Abstract
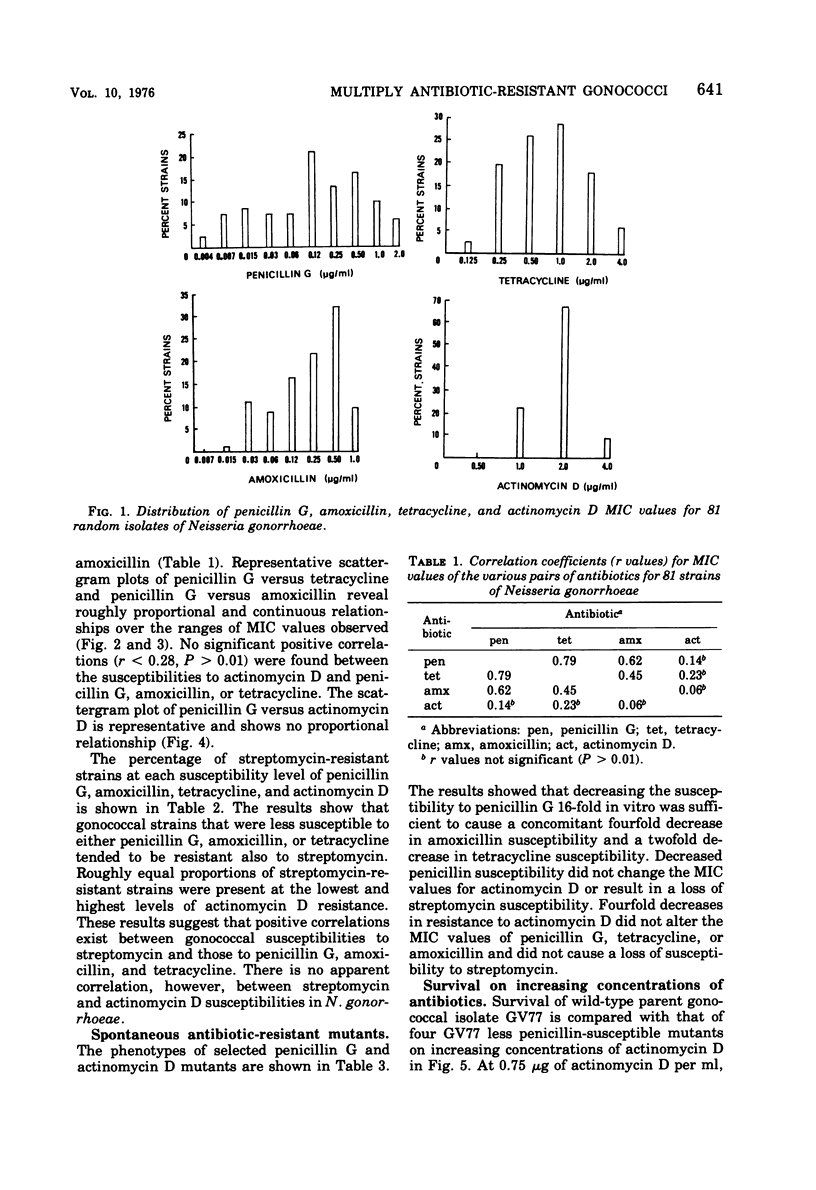
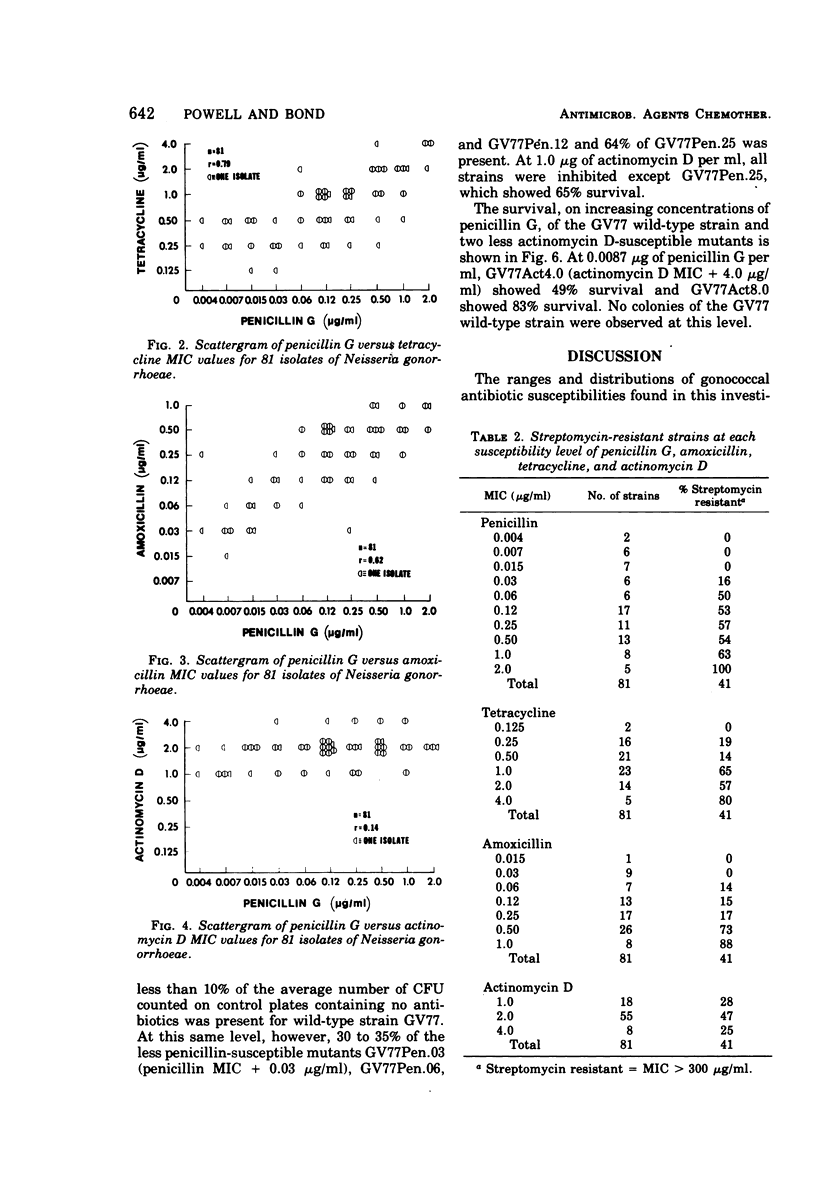
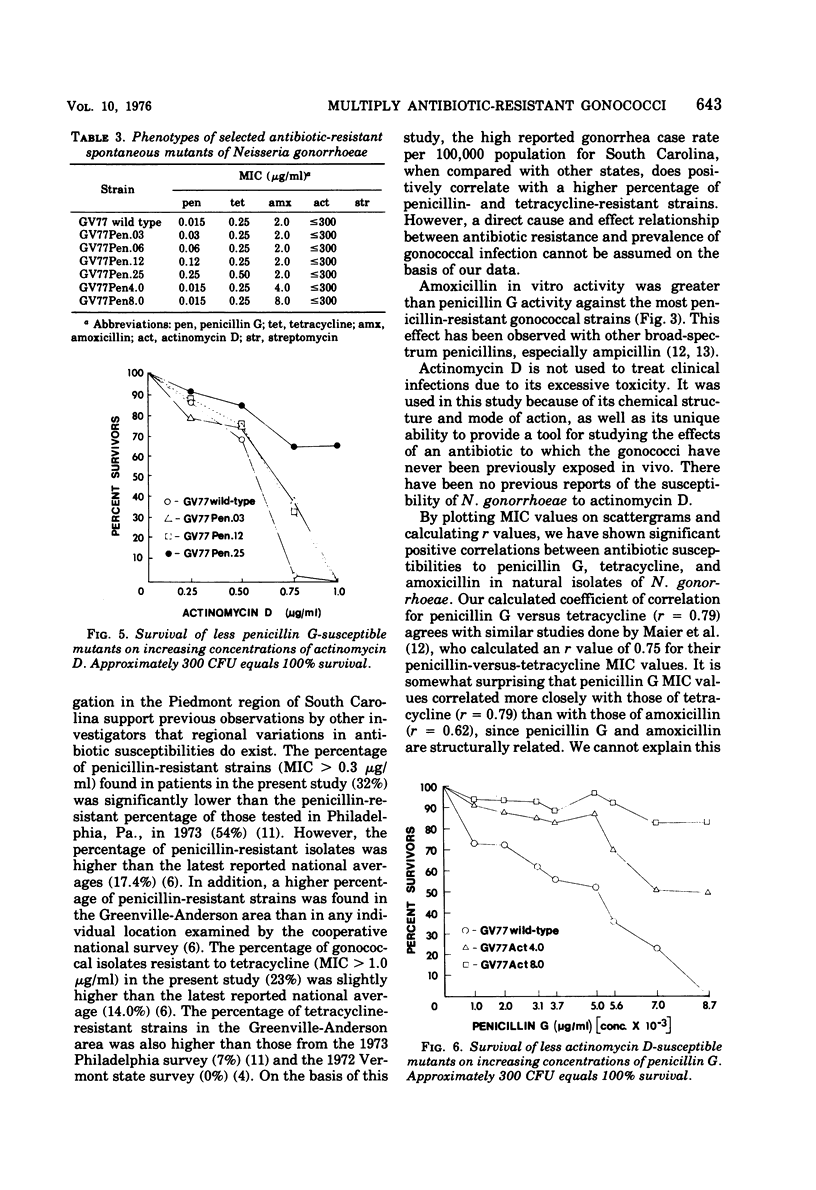
Minimal inhibitory concentration (MIC) values for penicillin G, tetracycline, amoxicillin, and actinomycin D were determined for 81 strains of Neisseria gonorrhoeae isolated from patients attending public health clinics in the Piedmont region of South Carolina. Gonococcal isolates were also screened for highlevel resistance to streptomycin. Significant positive correlations (r ≥ 0.45, P ≤ 0.01) were found between all possible pairs of the antibiotics penicillin G, tetracycline, and amoxicillin. The MIC values of actinomycin D showed no significant positive correlations with the MIC values of the other antibiotics. Gonococcal strains that were resistant to streptomycin tended to be resistant to penicillin G, tetracycline, and amoxicillin. Of the 81 isolates, 18.5% were multiply resistant to penicillin G, tetracycline, amoxicillin, and streptomycin. Spontaneous mutants with reduced antibiotic susceptibility, selected for decreased susceptibility to penicillin G, displayed small decreases in susceptibility to tetracycline, amoxicillin, and actinomycin D. Spontaneous mutants selected for decreased susceptibility to actinomycin D displayed small losses in susceptibility to penicillin G. The results show that multiple antibiotic resistance occurs in clinically isolated gonococcal strains in South Carolina. The results further suggest the presence of a common genetic mechanism determining antibiotic resistance in N. gonorrhoeae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornelius C. E., 3rd Six-city study of treatment of gonorrhoea in men using single oral doses of 1.5 or 3 g. tetracycline hydrochloride. Br J Vener Dis. 1970 Aug;46(4):330–333. doi: 10.1136/sti.46.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump D. W., Berry P. T. Increased susceptibility of Neisseria gonorrhoea from a rural area. Antimicrob Agents Chemother. 1973 Apr;3(4):503–505. doi: 10.1128/aac.3.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Sparling P. F. Altered crystal violet permeability and lytic behavior in antibiotic-resistant and -sensitive mutants of Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):757–763. doi: 10.1128/jb.124.2.757-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Cheng K. J. Effect of the removal of outer cell wall layers on the actinomycin susceptibility of a gram-negative bacterium. Antimicrob Agents Chemother. 1972 May;1(5):447–449. doi: 10.1128/aac.1.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Beilstein H. R., Zubrzycki L. Antibiotic disk susceptibility tests with Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1974 Mar;5(3):210–216. doi: 10.1128/aac.5.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Beilstein H. R., Zubrzycki L. Multiple antibiotic resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1974 Jul;6(1):22–28. doi: 10.1128/aac.6.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Zubrzycki L., Coyle M. B. Genetic analysis of drug resistance in Neisseria gonorrhoeae: identification and linkage relationships of loci controlling drug resistance. Antimicrob Agents Chemother. 1975 May;7(5):676–681. doi: 10.1128/aac.7.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Reyn A., Bentzon M. W. Relationships between the sensitivities in vitro of Neisseria gonorrhoeae to spiramycin, penicillin, streptomycin, tetracycline, and erythromycin. Br J Vener Dis. 1969 Sep;45(3):223–227. doi: 10.1136/sti.45.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W., Saz A. K. Possible mechanism of decreased susceptibility of Neisseria gonorrhoeae to penicillin. Antimicrob Agents Chemother. 1975 Jun;7(6):788–792. doi: 10.1128/aac.7.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Leive L. Actinomycin resistance and actinomycin excretion in a mutant of Escherichia coli. J Bacteriol. 1970 May;102(2):600–602. doi: 10.1128/jb.102.2.600-602.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox R. R. A survey of problems in the antibiotic treatment of gonorrhoea. With special reference to South-East Asia. Br J Vener Dis. 1970 Jun;46(3):217–242. doi: 10.1136/sti.46.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]


