Abstract
A fluorescence method was employed for studying the drying and swelling of PAAm–κC composite gels, which were formed from acrylamide (AAm) and N, N’- methylenebisacrylamide (BIS) with various κ–carrageenan (κC) contents by free radical crosslinking copolymerization in water. Composite gels were prepared at 80 °C with pyranine (Py) as a fluorescence probe. Scattered light, Isc, and fluorescence emission intensities, Iem, were monitored during drying and swelling of these gels. The fluorescence intensity of pyranine increased and decreased as drying and swelling time are increased, respectively, for all gel samples. The Stern–Volmer equation combined with moving boundary and Li-Tanaka models were used to explain the behavior of Iem during drying and swelling processes respectively. It is found that the desorption coefficient Dd decreased as κC contents were increased for a given temperature during drying. However, the cooperative diffusion coefficient, Ds presented exactly the opposite case. Conventional gravimetrical and volumetric experiments were also carried out during drying and swelling of PAAm–κC composite gels. It was observed that Dd and Ds values measured with the fluorescence method were found to be much larger than they were measured with the conventional methods.
Keywords: Acrylamide, Composite, Carrageenan, Fluorescence, Drying, Swelling
Introduction
Hydrogel composites have recently attracted a great deal of interest in biomedical and pharmaceutical systems and have been utilized in various applications including biomedicine, infant care, pharmaceuticals, and agriculture. In industrial applications, biomaterials are being substituted with synthetic polymers by improving the biocompatible for pharmaceutical and biomedical applications [1]. Hydrogels are synthetic polymers that can degrade or swell in response to internal or external stimulations. In general, a composite can be defined as a substance composed of two or more materials with different base structures combined in such a way that the end product has different properties than either of the parent materials. Chemical gels doped with biological gels are a new class of composite materials [2]. Polyacrylamide is a polymer that is formed from units of acrylamide, a known neurotoxin material. However, polyacrylamide itself is non-toxic, but it is a controversial ingredient because of its potential ability to secrete acrylamide [3], and also it renders highly attractive materials for a large variety of biomedical applications [4–6]. On the other hand, superabsorbent hydrogels are hydrophilic networks, which are capable of absorbing and retaining considerable amounts of water or physiological fluids. Because of their excellent characteristics, they are used in many applications such as in disposable diapers, agriculture, and medicine.
Due to their biocompatibility, biodegradability, and non-toxicity, polysaccharides are the main part of the natural-based superabsorbent hydrogels [7]. Carrageenans are biopolymers and/or bioactive compounds in seaweed [8] formed from linear polysaccharides made up of repeating galactose units and 3,6-anhydrogalactose, both sulfated and non-sulfated, joined by alternating α-1, 3-linked D-galactopyranose and β-, 1-linked 3, 6-anhydro-D-galactopyranose. Kappa and iota are the common carrageenan groups and are found in the gamet to phytic life phase of various seaweed species. There have been many articles published related to carrageenans such as on the biology of seaweeds and applications of marine polysaccharides [9–11]. Kappa-carrageenan (κC) can reduce or eliminate toxicity in biomedical applications, and for this reason κC has been applied for immobilizing protein and controlled drug-delivery systems [1].
Hysteresis [12] and percolative behavior [13] of κ-carrageenan were studied by the fluorescence method. The photon transmission technique was used to study the drying of PAAm gels with various crosslinker contents [14]. Steady-state fluorescence (SSF) technique was employed for studying the drying of polyacrylamide [15], κ-carrageenan [16] at various temperatures, and polyacrylamide hydrogels of various crosslinker contents [17]. Drying of polyacrylamide composite gels formed with various κC contents was investigated [18] and it was observed that the desorption coefficient decreased as κC contents were increased. Swelling of polyacrylamide (PAAm) hydrogel for various temperatures and crosslinker contents by using the fluorescence method was also reported [19, 20]. It was observed that cooperative diffusion coefficients increased and/or decreased as the swelling temperature and the crosslinker content were increased, respectively. The photon transmission technique was also used to study the swelling properties of κ-carrageenan (κC) gels prepared with various κC contents [21]. In these studies, it has been understood that gels with high carrageenan content possess more double helices and more lattice dislocations and swell slower than gels with low carrageenan content, which may contain less double helices and less lattice imperfections. The swelling of κ-carrageenan (κC) gels at various temperatures was studied by SSF technique [22]. The results in these preliminary works have shown that the fluorescence method can be used to measure diffusion coefficients at a molecular level during swelling of carrageenan gels. Swelling activation energies, ΔE of PAAm–κC composite were measured, which were found to be exothermic and endothermic in between 30 and 40 and 40 and 60 °C, respectively [23]. PAAm–κC composite gels are important for the use of sorbents in many applications of biomaterials and separation operations in biotechnology, drug-delivery systems, processing of agricultural products, sensors, and actuators. In addition, the presence of the natural parts guarantees biocompatibility, biodegradability, and non-toxicity of the super-absorbing materials. Super-absorbent PAAm–κC composites were used for swelling agents and devices in oral drug delivery and suggested as biocompatible and multifunctional polymers.
In this work, we aim to investigate the dynamical processes of PAAm–κC composites by using the steady-state fluorescence technique. By combining the Stern–Volmer equation with the moving boundary model, desorption coefficients Dd were determined for drying PAAm–κC composites. We observed that the desorption coefficients Dd decreased as κC contents were increased. The Li-Tanaka equation was used to determine the swelling time constants τs and cooperative diffusion coefficients Ds for the swelling processes. It was observed that the swelling time constant τs decreased and cooperative diffusion coefficients Ds increased as the κC contents were increased at given temperatures. Conventional gravimetrical and volumetric experiments were also carried out where similar behavior was observed for the measured parameters for PAAm–κC composites during drying and swelling processes.
Materials and methods
Materials
PAAm–κC composite gels were prepared by free-radical copolymerization using 0.71 g of AAm (Acrylamide, Merck), 0.01 g of BIS (N, N’-methylenebisacrylamide, Merck), 0.008 g of APS (ammonium persulfate, Merck) and 2 µl of TEMED (tetramethylethylenediamine, Merck) were dissolved in 5 ml of distilled water (pH 6.5) by heating. The heated mixture solution was held at 80 °C. Then different amounts of κ-carrageenan (0.5, 1, 1.5, 2, 2.5 and 3 (w/v) % κC) were added [24]. We used Py in the PAAm–κC composites as a fluorescence probe, which is a derivative of pyrene possessing three SO−3 groups, which can form bonds with positive charges on the gel. Py concentration was kept constant at 4 × 10−4 M, for all experiments. The solution was stirred (200 rpm) for 15 min to achieve a homogenous sample solution. All samples were deoxygenated by bubbling nitrogen for 10 min just before the polymerization process. The drying and swelling experiments of disc-shaped PAAm–κC composite gels prepared were performed in air and in water, respectively, at various temperatures (30, 40, 50, and 60 °C).
Fluorescence measurements
A model LS-50 spectrometer from Perkin-Elmer equipped with a temperature controller was used for fluorescence intensity measurements, which were made at a 90° position and spectral bandwidths were kept at 5 nm. Disc-shaped gel samples were placed on the wall of a 1-cm path-length square quartz cell filled with air and/or water for the drying and swelling experiments.
Results and discussion
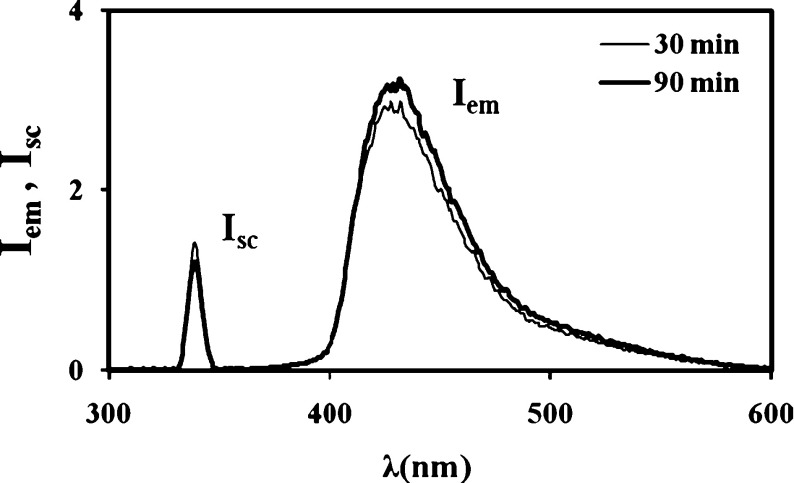
PAAm–κC gels were excited at 340 nm during in situ experiments and emission intensities of the pyranine were monitored at 427 nm as a function of drying and swelling time. As the water release began during drying, the fluorescence emission intensity, Iem, increased and the scattered light intensity Isc decreased. On the other hand, during swelling, Iem and Isc behaved the opposite, compared to the drying process. The position of the PAAm–κC gel was fixed behind the hole in the cell using stainless-steel wire so that the incoming light beam for the fluorescence measurements during drying and swelling travel in air and in distilled water respectively [25]. Here, one side of the quartz cell was covered by black cardboard with a circular hole, which was used to collimate the incoming light beam and limit its size to the dimensions of the gel disc as given in Fig. 1. Simultaneously, gravimetric measurement was performed by measuring weight. In the mean time, the thickness of the gel was also measured to calculate the volume of the PAAm–κC composite. The emission spectra of pyranine from the PAAm–κC composite gel, during the drying process at 40 °C for 1 (w/v) % κC-content sample, are given in Fig. 2. It can be seen that, as the water release increases, Iem increases and the scattered light intensity Isc decreases. It is known that the decrease in Isc corresponds to the decrease in turbidity of the drying gel, and then the corrected fluorescence intensity I is defined as Iem/Isc, i.e., as the drying time t is increased, quenching of excited pyranines decreases due to an increase in the water release from the drying PAAm–κC composite gel. These results can be quantified by considering the Stern–Volmer collisional type of quenching mechanism [26] for the fluorescence intensity I from the gel samples during both the drying and swelling processes. The following equation can be employed,
| 1 |
Fig. 1.
Position of PAAm–κC composite gels in the fluorescence cell during drying (in air) and swelling (in water). I 0 is excitation, I em is emission, and I sc is scattered light intensities at 340 and 427 nm, respectively
Fig. 2.
Emission spectra of pyranine from the hydrogel prepared with 1 (w/v) % κC-content samples at 40 °C during the drying process. Each curve corresponds to a different drying time
for the drying composite gel. Here kq is the quenching rate constant, τ0 is the lifetime of the fluorescence probe when no quencher exists, [Q] is the quencher concentration, and Io is the fluorescence intensity for no quencher-content samples. Since τ0 (=5 ns) is already known for Py, then water release can be calculated by using Eq. (2) by measuring I values at each drying step:
| 2 |
In Eq. (2), υ is the volume of the sample at the equilibrium state, which can be measured experimentally. Similarly kq was produced from a separate measurement using Eq. (1). On the other hand, it has been known that diffusion in a system with a moving boundary occurs in two distinct regions separated by a moving interface [27]. The moving interface can be marked by a discontinuous change in concentration as in the absorption by a liquid of a single component from a mixture of gases or by a discontinuity in the gradient of concentration, as in the progressive freezing of a liquid [27]. When the diffusion coefficient is discontinuous at a concentration c, i.e., the diffusion coefficient is zero below c and constant and finite above c, then the total amount, Mt of diffusing substance desorbed from unit area of a plane sheet of thickness a at time t, is given by
| 3 |
where D is a diffusion coefficient at concentration c1. Here Mf = ac1 is the equilibrium value of Mt. If it is assumed that Mt is proportional to the amount of water molecules released, W from the drying gel sample at time t, then Eq. (3) can be written as follows
| 4 |
The plots of W versus t according to Eq. (2) at 40 °C for 1.5 and 2.5 (w/v) % κC-content gel samples are presented in Fig. 3a, where the fit of the data to Eq. (4) produced the desorption coefficients DdI, which decrease by increasing the κC content at a given temperature, as given in Fig. 4. It is well known that the moisture-absorbing capacity of κC is much higher than PAAm, which results in a slower drying process in high κC-content composite gels. In other words, increasing the κC content in PAAm gels increases the water-absorbing capacity of the composite, causing the lower κC-content gel to dry faster than the high κC-content gel. One can compare these with the results of pure acrylamide with various crosslinker contents [17], where it was observed that the desorption coefficients of water molecules from the drying of pure acrylamide gels increased as the crosslinker content was increased. There, higher DdI values at high crosslinker content predicted that the gel segments move much faster in densely formed gels than they do in loosely formed gels. It was also observed that Ddw and Ddv coefficients were found to be much larger at high crosslinker content gels. On the other hand, the desorption coefficients for drying of PAAm hydrogels at various temperatures were found to be in the range of 1.8–52.9 × 10−9 m2/s, which is appropriately around the same range as our findings of 0.51–171 × 10−9 m2/s.
Fig. 3.
Plots of water release W, measured by a fluorescence, b gravimetrical, and c volumetric methods, versus drying time t, for PAAm–κC composite gels dried in air at 40 °C for 1.5 and 2.5 (w/v) % κC-content samples
Fig. 4.
Desorption coefficients versus (w/v) % κC content measured by fluorescence (D dI), gravimetric (D dW), and volumetric (D dV) methods at 40 °C
Water desorption, W, was also measured from the drying of PAAm–κC composite gel prepared with different κC content using the gravimetrical method. The plots of the data are presented in Fig. 3b at 40 °C for 1.5 and 2.5 (w/v) % κC-content composite gel. The fits of water release, W versus t1/2, according to Eq. (4) for the various κC-content gels produce the desorption coefficients DdW, which showed that the desorption coefficient decreases as the κC content is increased for each temperature as discussed earlier and shown in Fig. 4. The volume variations of PAAm–κC gels during the drying process are also monitored. The plots of the volume V versus drying time t, for 1.5 and 2.5 (w/v) % κC-content PAAm–κC composite gel dried at 40 °C, are shown in Fig. 3c. The data in Fig. 3c are again fitted to Eq. (4) for volume variation, and volumetric desorption coefficients DdV were produced. Again, it is seen from Fig. 4 that DdV values decreased as the κC content increased at each temperature; this is similar to DdW behavior, i.e., DdW and DdV coefficients are both found to be much smaller at high κC-content gels due to the high moisture-absorbing capacity of κC, as was predicted earlier.
As soon as drying was completed, the swelling experiment was started. The emission spectra of pyranine from the PAAm–κC composite during the swelling process at 40 °C for 2 (w/v) % κC-content gels presents the opposite behavior for Iem and Isc compared to drying of the PAAm–κC composite gels, as given in Fig. 5. In other words, as the swelling time t increased, the quenching rate of excited pyranines increased due to water uptake. Once kq values are measured, the water uptakes W can be calculated from the known τ0 values in each swelling step using Eq. (2). Here, it is assumed that the kq values do not vary during the swelling processes, i.e., the quenching process solely originates from the water molecules. Plots of water uptake W, calculated from the fluorescence measurements versus swelling time are presented in Fig. 6a. In order to quantify the result provided in Fig. 6a, the Li-Tanaka model was introduced as described below.
Fig. 5.
Emission spectra of pyranine from the hydrogel prepared with 2 (w/v) % κC-content samples at 40 °C during the swelling process. Each curve corresponds to a different drying time
Fig. 6.
Plots of water uptake, W, measured by a fluorescence, b gravimetrical, and c volumetric methods, versus swelling time, t, for PAAm–κC composite gels swollen in water at 40 °C for 1.5 and 2.5 (w/v) % κC-content samples
It has been known that the kinetics of the swelling of a gel is completely described by the behavior of the displacement vector as a function of space and time. Li and Tanaka [28] derived that the equation of motion is given by
| 5 |
Here, is the displacement vector measured from the final equilibrium location after the gel is totally swollen ( at t = ∞), σik is the stress tensor, and f is the friction coefficient between the network and solvent. If the shear modulus μ is not zero, the change of the total shear energy within the gel should be
| 6 |
Then, each small diffusion process determined by Eq. (5) must couple to a small shear process given by Eq. (6). A simultaneous solution of Eq. (5) and Eq. (6) produces equations for the swelling of a disc gel in axial and radial directions [28] where the axial and radial displacements are expressed as a series of components, each of them decaying exponentially with a time constant τn. The Li-Tanaka model assumed that the swelling rates of a disc in the axial (z) and radial (r) directions are the same. In the limit of large t, or if the time constant τs is much larger than the rest of τn, then the swelling kinetics is given by
| 7 |
where W and Wf are the water uptakes at time t and at equilibrium. B1 should be less than 1 is related to the shear modulus, μ and longitudinal osmotic modulus, M.
The data in Fig. 6 were fitted to the logarithmic relation produced from Eq. (7), where τs is the time constant, and can be measured by using fluorescence, gravimetric, and volumetric methods. τs is related to the cooperative diffusion coefficient, Ds, at the surface of a gel disc by
| 8 |
where α1 is a function of μ and M only [28], and af stands for the half thickness of the gel in the final equilibrium state. Hence, Ds can be calculated using Eq. (7) and employing linear regression of the curves in Fig. 6, which are typical solvent-uptake curves of each technique obeying the Li-Tanaka equation, which provided us with B1 and τs values. The experimental determination of these values was based on the method described by Li and Tanaka [28]. Then, using Eq. (8), cooperative diffusion coefficients Ds were determined for these disc-shaped composite gels for each technique and found to be around 10−9 m2/s, as given in Fig. 7. Each of the Ds values were produced from fluorescence, gravimetric, and volumetric measurements, respectively. It should be noticed that Ds values increased as the κC content was increased in Fig. 7. On the other hand, previously, the swelling of 3% κ-carrageenan in water vapor was studied using fluorescence spectroscopy [22], where the Li–Tanaka model was used to determine the swelling time constant (τsc) and cooperative diffusion coefficient (Ds) for the swelling processes. In that work, it was observed that, as τsc decreased, the Ds increased from 2.2 to 11.43 × 10−9 m2/s as the swelling temperature was increased from 30 to 60 °C. The difference between these values and our findings are in the range of 0.12–22.14 × 10−9 m2/s, most likely originated from the differences between the solvent and vapor penetration processes.
Fig. 7.
Cooperative diffusion coefficients versus (w/v) % κC content measured by fluorescence (DsI), gravimetric (DsW), and volumetric (DsV) methods at 40 °C
As discussed above, the water-absorbing capacity of κC is much higher than PAAm, which results in higher swelling rates in high κC-content composite gel. Then it is clear that lower κC-content gels swell much slower in comparison to a high κC-content gel. In other words, the presence of κC in the PAAm gel creates larger water-absorbing volumes, which then result in faster swelling of high κC-content composite gels having more double helices and more lattice dislocations. Therefore, PAAm–κC composites are important for the applications of agricultural purposes and oral drug-delivery systems.
Conclusions
The steady-state fluorescence technique was used to monitor drying and swelling behaviors of PAAm–κC composite gels prepared with various κC contents and measured at different temperatures. A moving boundary model combined with Stern–Volmer kinetics was used to measure desorption coefficients Dd for drying processes. It was observed that high κC-content composite gels dry much slower as a result of having smaller Dd coefficients for all measurements compared to low κC-content composite gels. A similar fluorescence method was employed to measure the swelling time constants τs, and the cooperative diffusion coefficients Ds, for composite gels prepared with various κC content. The Li-Tanaka Model combined with Stern–Volmer kinetics was used to measure the cooperative diffusion coefficients for the swelling process. The results were interpreted in terms of the swelling time constants: τs (decreased) and Ds (increased) as the κC contents increased. It was observed that high κC-content composite gels swell much faster as a result of having larger Ds coefficients for all measurements in comparison to low κC-content composites for a given temperature. These results have shown that the Li-Tanaka model can be used to describe the microstructure behavior of the system during drying and swelling processes of PAAm–κC composite gel. In conclusion, the Li-Tanaka model in conjunction with the fluorescence technique provided us with quite sensitive results to measure the drying and swelling parameters in air and water, respectively.
Acknowledgments
Experiments were done in the Spectroscopy Laboratory in the Department of Physics Engineering of Istanbul Technical University.
References
- 1.Hezaveh H, Muhamad II. Modification and swelling kinetic study of kappa carrageenan-based hydrogel for controlled release study. J. Taiwan Inst. Chem. Eng. 2013;44:182–191. doi: 10.1016/j.jtice.2012.10.011. [DOI] [Google Scholar]
- 2.Meena R, Prasad K, Mehta G, Siddhanta AK. Synthesis of the copolymer hydrogel κ-carrageenan-graft- PAAm: Evaluation of its absorbent and adhesive properties. J. Appl. Polym. Sci. 2006;102:5144–5153. doi: 10.1002/app.24703. [DOI] [Google Scholar]
- 3.Kadajji VG, Betageri GV. Water soluble polymers for pharmaceutical applications. Polymers. 2011;3:1972–2009. doi: 10.3390/polym3041972. [DOI] [Google Scholar]
- 4.Chiellini, E., Sunamoto, J., Migliaresi, C., Ottenbrite, R.M., Cohn, D. (eds.): Biomedical Polymers and Polymer Therapeutics, Springer Link, US, Kluwer Academic Publishers (2002)
- 5.Ottenbrite, R.M., Park, K., Okano T.: Biomedical Applications of Hydrogels Handbook. Springer Link, New York (2010)
- 6.Diederich VEG, Studer P, Kern A, Lattuada M, Storti G, Sharma RI, Snedeker JG, Morbidelli M. Bioactive polyacrylamide hydrogels with gradients in mechanical stiffness. Biotech. & Bioeng. 2013;110(5):1508–1511. doi: 10.1002/bit.24810. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi M, Soleimani F. Synthesis of novel polysaccharide based superabsorbent hydrogels via graft copolymerization of vinylic monomers onto kappa carrageenan. Int. J. Chem. Eng. Appl. 2011;2:304–306. [Google Scholar]
- 8.Holdt SL, Kraan S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- 9.Sadeghi M, Heidari B, Montazeri K. pH responsiveness properties of a biodegradable hydrogels based on carrageenan-g- poly(NaAA-co-NIPAM), World Academy Sci. Eng. Tech. 2011;52:417–420. [Google Scholar]
- 10.de Ruitter GA, Rudolph B. Carrageenan biotechnology. Trend Food Sci. Tech. 1997;8:389–395. doi: 10.1016/S0924-2244(97)01091-1. [DOI] [Google Scholar]
- 11.Falshaw R, Bixler HJ, Johndro K. Structure and performance of commercial kappa-2 carrageenan extracts:1. Structure analysis. Food Hydrocoll. 2001;15:441–452. doi: 10.1016/S0268-005X(01)00066-2. [DOI] [Google Scholar]
- 12.Kara S, Tamerler C, Bermek H, Pekcan Ö. Hysteresis during sol–gel and gel-sol phase transitions of κ-carrageenan: a photon transmission study. J. Bioact. Compat. Polym. 2003;18:33–44. doi: 10.1177/0883911503018001004. [DOI] [Google Scholar]
- 13.Tari Ö, Pekcan Ö. A percolation approach for investigating the sol–gel phase transition of κ-carrageenan: A steady state fluorescence study. J. Bioact. Compat. Polym. 2004;19(6):491–509. doi: 10.1177/0883911504048328. [DOI] [Google Scholar]
- 14.Kara S, Pekcan Ö. Photon transmission technique for monitoring drying process in acrylamide gels formed with various crosslinker contents. J. Appl. Polym. Sci. 2001;80:1898–1906. doi: 10.1002/app.1287. [DOI] [Google Scholar]
- 15.Aktaş DK, Evingür GA, Pekcan Ö. Drying of PAAm hydrogels at various temperatures: a fluorescence study. J. Macromol. Sci. Part B: Phys. 2007;46:581–590. doi: 10.1080/00222340701257950. [DOI] [Google Scholar]
- 16.Tari Ö, Pekcan Ö. Study of drying of κ-carrageenan gel at various temperatures using a fluorescence technique. Drying Tech. 2008;26:101–107. doi: 10.1080/07373930701781728. [DOI] [Google Scholar]
- 17.Evingür GA, Aktaş DK, Pekcan Ö. In situ steady state fluorescence (SSF) technique to study drying of PAAm hydrogels made of various cross-linker contents. Chem. Eng. Process. 2009;48:600–605. doi: 10.1016/j.cep.2008.07.003. [DOI] [Google Scholar]
- 18.Evingür GA, Pekcan Ö. Drying of polyacrylamide composite gels formed with various kappa-carrageenan content. J. Fluoresc. 2011;21:1531–1537. doi: 10.1007/s10895-011-0841-3. [DOI] [PubMed] [Google Scholar]
- 19.Aktaş DK, Evingür GA, Pekcan Ö. Study on swelling of hydrogels (PAAm) at various temperatures by using fluorescence technique. J. Mat. Sci. 2007;42:8481–8488. doi: 10.1007/s10853-007-1764-x. [DOI] [Google Scholar]
- 20.Aktaş DK, Evingür GA, Pekcan Ö. A fluorescence study on swelling of hydrogels (PAAm) at various crosslinker contents. Adv. Polym. Tech. 2009;28(4):215–223. doi: 10.1002/adv.20163. [DOI] [Google Scholar]
- 21.Kara S, Tamerler C, Arda E, Pekcan Ö. Photon Transmission study on swelling of κ-carrageenan gels prepared in various concentrations. Int. J. Bio. Macromol. 2003;33:235–243. doi: 10.1016/j.ijbiomac.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Tari Ö, Pekcan Ö. Swelling activation energy of κ-carrageenan in its gel state: A fluorescence study. J. Appl. Polym. Sci. 2007;106:4164–4168. doi: 10.1002/app.26980. [DOI] [Google Scholar]
- 23.Evingür GA, Pekcan Ö. Temperature effect on the swelling of PAAm–κ-carrageenan composites. J. Appl. Polym. Sci. 2012;123:1746–1754. doi: 10.1002/app.34632. [DOI] [Google Scholar]
- 24.Aktaş DK, Evingür GA, Pekcan Ö. Universal behaviour of gel formation from acrylamide-carrageenan mixture around the gel point: A fluorescence study. J. Bio. Strc. Dyn. 2006;24(1):83–90. doi: 10.1080/07391102.2006.10507102. [DOI] [PubMed] [Google Scholar]
- 25.Evingür GA, Pekcan Ö. Studies on drying and swelling of PAAm–NIPA composites in various compositions. Polym. Comp. 2011;32:928–936. doi: 10.1002/pc.21111. [DOI] [Google Scholar]
- 26.Birks JB. Photopyhsics of aromatic molecules. New York: Wiley, Interscience; 1971. [Google Scholar]
- 27.Crank J. The Mathematics of Diffusion. Oxford: Clarendon; 1975. [Google Scholar]
- 28.Li Y, Tanaka T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990;92:1365–1371. doi: 10.1063/1.458148. [DOI] [Google Scholar]