Abstract
Viruses cause most cases of bronchiolitis in infants; consequently the importance of other agents such as Mycoplasma pneumoniae (MP) in the etiology of bronchiolitis may not be fully recognized. We investigated the prevalence and seasonal distribution of bronchiolitis caused by MP in 674 children admitted to the Children's Hospital affiliated with Soochow University from January 2010 to December 2012. The presence of MP was confirmed by real-time PCR. During the 3 years, we identified MP in 17.2% of the children with bronchiolitis. The annual MP detection rates were 16.6% in 2010, 17.8% in 2011, and 17.2% in 2012. MP was detected throughout the year, with a peak from July to September. The median age of MP-positive children was 10 months. Common clinical manifestations included cough, wheezing, and high fever. Moist and/or wheezing rales were frequent, and pulmonary interstitial infiltration was seen in 66.4% of chest X-rays. Patients with MP infection were older, were more likely to have pulmonary interstitial infiltration, and had shorter hospital stays than those with respiratory syncytial virus infection. Our study revealed MP as an important cause of bronchiolitis, with peaks of occurrence during the summer and early autumn. Pulmonary interstitial infiltrations were a common event.
Bronchiolitis is an acute lower respiratory tract infection that primarily involves terminal and respiratory bronchioles. The disease may extend to the adjacent alveolar ducts and alveolar space1. Viral infection is the most common cause of bronchiolitis, and respiratory syncytial virus (RSV) is the most common pathogen2,3. Mycoplasma is the smallest free-living, self-replicating microorganism. It is highly transmissible and is a frequent cause of respiratory tract infection in children. The symptoms of MP upper respiratory tract infection are usually mild; one-fifth of infected individuals being asymptomatic, but in some cases, MP causes severe conditions such as organizing pneumonia4,5,6,7,8,9. Acute MP infection may also exacerbate asthma or cause asthmatic symptoms10,11.
Because MP is difficult to isolate in culture, infections are most often confirmed by polymerase chain reaction (PCR) gene amplification or serology. In children with bronchiolitis, detection rates of 75.8% have been reported for RSV and 2.7% for MP12. In recent years, the incidence of MP-caused bronchiolitis has been rising. A study of 211 cases of bronchiolitis reported an MP-positive rate of 7.1%; and as with RSV, it was frequently detected in moderate or severe cases13. In another report14, MP was identified in 34.3% of children with bronchiolitis who were between 6 months and 2 years of age, and who comprised 52.2% of the study subjects. The evidence shows that MP has become an important cause of bronchiolitis in infant patients. In the present study, we aimed to determine the prevalence and seasonal distribution of MP bronchiolitis among infant patients in Suzhou, China. Meanwhile, we compared the clinical characteristics of bronchiolitis caused by MP and by RSV.
Results
Patient characteristics
A total of 674 patients with bronchiolitis were studied, including 225 cases in 2010, 205 in 2011, and 244 in 2012. There were 457 (67.8%) male and 217 (32.2%) female patients. 247 (36.6%) were younger than 6 months of age, 234 (34.7%) were 6 months–1 year of age, 193 (28.6%) were 1–2 years of age. The youngest patient was 35 days old, and the oldest patient was 2 years of age.
Pathogens detected
Pathogens were identified in 586 of 674 specimens (86.9%). RSV was found in 343 cases (50.9%), MP in 116 cases (17.2%), parainfluenza virus (PIV) III in 41 cases (6.1%), human bocavirus (hBoV) in 36 cases (5.3%), human metapneumovirus (hMPV) in 34 cases (5%), influenza virus B (IVB) in nine cases (1.3%), influenza virus A (IVA) in six cases (0.9%), and PIV II in one case (0.1%). Mixed infection was observed in 35 cases, including 16 of MP + hBoV (45.7%), four of hMPV + hBoV (11.4%), four of hBoV + PIV III (11.4%), 3 cases of RSV + IVA (8.6%), three cases of RSV + hBoV (8.6%), three cases of hMPV + RSV (8.6%), and two cases of hMPV + PIV III (5.7%), respectively.
Prevalence of MP and RSV infections in different years and seasons
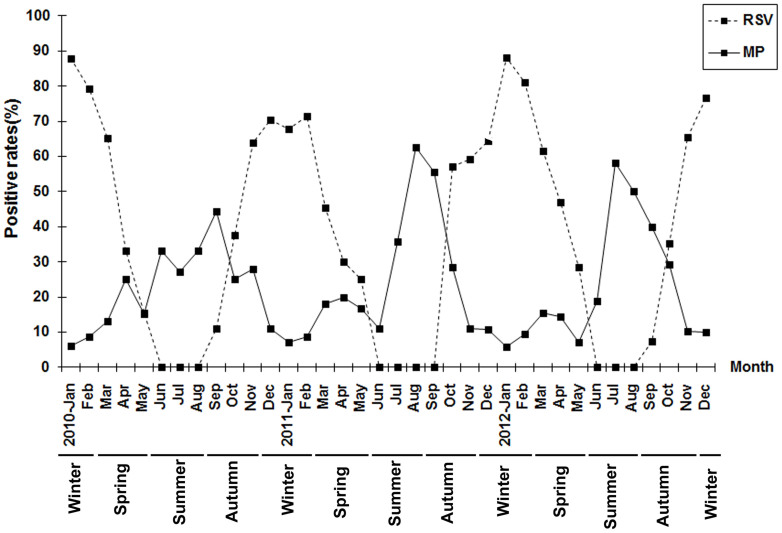
The annual MP-positive rates were 16.6% (2010), 17.8% (2011), and 17.2% (2012). MP was detected throughout the year with a epidemic peaks observed each year between July and September. The lowest MP-positive rates were January to February and November to December each year. The seasons in the Suzhou area of China were defined as spring (March–May), summer (June–August), autumn (September–November), and winter (December–February). The peaks of MP occurrence thus occurred in the summer and early autumn. The highest rates occurred in September 2010 (44.4%), August 2011 (62.5%), and July 2012 (58.3%), showing a peak that occurred earlier in successive years and higher infection rates in 2011 and 2012 than in 2010. The highest rates of RSV infection were seen to occur between November of one year and March of the following year. The lowest RSV-positive rates were observed each year between June and September. (Figure 1).
Figure 1. The prevalence and seasonal distribution of MP bronchiolitis in children in the Suzhou region between January 2010 and December 2012.
Prevalence of MP infection differed with age but not sex
Among the 116 cases of MP infection, the youngest patient was 2 months and the oldest was 2 years of age. The median age was 10 months (range: 6–15 months); 22 cases (19%) were less than 6 months of age, 56 (48.3%) were 6 months to 1 year, 38 (32.7%) were 1–2 years of age. The MP positivity rate in patients between 6 months and 1 year of age was significantly higher than that in other age groups (P < 0.01). Sixty cases occurred in males and 56 in females (M:F = 1.07:l). The difference was not statistically significant.
Clinical characteristics of patients infected with MP
Various degrees of fever were recorded in 38 cases (32.8%). The median duration of fever was 3.5 ± 1.0 days. Patients had differing severity of cough and wheezing; there were 13 cases (11.2%) of tachypnea or dyspnea, seven (6%) of O2 saturation < 90%, and 98 cases (84.5%) of lung rales and (or) wheezing.
The median white blood cell counts were 7.7 ± 3.5 × 109/l, mean C-reactive protein (CRP) was 8.5 ± 3.8 mg/l; 79 cases (68.1%) had peripheral blood platelet counts > 400 × 109/l. Thirty patients (25.8%) had elevated alanine aminotransferase (ALT) levels, 41 (35.5%) had elevated creatine kinase MB isoenzyme (CK-MB). The radiological analysis was performed by a radiologist blinded to the pathogens that had been isolated. Abnormal chest X-ray findings were seen in 332 patients (88.3%), including 77 cases (66.4%) of pulmonary interstitial infiltration, 14 (12.1%) of patchy shadows, and 19 (16.4%) of emphysema. Thirty-three cases had vomiting and/or diarrhea. All patients improved or were cured, and were discharged from hospital. The average hospital stay was 7 days (range, 6.3–9.0 days; Table 1).
Table 1. Overall characteristics of patients with MP bronchiolitis.
| Characteristics | No. of patients (%) |
|---|---|
| Age | |
| <6 months | 22 (8.9) |
| 6 months–1 year | 56 (23.9) |
| 1–2 years | 36 (18.6) |
| Sex | |
| Male | 60 (51.7) |
| Female | 56 (48.3) |
| Fever (°C) | |
| <38 | 1 (5.6%) |
| 38–39 | 5 (13.1%) |
| >39 | 32 (37.2%) |
| Symptom | |
| Cough | 116 (100) |
| Wheezing | 116 (100) |
| Tachypnea | 13 (11.2) |
| Dyspnea | 13 (11.2) |
| Vomiting/diarrhea | 33 (28.4) |
| Physical examination | |
| Lung wheezing rales | 89 (76.7) |
| Lung crackles | 9 (7.8) |
| SaO2 < 90% | 7 (6) |
| Lab test | |
| WBC (×109/l) | 7.7 ± 3.5 |
| CRP (mg/L) | 8.5 ± 3.8 |
| Blood platelet counts > 400 × 109/l | 79 (68.1) |
| ALT↑ | 30 (25.8) |
| CK-MB↑ | 41 (35.5) |
| Abnormal chest X-ray | |
| Interstitial infiltration | 77 (66.4) |
| Patchy shadows | 14 (12.1) |
| Emphysema | 19 (16.4) |
Data are expressed as number of patients (%) unless otherwise indicated.
WBC, white blood cell counts; CRP, C-reactive protein; ALT, alanine transaminase; CK-MB, creatine kinase-MB.
Characteristics of patients with MP or RSV infection
The average age was 10 months in MP patients and 5.2 months in RSV patients. Low fever was more common in children with RSV than MP infection, and high fever was more common in MP than RSV infection. SaO2 < 90% was more common in children with RSV than MP infection. Thrombocytosis, increased ALT and CK-MB were more common in children with MP infection than those with RSV infection. Pulmonary interstitial infiltration was more common in children infected with MP than those with RSV (Figure 2). Emphysema was more common in children with RSV than those with MP infection (Figure 3). The average hospital stay was longer in children with RSV than in those with MP infection (Table 2).
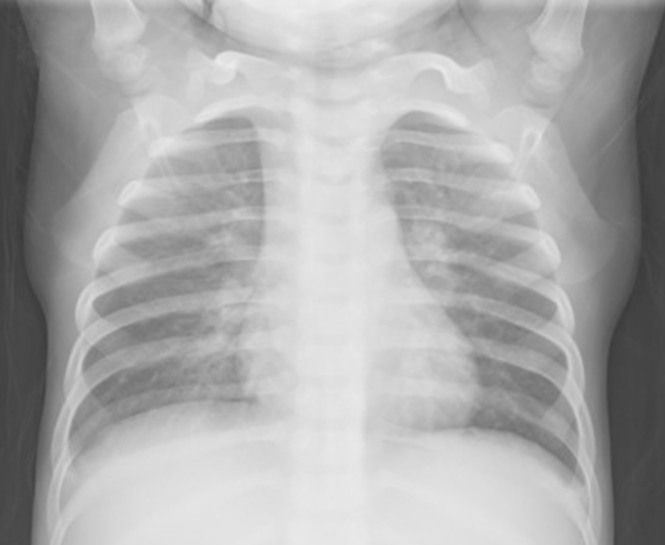
Figure 2. Radiographic characteristics of a patient with Mycoplasma bronchiolitis.
Thickened lung markings accompanied by fuzzy, messy, reticular high-density shadows, with prominence in the hilar region are seen on this chest X-ray.
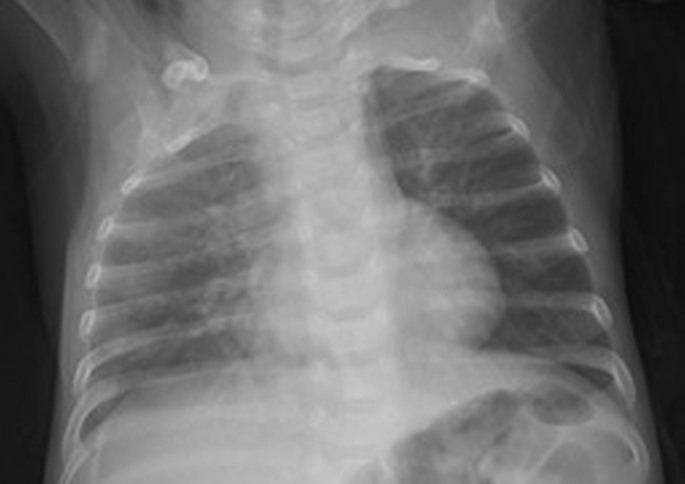
Figure 3. Radiographic characteristics of a patient infected with respiratory syncytial virus.
The chest X-ray shows increased brightness in both right and left lungs.
Table 2. Characteristics of patients with bronchiolitis caused by MP or RSV.
| Characteristic | MP (n = 116), n (%) | RSV (n = 343), n (%) | χ2/Z test | P |
|---|---|---|---|---|
| Mean age [months (range)] | 10.0 (6.0–15.0) | 5.2 (3.0–9.0) | 8.11 | <0.001 |
| <6 months | 22 (19) | 208 (60.6) | 60.224 | <.0001 |
| 6 months–1 year | 56 (48.3) | 84 (24.5) | 23.1362 | <.0001 |
| 1–2 years | 38 (32.7) | 51 (14.9) | 17.7494 | <.0001 |
| Sex | ||||
| Male | 60 (51.7) | 257 (74.9 | 21.84 | <0.001 |
| Female | 56 (48.3) | 86 (25.1) | 21.8426 | <0.0001 |
| Fever (°C) | 38 (32.8) | 92 (26.8) | 1.51 | >0.05 |
| <38 | 1 (5.6) | 80 (23.3) | 30.09 | <0.001 |
| 38–39 | 5 (13.1) | 11 (3.2) | 0.07 | >0.05 |
| >39 | 32 (27.6) | 1 (0.3) | 96.78 | <0.001 |
| Symptom | ||||
| Cough | 116 (100) | 340 (99.1) | Fisher's | 0.5751 |
| Wheezing | 116 (100) | 343 (100) | Fisher's | 1 |
| Tachypnea | 13 (11.2) | 98 (28.6) | 14.2557 | 0.0002 |
| Dyspnea | 13 (11.2) | 86 (25.1) | 9.8521 | 0.0017 |
| Vomiting/diarrhea | 33 (28.4) | 67 (19.8) | 4.0429 | 0.0444 |
| Physical examination | ||||
| Lung wheezing rales | 89 (76.7) | 270 (79.6) | 0.2021 | 0.6531 |
| Lung crackles | 9 (7.8) | 32 (9.3) | 0.2629 | 0.6081 |
| SaO2 < 90% | 7 (6.0) | 89 (25.9) | 20.78 | <0.001 |
| Lab tests | ||||
| WBC (×109/L) ± sem | 7.7 ± 3.5 | 7.2 ± 2.9 | 9.84 | >0.05 |
| CRP (mg/L) ± sem | 8.5 ± 3.8 | 7.6 ± 2.3 | 10.02 | >0.05 |
| Platelet count > 400 × 109/l | 79 (68.1) | 109 (31.7) | 15.3 | <0.05 |
| ALT↑ | 30 (25.8) | 31 (9) | 21.29 | <0.001 |
| CK-MB↑ | 41 (35.5) | 33 (9.6) | 42.42 | <0.001 |
| Chest X-ray | ||||
| Patchy shadow | 14 (12.1) | 68 (19.8) | 3.55 | >0.05 |
| Pulmonary emphysema | 19 (16.4) | 231 (67.3) | 90.79 | <0.001 |
| Pulmonary interstitial infiltration | 77 (66.4) | 44 (12.8) | 128.06 | <0.001 |
| Mean hospital stay [days (range)] | 7.0 (6.3–9.0) | 8.0 (7.0–9.0) | 5.73 | <0.001 |
| Medical history | ||||
| Premature birth | 18 (15.5) | 40 (11.7) | 1.17 | >0.05 |
| Breast feeding | 76 (65.5) | 198 (57.7) | 2.19 | >0.05 |
| Eczema | 38 (32.8) | 105 (30.6) | 0.19 | >0.05 |
| Family history of asthma | 14 (12.1) | 34 (9.9) | 0.43 | >0.05 |
Data are expressed as number of patients (%) unless otherwise indicated.
MP, Mycoplasma pneumonia; RSV, respiratory syncytial virus; WBC, white blood cell counts; CRP, C-reactive protein; ALT, alanine transaminase; CK-MB, creatine kinase-MB.
Discussion
In this study, MP was the second most frequently identified bronchiolitis pathogen after RSV, found in 17.2% of the cases. The detection rate of MP was higher than that in previous reports. Liu WK et al.15 reported an MP-positive rate of 11.3% in children with acute respiratory infections in Guangzhou. Pientong et al.16 studied 170 bronchiolitis cases with an MP detection rate of 8.2%. Our data suggest that the proportions of bronchiolitis cases in infants that are caused by MP, and the importance of MP as a bronchiolitis pathogen, are increasing.
We note that a PCR assay may reveal small numbers of organisms that are not the cause of infection. Therefore, a highly sensitive detection method such as non-quantitative PCR may overestimate the clinical importance of M pneumoniae as a pathogen. The results obtained with the method as used here (i.e., qRT-PCR, Ct curves) depended on the amount of target sequence in the starting (clinical) sample, and although there is no agreement on the CCU/ml indicative of infection, a cutoff value was chosen based on the available published data. The reasons we considered these patients were infected rather than colonized by M. pneumoniae are as follows. 1) The patients were being treated for a current diagnosis of bronchiolitis. All had ongoing clinical manifestations of lower respiratory tract infection. 2) We used quantitative PCR to detect MP-DNA in patient sputum. 3) The cutoff value for the detected copy number in the MP-PCR assay was set at >105 CCU/ml for MP infection. A specific threshold for Mycoplasma in the respiratory tract that can differentiate colonization from infection has not been established, however a cutoff value for the detected copy number in the MP-PCR assay was set at >105 CCU/ml. We believe that <102 CCU/ml is generally considered as indicative of colonization17,18. Skakni et al. used a semiquantitative PCR technique to detect M. pneumoniae DNA in clinical samples, and reported high loads (≥102 to ≥104 CCU/ml) of M. pneumoniae were found in 8 of 10 patients with acute pneumonia, and low loads (<102 CCU/ml) in were found in samples from asymptomatic patients17. Kleemola et al.18 used a commercial Gen-Probe probe test during an epidemic of M. pneumoniae infections among army conscripts. Comparison of the probe test results with the Mycoplasma culture and serologic results showed that the probe test was sensitive and specific for the rapid diagnosis of acute M. pneumoniae infection of the lower respiratory tract when sputum was used. It had good sensitivity (0.95) and specificity (0.85) among patients whose serologic results were consistent with their culture results18.
This is the largest study of MP-caused bronchiolitis in infants and included a series of patients in the Suzhou region over 3 consecutive years. Our data revealed that MP can be detected throughout the year with a peak prevalence between July and September each year, suggesting that the epidemic MP season in the Suzhou region is in summer and early autumn. A previous epidemiological study by Ji et al is consistent with the seasonal pattern presented in this study19. Our previous study suggested that the MP detection rate was positively correlated with environmental temperature. The higher the temperature, the higher the positivity rate20, which is consistent with other reports21,22. In temperate regions, outbreaks of MP pneumonia commonly occur in summer and early autumn. Respiratory tract infections caused by other pathogens are relatively infrequent at that time. Contrary to this finding, Hadil et al reported a higher prevalence of MP in autumn and only a few cases in winter and spring5. MP was not detected in summer. Defilippi et al.4 reported the first MP peak in June, and a second peak in December and January. Sidal et al.23 reported the highest prevalence of MP was in winter. But one report including data collected over 11 consecutive years showed that the prevalence of MP had no obvious seasonal differences24. Overall, the available studies suggest that the epidemiology of MP differs from region to region because of differences in climate.
Previous studies suggested that MP infections occurred mainly in school-age children and adolescents, with the highest prevalence in patients between 5 and 14 years of age25. MP infection in infants was relatively rare26. However, this study found that MP bronchiolitis was seen mainly in infants from 6 months to 1 year of age and had a detection rate 23.9% in that group of patients. Evidence that MP infection of infants is increasing comes from reports that 21.6% of patients 2 years of age had throat swabs that were MP-positive4. The highest prevalence age of MP infection ranges from 6 months to 1 year of age16. The data obtained in this study are consistent with those reports.
Cough, wheezing, and moderate to high fever of short duration were the main clinical manifestations of MP infection. Elevated ALT, increased myocardial enzymes, thrombocytosis and urticaria can appear in some patients. Chest X-ray examination shows pulmonary interstitial lesions, and both lungs can be involved. Xia et al.27 reported that wheezing and low fever are very common in infants with MP infection. Pulmonary signs and gastrointestinal involvement are common. A recent report suggests that thrombocytosis is very common in children with MP infection and may be related to different stages of inflammation28. In that series, 8% of children had elevated blood platelet counts when admitted to hospital, and 33% had elevated blood platelets on discharge. Similarly, in this study, 68.1% of children with MP infection had thrombocytosis.
Comparing MP with RSV bronchiolitis, Children with RSV-caused bronchiolitis were younger than those with MP infection. Fever was usually absent or mild in RSV-infected patients, but tachypnea, dyspnea, cyanosis was very common and extrapulmonary involvement was infrequent in those patients. Due to relatively severe symptoms of RSV infection in children, oxygen therapy is usually required, and the hospital stay is relatively long. Overall RSV infection causes more severe symptoms in children than MP does. The study results are consistent with the symptoms described by others16. We found that extrapulmonary complications, including thrombocytosis, and increased ALT and CK-MB, were more frequent in MP than in RSV infection. Development of autoantibodies is the main cause of extrapulmonary complications in patients with MP infection. Moreover, the chest X-ray had different features in patients with MP and RSV infection. Pulmonary interstitial infiltration was more common in patients with MP infection. Conversely, emphysema was more common in RSV infection. These findings are important in the differential diagnosis of MP and RSV infection.
The major limitation of this study was that serological examinations of MP and other pathogens were absent. Another limitation was the absence of asymptomatic control patients. There was also no standard scoring system to distinguish the different patterns in chest X-ray examinations of patients. We plan to conduct a more extensive study in the near future to address these issues.
Methods
Approvals
All experiments were performed following the relevant guidelines and regulations of Soochow University. The methods were carried out in accordance with the approved guidelines. The study was approved by the Medical Ethics Committee of Soochow University (No. Sdfey201005). The parents of all study participants gave both verbal and written informed consent before study enrollment.
Patients
This retrospective study was conducted from January 2010 to December 2012 in pediatric patients at the Department of Respiratory Disease of the Affiliated Children's Hospital, Suzhou University. The diagnosis of bronchiolitis was based on the following criteria29. (1) The disease occurred within two years of birth. (2) The onset was acute, accompanied by wheezing and dyspnea, and a previous history of upper respiratory tract infection. (3) The patient presented with restlessness, increased respiration and heart rate, nasal symptoms, and cyanosis. (4) Physical examination revealed widespread double lung wheeze during the onset of wheezing, accompanied by fine rales or crepitus. Patients with congenital heart disease, immune deficiency, bronchus, or pulmonary dysplasia were excluded in this study.
Sputum specimen collection
Nasopharyngeal secretions were collected from each study participant within 24 h after admission by a lab technician as previously described. Briefly, an aseptic plastic sputum catheter was inserted into the nostril to a depth of about 7–8 cm until reaching the pharynx. Approximately 2 ml of nasopharyngeal secretions was collected by applying negative pressure. The sample was mixed with 4–8 ml PBS, and centrifuged for 10 minutes at 300–500 rpm. The supernatant was discarded and the pellet was mixed with 4–8 ml PBS and centrifuged for an additional 10 minutes. The pellet was stored at −80°C until testing began.
Sputum MP-DNA detection and evaluation
DNA lysate (Shanghai Shenyou biotechnology company, Shanghai, China) was added to the sputum pellet following washing with PBS. The sample was heated to at 95°C for 10 min, centrifuged for 5 min at 12 000 rpm, and then the supernatant was collected. After extracting the DNA from the sputum specimen, MP DNA was detected by fluorescent real-time PCR (BIO-RAD iCycler, USA). The cyclic temperature settings were 93°C, 2 min; 93°C, 45 s; 55°C, 60 s → 10 cycles; 93°C 30 s → 55°C, 45 s → 30 cycles. The fluorescence collection point was set at the 55°C, 45 s. Ct value was used to quantify the fluorescence quantitative PCR results. The primer sequences and MP probe are shown in Table 3. The probe binding sequence was located between the upstream and downstream primer. The fluorescent reporter dye at the 5′ end of probe was 6-carboxyfluorescein (FAM), and the quencher at the 3′ end of the probe was 6-carboxytetramethylrhodamine (TAMRA). The primers and probe were purchased from Guangzhou Daan Gene Ltd. (Guangzhou, China). An MP-negative sample was defined as having an amplification curve that was not S-shaped or a Ct value = 30. Both results indicated that the MP DNA content was below the detection limit. A positive MP sample was defined as having an amplification curve was S-shaped and a Ct value < 30. The DNA content of the sputum was determined by the following criteria. If the sample C < 5.00 × 102, the DNA content was <2.5 × 103 gene copies/ml; if 5.00 × 102 ≤ C ≤ 5.00 × 108, the DNA content = 5 × 103 gene copies/ml; and if C > 5.00 × 108, the DNA content was >5 × 103 gene copies/ml.
Table 3. Amplification of MP, hMPV and hBoV genes.
| Gene | Sequence | Size (bp) | |
|---|---|---|---|
| MP | Forward | 5′-CCA ACCAAA CAA CAA CGT TCA-3′ | 76 |
| Reverse | 5′-ACC TTG ACTGGA GGC CGT TA-3′ | ||
| Probe | 5′-FAM-TCA ACT CGA ATA 'ACG GTG ACTTCT TAC CAC TG-3′-TAMRA | ||
| hMPV | Forward | 5′-AACCGTGTACTAAGTGATGCACTC-3′ | 213 |
| Reverse | 5′-CATTGTTTGACCGGCCCCATAA-3′ | ||
| hBoV | Forward | 5′-TGACATTCAACTACCAACAACCTG-3′ | 92 |
| Reverse | 5′-CAGATCCTTTTCCTCCTCCAATAC-3′ | ||
| Probe | 5′-FAM-AGCACCACAAAACACCTCAGGGG-3′-TAMRA |
MP, Mycoplasma pneumonia; hMPV, human metapneumovirus; hBoV, human bocavirus; FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Sputum respiratory virus detection
Direct immunofluorescence was used to detect syncytial virus infection (RSV), influenza virus A (IVA), influenza virus B (IVB), parainfluenza virus (PIV) I, PIV II, PIV III, and adenovirus (ADV). All assay kits were purchased from Chemicon (USA) and all staining procedures were performed according to the manufacture's instructions. Immunostained preparations were viewed with a fluorescence microscope (Leica 020-518.500, Germany).
RNA extraction and real-time PCR to detect the human metapneumovirus (hMPV) gene
RNA was extracted from sputum specimens using Trizol (Invitrogen, USA). cDNA was synthesized by reverse transcription. The cyclic temperature settings were 94°C, 30 s; 55°C, 30 s; 68°C, 30 s; amplified by 45 cycles with the last at 68°C for 7 min. hMPV was assayed by fluorescent real-time PCR (BIO-RAD iCycler). The cyclic temperature settings were 94°C, 30 s; 56°C, 30 s; 72°C, 30 s; amplified, 40 cycles. The primer sequences for hMPV are shown in Table 3.
DNA extraction and real-time PCR to detect the human bocavirus (hBoV) gene
Sputum DNA was extracted as described above, and hBoV-DNA was detected by real-time fluorescent PCR. The cyclic temperature settings were 94°C, 30 s; 56°C, 30 s; 72°C, 30 s; amplified by 40 cycles. The primer sequences and hBoV probe are shown in Table 3.
Statistical analysis
All data were analyzed using PASW 20.0 statistical software (IBM, USA). The comparisons among groups were performed using the chi square test. For data that did not meet the conditions of the chi square test, Fisher's exact probability test was used. Data with nonnormal distributions, were expressed as medians and quartile ranges (M; P25 P75) and differences were evaluated using the Mann–Whitney U test. P < 0.05 was considered significant.
Author Contributions
Y.Q.W. and C.L.H. wrote the main manuscript text and W.J. and Y.D.Y. collected and analyzed data. X.J.S. and J.X. detected Mycoplasma pneumoniae. All authors reviewed the manuscript.
Acknowledgments
This work was supported by Science and Technology Projects of Jiangsu Medical Department (Grant No. H201315).
References
- Zentz S. E. Care of infants and children with bronchiolitis: a systematic review. J Pediatr Nurs 26, 519–529 (2011). [DOI] [PubMed] [Google Scholar]
- Hervas D. et al. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis 31, 1975–1981 (2012). [DOI] [PubMed] [Google Scholar]
- Miller E. K. et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J 32, 950–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi A. et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med 102, 1762–1768 (2008). [DOI] [PubMed] [Google Scholar]
- Hadi N., Kashef S., Moazzen M., Pour M. S. & Rezaei N. Survey of Mycoplasma pneumoniae in Iranian children with acute lower respiratory tract infections. Braz J Infect Dis 15, 97–101 (2011). [PubMed] [Google Scholar]
- Hoffmann J. et al. Viral and atypical bacterial etiology of acute respiratory infections in children under 5 years old living in a rural tropical area of Madagascar. PLoS One 7, e43666 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libre J. M., Urban A., Garcia E., Carrasco M. A. & Murcia C. Bronchiolitis obliterans organizing pneumonia associated with acute Mycoplasma pneumoniae infection. Clin Infect Dis 25, 1340–1342 (1997). [DOI] [PubMed] [Google Scholar]
- Natori H. et al. Organizing pneumonia associated with Mycoplasma pneumoniae infection. Jpn J Radiol 28, 688–691 (2010). [DOI] [PubMed] [Google Scholar]
- Watanabe H., Uruma T., Nakamura H. & Aoshiba K. The role of Mycoplasma pneumoniae infection in the initial onset and exacerbations of asthma. Allergy Asthma Proc 35, 204–210 (2014). [DOI] [PubMed] [Google Scholar]
- Cosentini R. et al. Severe asthma exacerbation: role of acute Chlamydophila pneumoniae and Mycoplasma pneumoniae infection. Respir Res 9, 48 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. J. The Role of Mycoplasma pneumoniae Infection in Asthma. Allergy Asthma Immunol Res 4, 59–61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung R. Y., Chan R. C., Tam J. S., Cheng A. F. & Murray H. G. Epidemiology and aetiology of acute bronchiolitis in Hong Kong infants. Epidemiol Infect 108, 147–154 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra P. G. et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS One 6, e18928 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Subcommittee on, D. & Management of, B. Diagnosis and management of bronchiolitis. Pediatrics 118, 1774–1793 (2006). [DOI] [PubMed] [Google Scholar]
- Liu W. K. et al. Epidemiology of acute respiratory infections in children in Guangzhou: a three-year study. PLoS One 9, e96674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pientong C. et al. Atypical bacterial pathogen infection in children with acute bronchiolitis in northeast Thailand. J Microbiol Immunol Infect 44, 95–100 (2011). [DOI] [PubMed] [Google Scholar]
- Skakni L. et al. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J Clin Microbiol 30, 2638–2643 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemola S. R., Karjalainen J. E. & Raty R. K. Rapid diagnosis of Mycoplasma pneumoniae infection: clinical evaluation of a commercial probe test. J Infect Dis 162, 70–75 (1990). [DOI] [PubMed] [Google Scholar]
- Ji W. et al. Etiology of acute respiratory tract infection in hospitalized children in Suzhou from 2005 to 2011. Zhonghua Yu Fang Yi Xue Za Zhi 47, 497–503 (2013). [PubMed] [Google Scholar]
- Chen Z. et al. Epidemiology and associations with climatic conditions of Mycoplasma pneumoniae and Chlamydophila pneumoniae infections among Chinese children hospitalized with acute respiratory infections. Ital J Pediatr 39, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke L. Q., Wang F. M., Li Y. J. & Luo Y. C. Epidemiological characteristics of Mycoplasma pneumoniae pneumonia in children. Zhongguo Dang Dai Er Ke Za Zhi 15, 33–36 (2013). [PubMed] [Google Scholar]
- Waites K. B. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol 36, 267–278 (2003). [DOI] [PubMed] [Google Scholar]
- Sidal M. et al. Frequency of Chlamydia pneumoniae and Mycoplasma pneumoniae infections in children. J Trop Pediatr 53, 225–231 (2007). [DOI] [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K. & Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis 139, 681–687 (1979). [DOI] [PubMed] [Google Scholar]
- Nolevaux G. et al. Epidemiological and clinical study of Mycoplasma pneumoniae respiratory infections in children hospitalized in a pediatric ward between 1999 and 2005 at the Reims University Hospital. Arch Pediatr 15, 1630–1636 (2008). [DOI] [PubMed] [Google Scholar]
- Othman N., Isaacs D. & Kesson A. Mycoplasma pneumoniae infections in Australian children. J Paediatr Child Health 41, 671–676 (2005). [DOI] [PubMed] [Google Scholar]
- Xia Y., Wu C. K., Tang Y. Y. & Cao J. Differences in the clinical features of Mycoplasma pneumoniae pneumonia among children of different ages. Zhongguo Dang Dai Er Ke Za Zhi 15, 179–182 (2013). [PubMed] [Google Scholar]
- Youn Y. S. et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr 10, 48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. M. & Jiang Z. F. [Bronchiolitis]. Zhu Futang Practical Pediatrics [Hu, Y. M. (ed.)][1163–1199] (Peoples Health Publishing House, Beijing, 2002). [Google Scholar]