Abstract
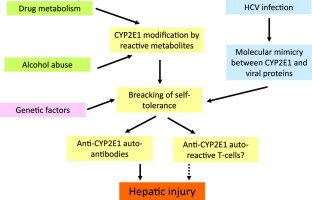
Autoimmune reactions involving cytochrome P4502E1 (CYP2E1) are a feature of idiosyncratic liver injury induced by halogenated hydrocarbons and isoniazid, but are also detectable in about one third of the patients with advanced alcoholic liver disease (ALD) and chronic hepatitis C (CHC). In these latter the presence of anti-CYP2E1 auto-antibodies is an independent predictor of extensive necro-inflammation and fibrosis and worsens the recurrence of hepatitis following liver transplantation, indicating that CYP2E1-directed autoimmunity can contribute to hepatic injury. The molecular characterization of the antigens recognized by anti-CYP2E1 auto-antibodies in ALD and CHC has shown that the targeted conformational epitopes are located in close proximity on the molecular surface. Furthermore, these epitopes can be recognized on CYP2E1 expressed on hepatocyte plasma membranes where they can trigger antibody-mediated cytotoxicity. This does not exclude that T cell-mediated responses against CYP2E1 might also be involved in causing hepatocyte damage. CYP2E1 structural modifications by reactive metabolites and molecular mimicry represent important factors in the breaking of self-tolerance against CYP2E1 in, respectively, ALD and CHC. However, genetic or acquired interferences with the mechanisms controlling the homeostasis of the immune system are also likely to contribute. More studies are needed to better characterize the impact of anti-CYP2E1 autoimmunity in liver diseases particularly in relation to the fact that common metabolic alterations such as obesity and diabetes stimulates hepatic CYP2E1 expression.
Keywords: Alcoholic liver disease, Hepatitis C, Autoimmunity, Oxidative stress, Liver injury
Abbreviations: ALD, alcoholic liver disease; CHC, chronic hepatitis C; CTLA-4, cytotoxic T lymphocyte associated antigen-4; CYP, cytochrome P450; DC, dendritic cell; HCV, hepatitis C virus; HER, hydroxyethyl free radical; LKM-1, anti-liver kidney microsome type I antibodies; OLT, orthotopic liver transplant; Tregs, regulatory T cells
Graphical abstract
Highlights
-
•
CYP2E1 is a frequent autoimmune target in alcoholic liver disease and hepatitis C.
-
•
Anti-CYP2E1 auto-antibodies mainly target conformational epitopes.
-
•
Molecular mimicry contribute to anti-CYP2E1 autoimmunity during HCV infection.
-
•
Anti-CYP2E1 autoimmunity contributes to the evolution of liver damage.
CYPs as targets of auto-immunity
Enzymes are common targets of immune-mediated reactions in liver autoimmune diseases. In these conditions, the breaking of self tolerance often involves various cytochrome P450 (CYP) isoenzymes [1]. CYPs are a large family of heme-containing proteins that are involved in the biotransformation of xenobiotics, but are also responsible for processing endogenous substrates and in the synthesis of steroid-derived hormones [2]. According to sequence homologies various CYPs are classified in families and sub-families identified by a combination of numbers and letters [2]. The involvement of CYPs as targets in autoimmunity streams from early reports showing that steroyl-21α hydroxylase (CYP21), cholesterol side-chain cleavage enzyme (CYP11A) and steroid-17α hydroxylase (CYP17) were the major adrenal cortex auto-antigens in idiopathic Addison's disease [3], while anti-CYP1A1 auto-antibodies are a characteristic of the hepatitis associated with type-1 polyendocrine syndrome [4]. From these observations, subsequent studies have implicated immunity against hepatic CYPs in idiosyncratic adverse reactions to drugs and in autoimmune hepatitis. In particular, antibodies against different CYP isoforms were detected in the case of dihydralazine- (anti-CYP1A2) or tienilic acid- (anti-CYP2C9) induced hepatitis as well as during hypersensitivity reactions to the aromatic anti-convulsants (anti-CYP3A) or in children treated with immunosuppressive drugs (CYP3A4, CYP2C9) [5–9]. The relevance of these observations in relation to the pathogenesis of drug-induced hepatitis was supported by the demonstration of that several functional CYP isoforms are transported from the Golgi apparatus via the secretory vesicles [10] to the plasma membrane of hepatocytes, where they and can be targeted by anti-CYP antibody [11,12].
A further aspect of the involvement of CYP in autoimmune reactions against the liver concerns anti-liver kidney microsome type I (LKM-1) antibodies which specifically target CYP2D6 [1] and are detectable in type II auto-immune hepatitis and in about 10% of patients with virus C (HCV) hepatitis [13]. Because of their prevalence, LKM-1 auto-antibodies have been extensively investigated showing that they recognize few immune-dominant epitopes formed by specific amino acid sequences located on the surface of the molecule [14–18]. CYP2D6, as other CYP isoenzymes, reaches the hepatocyte plasma membranes where can be targeted by circulating LKM-1 antibodies [19]. The clinical relevance of anti-CYP2D6 auto-reactivity is further supported by the detection of auto-reactive cytotoxic T-lymphocytes in patients with type-II autoimmune hepatitis and high titers of anti-CYP2D6 IgG [20,21]. Moreover, mice infection with CYP2D6-expressing Ad5 adenovirus leads to the production of anti-CYP2D6 IgG that cause immune-mediated liver injury by recognizing the same epitopes targeted by human auto-antibodies [22]. It is also noteworthy that beside CYP2D6, hepatitis C patients show auto-antibodies against other members of the CYP2 family, particularly CYP2A6 [23,24].
Autoimmune reactions targeting CYP2E1
Among the cytochrome P-450 family members, the isoenzyme 2E1 (CYP2E1) has been extensively investigated as it is a key enzyme in the metabolic activation of a variety of xenobiotics and carcinogens, including nitrosamines, benzene, vinyl chloride and halogenated solvents, and significantly contributes to the metabolism of ethanol to acetaldehyde [25]. As others CYPs, the 2E1 isoenzyme is readily inducible by the exposure to xenobiotics and alcohol consumption, but also by ketone bodies and hypoxia [25]. Furthermore, CYP2E1 has a high NADPH oxidase activity that leads to the generation of reactive oxygen species (ROS) and significantly contributes to the induction of oxidative stress in many pathological conditions [25]. Within the liver, CYP2E1 is mainly expressed in the endoplasmic reticulum of centrilobular hepatocytes, but two CYP2E1 variants accounting for 30–40% of the microsomal enzyme activity are also localized in the mitochondrial matrix (mtCYP2E1s) [25]. Moreover, a small CYP2E1 fraction (about 10% or the microsomal content) is also expressed on the outer layer of the hepatocyte plasma membranes [26]. Plasma membrane CYP2E1 is catalytically active [27], but this latter localization is particularly important in relation to auto-immune reactions.
The involvement of CYP2E1 in liver autoimmunity has emerged from studies by Bourdi and co-workers [28] and Eliasson and Kenna [29] who reported that about 70% of the patients suffering from hepatitis induced by the halogenated anesthetic halothane develop auto-antibodies specifically targeting CYP2E1. These auto-antibodies were identified as IgG isotype 4 [30]. Anti-CYP2E1 IgG were also detected in anesthesiologists exposed to halogenated hydrocarbon anesthetic gases [31], but not in other workers professionally exposed to similar gases used in refrigeration industry [32], in spite these latter may cause liver damage [33]. More recently, CYP2E1 has been recognized together with CYP3A4 and CYP2C9 as self-antigen in autoimmune reactions developing in subjects with idiosyncratic liver injury following isoniazid-based treatment for tuberculosis [34].
As mentioned above, CYP2E1 plays an important role in hepatic alcohol metabolism. During CYP2E1-mediated ethanol oxidation ethanol it self is converted to a free radical intermediate, known as hydroxyethyl free radicals (HER), that by reacting with CYP2E1 generate immunogenic adducts [35]. These anti-HER antibodies are detectable in the sera of either chronically ethanol-fed rats or patients with alcoholic liver disease (ALD) where they strictly correlate with CYP2E1 activity [36,37]. Moreover, human anti-HER IgG activate antibody-dependent cell-mediated cytotoxicity by selectively recognizing HER-modified CYP2E1 on the plasma membranes of ethanol-treated hepatocytes [38]. By extending these observations, Lytton and co-workers have reported that chronic intragastric alcohol-fed rats develop IgG directed towards CYP2E1 and CYP3A4 and that similar antibodies were also evident in the sera of alcohol abusers [39]. Interestingly, rats anti-CYP2E1 auto-reactivity correlates with the extent of hepatic injury [39]. In subsequent studies using a larger cohort of patients we have confirmed that high titers of anti-CYP2E1 auto-antibodies are present in about 40% of the subjects with advanced ALD and 11% of the heavy drinkers with fatty liver only [40]. In the former the presence of anti-CYP2E1 IgG correlates with the extent of lymphocyte infiltration and the number of apoptotic hepatocytes in liver biopsies [41], suggesting a possible contribution of auto-immune mechanisms in the pathogenesis of alcohol liver injury. The presence of anti-CYP2E1 immunity in ALD is not surprising, as these subjects frequently have signs of auto- and allo-immune reactions that are associated with the stimulation of lobular inflammation [42,43]. In particular, ALD patients show circulating anti-phospholipid auto-antibodies as well as IgG targeting proteins modified by the reaction with acetaldehyde, acetaldehyde/malondialdehyde (MAA) conjugates and lipid peroxidation end-products [44–48].
An other liver disease often associated with the presence of autoimmune responses involving CYP2E1 is chronic hepatitis C (CHC). Extrahepatic manifestations of autoimmunity are a common feature of CHC along with the presence of non-organ-specific auto-antibodies [14]. Furthermore, as mentioned above anti-CYP2D6 LKM-1 auto-antibodies are detectable in about 10% of CHC patients [14]. Using immuno-enzymatic tests we have observed that 38–40% of CHC patients have anti-CYP2E1 IgG which are unrelated to LKM-1 antibodies and do not cross-react with CYP2D6 [49,50]. The presence of these auto-antibodies is independent from circulating gamma-globulin levels and the viral genotype, indicating that they represent a general response to HCV infection [49,50]. It is interesting to note that, in spite both alcohol and HCV infection promote the development of anti-CYP2E1 autoimmunity, in our hands alcohol consumption by CHC patients did not increase the prevalence of anti-CYP2E1 auto-antibodies [49,50], suggesting that the breaking of self-tolerance promoted by alcohol and HCV infection are a rather independent and do not synergize each other. From the clinical point of view, anti-CYP2E1 auto-antibodies in CHC are associated with a 11 fold increase in the prevalence of severe necro-inflammation (grading ≥4 according to Ishack's criteria) and 4 fold increased risk of fibrosis. Moreover, multivariate analysis indicates anti-CYP2E1 IgG as an independent predictors of severe necro-inflammation in hepatitis C [49,50]. A further aspect of the involvement of anti-CYP2E1 auto-reactivity in CHC concerns the outcomes of the disease following liver transplantation. Now days end-stage CHC is becoming the main indication for orthotopic liver transplant (OLT) in Europe and United States. However, the recurrence of HCV infection in the transplanted organ is universal and the disease in OLT recipients follows a more aggressive course [51]. In this context, the development of autoimmune reactions after OLT is reported with rising frequency [52]. By investigating OLT recipients before and twelve months after transplantation we have observed that the prevalence of anti-CYP2E1 auto-antibodies in these patients is not appreciably modified by the liver transplant. However, the subjects who have elevated anti-CYP2E1 IgG before and maintained it after OLT have significantly higher incidence of recurrent hepatitis with severe necro-inflammation and fibrosis than those persistently negative or showing anti-CYP2E1 reactivity only before or after the transplant [53]. Moreover, the subjects persistently anti-CYP2E1-positive are at high risk of developing severe liver damage one year after OLT [53], indicating that autoimmune reactions involving CYP2E1 might contribute to hepatic injury in transplanted patients with recurrent hepatitis C.
Characterization of antigenic epitopes in CYP2E1
The analysis aimed to characterize the epitopes recognized by anti-CYP2E1 IgG associated with alcohol abuse, drug-induced hepatotoxicity and HCV infection have revealed that, independently from the etiology, these auto-antibodies are mainly unreactive toward the unfolded molecule, indicating a prevalent recognition of conformational antigens [54,55]. This make difficult to identify the specific epitopes as they can not be investigated by their cross-reaction with short peptides reproducing the protein sequence. To overcome such a difficulty we have used a methodology based on the combination of computer simulation and the analysis of the whole molecule antigenic properties following targeted single amino acid substitutions which do not alter CYP2E1 correct folding [54]. By this approach we have shown that anti-CYP2E1 IgG from patients with halothane hepatitis or ALD preferentially recognized two distinct epitopes on the CYP2E1 surface corresponding to the G-helix (Lys243 -Lys251) and an area (Lys324 -Glu421) formed by juxtaposition of the J′ and K″ helices, respectively (Fig. 1) [54]. In a similar manner anti-CYP2E1 antibodies associated with CHC target an area comprised between Lys324 and Glu346 at the juxtaposition of the J and J′ helices (Fig. 1) that partially overlaps with one the epitopes recognized by antibodies from ALD and halothane-induced hepatitis subjects [55]. This suggest the possible presence of antigenic hot-spots of CYP2E1 molecular structure. In the same vein, sequence alignments demonstrate that the CYP2E1324–346 epitope is has an amino acid sequence close to the peptides CYP2D6316–327 and CYP2D6321–379 that are among the most frequent targets of anti-CYP2D6 LKM-1 antibodies [17,56]. Interestingly, the J, K and L helices are also binding sites of anti-CYP2C9 and anti-CYP3A4 conformational antibodies associated with tienilic acid-induced autoimmune hepatitis and hypersensitivity reactions to aromatic anti-convulsing drugs, respectively [7,9]. These analogies are not surprising considering that CYP2 and CYP3 families have a high sequence homology and indicate that CYP auto-antigens are preferentially located in defined and rather conserved structures of the molecule.
Fig. 1.
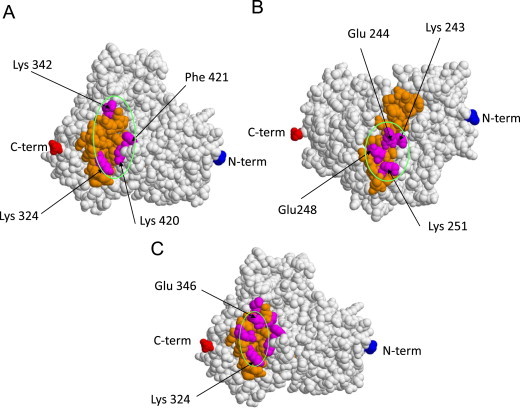
Tri-dimensional simulated structure of CYP2E1 showing the localization of the conformational epitopes recognized by anti-CYP2E1 IgG present in the sera of patients with alcoholic liver disease or halothane-induced hepatitis (panels A, B) or chronic hepatitis C (panel C). (Panels A, C) The area formed by the juxtaposition of J′ and K″ helices is evidenced in orange and specific amino acids are stained in purple. (Panel B) The epitope in the G helix comprised between Lys324 and Lys251 is shown in orange. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Possible mechanisms of liver injury by anti-CYP2E1 autoantibodies
As mentioned above, a small fraction of CYP2E1 is located on the outer layer of hepatocyte the plasma membranes [26,27]. Structural simulations based on CYP2E1 interaction with membrane lipids indicate that the conformational epitopes identified in G helix and in the area comprising the J–L helices are located on the outer portion of the molecule and are well accessible to the antibody binding [54]. In line with this, we have observed that anti-CYP2E1 auto-antibodies present in CHC [49] as well as those associated with ALD or halothane-induced hepatitis [29] bind to hepatocytes by recognizing plasma membrane CYP2E1. Thus, as in the case of LKM-1 antibodies [19], the binding of anti-CYP2E1 IgG to conformational epitopes on G and J–L helices has the capability to trigger antibody-mediated cytotoxicity, thus justifying the clinical association between anti-CYP2E1 reactivity and the severity of liver injury. Supporting this view we have observed that HER-modified CYP2E1 can mediate cytotoxic reactions by anti-HER antibodies from ALD patients [38]. This does not exclude that, as observed for anti-CYP2D6 auto-reactivity [20,21], T cell-mediated responses against CYP2E1 might also be involved in causing hepatocyte damage.
Mechanisms in the breaking of immune-tolerance toward CYP2E1
So far, the mechanisms leading to the breaking of self-tolerance toward hepatic CYP2E1 are still incompletely characterized. The liver is recognized to have unique immunological properties that modulate systemic tolerance to antigens from the portal circulation [57,58]. Under physiological conditions, Kupffer cells respond to low concentrations of bacterial products by producing interleukin-10 (IL-10) and low levels of pro-inflammatory mediators (TNF-α, ROS and prostanoids) that down-modulate the antigen presentation by dendritic and endothelial cells, thus suppressing T-cell activation [58]. Such an effect is lost following liver injury because of macrophage and dendritic cell (DC) activation in response to damage associated molecular patterns (PAMPs) [57]. Classically, B-cell activation, differentiation and proliferation occur in secondary lymphoid organs or in so-called “ectopic” germinal centers of non-lymphoid organs. In injured livers intra-portal lymphoid follicles acquire the features of active germinal centers [57], making possible the presentation of hepatocyte-derived antigens to B- and CD4+ T-lymphocytes as well as B-cell maturation to antibody producing plasma cells. Moreover, DCs can also cross-prime naïve CD8+ T-lymphocytes promoting cell-mediated responses. Beside DC, antigen presentation might also involve hepatic stellate cells [59] and possibly hepatocytes themselves, as pro-inflammatory cytokines have been shown to induce the hepatocyte expression of class II major histocompatibility complex (MHC) and co-stimulatory CD80 (B7.1) molecules [60]. Although during chronic liver injury CYP released from damaged hepatocytes might be a trigger for the breaking of self-tolerance, it should be noted that anti-CYP self-reactivity associated to CHC, alcohol abuse or adverse drug reactions is highly specific to individual CYPs [7–10] and there is no cross-reactivity between anti-CYP2E1 and anti-CYP2D6 antibodies [54,55] in HCV-infected patients. This indicates that additional and more specific mechanisms are likely involved.
Early studies have postulated that during drug-induced hepatitis the binding of reactive metabolites to CYPs would be able to promote immune responses against the drug-derived epitope(s) favouring at the same time the activation of normally quiescent auto-reactive lymphocytes recognizing native CYP molecules [61]. Although such a hypothesis has not been definitively proved, it is interesting to note that antibodies against CYP2E1 modified by the binding of drug-derived metabolites often accompany the presence of anti-CYP2E1 auto-antibodies in halothane and isoniazid idiosyncratic hepatitis [29,34] as well as in ALD [40]. In particular, we have observed that ALD patients with anti-HER antibodies have a 4 fold increased risk of developing anti-CYP2E1 auto-reactivity as compared to patients without anti-HER IgG [40], indicating that CYP2E1 structural modifications induced by HER are critical for the breaking of self-tolerance. This is consistent with the observation that small molecular difference are needed for the breaking of T-cells tolerance versus CYP2D6 in mice developing autoimmune hepatitis following infection with adenovirus expressing human CYP2D6 [62]. Furthermore, structural modifications of the pyruvate dehydrogenase complex consequent to biotinylation are required for the development auto-reactive B-cells and T-cells in an experimental model of biliary cirrhosis [63]. In this contest, genetic polymorphisms that influence the capacity of CYPs to generate reactive metabolites or that affect drug metabolite detoxification by phase II enzymes might likely influence inter-individual variability in the development of anti-CYP auto-reactivity [64]. However, in spite many genetic polymorphisms have been detected in CYP2E1 [65], at the moment no data has implicated their influence in the susceptibility to autoimmune responses.
Current researches on the mechanisms by which HCV infection promotes the onset of autoimmune reactions have focused on the peculiar ability of HCV to cause molecular mimicry [1,14]. Molecular mimicry defines the possibility that antigens from microorganisms can activate T-lymphocytes cross-reacting with self-antigens that share similar linear or conformational structures [66]. By now mimicry mechanisms involving viral proteins are recognized to play a key role in the pathogenesis of several human autoimmune diseases [66]. In the case of anti-CYP auto-reactivity, mimicry between HCV proteins E1, NS3, NS5a and NS5b and CYP2D6 accounts for the development of LKM-1 auto-antibodies [15,18,67] and T-cells antigens in CYP2D6, CYP2A6 and CYP2A7 [13,68]. Moreover, cross-reactivity between CYP2D6 and Cytomegalovirus and Herpes Simplex virus proteins has also been reported [16,18]. We have observed that the conformational epitopes in CYP2E1324–346 that are recognized by CHC-associated anti-CYP2E1 IgG have good sequence homology with two short peptides (NS5b438–449 and NS5b456–465) well conserved in NS5b RNA-dependent-DNA polymerase of most of HCV genotypes [55]. Furthermore, molecular imaging shows similarities in the tri-dimensional structure of these peptides and those of the epitopes in CYP2E1 molecule [55]. Accordingly, recombinant NS5b438–449 and NS5b456–465 peptides cross-react with CHC sera containing conformational anti-CYP2E1 IgG, while mice immunized with either the two viral peptides develop antibodies targeting human CYP2E1 [55]. Altogether, these data indicate molecular mimicry with HCV-NS5b a trigger for the development of auto-reactivity toward CYP2E1 in CHC. This might explain why the prevalence of anti-CYP2E1 auto-antibodies is unrelated to alcohol consumptions despite alcohol abuse is itself a trigger of autoimmune responses. More in general, mimicry mechanisms can be seen as a common mechanism in the breaking of self-tolerance toward CYPs in HCV infected patients.
Although CYP2E1 modifications by reactive metabolites and molecular mimicry can facilitate the selection of B- and T-cells with specificity toward CYP2E1 epitopes, additional factors may influence the large inter-individual variability that characterizes the presence of anti-CYP2E1 auto-reactivity in liver diseases. In this respect, the activity of regulatory T (Treg) cells is known to play an important role in the maintenance of immune homeostasis and in the prevention of autoimmune diseases [69,70]. In the liver, impairment of CD4+/CD25+ Tregs has been implicated as a contributor in the development of autoimmune hepatitis [71]. Among the mechanisms by which Tregs regulate immune functions a key role is played by cytotoxic T lymphocyte associated antigen-4 (CTLA-4) [72]. CTLA-4 is membrane protein that act as an inhibitory signal to T cells by competing with CD28 for interacting with CD80 (B7.1) and CD86 (B7.2) [73,74]. By this mechanism CTLA-4 interferes with important co-stimulatory signals essential for T-cell activation [73,74]. The immune-suppressor function of CTLA-4 is confirmed by observations that CD4+ and CD8+ T- and of B-cell clones rapidly expand in CTLA-4 knockout mice leading to lethal auto-reactive diseases [75]. In vitro data have shown that a human genetic polymorphisms involving a A→G base exchange at position 49 in exon 1 of the CTLA-4 gene is associated with a diminution of the normal inhibitory influence on T cell responses [73,74]. Moreover, primary biliary cirrhosis, type I auto-immune hepatitis, Graves' disease and type 1 diabetes mellitus have all been linked to the presence of such CTLA-4 polymorphisms [73,74]. We have observed that ALD patients have an increased prevalence CTLA-4 49 A/G substitution than healthy subjects. Moreover, in heavy drinkers the presence of the CTLA-4G allele increases by about four folds the risk of developing anti-CYP2E1 IgG, without influencing the prevalence of antibodies towards HER-antigens [40]. Interestingly, ALD patients having the combination both anti-HER IgG and the mutant CTLA-4 G allele have 23-fold higher risk of anti-CYP2E1 auto-reactivity than the subjects negative for both these factors [40]. Although ALD patients have also an increased prevalence of genetic polymorphisms (−627C→A and −1.117G→A) affecting IL-10 secretion by Tregs and macrophages, this does not influence the prevalence of anti-CYP2E1 IgG [40]. Thus, the antigenic stimulation by HER-modified CYP2E1 combined with an impaired control of T cell proliferation due to CTLA-4 mutation appears to specifically increase the probability to develop anti-CYP2E1 auto-reactivity. Altogether, these observations indicate that the breaking of self-tolerance toward CYPs is a multi-factorial event likely requiring structural modifications of the target molecule or molecular mimicry along with genetic or acquired interferences with the mechanisms controlling the homeostasis of the immune system.
Conclusions
Autoimmune reactions involving CYP2E1 play an important role in idiosyncratic liver injury induced by halogenated hydrocarbons and isoniazid, but are also detectable in about one third of the patients with advanced ALD or CHC. In these latter the presence of anti-CYP2E1 auto-antibodies is an independent predictor for extensive necro-inflammation and fibrosis. However, more studies are needed to better characterize the impact of anti-CYP2E1 autoimmunity in liver diseases particularly in relation to type II autoimmune hepatitis which is characterized by anti-CYP auto-reactivity [76] and nonalcoholic steatohepatitis (NASH), as in this later, insulin resistance associated to obesity stimulates hepatic CYP2E1 induction [77,78].
Conflict of interest
The authors have no conflict of interest.
References
- 1.Bogdanos D.P., Dalekos G.N. Enzymes as target antigens of liver-specific autoimmunity: the case of cytochrome P450s. Current Medicinal Chemistry. 2008;15(22):2285–2292. doi: 10.2174/092986708785747508. 18781950 [DOI] [PubMed] [Google Scholar]
- 2.Coon M.J. Cytochrome P450: nature's most versatile biological catalyst. Annual Review of Pharmacology and Toxicology. 2005;45:1–25. doi: 10.1146/annurev.pharmtox.45.120403.100030. 15832443 [DOI] [PubMed] [Google Scholar]
- 3.Betterle C. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocrine Reviews. 2002;23(3):327–354. doi: 10.1210/edrv.23.3.0466. 12050123 [DOI] [PubMed] [Google Scholar]
- 4.Lankisch T.O., Jaeckel E., Strassburg C.P. The autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy or autoimmune polyglandular syndrome type 1. Seminars in Liver Disease. 2009;29:307–314. doi: 10.1055/s-0029-1233535. 19676003 [DOI] [PubMed] [Google Scholar]
- 5.Bourdi M., Larrey D., Nataf J., Bernuau J., Pessayre D., Iwasaki M., Guengerich F.P., Beaune P.H. Anti-liver endoplasmic reticulum autoantibodies are directed against human cytochrome P-450IA2. A specific marker of dihydralazine-induced hepatitis. Journal of Clinical Investigation. 1990;85(6):1967–1973. doi: 10.1172/JCI114660. 2347920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaune P., Pessayre D., Dansette P., Mansuy D., Manns M. Autoantibodies against cytochromes P450: role in human diseases. Advances in Pharmacology. 1994;30:199–245. doi: 10.1016/s1054-3589(08)60175-1. 7833293 [DOI] [PubMed] [Google Scholar]
- 7.Leeder J.S., Gaedigk A., Lu X., Cook V.A. Epitope mapping studies with human anti-cytochrome P450 3A antibodies. Molecular Pharmacology. 1996;49(2):234–243. 8632755 [PubMed] [Google Scholar]
- 8.Belloc C., Gauffre A., André C., Beaune P.H. Epitope mapping of human CYP1A2 in dihydralazine-induced autoimmune hepatitis. Pharmacogenetics. 1997;7(3):181–186. doi: 10.1097/00008571-199706000-00002. 9241657 [DOI] [PubMed] [Google Scholar]
- 9.Lecoeur S., André C., Beaune P.H. Tienilic acid-induced autoimmune hepatitis: anti-liver and-kidney microsomal type 2 autoantibodies recognize a three-site conformational epitope on cytochrome P4502C9. Molecular Pharmacology. 1996;50(2):326–333. 8700140 [PubMed] [Google Scholar]
- 10.Neve E.P., Eliasson E., Pronzato M.A., Albano E., Marinari U., Ingelman-Sundberg M. Enzyme-specific transport of rat liver cytochrome P450 to the Golgi apparatus. Archives of Biochemistry and Biophysics. 1996;333(2):459–465. doi: 10.1006/abbi.1996.0415. 8809087 [DOI] [PubMed] [Google Scholar]
- 11.Loeper J., Descatoire V., Maurice M., Beaune P., Feldmann G., Larrey D., Pessayre D. Presence of functional cytochrome P-450 on isolated rat hepatocyte plasma membrane. Hepatology. 1990;11(5):850–858. doi: 10.1002/hep.1840110521. 2112112 [DOI] [PubMed] [Google Scholar]
- 12.Loeper J., Descatoire V., Maurice M., Beaune P., Belghiti J., Houssin D., Ballet F., Feldmann G., Guengerich F.P., Pessayre D. Cytochromes P-450 in human hepatocyte plasma membrane: recognition by several autoantibodies. Gastroenterology. 1993;104(1):203–216. doi: 10.1016/0016-5085(93)90853-5. 7678237 [DOI] [PubMed] [Google Scholar]
- 13.Bogdanos D., Mieli-Vergani G., Vergani D. Autoantibodies and their antigens in autoimmune hepatitis. Seminars in Liver Disease. 2009;29(03):241–253. doi: 10.1055/s-0029-1233533. [DOI] [PubMed] [Google Scholar]
- 14.Ferri S., Muratori L., Lenzi M., Granito A., Bianchi F., Vergani D. HCV and autoimmunity. Current Pharmaceutical Design. 2008;14(17):1678–1685. doi: 10.2174/138161208784746824. [DOI] [PubMed] [Google Scholar]
- 15.Manns M.P., Griffin K.J., Sullivan K.F., Johnson E.F. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. Journal of Clinical Investigation. 1991;88(4):1370–1378. doi: 10.1172/JCI115443. 1717511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto A.M., Cresteil D., Homberg J.C., Alvarez F. Characterization of anti-liver-kidney microsome antibody (anti-LKM1) from hepatitis C virus-positive and -negative sera. Gastroenterology. 1993;104(6):1762–1767. doi: 10.1016/0016-5085(93)90657-x. 7684716 [DOI] [PubMed] [Google Scholar]
- 17.Sugimura T., Obermayer-Straub P., Kayser A., Braun S., Loges S., Alex B., Lüttig B., Johnson E.F., Manns M.P., Strassburg C.P. A major CYP2D6 autoepitope in autoimmune hepatitis type 2 and chronic hepatitis C is a three-dimensional structure homologous to other cytochrome P450 autoantigens. Autoimmunity. 2002;35(8):501–513. doi: 10.1080/0891693021000069556. 12765476 [DOI] [PubMed] [Google Scholar]
- 18.Kerkar N., Choudhuri K., Ma Y., Mahmoud A., Bogdanos D.P., Muratori L., Bianchi F., Williams R., Mieli-Vergani G., Vergani D. Cytochrome P4502D6193–212: a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. Journal of Immunology. 2003;170(3):1481–1489. doi: 10.4049/jimmunol.170.3.1481. 12538711 [DOI] [PubMed] [Google Scholar]
- 19.Muratori L., Parola M., Ripalti A., Robino G., Muratori P., Bellomo G., Carini R., Lenzi M., Landini M.P., Albano E., Bianchi F.B. Liver/kidney microsomal antibody type 1 targets CYP2D6 on hepatocyte plasma membrane. Gut. 2000;46(4):553–561. doi: 10.1136/gut.46.4.553. 10716687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y., Bogdanos D.P., Hussain M.J., Underhill J., Bansal S., Longhi M.S., Cheeseman P., Mieli-Vergani G., Vergani D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130(3):868–882. doi: 10.1053/j.gastro.2005.12.020. 16530525 [DOI] [PubMed] [Google Scholar]
- 21.Longhi M.S., Hussain M.J., Bogdanos D.P., Quaglia A., Mieli-Vergani G., Ma Y., Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46(2):472–484. doi: 10.1002/hep.21658. 17559153 [DOI] [PubMed] [Google Scholar]
- 22.Lapierre P., Djilali-Saiah I., Vitozzi S., Alvarez F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology. 2004;39(4):1066–1074. doi: 10.1002/hep.20109. [DOI] [PubMed] [Google Scholar]
- 23.Dalekos G.N., Obermayer-Straub P., Bartels M., Maeda T., Kayser A., Braun S., Loges S., Schmidt E., Gershwin M.E., Manns M.P. Cytochrome P450 2A6: a new hepatic autoantigen in patients with chronic hepatitis C virus infection. Journal of Hepatology. 2003;39(5):800–806. doi: 10.1016/s0168-8278(03)00356-8. 14568264 [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa H., Kitazawa E., Kikuchi K., Fujikawa H., Kawaguchi N., Abe K., Matsushita M., Matsushima H., Igarashi T., Hankins R.W., Kako M. Immunoreactivity to various human cytochrome P450 proteins of sera from patients with autoimmune hepatitis, chronic hepatitis B, and chronic hepatitis C. Autoimmunity. 2000;33(1):23–32. doi: 10.3109/08916930108994106. 11204250 [DOI] [PubMed] [Google Scholar]
- 25.Lu Y., Cederbaum A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radical Biology and Medicine. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. 18078827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neve E.P., Ingelman-Sundberg M. Molecular basis for the transport of cytochrome P450 2E1 to the plasma membrane. Journal of Biological Chemistry. 2000;275(22):17130–17135. doi: 10.1074/jbc.M000957200. 10747972 [DOI] [PubMed] [Google Scholar]
- 27.Wu D.F., Cederbaum A.I. Presence of functionally active cytochrome P-450IIE1 in plasma membrane of rat hepatocytes. Hepatology. 1992;15:515–524. doi: 10.1002/hep.1840150326. [DOI] [PubMed] [Google Scholar]
- 28.Bourdi M., Chen W., Peter R.M., Martin J.L., Buters J.T., Nelson S.D., Pohl L.R. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chemical Research in Toxicology. 1996;9(7):1159–1166. doi: 10.1021/tx960083q. 8902272 [DOI] [PubMed] [Google Scholar]
- 29.Eliasson E., Kenna J.G. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Molecular Pharmacology. 1996;50(3):573–582. 8794896 [PubMed] [Google Scholar]
- 30.Njoko D.B., Mellerson J.L., Talor M.V., Kerr D.R., Faraday N.R., Outshoor I., Rose N.R. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in the pathogenesis of idiosyncratic drug-induced hepatitis. Clinical and Vaccine Immunology. 2006;13:258–265. doi: 10.1128/CVI.13.2.258-265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Njoku D.B., Greenberg R.S., Bourdi M., Borkowf C.B., Dake E.M., Martin J.L., Pohl L.R. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesthesia & Analgesia. 2002;94(2):243–249. doi: 10.1097/00000539-200202000-00003. 11812677 [DOI] [PubMed] [Google Scholar]
- 32.Gunnare S., Vidali M., Lillienberg L., Ernstgård L., Sjögren B., Hagberg M., Albano E., Johanson G. Non-positive autoimmune responses against CYP2E1 in refrigeration mechanics exposed to halogenated hydrocarbons. Science of the Total Environment. 2007;383(1–3):90–97. doi: 10.1016/j.scitotenv.2007.05.005. 17582468 [DOI] [PubMed] [Google Scholar]
- 33.Hoet P., Graf M.L., Bourdi M., Pohl L.R., Duray P.H., Chen W., Peter R.M., Nelson S.D. Epidemic of liver disease caused by hydrochlorofluorocarbons used as ozone-sparing substitutes of chlorofluoro carbons. Lancet. 1997;350(9077):556–559. doi: 10.1016/S0140-6736(97)03094-8. 9284778 [DOI] [PubMed] [Google Scholar]
- 34.Metushi I.G., Sanders C., Acute Liver Study Group. Lee W.M., Uetrecht J. Detection of anti-isoniazid and anti-cytochrome P450 antibodies in patients with isoniazid-induced liver failure. Hepatology. 2014;59(3):1084–1093. doi: 10.1002/hep.26564. 23775837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albano E., French S.W., Ingelman-Sundberg M. Hydroxyethyl radicals in ethanol hepatotoxicity. Frontiers in Bioscience. 1999;4:D533–D540. doi: 10.2741/albano. 10369806 [DOI] [PubMed] [Google Scholar]
- 36.Albano E., Clot P., Morimoto M., Tomasi A., Ingelman-Sundberg M., French S.W. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23(1):155–163. doi: 10.1002/hep.510230121. 8550035 [DOI] [PubMed] [Google Scholar]
- 37.Clot P., Albano E., Eliasson E., Tabone M., Aricò S., Israel Y., Moncada C., Ingelman-Sundberg M. Cytochrome P4502E1 hydroxyethyl radical adducts as the major antigen in autoantibody formation among alcoholics. Gastroenterology. 1996;111(1):206–216. doi: 10.1053/gast.1996.v111.pm8698201. 8698201 [DOI] [PubMed] [Google Scholar]
- 38.Clot P., Parola M., Bellomo G., Dianzani U., Carini R., Tabone M., Aricò S., Ingelman-Sundberg M., Albano E. Plasma membrane hydroxyethyl radical adducts cause antibody-dependent cytotoxicity in rat hepatocytes exposed to alcohol. Gastroenterology. 1997;113(1):265–276. doi: 10.1016/s0016-5085(97)70104-5. 9207287 [DOI] [PubMed] [Google Scholar]
- 39.Lytton S.D., Berg U., Nemeth A., Ingelman-Sundberg M. Autoantibodies against cytochrome P450s in sera of children treated with immunosuppressive drugs. Clinical & Experimental Immunology. 2002;127(2):293–302. doi: 10.1046/j.1365-2249.2002.01754.x. 11876753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidali M., Stewart S.F., Rolla R., Daly A.K., Chen Y., Mottaran E., Jones D.E., Leathart J.B., Day C.P., Albano E. Genetic and epigenetic factors in autoimmune reactions toward cytochrome P4502E1 in alcoholic liver disease. Hepatology. 2003;37(2):410–419. doi: 10.1053/jhep.2003.50049. 12540792 [DOI] [PubMed] [Google Scholar]
- 41.Stewart S.F., Jones D.E.J., Vidali M., Haugk B., Brunt A.D., Albano E., Day C.P. Correlation between anti-CYP2E1 titre and lymphocyte infiltration in ALD- an autoimmune disease in some patients. Journal of Hepatology. 2004;40(Suppl. 1):176. [Google Scholar]
- 42.McFarlane I.G. Autoantibodies in alcoholic liver disease. Addiction Biology. 2000;5(2):141–151. doi: 10.1080/13556210050003720. 20575828 [DOI] [PubMed] [Google Scholar]
- 43.Vidali M., Stewart S.F., Albano E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends in Molecular Medicine. 2008;14(2):63–71. doi: 10.1016/j.molmed.2007.12.005. 18222109 [DOI] [PubMed] [Google Scholar]
- 44.Vay D., Rigamonti C., Vidali M., Mottaran E., Alchera E., Occhino G., Sartori M., Albano E. Anti-phospholipid antibodies associated with alcoholic liver disease target oxidized phosphatidylserine on apoptotic cell plasma membranes. Journal of Hepatology. 2006;44(1):183–189. doi: 10.1016/j.jhep.2005.06.010. 16143424 [DOI] [PubMed] [Google Scholar]
- 45.Thiele G.M., Freeman T.L., Klassen L.W. Immunologic mechanisms of alcoholic liver injury. Seminars in Liver Disease. 2004;24(3):273–287. doi: 10.1055/s-2004-832940. 15349805 [DOI] [PubMed] [Google Scholar]
- 46.Rolla R., Vay D., Mottaran E., Parodi M., Traverso N., Aricò S., Sartori M., Bellomo G., Klassen L.W., Thiele G.M., Tuma D.J., Albano E. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31(4):878–884. doi: 10.1053/he.2000.5373. 10733543 [DOI] [PubMed] [Google Scholar]
- 47.Mottaran E., Stewart S.F., Rolla R., Vay D., Cipriani V., Moretti M., Vidali M., Sartori M., Rigamonti C., Day C.P., Albano E. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radical Biology and Medicine. 2002;32(1):38–45. doi: 10.1016/s0891-5849(01)00757-2. 11755315 [DOI] [PubMed] [Google Scholar]
- 48.Stewart S.F., Vidali M., Day C.P., Albano E., Jones D.E. Oxidative stress as a trigger for cellular immune responses in patients with alcoholic liver disease. Hepatology. 2004;39(1):197–203. doi: 10.1002/hep.20021. 14752838 [DOI] [PubMed] [Google Scholar]
- 49.Vidali M., Occhino G., Ivaldi A., Serino R., Moia S., Alchera E., Carini R., Rigamonti C., Sartori M., Albano E. Detection of auto-antibodies against cytochrome P4502E1 (CYP2E1) in chronic hepatitis C. Journal of Hepatology. 2007;46(4):605–612. doi: 10.1016/j.jhep.2006.11.009. 17196701 [DOI] [PubMed] [Google Scholar]
- 50.Sutti S., Vidali M., Mombello C., Sartori M., Albano E. Conformational anti-cytochrome P4502E1 (CYP2E1) auto-antibodies contribute to necro-inflammatory injury in chronic hepatitis C. Journal of Viral Hepatitis. 2010;17(10):685–690. doi: 10.1111/j.1365-2893.2010.01359.x. 20738774 [DOI] [PubMed] [Google Scholar]
- 51.Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? Journal of Hepatology. 2005;42(4):448–456. doi: 10.1016/j.jhep.2005.01.011. 15763325 [DOI] [PubMed] [Google Scholar]
- 52.Vergani D., Mieli-Vergani G. Autoimmunity after liver transplantation. Hepatology. 2002;36(2):271–276. doi: 10.1053/jhep.2002.35339. 12143033 [DOI] [PubMed] [Google Scholar]
- 53.Rigamonti C., Vidali M., Donato M.F., Sutti S., Occhino G., Ivaldi A., Arosio E., Agnelli F., Rossi G., Colombo M., Albano E. Serum autoantibodies against cytochrome P450 2E1 (CYP2E1) predict severity of necroinflammation of recurrent hepatitis C. American Journal of Transplantation. 2009;9(3):601–609. doi: 10.1111/j.1600-6143.2008.02520.x. 19191768 [DOI] [PubMed] [Google Scholar]
- 54.Vidali M., Hidestrand M., Eliasson E., Mottaran E., Reale E., Rolla R., Occhino G., Albano E., Ingelman-Sundberg M. Use of molecular simulation for mapping conformational CYP2E1 epitopes. Journal of Biological Chemistry. 2004;279(49):50949–50955. doi: 10.1074/jbc.M407329200. 15456790 [DOI] [PubMed] [Google Scholar]
- 55.Sutti S., Vidali M., Mombello C., Sartori M., Ingelman-Sundberg M., Albano E. Breaking self-tolerance toward cytochrome P4502E1 (CYP2E1) in chronic hepatitis C: possible role for molecular mimicry. Journal of Hepatology. 2010;53(3):431–438. doi: 10.1016/j.jhep.2010.03.030. 20576306 [DOI] [PubMed] [Google Scholar]
- 56.Ma Y., Thomas M.G., Okamoto M., Bogdanos D.P., Nagl S., Kerkar N., Lopes A.R., Muratori L., Lenzi M., Bianchi F.B., Mieli-Vergani G., Vergani D. Key residues of a major cytochrome P4502D6 epitope are located on the surface of the molecule. Journal of Immunology. 2002;169(1):277–285. doi: 10.4049/jimmunol.169.1.277. 12077255 [DOI] [PubMed] [Google Scholar]
- 57.Racanelli V., Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl. 1):S54–S62. doi: 10.1002/hep.21060. 16447271 [DOI] [PubMed] [Google Scholar]
- 58.Crispe I.N. Immune tolerance in liver disease. Hepatology. 2014 doi: 10.1002/hep.27254. 24913836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winau F., Quack C., Darmoise A., Kaufmann S.H. Starring stellate cells in liver immunology. Current Opinion in Immunology. 2008;20(1):68–74. doi: 10.1016/j.coi.2007.10.006. 18068343 [DOI] [PubMed] [Google Scholar]
- 60.Herkel J., Jagemann B., Wiegard C., Lazaro J.F., Lueth S., Kanzler S., Blessing M., Schmitt E., Lohse A.W. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology. 2003;37(5):1079–1085. doi: 10.1053/jhep.2003.50191. [DOI] [PubMed] [Google Scholar]
- 61.Van Pelt F.N., Straub P., Manns M.P. Molecular basis of drug-induced immunological liver injury. Seminars in Liver Disease. 1995;15(3):283–300. doi: 10.1055/s-2007-1007281. 7491507 [DOI] [PubMed] [Google Scholar]
- 62.Ehser J., Holdener M., Christen S., Bayer M., Pfeilschifter J.M., Hintermann E., Bogdanos D., Christen U. Molecular mimicry rather than identity breaks T-cell tolerance in the CYP2D6 mouse model for human autoimmune hepatitis. J. Autoimmune. 2013;42:39–49. doi: 10.1016/j.jaut.2012.11.001. 23200317 [DOI] [PubMed] [Google Scholar]
- 63.Palmer J.M., Robe A.J., Burt A.D., Kirby J.A., Jones D.E. Covalent modification as a mechanism for the breakdown of immune tolerance to pyruvate dehydrogenase complex in the mouse. Hepatology. 2004;39(6):1583–1592. doi: 10.1002/hep.20248. 15185299 [DOI] [PubMed] [Google Scholar]
- 64.Czaja A.J. Drug-induced autoimmune-like hepatitis. Digestive Diseases and Sciences. 2011;56(4):958–976. doi: 10.1007/s10620-011-1611-4. 21327704 [DOI] [PubMed] [Google Scholar]
- 65.Neafsey P., Ginsberg G., Hattis D., Johns D.O., Guyton K.Z., Sonawane B. Genetic polymorphism in CYP2E1: population distribution of CYP2E1 activity. Journal of Toxicology and Environmental Health B Critical Reviews. 2009;12(5–6):362–388. doi: 10.1080/10937400903158359. 20183527 [DOI] [PubMed] [Google Scholar]
- 66.Münz C., Lünemann J.D., Getts M.T., Miller S.D. Antiviral immune responses: triggers of or triggered by autoimmunity? Nature Reviews Immunology. 2009;9(4):246–258. doi: 10.1038/nri2527. 19319143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marceau G., Lapierre P., Béland K., Soudeyns H., Alvarez F. LKM1 autoantibodies in chronic hepatitis C infection: a case of molecular mimicry? Hepatology. 2005;42(3):675–682. doi: 10.1002/hep.20816. 16037945 [DOI] [PubMed] [Google Scholar]
- 68.Kammer A.R., van der Burg S.H., Grabscheid B., Hunziker I.P., Kwappenberg K.M., Reichen J., Melief C.J. Molecular mimicry of human cytochrome P450 by hepatitis C virus at the level of cytotoxic T cell recognition. Journal of Experimental Medicine. 1999;190(2):169–176. doi: 10.1084/jem.190.2.169. 10432280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shevach E.M., DiPaolo R.A., Andersson J., Zhao D.M., Stephens G.L., Thornton A.M. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunological Reviews. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. 16903906 [DOI] [PubMed] [Google Scholar]
- 70.Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nature Reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. 18566595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Longhi M.S., Hussain M.J., Kwok W.W., Mieli-Vergani G., Ma Y., Vergani D. Autoantigen-specific regulatory T cells, a potential tool for immune-tolerance reconstitution in type-2 autoimmune hepatitis. Hepatology. 2011;53(2):536–547. doi: 10.1002/hep.24039. [DOI] [PubMed] [Google Scholar]
- 72.Walker L.S. Treg and CTLA-4: two intertwining pathways to immune tolerance. Journal of Autoimmunity. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. 23849743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scalapino K.J., Daikh D.I. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunological Reviews. 2008;223:143–155. doi: 10.1111/j.1600-065X.2008.00639.x. 18613834 [DOI] [PubMed] [Google Scholar]
- 74.Gough S.C., Walker L.S., Sansom D.M. CTLA4 gene polymorphism and autoimmunity. Immunological Reviews. 2005;204:102–115. doi: 10.1111/j.0105-2896.2005.00249.x. 15790353 [DOI] [PubMed] [Google Scholar]
- 75.Kim J.M., Rasmussen J.P., Rudensky A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunology. 2007;8(2):191–197. doi: 10.1038/ni1428. 17136045 [DOI] [PubMed] [Google Scholar]
- 76.Liaskou E., Hirschfield G.M., Gershwin M.E. Mechanisms of tissue injury in autoimmune liver diseases. Seminars in Immunopathology. 2014;36(5):553–568. doi: 10.1007/s00281-014-0439-3. 25082647 [DOI] [PubMed] [Google Scholar]
- 77.Chalasani N., Gorski J.C., Asghar M.S., Asghar A., Foresman B., Hall S.D., Crabb D.W. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37(3):544–550. doi: 10.1053/jhep.2003.50095. 12601351 [DOI] [PubMed] [Google Scholar]
- 78.Emery M.G., Fisher J.M., Chien J.Y., Kharasch E.D., Dellinger E.P., Kowdley K.V., Thummel K.E. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38(2):428–435. doi: 10.1053/jhep.2003.50342. 12883487 [DOI] [PubMed] [Google Scholar]


