Abstract
Nonstructural protein 1 (NS1) is secreted by dengue virus in the first days of infection and acts as an excellent dengue biomarker. Here, the direct electrical detection of NS1 from dengue type 2 virus has been achieved by the measurement of variations in open circuit potential (OCP) between a reference electrode and a disposable Au electrode containing immobilized anti-NS1 antibodies acting as immunosensor. Egg yolk immunoglobulin (IgY) was utilized for the first time as the biological recognition element alternatively to conventional mammalian antibodies in the detection of dengue virus NS1 protein. NS1 protein was detected in standard samples in a 0.1 to 10 µg.mL−1 concentration range with (3.2 ± 0.3) mV/µg.mL−1 of sensitivity and 0.09 µg.mL−1 of detection limit. Therefore, the proposed system can be extended to detect NS1 in real samples and provide an early diagnosis of dengue.
Dengue is an infectious disease caused by a Flavivirus with four different serotypes transmitted among humans by a mosquito of the Aedes genus mainly in tropical and subtropical regions1. In some cases, dengue develops into severe forms, such as shock (dengue shock syndrome) and haemorrhage1. Although dengue has been intensively studied, its diagnosis can be difficult due to the non-specific symptoms. According to recent studies, many cases of dengue have been underestimated four times more than the confirmed cases2, indicating that this disease lacks effective identification methods, mainly in the first days of infection when the symptoms are commonly mistaken for other infectious diseases2.
Presently, the detection of dengue virus nonstructural protein 1 (NS1) is the preferred method for an early dengue diagnosis because it is secreted by dengue virus in the first days of infection3, and also because NS1 can be detected in patients with both primary and secondary dengue infections up to 9 days after the onset of the infection4. Conventional tests to detect NS1 include Enzyme Linked Immunosorbent Assay (ELISA)4, which has been adopted as a routine test, as well as the Polymerase Chain Reaction (PCR) method5. However, the latter techniques are not well suited for a rapid test for NS1 detection since they are multi-steps, expensive methods, requiring trained personnel for implementation.
On the other hand, immunosensors are promising devices used to detect antigens in a simple, rapid and economical way. Immunosensors comprise biosensors based on specific antigen-antibody interactions. Usually antibodies are immobilized on a solid support (transducer) in order to detect either directly or indirectly the specific antigen6.
Over the past years, some research groups have devoted efforts to propose immunosensors to detect NS1 protein and consequently provide a diagnosis of dengue. Some immunosensors that detect NS1 using different materials and methodologies can be found in the literature, including optical7, piezoelectric8 and electrochemical methods9,10. In all cases, antibodies from mammalians, such as immunoglobulin G (IgG) are used as biological recognition elements (receptors) in the immunosensor configuration for the specific recognition of NS1. However, egg yolk immunoglobulin (IgY) can also be used as a receptor in immunoassays. Structurally, the IgY molecule exhibits the same form as IgG, with both containing heavy and light variable chains and constant domains, but IgY has a heavier domain, hence, a slightly higher molecular weight11,12. These antibodies represent an alternative to conventional antibodies from mammalian blood. They have been obtained in a non-invasive procedure and purified in larger amounts from chicken eggs11,12. Moreover, the recognition of epitopes by IgY antibodies is also higher in comparison with the IgG antibodies for the same antigen11,12, which makes IgY an ideal system to be applied in immunosensors.
Sudjarwo et. al. have purified and characterized IgY antibodies with potential application in diagnostic kits of dengue13. In this case, laying hens were immunized intramuscularly with inactivated dengue virus, creating antibodies against all virus proteins13. This approach, although effective, may increase the risk of non-specific reactions in dengue immunoassays since various IgY antibodies are produced for different viral proteins. On the other hand, when laying hens are immunized with only NS1 protein, IgY antibodies are created only against NS1, increasing the specificity of the immunoassays.
Here, we have detected NS1 protein from dengue type 2 virus using IgY antibodies from chicken as a new biological recognition element. The measurement system, i.e., a potentiometric immunosensor comprises a disposable Au electrode containing immobilized anti-NS1 IgY antibodies. A high accuracy instrumentation amplifier was applied as a readout circuit of antibody-antigen interactions. The disposable electrode was characterized by electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). The immunosensor measurements provided an efficient detection of NS1 protein.
Results
Characterization of the electrode
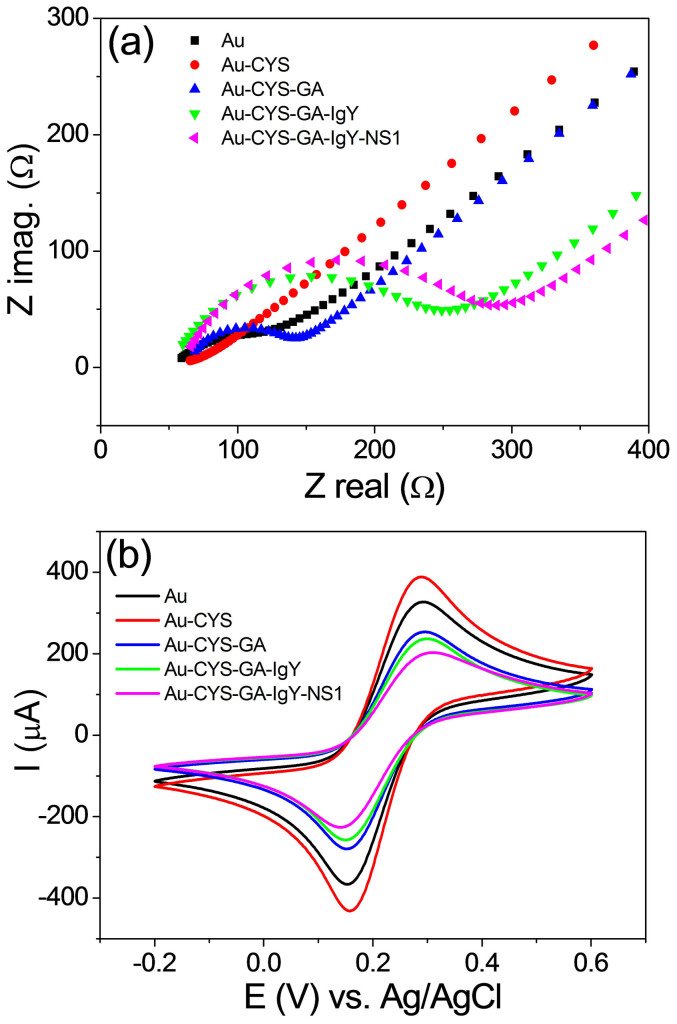
The electrodes were characterized by EIS and CV in order to verify the steps of the immobilization process. EIS and CV measurements were taken in the presence of 1.0 mM [Fe(CN)6]3−/4− in a 0.1 M KCl solution. Figure 1 shows the Nyquist diagrams (a) and the cyclic voltammograms (b) obtained using the Au electrode and the modified Au electrode: Au-CYS, Au-CYS-GA, Au-CYS-GA-IgY and Au-CYS-GA-IgY-NS1. The black diagram ( ) and voltammogram (—) obtained using the Au electrode presented a very low resistance, suggesting a fast electron transfer process of Fe(CN)63−/4− at the electrode surface. The red diagram (
) and voltammogram (—) obtained using the Au electrode presented a very low resistance, suggesting a fast electron transfer process of Fe(CN)63−/4− at the electrode surface. The red diagram ( ) and voltammogram (
) and voltammogram ( ) correspond to the formation of the SAM of CYS on the Au electrode and show a predominance of the diffusive effect over the resistive one. This effect is due to amine protonation, which confers a positive charge layer, promoting the attraction of FeCN6−3/−4 and, hence, a small decrease in the resistance (impedance) and an increase of the peak currents (voltammetry). The following immobilization steps, corresponding to the attachment of GA [blue diagram and voltammogram(
) correspond to the formation of the SAM of CYS on the Au electrode and show a predominance of the diffusive effect over the resistive one. This effect is due to amine protonation, which confers a positive charge layer, promoting the attraction of FeCN6−3/−4 and, hence, a small decrease in the resistance (impedance) and an increase of the peak currents (voltammetry). The following immobilization steps, corresponding to the attachment of GA [blue diagram and voltammogram( ,
,  )], IgY antibodies [green diagram and voltammogram (
)], IgY antibodies [green diagram and voltammogram ( ,
,  )] and NS1 antigen [pink diagram and voltammogram (
)] and NS1 antigen [pink diagram and voltammogram ( ,
,  )] showed a growth of the resistive arch (impedance) and a decrease in the oxidation and reduction peaks (voltammetry). The difference in these diagrams/voltammograms can be related to each immobilization step, since the molecules have blocked the electron transfer between the disposable Au electrode surface and the electrolyte.
)] showed a growth of the resistive arch (impedance) and a decrease in the oxidation and reduction peaks (voltammetry). The difference in these diagrams/voltammograms can be related to each immobilization step, since the molecules have blocked the electron transfer between the disposable Au electrode surface and the electrolyte.
Figure 1. Nyquist diagrams at a potential of 0.22 V in the presence of 1.0 mM [Fe(CN)6]3−/4− in a 0.1 M KCl of Au electrode ( ), Au-CYS (
), Au-CYS ( ), Au-CYS-GA (
), Au-CYS-GA ( ) Au-CYS-GA-IgY (
) Au-CYS-GA-IgY ( ) and Au-CYS-GA-IgY-NS1 (
) and Au-CYS-GA-IgY-NS1 (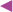 ) (a).
) (a).
Cyclic voltammograms obtained for the Au electrode ( ), Au-Cys (
), Au-Cys ( ), Au-Cys-GA (
), Au-Cys-GA ( ) Au-Cys-GA-Ab (
) Au-Cys-GA-Ab ( ) and Au-Cys-GA-IgY-NS1 (
) and Au-Cys-GA-IgY-NS1 ( ) at 100 mV s-1 in the presence of 1.0 mM [Fe(CN)6]3-/4– in 0.1 M KCl (b).
) at 100 mV s-1 in the presence of 1.0 mM [Fe(CN)6]3-/4– in 0.1 M KCl (b).
pH sensitivity of the unmodified electrode
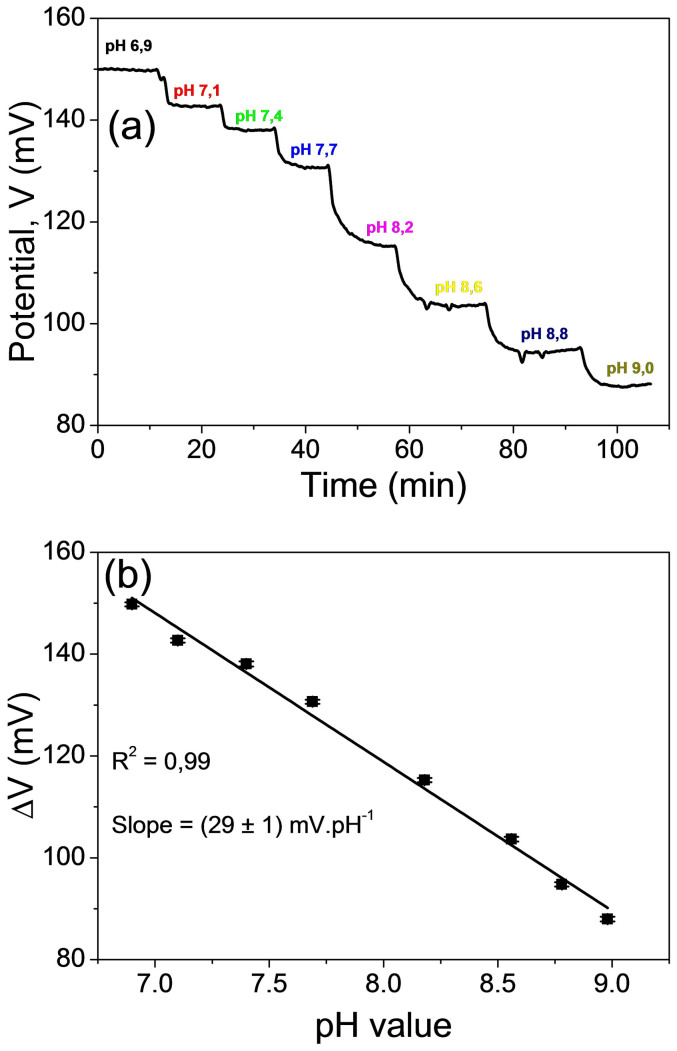
Since our system represents a surface-charge measuring, a study of pH sensitivity is very important. Measurements of the pH sensitivity were performed by subsequent additions of NaOH (0.1 M) at a certain time interval and using the disposable Au electrode without modification with antibodies. A pH meter was placed in contact with the solution (0.01X PBS). Changes in the pH value were recorded with the changes in the output potential in the course of time. Figure 2a shows the dynamic response of the unmodified disposable Au electrode to the pH changes. A decrease in the output potential due to an increase in the negative charges in the solution was observed. In fact, the addition of NaOH leads to an increase in negative charges (OH−) in the solution and a reduction in the positive charges (H+) on the surface of the disposable Au electrode. In other words, the positive ions recombine with the negative ones, therefore, the density of the positive charges decreases, decreasing the potential on the Au electrode surface. A pH sensitivity of (29 ± 1) mV.pH−1 was obtained (Fig. 2b), which is in agreement with the literature14,15.
Figure 2.
(a) Dynamic response of the unmodified gold electrode upon successive additions of NaOH. (b) pH sensitivity of the unmodified gold electrode.
NS1 protein detection
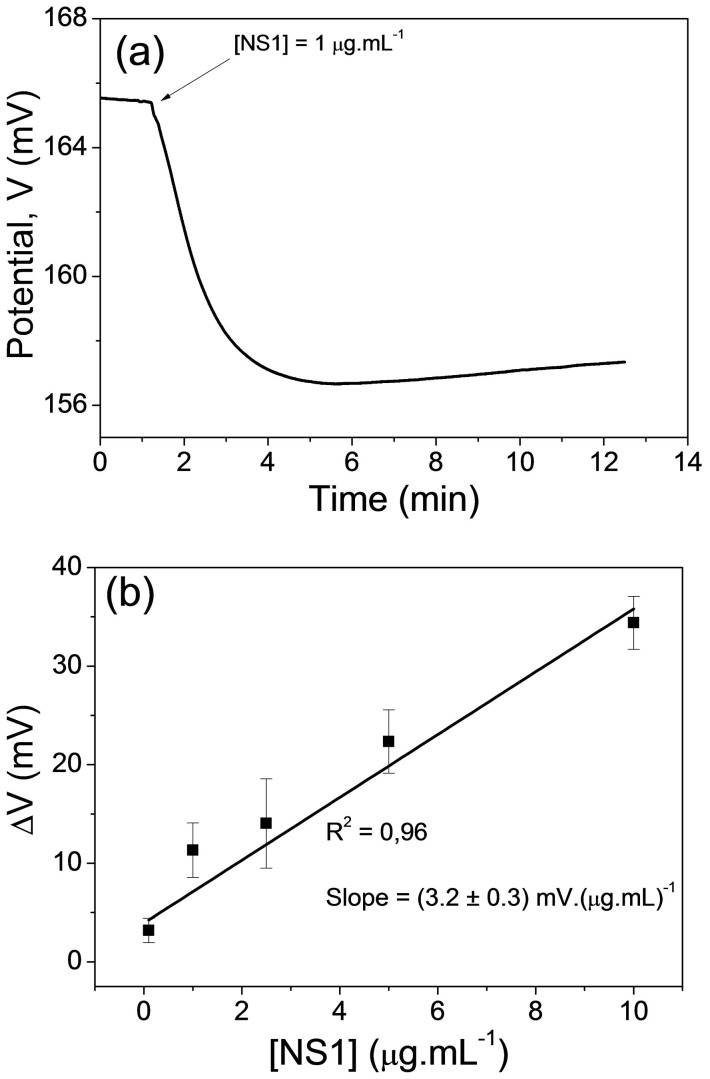
Figure 3a shows the dynamic response of the immunosensor for the detection of 1 μg.mL−1 NS1. The behaviour of the output potential over time for the NS1 detection is similar to that observed for the detection of OH− ions (Fig. 2a), which exhibit a decrease due to the accumulation of negative charge on the active layer of the disposable Au electrode. In this case, OCP variation is due only to IgY-NS1 interactions since the NS1 was solubilized in 0.01X PBS. The isoelectric point of NS1 protein is approximately 5.716. As NS1 is negatively charged at pH 7.4, it induces negative charges in the immobilized IgY antibodies on the disposable Au electrode when NS1-IgY coupling occurs.
Figure 3.
(a) Dynamic response of the proposed immunosensor upon the addition of 1 mg.mL−1 NS1 in the measuring cell. (b) Analytical curve of the FET immunosensor. Measurement conditions: 0.01X PBS, 25°C.
Measurements were taken at various NS1 concentrations (0.1 to 10 µg.mL−1) and the analytical curve of the proposed immunosensor was obtained. The OCP variation (ΔV) from the point of injection of NS1 up to the stabilization point of the potential after its decrease was used as a standard. This curve is shown in Figure 3b, where the error bars represent the average of three measurements (each measurement corresponded to a different electrode). The analytical curve shows a sensitivity of (3.2 ± 0.3) mV.µg.mL−1 and a correlation coefficient (R2) of 0.96. The limit of detection was 0.09 µg.mL−1(obtained by using three times the standard deviation of the signal blank/slope of the calibration curve).
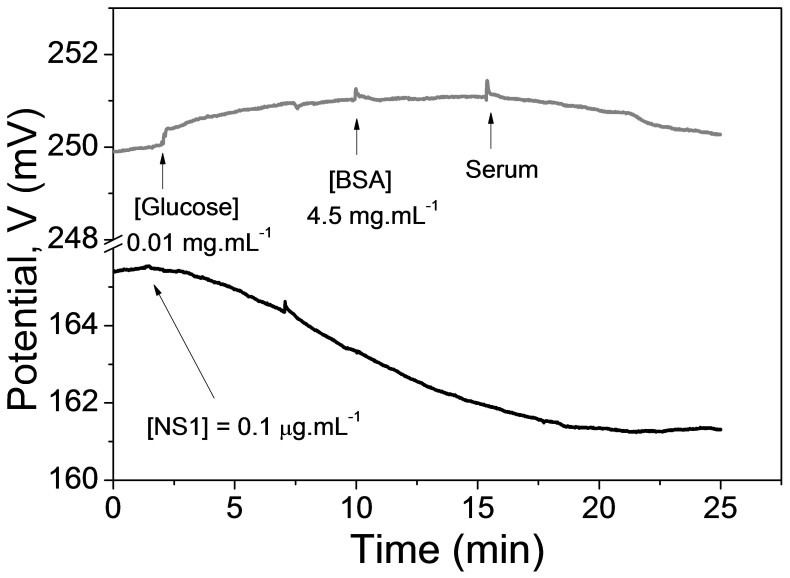
Negative tests with other substances were performed using BSA, glucose and desalted human serum samples. The study was approved by the Ethics Committee of the Federal University of São Carlos (UFSCar) in keeping with the principles enunciated in the Declaration of Helsinki. Figure 4 shows the response of the immunosensor after successive additions of these substances (gray line). The signal containing the response to the detection of the lowest NS1 concentration is shown for comparison (black line). The Y-axis was broken and shifted so as to reveal the effects on the response. The response to BSA, glucose and serum is very small and can be neglected in comparison to the detection of NS1. It is noteworthy that the signal related to the human serum that is rich in protein is also small, even for a long period of time.
Figure 4. Dynamic response of the proposed immunosensor upon the addition of interfering substances.
The signal containing the response to the detection of NS1 is shown for comparison (black) and the Y-axis was broken so as to reveal the effects on the response.
Discussion
The attachment of IgY antibodies on the Au electrode surface were demonstrated in the Au electrode characterization study. Electrochemical measurements (EIS and CV) validated the immobilization protocol, including the antibody-antigen interaction (Fig. 1). According to the literature, the detection of antigen-antibody interactions by a potentiometric immunosensor is possible with two restrictions17: (i) measurements must be taken in low ionic strength solutions, providing a high Debye length and preventing the screening effect of the counter-ions of the solution and (ii) electrodes that present non-Nernstian behaviour (pH sensitivity < 59.15 mV.pH−1) must be used. All measurements in the immunosensor were taken using a low ionic strength PBS solution (PBS diluted 100 times or 0.01X PBS). In this condition, a Debye length (λD) of ca. 7.3 nm is achieved18. It is noteworthy that 29 mV.pH−1 is sub-Nernstian (Fig. 2b), a crucial requirement according to the theory for the construction of potentiometric immunosensors17.
In the proposed immunosensor, the output signal (OCP variation) is due only to IgY-NS1 interactions since the NS1 was solubilized in 0.01X PBS. The proposed immunosensor showed high specificity to NS1 (Fig. 4). This fact can be related to the genetic differences in species (human/chicken) when IgY antibodies are used as biological recognition elements. Since NS1 circulates in high levels in the blood of patients infected with dengue3,4,19,20 and the analytical curve covers this range of NS1 levels, the proposed immunosensor can provide a positive result even in mild cases of infection.
PCR method has been applied in dengue diagnosis in a very efficient way5,21. However, as previously mentioned, PCR is not appropriate for a rapid, simple and routinely test due to its needs specialized equipment, people, expense of time and presents a high cost when compared with the our proposed NS1 sensitive electrode.
Recently, Jahanshahi and coworkers22 have developed a new method based on surface plasmon resonance (SPR) for the determination of the anti-dengue virus (IgM) in human serum samples, with a response time of 10 min. The authors stated that only 1 µl of sample from a dengue patient is necessary. However, it is important to mention that the SPR apparatus may not be cost-effective if compared with the system presented here.
For sake of comparison, we shall highlight some aspects from recent published papers for detection of NS1: The disposable compact disk biosensor proposed by Cavalcanti and coworkers9 presented a detection limit of 0.33 ng.mL−1. In this case, however, a potentiostat is still necessary. Dias and coworkers10 have proposed a NS1 protein immunosensor based on carbon nanotube-ink printed electrode and the anti-NS1 antidodies using a potentiostat/galvanostat as measurement system. In contrast to the our methodology, which detects NS1 directly, the system proposed by Dias and coworkers refers to an indirect amperometric detection in which the signal was generated by a catalytic reaction from the peroxidase enzyme (conjugated with anti-NS1) and H2O2. This system presented a detection limit of 12 ng.mL−1 for NS110.
In summary, the direct electrical detection of dengue type 2 virus NS1 protein has been achieved applying the measurement of variations in OCP and using a disposable Au electrode containing immobilized anti-NS1 antibodies purified from chickens (IgY antibodies). The immunosensor exhibited a fast response, high sensitivity and good stability for the electrical detection of NS1. The method can be performed for the immobilization of any IgY antibodies and applied for the evaluation of other antigenic proteins that represent potential disease biomarkers. In addition, the immunosensor is relatively cheap, easy to construct and selective for NS1 detection in low concentrations.
Methods
Chemicals
Dengue type virus 2 NS1 glycoprotein and chicken polyclonal IgY anti-NS1 antibodies were fabricated and provided by DNAPTA Biotecnologia (Brazil). Cysteamine (CYS), glutaraldehyde (GA) and ethanolamine were purchased from Sigma-Aldrich. Human serum samples used as negative tests were provided by anonymous donors, and all the procedures were approved by the Ethics Committee of the Federal University of São Carlos. All chemicals were used without further purification, except that human serum was desalted. The other chemical reagents were of analytical grade. All solutions were prepared with Millipore Milli-Q water (resistivity >18.3 MΩ.cm).
Fabrication and characterization of the electrode
In the construction of the disposable Au electrode, BK7 glasses were coated with 20 nm of chromium for adherence and covered with 130 nm of gold (Au) by high vacuum evaporation. Self-assembled monolayers (SAMs) of CYS along GA were used to modify the disposable Au electrode. First, a SAM was formed by the immersion of the Au electrode in a CYS ethanolic solution (10 mM) for 24 hours at room temperature. The electrode was washed with ethanol and water, immersed in a GA solution (2.5% v/v) for 1 hour and washed with water. Antibody immobilization consisted of dropping 10 µL of 200 µg.mL−1 IgY solution in 1X PBS (phosphate buffered saline) directly over the Au electrode (previously modified) for 1 hour. The blocking step was performed using a 50 mM ethanolamine solution in 1X PBS for 30 min to prevent non-specific reactions. The immunosensors were stored in a 1X PBS buffer solution at 4°C until use. Figure 5 shows the schematic representation of the immunosensor preparation.
Figure 5. Schematic representation of the immunosensor preparation.
Functionalization with CYS, subsequent activation with GA, IgY antibodies immobilization, blocking with ethanolamine and NS1 addition.
The immobilization of the IgY antibodies was followed by electrochemical measurements (EIS and CV). The measurements were taken in an electrochemical microcell (300 µL) at room temperature by a three-electrode system: the disposable Au electrode as a working electrode, a platinum wire as a counter electrode and Ag/AgCl (3.0 mol L−1KCl) as the reference electrode. An Autolab Eco Chemie PGSTAT12 potentiostat/galvanostat was employed in all electrochemical measurements, using a 0.1 M KCl solution containing 1.0 mM Fe(CN)6−3/−4 as redox pair. The impedance measurements were recorded under an applied potential of 0.22 V.
OCP experimental setup
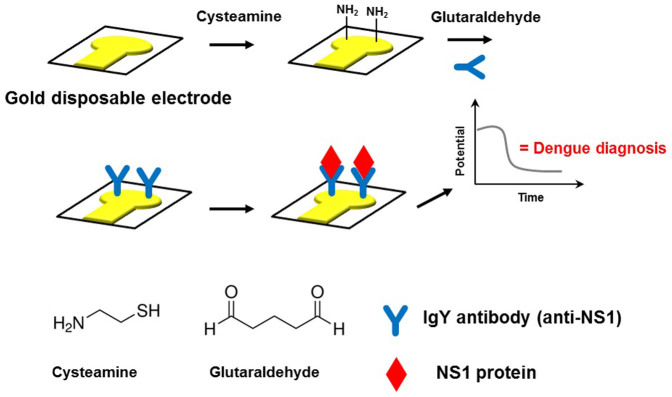
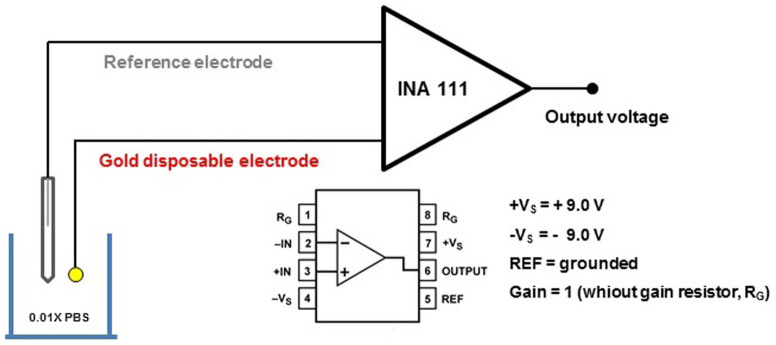
The immunosensor measurements were carried out in an open circuit potential (OCP) configuration. An INA 111 commercial instrumental amplifier (Texas Instruments, USA) operating as a unity gain buffer was utilized as the readout circuit23. The immunosensor was connected to the INA according to the schematic diagram shown in Figure 6. An Ag/AgCl (3.0 mol L−1 KCl) reference electrode was used to maintain a constant voltage and was connected to the reference pin of INA 111. A low ionic strength buffer solution (0.01X PBS, pH 7.4) was used in all measurements to provide a λD of ca. 7.3 nm from the electrode surface. The immunoassays were conducted by adding known aliquots of NS1 protein diluted in the same buffer (0.01X PBS, pH 7.4) in the measuring microcell (300 µL).
Figure 6. Schematic diagram of the proposed immunosensor showing the connection pins of the INA 111 instrumentation amplifier used as a readout circuit in the OCP measurements.
Author Contributions
N.C.S.V. conceived the idea for this study, contributed to its design and coordination and developed the experimental setup of the immunosensor. A.F. constructed the NS1-sensitive electrode and performed the electrical immunoassays. J.F.S. helped in the electrical immunoassays and performed the pH-sensing measurements. B.C.J. conducted the electrochemical measurements. S.A., P.P.J., R.L.L. and M.L.N. produced the IgY antibodies and the NS1 protein. F.E.G.G. and V.Z. gave advice and guided the experiments. All authors contributed to the writing of this manuscript and approved the final manuscript.
Acknowledgments
The authors are grateful to CAPES, CNPq and FAPESP (2011/20110-0) for the financial support.
References
- Guzman M. G. et al. Dengue: a continuing global threat. Nature Rev. Microbiol. 8, S7–S16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapphra K. et al. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn. Micr. Infec. Dis. 60, 387–391 (2008). [DOI] [PubMed] [Google Scholar]
- Dussart P. et al. Evaluation of an enzyme immunoassay for detection of dengue virus NS1 antigen in human serum. Clin. Vaccine Immunol. 13, 1185–1189 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkham T. M., Chung Y. K., Tang K. F. & Ooi E. E. The performance of RT-PCR compared with a rapid serological assay for acute dengue fever in a diagnostic laboratory. Trans. Roy. Soc. Trop. Med. Hyg. 100, 142–148 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquette C. A. & Blum L. J. State of the art and recent advances in immunoanalytical systems. Biosens. Bioelectron. 21, 1424–1433 (2006). [DOI] [PubMed] [Google Scholar]
- Camara A. R. et al. Dengue immunoassay with an LSPR fiber optic sensor. Opt. Express 21, 27023–27031 (2013). [DOI] [PubMed] [Google Scholar]
- Tai D.-F., Lin C.-Y., Wu T.-Z., Huang J.-H. & Shu P.-Y. Artificial receptors in serologic tests for the early diagnosis of dengue virus infection. Clin. Chem. 52, 1486–1491 (2006). [DOI] [PubMed] [Google Scholar]
- Cavalcanti I. T., Guedes M. I. F., Sotomayor M. D. P. T., Yamanaka H. & Dutra R. F. A label-free immunosensor based on recordable compact disk chip for early diagnostic of the dengue virus infection. Biochem. Eng. J. 67, 225–230 (2012). [Google Scholar]
- Dias A. C. M. S., Gomes-Filho S. L. R., Silva M. M. S. & Dutra R. F. A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosens. Bioelectron. 44, 216–221 (2013). [DOI] [PubMed] [Google Scholar]
- Dias da Silva W. & Tambourgi D. V. IgY: A promising antibody for use in immunodiagnostic and in immunotherapy. Vet. Immunol. Immunop. 135, 173–180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillner E. et al. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals 40, 313–322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudjarwo S. A. & Sudjarwo K. E. Purification and characterization protein of anti-dengue specific immunoglobulin Y for diagnostic kit of dengue. J. App. Pharm. Sci. 2, 007–012 (2012). [Google Scholar]
- Vieira N. C. S., Figueiredo A., Fernandes E. G. R., Guimaraes F. E. G. & Zucolotto V. Nanostructured polyaniline thin films as urea-sensing membranes in field-effect devices. Synthetic Met. 175, 108–111 (2013). [Google Scholar]
- Arruda I. G. et al. Layer-by-Layer Films Containing SiO2 Nanoparticles as Field-Effect pH Sensors. Sensor Lett. 11, 348–351 (2013). [Google Scholar]
- Bletchly C. Antigenic and structural analysis of the NS1 glycoprotein of dengue virus. PhD thesis, Univ. of Queensland (2002). [Google Scholar]
- Schasfoort R. B. M., Bergveld P., Kooyman R. P. H. & Greve J. Possibilities and limitations of direct detection of protein charges by means of an immunological field-effect transistor. Anal. Chim. Acta 238, 323–329 (1990). [Google Scholar]
- Elnathan R. et al. Biorecognition Layer Engineering: Overcoming Screening Limitations of Nanowire-Based FET Devices. Nano Lett. 12, 5245–5254 (2012). [DOI] [PubMed] [Google Scholar]
- Alcon S. et al. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40, 376–381 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty D. H. et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186, 1165–1168 (2002). [DOI] [PubMed] [Google Scholar]
- Prada-Arismendy J. & Castellanos J. E. Real time PCR. Application in dengue studies. Colomb. Med. 42, 243–258 (2011). [Google Scholar]
- Jahanshahi P., Zalnezhad E., Sekaran S. D. & Adikan F. R. M. Rapid Immunoglobulin M-Based Dengue Diagnostic Test Using Surface Plasmon Resonance Biosensor. Sci. Rep. 4 10.1038/srep03851 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrela P. et al. Label-Free Detection of Protein interactions with peptide aptamers by open circuit potential measurement. Electrochim. Acta 53, 6489–6496 (2008). [Google Scholar]