Abstract
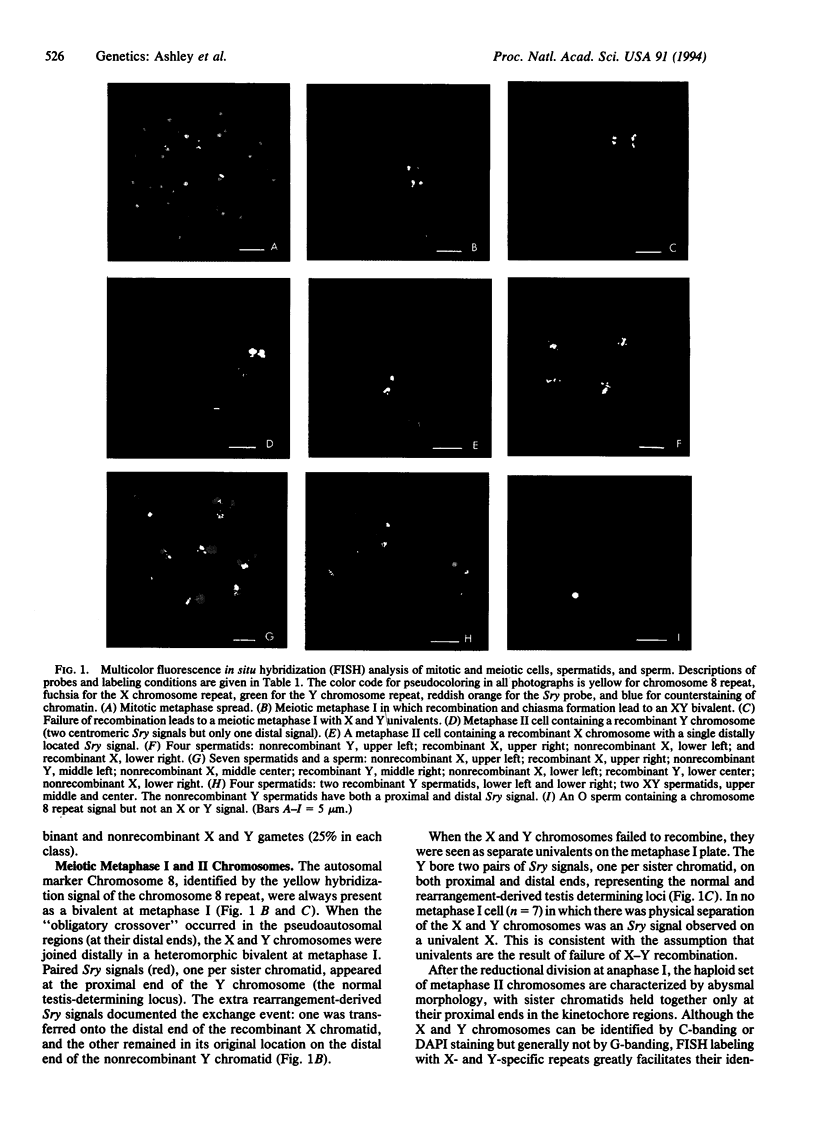
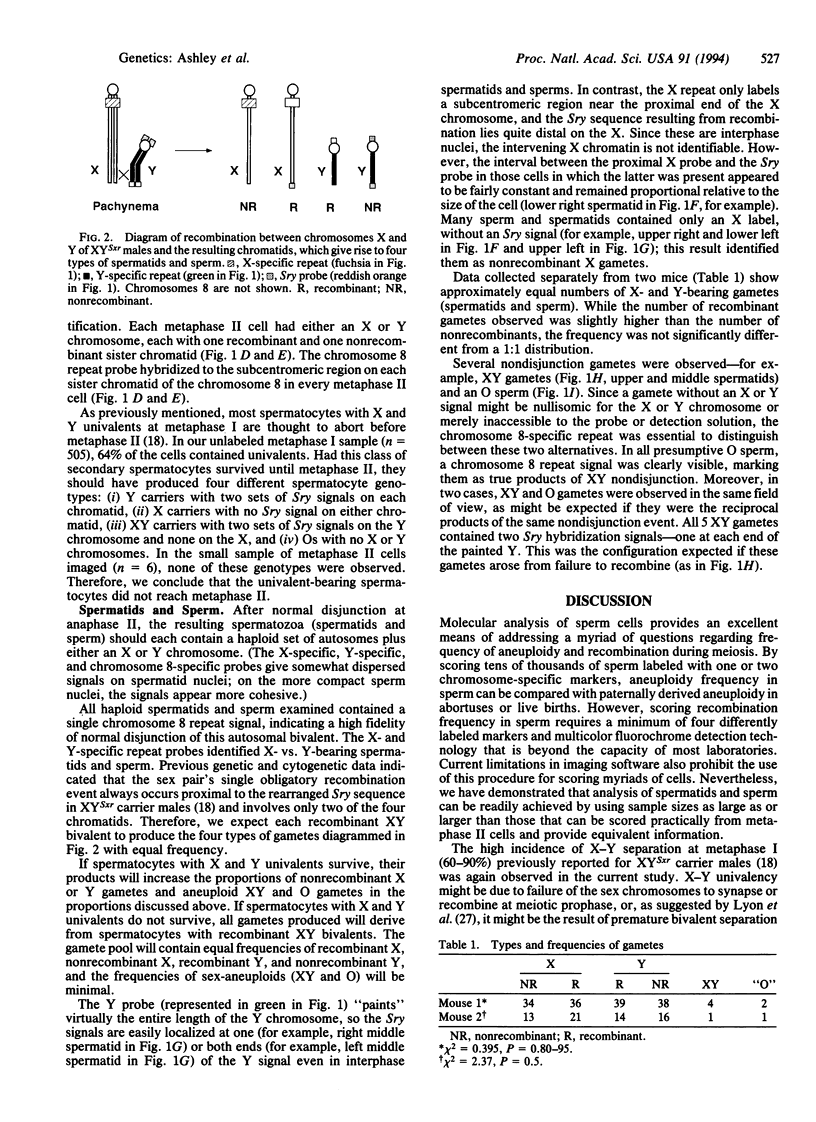
Current meiotic dogma holds that synapsis is required for recombination and that recombination is required for proper disjunction. The mouse chromosome aberration XYSxr [sex reversal; redesignated XY,Tp(Y)1Ct] appears to challenge this assumption, for although chromosomes X and Y often fail to synapse and recombine, there is no dramatic increase in aneuploid progeny. An explanation of this conundrum might be that X-Y univalent spermatocytes do not survive. The phenotype of sex reversal is generated by the "obligatory" crossover between the X and Y chromosomes, which always occurs proximal to a duplicated copy of the testis-determining gene Sry and transfers one copy from one chromatid of the Y chromosome to one chromatid of the X. Animals that inherit an X chromosome with the Sry gene are chromosomally female but phenotypically male. We have used fluorescence in situ hybridization (FISH) to visualize probes for the X and Y chromosomes and for the Sry sequence and chromosome 8 to track the fate of both recombinant and nonrecombinant chromosomes through metaphases I and II into spermatids and sperm. In the 219 gametes examined by multicolor FISH, the frequency of aneuploid products (XY or "O") was low (3.7%) despite a high frequency (66%) of X-Y separation at metaphase I. In balanced gametes, X and Y recombinant chromosomes slightly exceeded nonrecombinants. Both of these observations support the earlier proposal that asynapsis and nondisjunction in primary spermatocytes lead to their developmental arrest and degeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechey C. V. X-Y chromosome dissociation and sterility in the mouse. Cytogenet Cell Genet. 1973;12(1):60–67. doi: 10.1159/000130439. [DOI] [PubMed] [Google Scholar]
- Bishop C. E., Hatat D. Molecular cloning and sequence analysis of a mouse Y chromosome RNA transcript expressed in the testis. Nucleic Acids Res. 1987 Apr 10;15(7):2959–2969. doi: 10.1093/nar/15.7.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle A. L., Ballard S. G., Ward D. C. Differential distribution of long and short interspersed element sequences in the mouse genome: chromosome karyotyping by fluorescence in situ hybridization. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7757–7761. doi: 10.1073/pnas.87.19.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle A. L., Ward D. C. Isolation and initial characterization of a large repeat sequence element specific to mouse chromosome 8. Genomics. 1992 Mar;12(3):517–525. doi: 10.1016/0888-7543(92)90443-v. [DOI] [PubMed] [Google Scholar]
- Brandriff B., Gordon L., Ashworth L., Watchmaker G., Carrano A., Wyrobek A. Chromosomal abnormalities in human sperm: comparisons among four healthy men. Hum Genet. 1984;66(2-3):193–201. doi: 10.1007/BF00286600. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Pollard C. E., Hawker S. G. Sex-reversed mice: XX and XO males. Cytogenetics. 1971;10(5):318–337. doi: 10.1159/000130151. [DOI] [PubMed] [Google Scholar]
- Chandley A. C., Speed R. M. Cytological evidence that the Sxr fragment of XY,Sxr mice pairs homologously at meiotic prophase with the proximal testis-determining region. Chromosoma. 1987;95(5):345–349. doi: 10.1007/BF00293181. [DOI] [PubMed] [Google Scholar]
- Disteche C. M., Tantravahi U., Gandy S., Eisenhard M., Adler D., Kunkel L. M. Isolation and characterization of two repetitive DNA fragments located near the centromere of the mouse X chromosome. Cytogenet Cell Genet. 1985;39(4):262–268. doi: 10.1159/000132155. [DOI] [PubMed] [Google Scholar]
- EVANS E. P., BRECKON G., FORD C. E. AN AIR-DRYING METHOD FOR MEIOTIC PREPARATIONS FROM MAMMALIAN TESTES. Cytogenetics. 1964;3:289–294. doi: 10.1159/000129818. [DOI] [PubMed] [Google Scholar]
- Estop A. M., Cieply K., Vankirk V., Munne S., Garver K. Cytogenetic studies in human sperm. Hum Genet. 1991 Aug;87(4):447–451. doi: 10.1007/BF00197166. [DOI] [PubMed] [Google Scholar]
- Evans E. P., Burtenshaw M. D., Cattanach B. M. Meitoic crossing-over between the X and Y chromosomes of male mice carrying the sex-reversing (Sxr) factor. Nature. 1982 Dec 2;300(5891):443–445. doi: 10.1038/300443a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. J., Darling S. M., Thomas N. S., Goodfellow P. N. A pseudoautosomal gene in man. Science. 1986 Nov 7;234(4777):740–743. doi: 10.1126/science.2877492. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Guttenbach M., Schmid M. Determination of Y chromosome aneuploidy in human sperm nuclei by nonradioactive in situ hybridization. Am J Hum Genet. 1990 Mar;46(3):553–558. [PMC free article] [PubMed] [Google Scholar]
- Guttenbach M., Schmid M. Non-isotopic detection of chromosome 1 in human meiosis and demonstration of disomic sperm nuclei. Hum Genet. 1991 Jul;87(3):261–265. doi: 10.1007/BF00200901. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Jacobs P. A. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Sherman S. L., Pettay D., Page D. C., Jacobs P. A. XY chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am J Hum Genet. 1991 Aug;49(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Joseph A. M., Gosden J. R., Chandley A. C. Estimation of aneuploidy levels in human spermatozoa using chromosome specific probes and in situ hybridisation. Hum Genet. 1984;66(2-3):234–238. doi: 10.1007/BF00286608. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Rademaker A. The frequency of aneuploidy among individual chromosomes in 6,821 human sperm chromosome complements. Cytogenet Cell Genet. 1990;53(2-3):103–107. doi: 10.1159/000132905. [DOI] [PubMed] [Google Scholar]
- Miklos G. L. Sex-chromosome pairing and male fertility. Cytogenet Cell Genet. 1974;13(6):558–577. doi: 10.1159/000130307. [DOI] [PubMed] [Google Scholar]
- Nederlof P. M., van der Flier S., Wiegant J., Raap A. K., Tanke H. J., Ploem J. S., van der Ploeg M. Multiple fluorescence in situ hybridization. Cytometry. 1990;11(1):126–131. doi: 10.1002/cyto.990110115. [DOI] [PubMed] [Google Scholar]
- Page D. C., Bieker K., Brown L. G., Hinton S., Leppert M., Lalouel J. M., Lathrop M., Nystrom-Lahti M., de la Chapelle A., White R. Linkage, physical mapping, and DNA sequence analysis of pseudoautosomal loci on the human X and Y chromosomes. Genomics. 1987 Nov;1(3):243–256. doi: 10.1016/0888-7543(87)90051-6. [DOI] [PubMed] [Google Scholar]
- Ried T., Baldini A., Rand T. C., Ward D. C. Simultaneous visualization of seven different DNA probes by in situ hybridization using combinatorial fluorescence and digital imaging microscopy. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1388–1392. doi: 10.1073/pnas.89.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried T., Lengauer C., Cremer T., Wiegant J., Raap A. K., van der Ploeg M., Groitl P., Lipp M. Specific metaphase and interphase detection of the breakpoint region in 8q24 of Burkitt lymphoma cells by triple-color fluorescence in situ hybridization. Genes Chromosomes Cancer. 1992 Jan;4(1):69–74. doi: 10.1002/gcc.2870040109. [DOI] [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Johnsson C., Vergnaud G., Cooke H. J., Weissenbach J. A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature. 1986 Jan 23;319(6051):291–295. doi: 10.1038/319291a0. [DOI] [PubMed] [Google Scholar]
- Rudak E., Jacobs P. A., Yanagimachi R. Direct analysis of the chromosome constitution of human spermatozoa. Nature. 1978 Aug 31;274(5674):911–913. doi: 10.1038/274911a0. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Takaesu N., Freeman S. B., Grantham M., Phillips C., Blackston R. D., Jacobs P. A., Cockwell A. E., Freeman V., Uchida I. Trisomy 21: association between reduced recombination and nondisjunction. Am J Hum Genet. 1991 Sep;49(3):608–620. [PMC free article] [PubMed] [Google Scholar]
- Warren A. C., Chakravarti A., Wong C., Slaugenhaupt S. A., Halloran S. L., Watkins P. C., Metaxotou C., Antonarakis S. E. Evidence for reduced recombination on the nondisjoined chromosomes 21 in Down syndrome. Science. 1987 Aug 7;237(4815):652–654. doi: 10.1126/science.2955519. [DOI] [PubMed] [Google Scholar]
- Wiegant J., Ried T., Nederlof P. M., van der Ploeg M., Tanke H. J., Raap A. K. In situ hybridization with fluoresceinated DNA. Nucleic Acids Res. 1991 Jun 25;19(12):3237–3241. doi: 10.1093/nar/19.12.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]




