Abstract
Introduction
The objective of this study was to investigate the possible role of UDP-glucose dehydrogenase (UGDH) in osteoarthritis (OA) and uncover whether, furthermore how interleukin-1beta (IL-1β) affects UGDH gene expression.
Methods
UGDH specific siRNAs were applied to determine the role of UGDH in proteoglycan (PG) synthesis in human articular chondrocytes. Protein levels of UGDH and Sp1 in human and rat OA cartilage were detected. Then, human primary chondrocytes were treated with IL-1β to find out whether and how IL-1β could regulate the gene expression of UGDH and its trans-regulators, that is Sp1, Sp3 and c-Krox. Finally, p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 and stress-activated protein kinase/c-Jun N-terminal kinase (SAP/JNK) inhibitor SP600125 were used to pick out the pathway that mediated the IL-1β-modulated PGs synthesis and gene expression of UGDH, Sp1, Sp3 and c-Krox.
Results
UGDH specific siRNAs markedly inhibited UGDH mRNA and protein expression, and thus led to an obvious suppression of PGs synthesis in human articular chondrocytes. UGDH protein level in human and rat OA cartilage were much lower than the corresponding controls and negatively correlated to the degree of OA. Decrease in Sp1 protein level was also observed in human and rat OA cartilage respectively. Meanwhile, IL-1β suppressed UGDH gene expression in human articular chondrocytes in the late phase, which also modulated gene expression of Sp1, Sp3 and c-Krox and increased both Sp3/Sp1 and c-Krox/Sp1 ratio. Moreover, the inhibition of SAP/JNK and p38 MAPK pathways both resulted in an obvious attenuation of the IL-1β-induced suppression on the UGDH gene expression.
Conclusions
UGDH is essential in the PGs synthesis of articular chondrocytes, while the suppressed expression of UGDH might probably be involved in advanced OA, partly due to the modulation of p38 MAPK and SAP/JNK pathways and its trans-regulators by IL-1β.
Electronic supplementary material
The online version of this article (doi:10.1186/s13075-014-0484-2) contains supplementary material, which is available to authorized users.
Introduction
Proteoglycans (PGs) are glycoconjugates composed of a core protein backbone and numerous glycosaminoglycan (GAG) side chains, which determine the fluid and electrolyte balance and the elasticity of articular cartilage and provide the living space for chondrocytes through interaction with the collagen network. Thus, PGs are essential in maintaining cartilage homeostasis [1]. Loss of PGs would lead to the imbalance of cartilage homeostasis, which further accelerates the degeneration of cartilage matrix and the apoptosis of chondrocytes, and finally triggers the pathogenesis of osteoarthritis (OA), a chronic and degenerative arthritis with a high prevalence in the elderly [1,2].
UDP-glucose dehydrogenase (UGDH) catalyzes the transformation of UDP-glucose to UDP-glucuronic acid, a key precursor for the synthesis of the GAG chain in PGs [3-6]. Stimulating UGDH enzyme activity with transforming growth factor β (TGF-β) resulted in the enhanced GAG synthesis in articular chondrocytes [7]. However, whether UGDH is indispensable in the PG synthesis of articular chondrocytes and whether UGDH is also involved in the pathogenesis of OA still remain unclear.
IL-1β is a key pro-inflammatory cytokine in the progression of OA, which attenuates the anabolism but enhances the catabolism in the chondrocytes, through activating the downstream signaling pathways, including those of stress-activated protein kinase/c-Jun N-terminal kinase (SAP/JNK) and p38 mitogen-activated protein kinase (p38 MAPK) [8-10]. It is well-known that IL-1β is one of the key pro-inflammatory factors responsible for the PG loss in OA pathogenesis. However, whether UGDH is involved in the IL-1β-induced PG loss is unknown.
Specificity protein 1 (Sp1), Sp3 and Krueppel-related zinc finger protein cKrox (c-Krox) are trans-regulators sharing almost the same binding sites located in the promoter region of UGDH gene [11,12]. Sp1 recognizes GC- or GT-rich motifs and presents positive regulatory effects on the transcriptional activity of the UGDH gene [13,14]. Sp3 is another member of the Sp family, which represses Sp1-mediated activation of gene transcription due to the competition for their common binding sites [12]. Meanwhile, c-Krox, the key trans-regulator of type 1 collagen [15], inhibits gene transcription of UGDH in chondrocytes [11].
So, we hypothesized that UGDH is essential in the PG synthesis of articular chondrocytes, and that IL-1β inhibits UGDH gene expression through modulating UGDH trans-regulators and the downstream signaling cascades, including the SAP/JNK and p38 MAPK pathways, which might be involved in the PGs loss of OA cartilage and contribute to the OA pathogenesis. So, we detected PG content in human primary chondrocytes treated with UGDH-specific siRNA, measured the protein level of UGDH and Sp1 in human and rat OA cartilage and detected the influence of the activation and inhibition of SAP/JNK or p38 MAPK pathways on the gene expression of UGDH and its trans-regulators in human articular chondrocytes, in an attempt to uncover the role of UGDH in the PG synthesis of articular chondrocytes and the pathogenesis of OA.
Methods
Cartilage specimens
Human articular cartilage specimens from the knee joints were obtained from OA patients diagnosed with advanced OA using the criteria of the American College of Rheumatology for OA undergoing total knee replacement surgery (21 knees from 15 female patients, aged 66 ± 10 years) with signed informed consent [16]. The procedures were in accordance to the ethical guidelines of the Helsinki Declaration of 1975 (as revised in 2000) and approved by Medical Ethics Committee of the Zhongnan Hospital of Wuhan University (number 2012030). Microscopically normal cartilage (MNC) and degenerative cartilage (DC) from the same patient was collected respectively from the tibial plateau using a surgical microscope with 8-fold amplification, paired and numbered [17].
Pathogen-free adult Wistar rats (weight 220 to 280 g) were supplied by Experimental Centre of Medical Scientific Academy of Hubei province, which also approved the animal study protocol applied in the study (number 2008–0005). The protocol was in accordance with the Guide for the Care and Use of Laboratory Animals (eighth edition) by the National Research Council of the United States National Academies. The animal study was performed in the Animal Biosafety Level-3 Laboratory of Wuhan University (Wuhan, China) accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The OA model was induced as described previously [18]. Sixteen rats were grouped into the control group and the OA group, which were intra-articularly injected respectively with 20 μL of sterile 0.9% saline or 4% (w/v) papain (Sigma-Aldrich, MO, USA) solution in saline to the right knees of the rats on days 1, 4 and 7. Two weeks after the last injection, all the rats were sacrificed under anesthesia for the knee joints.
Histopathology assay
Cartilage samples from the weight-bearing area of the knee joint were used in pathological testing. Human MNC samples were defined as the control, while the DC samples were defined as the OA cartilage. Samples of human and rat cartilage were fixed in 4% paraformaldehyde overnight and embedded in paraffin wax, successively. Then, sections of 5 μm were obtained perpendicularly to the surface of articular cartilage. HE and Safranin O staining was performed according to the standard protocol. The degree of OA was presented independently by three observers according to the modified Mankin scoring system using a blinded method [19]. Moreover, protein expression of UGDH and Sp1 in the chondrocytes was also detected using immunohistochemical (IHC) assay with anti-UGDH (1:150, Proteintech, Chicago, IL, USA) and anti-Sp1 (1:150, Proteintech) antibodies. The relative protein level of UGDH and Sp1 was presented as the mean absorbance of each positively stained chondrocyte using NIS-elements software (Nikon, Tokyo, Japan).
Chondrocyte isolation, culture and treatment
Human cartilage samples without microscopically visible degeneration were dissected and digested with 0.25% trypsin (Sigma-Aldrich, MO, USA) for 30 minutes and 0.2% collagenase typeII (Sigma-Aldrich) for 12 h in serum-free DMEM/F-12 (1:1 v/v) (Thermo Fisher, Beijing, China). Then, chondrocytes were collected and cultured as a monolayer in DMEM/F12 (1:1 v/v) with 10% (v/v) fetal bovine serum (Thermo Fisher), 100 IU/mL penicillin (Biyotime, Haimen, China), 100 μg/mL streptomycin (Biyotime), and 2 mM glutamine (Biyotime) at 37°C with 5% CO2. Hereafter, the chondrocytes were treated with UGDH-specific siRNA for 4 h using Lipofectamine 2000 Reagent (Life technologies, Grand Island, NY, USA) and cultured for another 48 h following the manufacturer’s protocol. Details of the UGDH-specific siRNA are listed in Table 1. Chondrocytes were also treated with human recombinant IL-1β (10, 20, 40 ng/mL, PeproTech, Rocky Hill, NJ, USA) for 12, 24 and 48 h, and were also pretreated with p38 MAPK inhibitor SB203580 (20 μM, Sigma-Aldrich) or SAP/JNK inhibitor SP600125 (10 μM, Sigma-Aldrich) for 0.5 h and subsequently co-treated with 10 ng/mL IL-1β for another 48 h, to detect the mRNA and protein level of the genes of interest. Meanwhile, chondrocytes were also treated with IL-1β (10 ng/mL) for 0 to 120 minutes or pretreated with SP600125 or SB203580 for 30 miutes and then treated with 10 ng/mL IL-1β for another 30 minutes for the phosphorylation status of JNK and p38 MAPK. Chondrocytes from at least three individuals were applied in every in vitro experiment.
Table 1.
The small interfering RNA applied in the study
| Target genes | Gene ID | siRNA pairs | Sequence |
|---|---|---|---|
| UGDH | 2582 | Pair 1 | F: 5′-CUGAGUGGGACAUGUUUAATT -3′ |
| R: 5′-UUAAACAUGUCCCACUCAGTT -3′ | |||
| Pair 2 | F: 5′-CAGCCAUCAAGGACCUAAATT -3′ | ||
| R: 5′-UUUAGGUCCUUGAUGGCUGTT -3′ | |||
| Pair 3 | F: 5′-GCCAGAAGUAGCUCGUUAUTT -3′ | ||
| R: 5′-AUAACGAGCUACUUCUGGCTT -3′ | |||
| Negative control | NA | Pair 1 | F: 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| R: 5′-ACTUGACACGUUCGGAGAATT-3′ |
UGDH, UDP-glucose dehydrogenase; NA, not applicable.
GAG detection
GAG content was detected using 1,9-dimethylmethylene blue (DMB, Sigma-Aldrich) reagent as reported [20-22]. Absorbance at 570 nm was measured using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan). A standard curve constructed with chondroitin sulfate sodium salt from shark cartilage (Sigma-Aldrich) was used to quantify GAG content in the chondrocyte cultures. Then, total GAG was determined as GAG content versus protein content of the same culture. Meanwhile, chondrocytes were cultured on coverslips, fixed in 10% (w/v) neutral formalin for 15 minutes, stained with 0.5% (w/v) Alcian blue dye and photographed using an AZ100 Microscopes (Nikon, Tokyo, Japan). Relative GAG content was determined as mean absorbance of each positively stained chondrocyte.
Real-time quantitative PCR assay
Real-time quantitative PCR assay was performed as previously described [23]. Total RNA was isolated using TRIzol reagent (Life Technologies). Single-strand cDNA was obtained using a First Strand cDNA Synthesis Kit (Takara, Dalian, China). Primer Premier 5.0 (Premier Biosoft, Palo Alto, CA, USA) and the NCBI BLAST database were applied to design the primers for the genes of interest. The primers used in this study are listed in Table 2. The RT-PCR assay was performed on a StepOne thermal cycler (Applied Biosystems, Grand Island, NY, USA) using reverse-transcription (RT)-PCR kits (Takara) using the following procedure: pre-denaturation at 95°C for 30 sec, denaturation at 95°C for 5 sec, annealing at Tm for 30 sec, and extension at 72°C for 30 sec. The last three steps ran for 40 cycles. Relative standard curves were constructed for relative quantification. The expression of all the target genes was normalized to the GAPDH gene to standardize comparison.
Table 2.
The primers used in the study
| Target genes | Gene ID | Sequence | Annealing (°C) | Product size (bp) |
|---|---|---|---|---|
| UGDH | 2582 | F: 5′-CAGGCTATGTTGGAGGACCC-3′ | 60 | 162 |
| R: 5′-TCGACAGGATTCTACCACTTCTT-3′ | ||||
| Sp1 | 6667 | F: 5′-ATGGACAGGTCAGTTGGCAG-3′ | 60 | 89 |
| R: 5′-CTGCATTGGGGCTAAGGTGA-3′ | ||||
| Sp3 | 6670 | F: 5′-CAGTCAGCAGATGGTCAGCA-3′ | 60 | 185 |
| R: 5′-CCCTGAACCTGGACTTGACC-3′ | ||||
| c-Krox | 51043 | F: 5′-CGGTGTTCGATTCACCAGGA-3′ | 60 | 134 |
| R:5′-GCAGGTGCATGTGGTTCTTG-3′ | ||||
| GAPDH | 2597 | F: 5′-GAAATCCCATCACCATCTTCCAG-3′ | 60 | 313 |
| R:5′-GAGTCCTTCCACGATACCAAAG-3′ |
UGDH, UDP-glucose dehydrogenase; Sp1, specificity protein 1; Sp3, specificity protein 3; c-Krox, krueppel-related zinc finger protein cKrox; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blotting assay
Total proteins were obtained from human cartilage samples and chondrocyte cultures using radioimmunoprecipitation assay (RIPA) lysis buffer (Biyotime), while nuclear proteins were extracted using a nuclear protein extraction kit (Biyotime). Then, proteins were size-fractionated by SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Bellerica, MA, USA). The target proteins were probed with anti-UGDH (1:1,000, Proteintech), anti-Sp1 (1:800, Proteintech), anti-Phospho-SAPK/JNK (Tyr185) (1:500, Enogene, Nanjing, China), anti-Phospho-p38 MAPK (Thr180) (1:500, Enogene), anti-SAPK/JNK (1:1000, Sangon, Shanghai, China), anti-p38 MAPK (1:1000, Sangon), anti-GAPDH (1:1,000, Proteintech) and anti-lamin A/C (1:1,000, Proteintech) primary antibodies, incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000, Proteintech). Blots were developed using ECL reagent (Advansta, Menlo Park CA, USA). A Fusion FX system (Vilber Lourmat, Marne-la-Vallée, France) was applied to photograph the blots. Then, relative the protein level of UGDH and SP1 was obtained using Quantity One software (Version 4.6, Bio-Rad, Berkeley, CA, USA), compared with the corresponding controls and standardized to GAPDH.
Statistical analysis
Data analysis was performed using SPSS 17.0 (SPSS Science Inc., Armonk, NY, USA) and Prism 5.0 (GraphPad Software, San Diego, CA, USA). Results were presented as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) or Student’s t-test, as appropriate, after testing the homogeneity of variances, were performed to analyze the data. The Wilcoxon rank-sum test was applied to analyze the difference between Mankin scores for cartilage from the control and the OA group. Spearman rank correlation analysis was performed to test the correlation between Mankin score and UGDH protein level in human and rat cartilage. Values of P <0.05 were considered statistically significant.
Results
UGDH was essential in PG synthesis of human articular chondrocytes
Obvious decreases in UGDH mRNA and protein levels were observed in human articular chondrocytes treated with three different UGDH-specific siRNA (Figure 1A, B and C, P <0.05), which was accompanied by a decrease in total GAG content in the chondrocyte cultures (Figure 1D, P <0.05). Meanwhile, the staining of chondrocytes treated with UGDH-specific siRNA by Alcian blue was much lower than the control (Figure 1E and F, P <0.05), which also indicated the suppression of PG synthesis in the chondrocytes due to inhibited UGDH gene expression.
Figure 1.

UDP-glucose dehydrogenase ( UGDH )-specific siRNA suppressed glycosaminoglycan (GAG) synthesis in human articular chondrocytes. Human articular chondrocytes were treated with UGDH-specific siRNA for 48 h. Then, mRNA and the protein level of UGDH were detected using real-time PCR (A) and western blotting assay (B, C), while GAG content of these chondrocyte cultures were detected with 1,9-dimethylmethylene blue dye (D) and Alcian blue dye (E, F). The protein level of UGDH was presented as gray level of the blots quantified using Quantity One software (Version 4.6, Bio-Rad). Values are presented as mean ± standard error of the mean from at least three independent experiments. *P <0.05 and **P <0.01 versus control.
UGDH protein expression was decreased in OA cartilage
Serious degenerative features of human OA cartilage, namely extensive surface irregularities, clefts to calcified zone, and even complete disorganization, were observed using HE staining (Figure 2A and B). A marked decrease in GAG content was observed in DC by safranin O staining, when compared with MNC from the same OA patient (Figure 2E and F). Mankin scores of MNC were also much lower than those for DC (P <0.05), while the UGDH protein level of DC was also significantly lower than that of MNC (P <0.05). An additional figure file shows this in more detail (see Additional file 1A). Similar degenerative features were also observed in rat OA cartilage, together with an increase of chondrocyte numbers (Figure 2C and D). Safranin O staining of rat OA cartilage was also much lighter (Figure 2G and H). Moreover, Mankin scores for rat OA cartilage were much higher (P <0.05), while UGDH protein level was also lower when compared with normal control (P <0.05). An additional figure file shows this in more detail (see Additional file 1B). Further correlation analysis showed that UGDH protein level in both human and rat cartilage was negatively correlated with the Mankin score (P <0.05, Figure 2M and N), which indicated a strong correlation between the suppressed UGDH protein level with the stimulated cartilage degeneration during OA.
Figure 2.

Histopathology assay of human and rat osteoarthritis (OA) cartilage. Human cartilage from the tibial plateau of OA patients was collected, and the rat OA model was induced. Human OA cartilage was divided as microscopically normal cartilage (MNC) and degenerative cartilage (DC). The former was defined as the control, and the latter as OA cartilage. HE staining (A-D) and Safranin O staining (E-H) was performed. UDP-glucose dehydrogenase (UGDH) protein level was detected using immunohistochemical (IHC) assay (I, L) and presented as mean optical density of each chondrocytes using NIS-Elements software (Nikon, Tokyo, Japan), and the modified Mankin score system was applied to grade the degree of OA. Cartilage samples from 11 knees were included in the pathological analysis. Then, correlation between the Mankin score and UGDH protein level of human and rat cartilage (M, N) was performed. Scale bars, 100 μm.
IL-1β decreased UGDH gene expression and inhibited GAG synthesis
To determine whether IL-1β was involved in the suppression of UGDH protein expression in OA cartilage, we treated human articular chondrocytes with recombinant IL-1β and found that IL-1β decreased the total GAG content of chondrocyte cultures in a concentration- and time-dependent manner (all P <0.05, Figure 3A). Although mRNA level of UGDH was increased after 12 h, IL-1β downregulated UGDH mRNA level in a concentration-dependent manner after 24 h or 48 h of treatment (P <0.05, Figure 2B). Moreover, it also turned out that UGDH protein level was downregulated by IL-1β treatment for 48 h (all P <0.05, Figure 3C and D).
Figure 3.
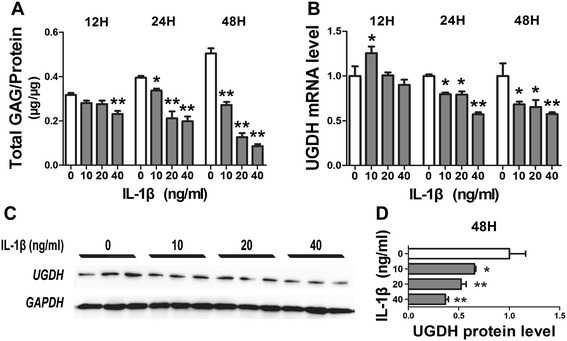
Interleukin 1β (IL-1β) modulated glycosaminoglycan (GAG) synthesis and UDP-glucose dehydrogenase ( UGDH ) gene expression. Human articular chondrocytes were treated with recombinant IL-1β for 12 h, 24 h and 48 h. Then, GAG content (A) and the mRNA level of UGDH (B) of chondrocyte cultures was detected. Meanwhile, UGDH protein level from chondrocytes treated with IL-1β for 48 h was detected using western blotting assay (C, D). Values were presented as mean ± standard error of the mean from at least three independent experiments. *P <0.05 and **P <0.01 versus control.
Transcriptional regulation of UGDH
Sp1, Sp3 and c-Krox are the key trans-regulators of the UGDH gene [11-13]. Here, Sp1 protein level in human DC was markedly lower than the MNC of the same patient (P <0.05, Figure 4A and B). Meanwhile, a notable decrease of Sp1 protein level in OA rat cartilage was also observed (P <0.05, Figure 4A and B). Moreover, the mRNA expression of Sp1 in human primary chondrocytes was downregulated after IL-1β treatment, while c-Krox mRNA levels were increased (P <0.05, Figure 4C and E). Sp3 mRNA expression was stimulated by IL-1β after 12-h and 24-h treatment, while an obvious decrease in Sp3 mRNA level was detected after 48 h (P <0.05, Figure 4D). A concentration-dependent suppression of protein expression and nuclear translocation of Sp1 were also observed in chondrocytes treated with IL-1β for 48 h (all P <0.05, Figure 4F and G). Moreover, the ratio of Sp3/Sp1 and c-Krox/Sp1 was markedly increased after IL-1β treatment (P <0.05). An additional figure file shows this in more detail (see Additional file 2).
Figure 4.

Gene expressions of trans-regulators of UDP-glucose dehydrogenase ( UGDH ). Specificity protein 1 (Sp1) protein level in was detected in both human and rat cartilage samples (A, B). Relative mRNA levels of Sp1, Sp3 and Krueppel-related zinc finger protein c-Krox (c-Krox) were also detected in human primary chondrocyte treat with IL-1β (C-E). Total and nuclear Sp1 protein level was also detected and quantified (F, G). MNC, microscopically normal cartilage; DC, degenerative cartilage. Scale bars, 100 μm. Values are presented as mean ± standard error of the mean from at least three independent experiments. *P <0.05 and **P <0.01 versus control.
IL-1β modulated the expression of UGDH through the p38 MAPK and SAP/JNK pathway
IL-1β obviously increased the phosphorylation levels of both SAP/JNK and p38 MAPK from 5 minutes to 1 h, or even longer in human articular chondrocytes, while the peak of phosphorylation last from 15 to 60 minutes (Figure 5A). Then, the phosphorylation status of both SAP/JNK and p38 MAPK started to fade after 60 minutes (Figure 5A). However, the activation of phosphorylation of SAP/JNK and p38 MAPK by IL-1β were obviously reduced by the pretreated SP600125 and SB203580, respectively (Figure 5B). Further, SB203580 promoted GAG synthesis and UGDH mRNA expression but did not affect the trans-regulators, while SP600125 affected none of these process (P <0.05, Figure 6A-D). However, IL-1β inhibited GAG synthesis and gene expression of UGDH, Sp1 and Sp3, but stimulated c-Krox gene expression (P <0.05, Figure 6A-D), while both SB203580 and SP600125 attenuated the effect of IL-1β on these process (P <0.05, Figure 6A-D), which indicated that both p38 MAPK pathway and SAP/JNK pathway were involved in the IL-1β-modulated UGDH gene expression.
Figure 5.
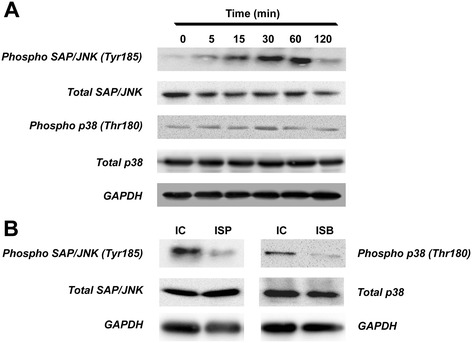
Phosphorylation of stress-activated protein kinase/c-Jun N-terminal kinase (SAP/JNK) and p38 mitogen-activated protein kinase (MAPK) protein in human primary chondrocytes. Human primary chondrocytes were treated with IL-1β treatment (10 ng/mL) for 0 to 120 mintes (A) or pretreated with SP600125 or SB203580 for 30 minutes and then treated with 10 ng/mL IL-1β for 30 minutes (B). Then, phosphorylated and total protein level of SAP/JNK and p38 MAPK, as well as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein level, were detected using western blotting assay. IC, IL-1β control, namely chondrocytes treated with 10 ng/mL IL-1β for 30 minutes; ISP, chondrocytes pretreated with SP600125 for 30 minutes and subsequently co-treated with the SP600125 and 10 ng/mL IL-1β for another 30 minutes; ISB, chondrocytes pretreated with SB203580 for 30 minutes and then co-treated with the SB203580 and 10 ng/mL IL-1β for another 30 minutes.
Figure 6.
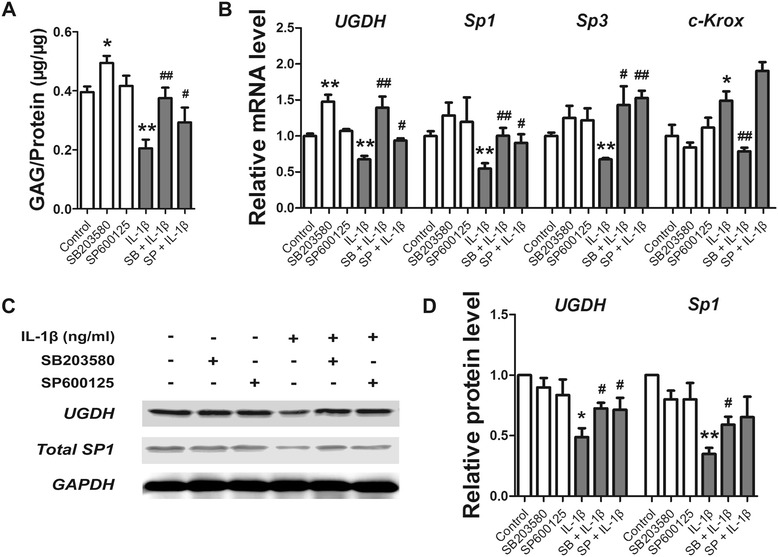
IL-1β, SB203580 and SP600125 modulated UDP-glucose dehydrogenase ( UGDH ) gene expression. Human articular chondrocytes were pretreated with SB203580 (20 μM) and SP600125 (10 μM) for 30 minutes and/or subsequently treated with IL-1β (10 ng/mL) for another 48 h. Total GAG (A), the mRNA level of UGDH, specificity protein 1 (Sp1), Sp3 and Krueppel-related zinc finger protein c-Krox (c-Krox) (B), and the protein level of UGDH and Sp1 (C, D) was also detected. Values are presented as mean ± standard error of the mean from at least three independent experiments. *P <0.05, **P <0.01 versus control, # P <0.05, ## P <0.01 versus the IL-1β group.
Discussion
It is well-known that the content of PG is most abundant in the mid-zone of articular cartilage, rather than the superficial or deep zones; chondrocytes in the mid-zone highly synthesize both PGs and collagens, while chondrocytes in the superficial and deep zones mainly synthesize collagens (type II and type X, respectively) instead of PG [24]. Meanwhile, chondrocytes in the mid-zone but not the superficial or deep zones of articular cartilage have high UGDH activity [25], which indicates a possible correlation between UGDH enzyme activity and PG synthesis in articular chondrocytes. Moreover, evidence also indicates that UGDH determines hyaluronan synthesis in prostate cancer cells, which thus promotes the metastasis progression of the cancer cells [26], while the stimulated UGDH expression by TGF-β promotes hyaluronan production in articular surface cells in chicks [6,7]. In the present study, suppressing UGDH gene expression led to an obvious decrease in PG synthesis in human articular chondrocytes. Taken together, these findings suggest that UGDH plays a critical role in the PG synthesis of articular chondrocytes, although the intracellular synthesis of UDP-glucuronic acid was not measured in the present study. As PG are the key components in the cartilage matrix, which maintain the fluid and electrolyte balance, and provide the living space of chondrocytes and the elasticity of the cartilage, we speculate that UGDH might further be an essential player in maintaining cartilage homeostasis.
As a typical degenerative disease of articular cartilage, OA starts with the disturbance of cartilage homeostasis, which leads to the subsequent loss of cartilage matrix and disorganization of articular cartilage. However, no correlation between the PGs loss and UGDH in OA has been reported, except Zemel et al. who indicated that no significant increase in UGDH activity was observed between human normal and OA chondrocytes, and that the lack of significantly enhanced UGDH activity could contribute to continuous GAG loss during OA progress [27]. In the present study, we found, for the first time, that protein level of UGDH is obviously lower in DC than that of MNC from the same OA patient, while chondrocytes also expressed less UGDH protein in rat OA cartilage than that of the normal cartilage. Taken together, the suppressed protein expression and the unchanged enzyme activity of UGDH help to explain the inability of chondrocytes to handle the continuous GAG loss in the advanced OA. However, the OA cartilage samples from either the OA patients undergoing total knee replacement or the rats with papain-induced OA, an aggressive model with an acute local inflammation in the joints and a rapid progress to the terminal stage of OA, were all at their advanced stages, which could not fully replicate the natural pathogenesis of OA dynamically. Other milder models with a more natural and mimic process, like the aging model and running model etc., would be better for the investigation in the role of UGDH in OA. Meanwhile, how the expression of UGDH was suppressed in articular chondrocytes still remained unclear.
IL-1β is one of the major pro-inflammatory factors highly expressed in cartilage and synovium throughout the OA pathogenesis and responsible for the PGs loss and cartilage degeneration [8,9,28-30]. However, Maneix et al. reported that exogenous IL-1β failed to modulate UGDH enzyme activity in articular chondrocytes [7], while Hickery et al. also found that IL-1α, another member of the IL-1 family, could neither modulate UGDH activity [25]. In the present study, we observed that UGDH gene expression was stimulated by IL-1β (10 ng/ml) after a 12-hour exposure, which was in accordance with the results from Maneix et al. [7], while obvious inhibitions of UGDH gene expression were observed after IL-1β treatment at higher concentrations or for longer time, which thus resulted in the suppressed synthesis of GAG in the chondrocytes. All these findings indicated that IL-1β might possibly be involed in the suppression of UGDH protein expression in OA cartilage, and that the restricted UGDH expression induced by IL-1β, rather than the negligible alteration of UGDH enzyme activity, that might participate in the compensation and decompensation of cartilage matrix during OA pathogenesis. However, as IL-1β presents plentiful effects on cartilage, the functional measurement of IL-1β on GAG-precursor synthesis would further strengthen the evidence in the present study. Meanwhile, as there are multiple factors involved in OA pathogenesis, other stimuli including 17β-oestradiol, TGF-β and IGF-1 could also be involved in this process through modulate either the enzyme activity or gene expression of UGDH [6,7,13,25]. Combining the evidences that UGDH plays an essential role in GAG synthesis and cartilage homeostasis, we suggest that UGDH might be possibly a novel target for OA therapy.
Previous studies have demonstrated that IL-1β acts through the activation of downstream signaling cascades. IL-1β binds to type 1 IL-1 receptor (IL-1R1) and then triggers the downstream cascade reaction, which finally leads to the activation of the SAP/JNK, p38 MAPK and NF-κB signaling pathways [10,31,32]. However, although all the three pathways are involved in the metabolic disturbance induced by IL-1β, NF-κB signaling is believed to be mainly responsible for the inflammatory activity of IL-1β. Meanwhile, recent data suggests that it the SAP/JNK and p38 signaling pathway mediatesd the IL-1β-induced suppression of xylosyltransferase I gene expression and the subsequent GAG synthesis in human articular chondrocytes [33]. In the present study, we also observed that inhibition of both p38 MAPK and SAP/JNK led to obvious attenuation of the IL-1β-induced suppression of the gene expression of UGDH and its trans-regulators; this indicates that IL-1β could suppress UGDH gene expression and consequently inhibit PG synthesis in articular chondrocytes, which might suppress matrix restoration and contribute to the OA progression.
Sp1 binds to the GC or GT rich motifs of UGDH promoter sequence and promote transcriptional activity of UGDH gene, while Sp3 and c-Krox were suggested to be playing the negative regulatory roles [11-13]. Inhibition of Sp1 expression with siRNA resulted in attenuation of UGDH enzyme activity, reduction of UGDH gene promoter activity and consequent depression of UGDH mRNA levels [13]. Meanwhile, TGF-β stimulated UGDH gene expression through increasing DNA binding of Sp1 to the sequences located in UGDH promoter [13]. It was also reported that IL-1β inhibited COL2A1 gene transcription by increasing the Sp3/Sp1 ratio and inhibiting the binding of Sp1 and Sp3 to the promoter [34]. Binding to the same sequence that binds Sp1 and Sp3, c-Krox was suggested to act in concert with Sp1 and Sp3 to modulate UGDH gene expression [11]. Overexpression of c-Krox gene in rabbit articular chondrocytes leads to marked decrease in mRNA and protein level of UGDH gene, which is mediated by the increased binding of c-Krox to the cis-sequence located in the UGDH promoter [11]. In the present study, IL-1β altered the gene expression of Sp1, Sp3 and c-Krox, decreased the nuclear translocation of Sp1 protein, and increased the Sp3/Sp1 ratio, as well as c-Krox/Sp1 ratio. Altogether, it suggests that Sp1, Sp3 and c-Krox mediated the modulation of IL-1β on UGDH gene expression. Sp3/Sp1 ratio and c-Krox/Sp1 ratio in chondrocytes might be helpful in estimating the effects of drugs, cytokines or growth factors on cartilage homeostasis. Moreover, decreasing Sp3/Sp1 and c-Krox/Sp1 ratio could help to restore the cartilage phenotype in osteoarthritic joints.
Conclusions
In conclusion, UGDH plays a critical role in the PG synthesis of articular chondrocytes, of which the expression is suppressed in advanced OA. Meanwhile, IL-1β suppresses UGDH gene expression through activating SAP/JNK and p38 MAPK pathways and subsequently modulating the gene expression of UGDH’s trans-regulators including Sp1, Sp3 and c-Krox. Accordingly, we speculate that IL-1β might be involved in the suppression of UGDH gene expression in OA, which would probably contribute to the OA pathogenesis.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (number 81371940, 81220108026, 81430089, 81401832), international science and technology cooperation projects of Hubei province (2012IHA01202), and the Specialized Research Fund for the Doctoral Program of Higher Education (20130141110037).
Abbreviations
- Bp
base pairs
- c-Krox
Krueppel-related zinc finger protein cKrox
- DC
degenerative cartilage
- DMB
1,9-dimethylmethylene blue
- DMEM
Dulbecco’s modified Eagle’s medium
- GAG
glycosaminoglycan
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HE
haematoxylin-eosin
- IHC
immunohistochemical
- IL-1β
interleukin-1 beta
- MNC
microscopically normal cartilage
- OA
osteoarthritis
- p38 MAPK
p38 mitogen-activated protein kinase
- PG
proteoglycans
- RT-PCR
reverse transcription polymerase chain reaction
- SAP/JNK
stress-activated protein kinase/c-Jun N-terminal kinase
- SEM
standard error of the mean
- siRNA
small interfering RNA
- Sp
specificity protein
- TGF-β
transforming growth factor β
- UGDH
UDP-glucose dehydrogenase
Additional files
Additional figure that shows relative protein expression of UDP-glucose dehydrogenase (UGDH) and Mankin score of human and rat cartilage.
Additional figure showing that IL-1β modulated the mRNA expression ratio of specificity protein 3 ( Sp3) and Sp1, as well as the ratio of Krueppel-related zinc finger protein c-Krox ( c-Krox ) and Sp1 in human primary chondrocytes.
Footnotes
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
YXW designed the study, carried out the experimental work, collected, analyzed and interpreted the data, and drafted the manuscript. JL, LLW and KT participated in the design and experimental work, data analysis and interpretation, and manuscript preparation. JM helped out in the experimental design, data analysis and interpretation, and manuscript preparation. HW and LBC obtained the funds, designed the study, interpreted the data, and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Yinxian Wen, Email: wenyinxian@whu.edu.cn.
Jing Li, Email: ljzyd2010@163.com.
Linlong Wang, Email: wang-lin-long@163.com.
Kai Tie, Email: sanitdiego2001@sina.com.
Jacques Magdalou, Email: Jacques.Magdalou@univ-lorraine.fr.
Liaobin Chen, Email: lbchen@whu.edu.cn.
Hui Wang, Email: wanghui19@whu.edu.cn.
References
- 1.Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egger S, Chaikuad A, Kavanagh KL, Oppermann U, Nidetzky B. Structure and mechanism of human UDP-glucose 6-dehydrogenase. J Biol Chem. 2011;286:23877–23887. doi: 10.1074/jbc.M111.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger S, Chaikuad A, Kavanagh KL, Oppermann U, Nidetzky B. UDP-glucose dehydrogenase: structure and function of a potential drug target. Biochem Soc Trans. 2010;38:1378–1385. doi: 10.1042/BST0381378. [DOI] [PubMed] [Google Scholar]
- 5.Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113:193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 6.Clarkin CE, Allen S, Kuiper NJ, Wheeler BT, Wheeler-Jones CP, Pitsillides AA. Regulation of UDP-glucose dehydrogenase is sufficient to modulate hyaluronan production and release, control sulfated GAG synthesis, and promote chondrogenesis. J Cell Physiol. 2011;226:749–761. doi: 10.1002/jcp.22393. [DOI] [PubMed] [Google Scholar]
- 7.Maneix L, Beauchef G, Servent A, Wegrowski Y, Maquart FX, Boujrad N, Flouriot G, Pujol JP, Boumediene K, Galera P, Moslemi S. 17Beta-oestradiol up-regulates the expression of a functional UDP-glucose dehydrogenase in articular chondrocytes: comparison with effects of cytokines and growth factors. Rheumatology (Oxford) 2008;47:281–288. doi: 10.1093/rheumatology/kem323. [DOI] [PubMed] [Google Scholar]
- 8.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 9.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427:S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 10.Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3:cm2. doi: 10.1126/scisignal.3105cm2. [DOI] [PubMed] [Google Scholar]
- 11.Beauchef G, Kypriotou M, Chadjichristos C, Widom RL, Porée B, Renard E, Moslemi S, Wegrowski Y, Maquart FX, Pujol JP, Galéra P. c-Krox down-regulates the expression of UDP-glucose dehydrogenase in chondrocytes. Biochem Biophys Res Commun. 2005;333:1123–1131. doi: 10.1016/j.bbrc.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Hagen G, Muller S, Beato M, Suske G. Sp1-mediated transcriptional activation is repressed by Sp3. Embo J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bontemps Y, Vuillermoz B, Antonicelli F, Perreau C, Danan JL, Maquart FX, Wegrowski Y. Specific protein-1 is a universal regulator of UDP-glucose dehydrogenase expression: its positive involvement in transforming growth factor-beta signaling and inhibition in hypoxia. J Biol Chem. 2003;278:21566–21575. doi: 10.1074/jbc.M209366200. [DOI] [PubMed] [Google Scholar]
- 14.Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Grave’s disease. J Biol Chem. 2011;286:24487–24499. doi: 10.1074/jbc.M111.241166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galera P, Musso M, Ducy P, Karsenty G. c-Krox, a transcriptional regulator of type I collagen gene expression, is preferentially expressed in skin. Proc Natl Acad Sci U S A. 1994;91:9372–9376. doi: 10.1073/pnas.91.20.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, Mankin H, McShane DJ, Medsger TJR, Meenan R, Mikkelsen W, Mqskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 17.Vignon E, Arlot M. Macroscopically normal cartilage from the human osteoarthritic femoral head. II. Measurement of cartilage thickness and cell density. J Rheumatol. 1981;8:447–450. [PubMed] [Google Scholar]
- 18.Qin J, Liu YS, Liu J, Li J, Tan Y, Li XJ, Magdalou J, Mei QB, Wang H, Chen LB. Effect of Angelica sinensis Polysaccharides on Osteoarthritis In Vivo and In Vitro: A Possible Mechanism to Promote Proteoglycans Synthesis. Evid Based Complement Alternat Med. 2013;2013:794761. doi: 10.1155/2013/794761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 20.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 21.Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1, 9-dimethylmethylene blue. Anal Biochem. 1996;243:189–191. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 22.Dey P, Saphos CA, McDonnell J, Moore VL. Studies on the quantification of proteoglycans by the dimethylmethylene blue dye-binding method. Specificity, quantitation in synovial lavage fluid, and automation. Connect Tissue Res. 1992;28:317–324. doi: 10.3109/03008209209016823. [DOI] [PubMed] [Google Scholar]
- 23.Tan Y, Liu J, Deng Y, Cao H, Xu D, Cu F, Lei Y, Magdalou J, Wu M, Chen L, Wang H. Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol Lett. 2012;214:279–287. doi: 10.1016/j.toxlet.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 25.Hickery MS, Bayliss MT, Dudhia J, Lewthwaite JC, Edwards JC, Pitsillides AA. Age-related changes in the response of human articular cartilage to IL-1alpha and transforming growth factor-beta (TGF-beta): chondrocytes exhibit a diminished sensitivity to TGF-beta. J Biol Chem. 2003;278:53063–53071. doi: 10.1074/jbc.M209632200. [DOI] [PubMed] [Google Scholar]
- 26.Wei Q, Galbenus R, Raza A, Cerny RL, Simpson MA. Androgen-stimulated UDP-glucose dehydrogenase expression limits prostate androgen availability without impacting hyaluronan levels. Cancer Res. 2009;69:2332–2339. doi: 10.1158/0008-5472.CAN-08-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zemel E, Nahir AM. Uridine diphosphoglucose dehydrogenase activity in normal and osteoarthritic human chondrocytes. J Rheumatol. 1989;16:825–827. [PubMed] [Google Scholar]
- 28.Wang Z, Qiu Y, Lu J, Wu N. Connective tissue growth factor promotes interleukin-1beta-mediated synovial inflammation in knee osteoarthritis. Mol Med Rep. 2013;8:877–882. doi: 10.3892/mmr.2013.1570. [DOI] [PubMed] [Google Scholar]
- 29.McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31:1039–1045. doi: 10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelletier JP, Kapoor M, Fahmi H, Lajeunesse D, Blesius A, Maillet J, Martel-Pelletier J. Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1beta in the synovium. Ann Rheum Dis. 2013;72:250–257. doi: 10.1136/annrheumdis-2012-201710. [DOI] [PubMed] [Google Scholar]
- 31.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/S0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 33.Khair M, Bourhim M, Barre L, Li D, Netter P, Magdalou J, Fournel-Gigleux S, Ouzzine M. Regulation of xylosyltransferase I gene expression by interleukin 1beta in human primary chondrocyte cells: mechanism and impact on proteoglycan synthesis. J Biol Chem. 2013;288:1774–1784. doi: 10.1074/jbc.M112.419887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadjichristos C, Ghayor C, Kypriotou M, Martin G, Renard E, Ala-Kokko L, Suske G, de Crombrugghe B, Pujol JP, Galera P. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J Biol Chem. 2003;278:39762–39772. doi: 10.1074/jbc.M303541200. [DOI] [PubMed] [Google Scholar]


