Abstract
Gastroesophageal reflux disease (GERD) has a high prevalence worldwide. Recent reports have noted a high prevalence even in Asian countries. GERD significantly affects the quality of life and can present with a wide variety of symptoms. Not all reflux is acid, and non-acid reflux disease can be more difficult to diagnose and can lead to a variety of extra-esophageal symptoms. Although proton pump inhibitors (PPIs) are effective in the majority of patients, but they are not without side effects, and their effect often diminishes with time. For patients who do not desire to be on long-term PPIs or have incomplete symptom resolution with medication, various endoscopic and minimally invasive treatment modalities are now available. The etiology of GERD can be multifactorial including dysfunctional LES, presence of a hiatal hernia, and transient lower esophageal sphincter relaxations (TLESRs). We hence believe that the treatment should be individualized to the cause of the reflux. In the following review, we describe the etiology of reflux disease and attempt to lay a framework for the diagnosis and selection of patients for the various interventions available for treatment, along with their evidence base.
Keywords: Gastroesophageal reflux disease, Lower esophageal sphincter, Hiatal hernia, Nissen fundoplication, Endoscopic treatments
Gastroesophageal Reflux Disease: Diagnosis and Patient Selection
Gastroesophageal reflux disease (GERD) is defined per the Montreal definition as “reflux that causes troublesome symptoms, mucosal injury in the esophagus, or both of these” [1].
The prevalence of GERD has been noted to be extremely high in Western countries. In 2004, approximately 20 % of the US population reported reflux symptoms that occurred at least weekly [2]. According to data from the Gallup organization (1988), approximately 44 % of Americans experience heartburn at least once per month. Almost 18 % of Americans take non-prescription drugs for reflux–related symptoms [3].
There are a few recent studies looking at the prevalence of GERD in Asian countries, and a high prevalence even in Asian countries has been noted. Sharma et al. in their study published in the Indian journal of gastroenterology found that the prevalence of GERD in an urban adult population from northern India was 16.2 % [4].
Symptoms related to reflux disease are extensive. Patients can present with the most typical symptoms of GERD, which are heartburn, regurgitation, and epigastric pain. Atypical symptoms of GERD are many including dysphagia, oropharyngeal symptoms such as hoarseness, globus, and chronic cough. Patients can also have chest symptoms such as chest pain, pneumonias, chronic aspiration, and asthma. Other manifestations can be dental erosions, sinusitis, otitis media, sleep apnea. And finally, patients can have complications of GERD that include stricture, ulceration, inflammation, and Barrett’s esophagus (Table 1).
Table 1.
Various manifestations of GERD
| Typical symptoms | Atypical symptoms | Complications |
|---|---|---|
| Heartburn | Chronic cough | Stricture |
| Regurgitation | Hoarseness | Esophagitis |
| Epigastric pain | Globus | Ulceration |
| Dysphagia | Barrett’s esophagus | |
| Chest pain | Adenocarcinoma | |
| Chronic aspiration | ||
| Bronchitis | ||
| Sinusitis |
Etiology of GERD
Stein and coworkers gave us the concept of the plumbing circuit where the esophagus functions as an antegrade pump, the lower esophageal sphincter (LES) as a valve, and stomach as a reservoir [5]. Problems with any component of the circuit (either poor esophageal motility or a dysfunctional LES or delayed gastric emptying) can lead to GERD. From a medical or surgical standpoint, it is extremely important to identify which of these components is defective so that effective therapy can be applied.
The LES is defined by manometry as a zone of elevated intraluminal pressure at the esophagogastric junction. It is a 3–5-cm segment of contracted circular smooth muscle at the distal end of the esophagus, the resting tone of which varies from 10 to 35 mmHg when measured end expiratory. A hypotensive pressure within the sphincter is often a cause of severe reflux, and the etiology of hypotensive LES is unknown.
Another cause of reflux is transient inappropriate LES relaxations (relaxation in the absence of swallowing). These transient lower esophageal sphincter relaxations (TLESRs) are vagally mediated reflexes triggered by gastric distension and serve to vent the stomach. Prolonged or more frequent TLESRs are now thought to be the most common cause of reflux disease. These patients are noted to have a very low gastric yield pressure such that patients may experience reflux even with a glass of water.
Apart from the LES itself, there are two other anatomical structures that contribute to preventing reflux at the esophagogastric junction which are the diaphragmatic crura and the phrenoesophageal ligament which helps form the angle of His. For proper LES function, this junction must be located in the abdomen so that the diaphragmatic crura can assist the action of the LES, thus functioning as an extrinsic sphincter. If this mechanism is defective, GERD is exacerbated. A hiatal hernia may contribute to reflux as proximal migration of the LES may result in loss of its abdominal high-pressure zone (HPZ) or the length of the HPZ may decrease. Also the crural mechanisms will not be effective in preventing reflux. Hence, the reduction of the hiatal hernia with reestablishing the intra-abdominal length of the esophagus, with proper crural closure, apart from a fundic wrap, are key components to the surgical correction of GERD.
The patient factors related to reflux also cannot be ignored. These include eating refluxogenic foods, overeating, and eating immediately before laying supine. These can be easily rectified with lifestyle changes. Obesity also is a significant risk factor for GERD. This used to be a disease of Western nations but now is rampantly spreading worldwide. A chronically increased intra-gastric pressure and increased frequency of transient LES relaxations (TLESRs) are thought to play the major role in obesity related GERD.
Non-acid Reflux Disease (NARD)
A newer line of investigation is the realization that not all reflux is acid. Other components of the reflux fluid such as bile acids, pepsin, gas etc, apart from hypersensitivity to the volume of reflux itself have all been thought to contribute to reflux disease, especially reflux with atypical symptoms and those symptoms that do not respond to proton pump inhibitors.
Patient Evaluation
Patients with reflux symptoms are frequently started on anti-acid medications as a diagnostic and therapeutic maneuver. This has been the standard for several years. These medications are extremely effective at blocking acid and have few side effects. It is recommended that patients also have an upper endoscopy as part of their evaluation to determine if they have a complication of reflux such as inflammation, strictures, or even Barrett’s type changes. There is now a movement to say that the endoscopy should really be done first before the medications are started to determine the patient’s true baseline. This can help to guide further treatment. This is particularly important if someone has Barrett’s as they can get diagnosed earlier.
If further evaluation is warranted or a procedure for reflux is being considered, then an ambulatory pH study needs to be done to objectively measure the patient’s reflux. There are several reasons why a patient might be considered for a procedure for treatment of their reflux. Patients for whom the medications no longer work, those with side effects to the medications, those with atypical symptoms, or those wishing to no longer be on medications are candidate for an anti-reflux procedure. Also, patients with complications of reflux should be considered for a procedure.
Ambulatory pH monitoring is considered the gold standard confirmatory test. The pH study should ideally be performed off of medications. The gold standard is a catheter-based study first described by DeMeester et al. The total time with pH <4 is recorded by a probe placed 5 cm above the LES, and a composite score (six variables) is calculated. The testing is done with patients off PPIs for 7 days and H2 receptor blockers for 3 days. The components of the Jamieson-DeMeester score are (Normal <14.72) as follows:
Total esophageal acid exposure time
Upright acid exposure time
Supine acid exposure time
Number of episodes of reflux
Number of reflux episodes lasting more than 5 min
The duration of the longest reflux episode
The patients chart their symptoms during the period of testing, and the symptoms are then correlated with periods of acid reflux on pH testing, and a symptom-associated probability is hence calculated (SAP).
Now, the wireless pH monitoring system has risen in popularity (Bravo capsule). This study is 48 h and is considered to be more accurate as the patient is more likely to go about their daily activities without a catheter in place. This sensor can be placed endoscopically with sedation or without sedation transorally. One of the most important pieces of data gleaned from the pH study is the symptom correlation. This can really guide the physician doing the surgical procedure and be used to establish expectations and outcomes for the patient.
If non-acid reflux is suspected, the pH study can be combined with intraluminal impedance to determine the presence of non-acid reflux. In this study, the symptom correlation is particularly important.
The next study that every patient being considered for a procedure should have is an esophageal function study. Esophageal manometry is the gold standard. When combined with multichannel intraluminal impedance, it is referred to as esophageal function testing. This study allows evaluation of peristalsis and contraction amplitudes within the esophageal body, the pressure, relaxation, and length of the LES and bolus transit through the esophagus. It is imperative to rule out achalasia before proceeding with a procedure for reflux disease. This will also help to guide further therapy based on the esophageal motility and the function of the lower esophageal sphincter.
An esophagogram is helpful in determining the presence of a hiatal hernia and gives a general idea of esophageal motility disorders. A gastric emptying study may also be helpful if a patient has bloating or retained food on endoscopy.
Treatment Options for GERD
Once the studies are done, the results can be carefully analyzed to tailor treatment of the patients reflux. The treatment options follow. Multiple lifestyle modifications have been suggested although they are not backed by substantial evidence but can be tailored to patient symptoms.
Losing weight (if overweight)
Avoiding alcohol, chocolate, citrus juice, and tomato-based products, spicy meals may benefit few patients who find these to trigger symptoms
Avoiding large meals
Waiting 2 to 3 h after a meal before lying down in patients with nocturnal symptoms
Medical Treatment
The recent American College of Gastroenterology (ACG) guidelines recommend that in patients with symptoms and history consistent with uncomplicated GERD, the diagnosis of GERD may be assumed and empirical therapy begun. Patients who show signs of GERD complications or other illness or who do not respond to therapy should be considered for further diagnostic testing [6]. That being said, these recommendations are now being challenged as there is growing evidence that an endoscopy at baseline can be very helpful in determining further management of the patient’s GERD.
The LOTUS trial, a 5-year exploratory randomized, open, parallel group trial demonstrated that with anti-reflux therapy for GERD, either using drug-induced acid suppression with esomeprazole or laparoscopic anti-reflux surgery, most patients achieve and remain in remission at 5 years [7].
H2 Receptor Blockers Versus PPIs
The recommendations are an 8-week course of treatment starting with either a proton pump inhibitor (PPI) or an H2 receptor blocker. A step-up or step-down approach is accordingly followed, the goal eventually being to achieve symptom remission and stop therapy. However, for patients who require long-term maintenance PPIs, the lowest effective dose, preferably an on-demand or intermittent therapy, should be recommended to avoid long-term complications of the PPIs. H2 receptor blocker therapy can be used as a maintenance option in patients without erosive disease if they experience heartburn relief.
Evidence on Potential Risks Associated with PPIs
Patients with known osteoporosis can remain on PPI therapy. Concern for hip fractures and osteoporosis should not affect the decision to use PPI long-term except in patients with other risk factors for hip fracture.
PPI therapy can be a risk factor for Clostridium difficile infection and should be used with care in patients at risk.
Short-term PPI usage may increase the risk of community-acquired pneumonia. The risk does not appear elevated in long-term users.
PPI therapy does not need to be altered in concomitant clopidogrel users as clinical data does not support an increased risk for adverse cardiovascular events.
Surgical Treatments of GERD
Indications for surgical therapy as recommended by the Society for Gastrointestinal and Endoscopic Surgeons (SAGES) are the following:
When the diagnosis of reflux is objectively confirmed, surgical therapy should be considered in individuals who
Have failed medical management (inadequate symptom control, severe regurgitation not controlled with acid suppression, or medication side effects) or
Opt for surgery despite successful medical management (due to quality of life considerations, lifelong need for medication intake, expense of medications, etc.) or
Have complications of GERD (e.g., Barrett’s esophagus, peptic stricture) or
Have extra-esophageal manifestations (asthma, hoarseness, cough, chest pain, aspiration)
If patients decide to proceed with a procedure for their reflux, there are several options available. The surgical procedures are the Nissen fundoplication and the LINX procedure (Torax). The endoscopic procedures with the most data are the Stretta procedure (Medieri Therapeutics) and the trans-oral fundoplication (Endogastric Solutions). The procedures, indications, and outcomes are reviewed below.
Laparoscopic Nissen Fundoplication
Laparoscopic fundoplication is still considered the gold standard therapy for the treatment of GERD in patients needing invasive treatment. The fundus of the stomach is wrapped around the esophagus to create a new valve at the level of the esophagogastric junction. The success rate of this procedure is 95 %.
Based on a consensus of 40 experienced foregut surgeons, the following standardized approach to Nissen fundoplication has been recommended:
Opening of the phrenoesophageal ligament
Preservation of the hepatic branch of the anterior vagus nerve
Dissection of both crura
Transhiatal mobilization to allow approximately 3 cm of intra-abdominal esophagus
Short gastric vessel division
Crural closure posteriorly with non-absorbable sutures
Creation of a 1.5 to 2-cm wrap with the most distal suture incorporating the anterior muscular wall of the esophagus, with bougie placement at the time of wrap construction
This procedure allows for a concurrent hiatal hernia repair. For anyone with a hiatal hernia greater than 2 cm or where the gastroesophageal junction is in the chest, this is the best procedure. The esophagus can be mobilized circumferentially to allow good reduction into the peritoneal cavity and allow the diaphragm to participate in the reflux mechanism.
A rigorous randomized study by Anvari et al. reestablished Nissen fundoplication as the gold standard in treating GERD. The investigators showed that at 1 year, the outcome and the symptom control in the surgical group was better than that in the medical group [8].
The authors felt that fundoplication was preferable to PPIs, since PPIs, although effective in controlling the acid component of the refluxate, do not eliminate the reflux of bile, which some believe to be a major contributor to the pathogenesis of Barrett’s epithelium.
The Nissen fundoplication is also considered a durable procedure. A study by Kelly et al. demonstrated durability to 10 years when performed by an experienced surgeon [9].
The downside of Nissen fundoplication is the recovery time, which can be 4 to 6 weeks, and the potential for failures and dysphagia. As a result, other treatments for reflux have been sought.
The LINX Reflux Management System (Torax Medical)
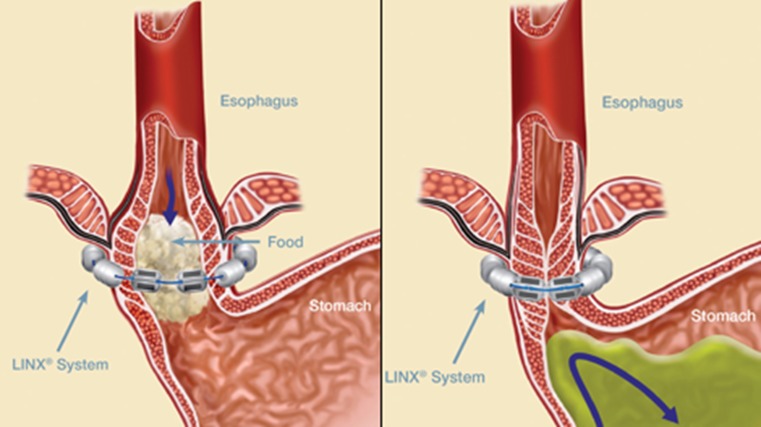
The US Food and Drug Administration approved the LINX Reflux Management System in March 2012. The LINX System is a small flexible band of interlinked titanium beads with magnetic cores (Fig. 1). The magnetic attraction between the beads is intended to help the LES resist opening to gastric pressures, preventing reflux from the stomach into the esophagus. LINX is designed so that swallowing forces temporarily break the magnetic bond, allowing food and liquid to pass normally into the stomach. Magnetic attraction of the device is designed to close the LES immediately after swallowing, restoring the body's natural barrier to reflux. The device is designed to augment the lower esophageal sphincter pressure and gastric yield pressure.
Fig. 1.
The LINX reflux management system
The LINX device is placed laparoscopically around the GE junction. The setup is similar to that of a Nissen fundoplication in case on laparoscopy the patient anatomy is found less suitable for placement of the LINX device. It is done under general anesthesia. A narrow window is dissected immediately posterior at the GE junction. A sizer is then placed to ensure that the device will sit in good approximation. The sizer is then replaced for the device which is then locked in place. In our practice, we do a routine intra-op upper endoscopy to confirm that the scope can be easily passed through the GE junction and device has been placed appropriately. We observe patients overnight; however, typically the hospital stay does not exceed 24 h. The advantage of the LES augmentation procedure with LINX is that patients can be started on a solid diet right away, in fact it is recommended to “exercise” the device. Patients have to be advised that they cannot have a MRI in the future.
In a multicenter study of 44 patients who underwent laparoscopic placement of the LINX device, at 4 years, the device provided long-term clinical benefits with no safety issues, as demonstrated by reduced esophageal acid exposure, improved GERD-related quality of life, and cessation of dependence on PPIs, with minimal side effects and no safety issues [10]. There have also been two other large studies demonstrating excellent results with the LINX device (85 % success rate) [11, 12].
Endoluminal Therapies
Risks of dysphagia, bloating, and adverse outcomes are sometimes associated with surgery. For these reasons, patients have desired less invasive techniques. Over the last decades, several endoluminal therapies have been tried and failed. The two that have survived the test of time thus far include the trans-oral fundoplication and radiofrequency ablation of the LES.
Advantages of endoluminal techniques are as follows:
Great in patients with co-morbidities especially respiratory or cardiac risk factors
Often can be done on an outpatient basis in under an hour
Low risk procedures
If fails patients can still proceed with Nissen fundoplication
Can be done in patients with previous failed anti-reflux procedures
Contraindications for endoluminal therapies are as follows:
Severe dysphagia
Esophagitis greater than grade 2
Body mass index >40
Gastroesophageal reflux disease refractory to proton pump inhibitor
Hiatal hernia >2 cm
Trans-oral Incisionless Fundoplication (TIF)
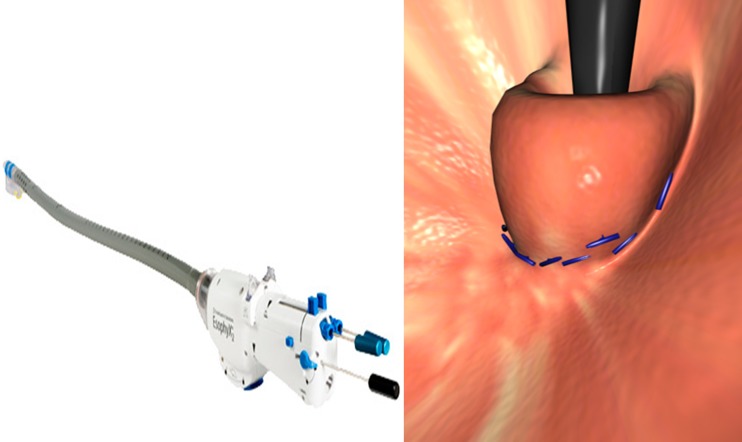
The EsophyX device is inserted transorally using visual guidance from a flexible endoscope. The esophagogastric junction is plicated with multiple full-thickness polypropylene sutures along the lesser curvature starting 1 cm below the Z line to create a fundoplication in the gastric cardia, and a 270° valve of 2–3 cm in length can be achieved (Fig. 2). The procedure is typically performed under general anesthesia.
Fig. 2.
The EsophyX device for TIF with multiple fasteners deployed to create a trans-oral incisionless fundoplication
According to the recent SAGES guidelines on endoluminal procedures, it was felt that although long-term data is not yet available for EsophyX, in short-term follow-up, from 6 months to 2 years, EsophyX may be effective in patients with a hiatal hernia <2 cm with typical and atypical GERD. Further studies are required to define optimal techniques and most appropriate patient selection criteria and to further evaluate device and technique safety. Due to a low quality of evidence, the strength of the recommendation was graded as weak [13].
Bell et al. in a study of 100 consecutive patients found that GERD Health-Related Quality of Life (GERD-HRQL) total score was normalized in 73 %, and major adverse events occurred [14]. A randomized controlled trial of TIF versus PPI was presented at the Digestive Disease Week conference this year. At a 6-month follow-up, 90 % of patients in the TIF group were off of PPI medications, and 54 % had normalization of acid exposure in the esophagus. A sham, controlled study is also soon to be reported.
This procedure is most helpful in the patient with a patulous flat LES seen on retroflexion with endoscopy. This procedure helps to reconstruct the angle of His. As more data emerges, this will be important in the armamentarium of treatments for GERD.
Radiofrequency (RF) Ablation of the LES-Stretta System
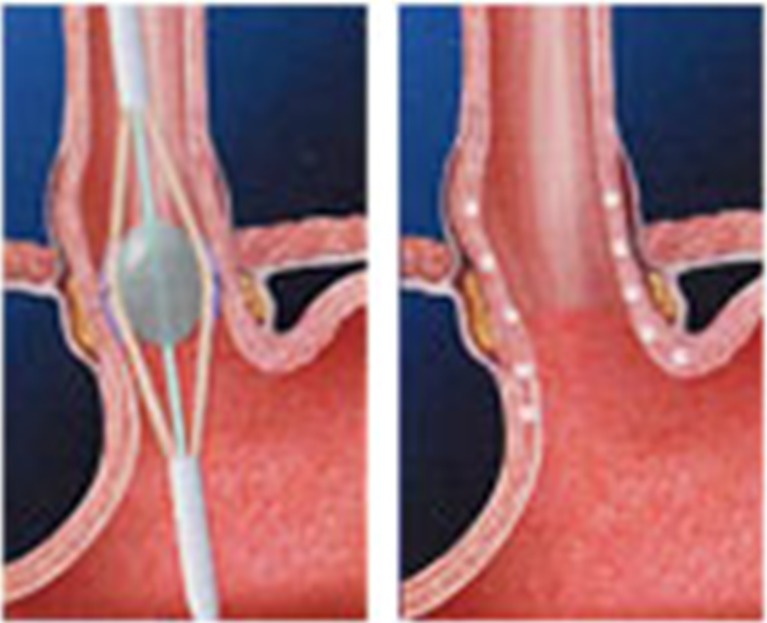
The FDA originally cleared Stretta for use in 2000 and issued an updated clearance on the RF generator in 2011. The trans-oral Stretta catheter system uses a proprietary algorithmic application of low power (5 W) RF energy and generates low tissue temperatures (65 to 85 °C) during a series of 1-min treatment cycles. The procedure can be performed on an outpatient basis under conscious sedation. A flexible endoscope is first passed, and the anatomy is assessed. The distance of the GE junction from the incisors is measured. The scope is then withdrawn, and the Stretta catheter is passed over a guide wire and used in conjunction with the radiofrequency generator control module to deliver temperature-controlled radiofrequency energy circumferentially at the GE junction. Balloon inflation places the electrodes into the muscle layer of the lower esophagus, and radiofrequency energy is delivered (Fig. 3). Eight lesions are made in a radial manner by rotating the balloon 45°.
Fig. 3.
Stretta procedure
Stretta therapy remodels the musculature of the lower esophageal sphincter (LES) and gastric cardia. Clinical studies demonstrate that the Stretta RF treatment results in significant reductions in tissue compliance and transient LES relaxations. These mechanisms act to restore the natural barrier function of the LES as well as to significantly reduce spontaneous regurgitation caused by transient inappropriate relaxations of the sphincter. Overall, this technology appears to be successful in 67 % of patients.
The SAGES guidelines on endoluminal therapies recommend that Stretta is an appropriate therapy for patients being treated for GERD who are 18 years of age or older; who have had symptoms of heartburn, regurgitation, or both for 6 months or more; who have been partially or completely responsive to anti-secretory pharmacologic therapy; and who have declined laparoscopic fundoplication. The quality of evidence was high, and recommendation was graded as strong [13]. This is the only endoluminal procedure that has undergone rigorous evaluation with randomized trials. Case series with 48 months of follow-up also demonstrate excellent results with this procedure in the proper patient population.
I find this procedure particularly helpful in patients with transient inappropriate LES relaxations. It is also helpful in patients with recurrent reflux following Nissen fundoplication.
How to Choose the Proper Treatment
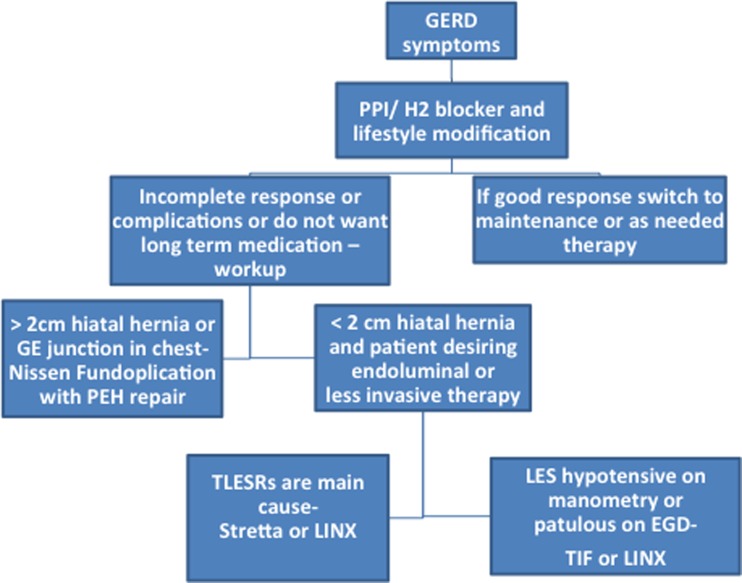
Medications are extremely effective at blocking acid in the stomach. For patients with reflux symptoms, these should be the first line of therapy in conjunction with lifestyle changes. If for some reason the patient cannot take the medications, is less responsive to therapy, or has complications of their reflux disease, a procedure should be considered. The Nissen fundoplication is the gold standard surgery for reflux. If a patient has a hiatal hernia greater than 2 cm, this is the procedure of choice. This procedure can work for any etiology of reflux. However, if the patient has a small hiatal hernia, then it should be determined whether the endoscopic view of the valve is abnormal, there is a hypotensive LES, or the patient has TLESRs (Fig. 4). For hypotensive LES, the patient has the options of TIF or LINX. If TLESRs are the cause, then the Stretta and LINX are good choices. The LINX is 85 % effective with a low-risk profile and quick recovery. The endoluminal therapies are effective in around 2/3 of patients overall. The symptom correlation on the pH study should be discussed with the patient prior to procedure and the reflux objectively documented.
Fig. 4.
The decision tree in anti-reflux therapy
Conclusion
In conclusion, GERD is far more common not just in Western countries but across the globe than previously thought to be. It can significantly affect the quality of life. Not all reflux is acid and non-acid reflux disease can be more difficult to diagnose and can lead to a variety of extra-esophageal symptoms. PPIs are effective in the majority of patients, but they are not without side effects. For patients who do not desire to be on long-term PPIs or have incomplete symptoms resolution with medication, the treatment should be tailored to the cause of the reflux.
References
- 1.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Digestive Diseases Statistics for the United States-National Digestive Diseases Information Clearinghouse. (n.d.). Home-National Digestive Diseases Information Clearinghouse
- 3.Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis. 1976;21(11):953–956. doi: 10.1007/BF01071906. [DOI] [PubMed] [Google Scholar]
- 4.Sharma PK, Ahuja V, Madan K, et al. Prevalence, severity, and risk factors of symptomatic gastroesophageal reflux disease among employees of a large hospital in northern India. Indian J Gastroenterol. 2011;30(3):128–134. doi: 10.1007/s12664-010-0065-5. [DOI] [PubMed] [Google Scholar]
- 5.Stein HJ, DeMeester TR. Outpatient physiologic testing and surgical management of foregut motility disorders. Curr Probl Surg. 1992;29(7):413–555. doi: 10.1016/0011-3840(92)90036-3. [DOI] [PubMed] [Google Scholar]
- 6.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 7.Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305(19):1969–1977. doi: 10.1001/jama.2011.626. [DOI] [PubMed] [Google Scholar]
- 8.Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: one-year follow-up. Surg Innov. 2006;13(4):238–249. doi: 10.1177/1553350606296389. [DOI] [PubMed] [Google Scholar]
- 9.Kelly JJ, Watson DI, Chin KF, Devitt PG, Game PA, Jamieson GG. Laparoscopic Nissen fundoplication: clinical outcomes at 10 years. J Am Coll Surg. 2007;205(4):570–575. doi: 10.1016/j.jamcollsurg.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Lipham JC, Demeester TR, Ganz RA, et al. The LINX(R) reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc. 2012;26(10):2944–2949. doi: 10.1007/s00464-012-2289-1. [DOI] [PubMed] [Google Scholar]
- 11.Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. 2010;252(5):857–862. doi: 10.1097/SLA.0b013e3181fd879b. [DOI] [PubMed] [Google Scholar]
- 12.Bonavina L, Saino G, Bona D, Sironi A, Lazzari V. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg. 2013;217(4):577–585. doi: 10.1016/j.jamcollsurg.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 13.SAGES guidelines committee: endoluminal treatments for gastroesophageal reflux disease (GERD)
- 14.Bell RC, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215(6):794–809. doi: 10.1016/j.jamcollsurg.2012.07.014. [DOI] [PubMed] [Google Scholar]