells. Nature. 2011;469(7331):498–503. doi: 10.1038/nature09702. 21270888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Ooijen G., Millar A.J. Non-transcriptional oscillators in circadian timekeeping. Trends in Biochemical Sciences. 2012;37(11):484–492. doi: 10.1016/j.tibs.2012.07.006. 22917814 [DOI] [PubMed] [Google Scholar]
- 93.Tang Y., Forsyth C.B., Farhadi A., Rangan J., Jakate S., Shaikh M., Banan A., Fields J.Z., Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcoholism, Clinical and Experimental Research. 2009;33(7):1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. 19389191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenwasser A.M. Alcohol, antidepressants, and circadian rhythms. Human and animal models. Alcohol Research and Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25(2):126–135. 11584551 [PMC free article] [PubMed] [Google Scholar]
- 95.Bull-Otterson L., Feng W., Kirpich I., Wang Y., Qin X., Liu Y., Gobejishvili L., Joshi-Barve S., Ayvaz T., Petrosino J., Kong M., Barker D., McClain C., Barve S. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS One. 2013;8(1):e53028. doi: 10.1371/journal.pone.0053028. 23326376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voigt R.M., Forsyth C.B., Green S.J., Mutlu E., Engen P., Vitaterna M.H., Turek F.W., Keshavarzian A. Circadian disorganization alters intestinal microbiota. PloS One. 2014;9(5):e97500. doi: 10.1371/journal.pone.0097500. 24848969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang Y., Forsyth C.B., Banan A., Fields J.Z., Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. Journal of Pharmacology and Experimental Therapeutics. 2009;329:952–958. doi: 10.1124/jpet.108.148643. 19276402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forsyth C.B., Farhadi A., Jakate S.M., Tang Y., Shaikh M., Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol (Fayetteville, N.Y.) 2009;43(2):163–172. doi: 10.1016/j.alcohol.2008.12.009. 19251117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matuskova Z., Siller M., Tunkova A., Anzenbacherova E., Zacharova A., Tlaskalova-Hogenova H., Zidek Z., Anzenbacher P. Effects of Lactobacillus casei on the expression and the activity of cytochromes P450 and on the CYP mRNA level in the intestine and the liver of male rats. Neuro Endocrinology Letters. 2011;32(Suppl. 1):8–14. 22167211 [PubMed] [Google Scholar]


