Abstract
Many forms of hypersensitivity reactions and allergic responses depend on deregulated mast cell activity. Several reports suggested that the antiapoptotic Bcl-2 family protein Bcl2a1/Bfl-1/A1 plays a critical role in mast cell survival upon activation. However, its in vivo relevance is poorly understood because of quadruplication of the Bcl2a1 gene locus in mice, hindering conventional knockout studies. In this study, we used a mouse model allowing traceable constitutive knockdown of all A1 isoforms expressed in the hematopoietic system by RNA interference. Knockdown of A1 reduced mast cell numbers in the skin and impaired connective tissue–like mast cell survival upon FcεRI-mediated activation in vitro. In contrast, A1 was dispensable for mucosa-like mast cell differentiation and survival. Moreover, knockdown of A1 prevented IgE-mediated passive systemic and cutaneous anaphylaxis in vivo. Our findings demonstrate that A1 is essential for the homeostasis of connective tissue mast cells, identifying A1 as a possible therapeutic target for therapy of certain types of mast cell–driven allergy symptoms.
Introduction
Mast cells are granulated tissue-resident cells of myeloid lineage that constitute a major sensory arm of the innate immune system (1). Mast cells can respond to a broad range of pathogens, as well as physical cues, and are responsible for the production and release of a vast array of effector molecules, including proteases, histamines, cytokines, and chemokines, as well as inflammatory lipids like leukotrienes or PGs (2, 3). In addition to their protective role against microbial pathogens and parasites, deregulated mast cell activation is frequently associated with pathological conditions and hypersensitivity reactions, such as allergy, asthma, or atopic eczema (4). In these diseases, mast cell numbers are usually increased, and enhanced levels of mast cell mediators can be measured in plasma or other body fluids (5). In particular, during an allergic reaction, mast cells are activated by IgE/allergen-mediated stimulation through high-affinity FcεRI, resulting in the release of the proinflammatory mediators. A unique feature of mast cells is that, once activated, they withstand the exhaustive degranulation process, survive, regranulate, and can become activated again, allowing perpetuation of the inflammatory condition or allergic reaction (6, 7).
Mast cells are heterogeneous in phenotype and function. These cells derive from bone marrow progenitors, egress, and circulate in the peripheral blood and lymphatic organs but mature within the tissue in response to SCF and other locally produced cytokines (8, 9). Historically, two main mast cell subtypes have been described in mice based on their phenotype and tissue localization: T cell–dependent mucosal mast cells (MMCs), which are found primarily in the mucosa of the gastrointestinal system and in the lamina propria of the respiratory tract, and T cell–independent connective tissue mast cells (CTMCs), which are localized in the submucosa of the gastrointestinal tract, in the skin, and in the peritoneum. By analogy to rodents, two main mast cell subtypes have been described in humans, but they are distinguished based on their distinct protease-expression patterns: one resides preferentially in mucosal tissues containing mainly mast cell tryptase, and the other is found in connective tissues containing tryptase, chymase, cathepsin G, and carboxypeptidase (10, 11).
MMCs and CTMCs differ in aspects other than localization and protease-expression pattern. In mice, for example, the lifespan of MMCs is ∼2 wk but that of CTMCs is >2 mo (12). Hence, a fundamental question in mast cell biology is how cell survival is controlled. The antiapoptotic Bcl-2 family protein Mcl-1 was shown to be essential for mast cell survival, because Cre recombinase expression under control of portions of the mast cell–specific carboxypeptidase-3 promoter (Cpa3) leads to near-complete mast cell loss in mice carrying floxed alleles of this survival gene (13). Although this confirms that Mcl-1 is critical for homeostatic mast cell survival, most likely by preventing BH3-only protein–driven Bax activation in mice (14), human A1/Bfl-1 was suggested to be the major effector in activation-induced survival of mast cells (7, 15). In addition, A1/Bfl-1 has been implicated in mast cell–driven pathologies, as the percentage of Bfl-1+ mast cells was increased significantly in skin biopsies from patients with dermatitis or psoriasis compared with lesion-free skin samples (15).
Despite the high interest in A1/Bfl-1–regulated cell death mechanisms, their role in mast cell biology and pathology is only poorly understood because quadruplication of its gene locus in mice has hindered conventional knockout studies. Indeed, A1 is encoded in three individual genes in the mouse genome (A1-a, A1-b, and A1-d), whereas A1-c is a pseudogene, carrying a premature STOP codon (16). Selective deletion of A1-a in mice by gene targeting uncovered an augmented apoptosis susceptibility of granulocytes, as well as of allergen-sensitized and activated mast cells, in vitro. A1a-knockout animals appeared otherwise normal, most likely as the result of functional redundancies of A1-a with A1-b and A1-d isoforms (7, 17). We recently described a mouse model using RNA interference that allows traceable constitutive ablation of all A1 mRNA species found in the hematopoietic system, demonstrating that A1/Bfl-1 is involved in the normal development, maturation, and survival of lymphocytes and granulocytes (18). We took advantage of this model to characterize the role of A1 in the different mast cell subtypes by analyzing their activation-induced cell survival ex vivo and explored its role in IgE-dependent anaphylaxis models of allergic reaction in vivo.
Materials and Methods
Transgenic mice
The generation of VVA1 mice (strain VVA1.2) and VVFF-transgenic mice was described previously (18). Mice were maintained on a C57BL/6N genetic background. Animal experiments were performed using 6–12-mo-old mice, in accordance with Austrian legislation (BMWF-66.011/0112II/3b/2012). Because of the variegation in transgene expression in VVA1 mice, only mice that exhibited >60% Venus+ peripheral blood leukocytes were used for analysis.
Immunofluorescence analysis, cell staining, cell sorting, and Abs used
The mAbs used were purchased from either eBioscience or BioLegend. Their specificities are RA3-6B2, anti-B220; RB6-8C5, anti–Gr-1; RMM-1, anti-IgM; 11-26C, anti-IgD; MI/70, anti–Mac-1; 6D5, anti-CD19; 2B8, anti-CD117 (c-Kit); Mar-1, anti-FcεRI; and DX5, anti-CD49b. The analysis was performed using a FACSCalibur or LSR Fortessa cell analyzer (BD). For the morphological characterization of mast cells, May-Grünwald-Giemsa staining was used on cytospins from peritoneal lavage fluid.
Histology
Tissue specimens were fixed with 2% paraformaldehyde and embedded in paraffin. Four-micrometer sections were stained with 0.1% toluidine blue and fast green for histological examination and enumeration of mast cells.
Cell culture
Connective tissue–like mast cells (CTLMCs) were derived from bone marrow by cultivation of the cells in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 U/ml penicillin, 50 μg/ml streptomycin, 50 μM 2-ME, 50 ng/ml SCF, and 1 ng/ml murine (m)IL-4 (PeproTech). Mucosal-like mast cells (MLMCs) were generated by culturing bone marrow cells in DMEM 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, 50 ng/ml SCF, 1 ng/ml human TGF-β1, 5 mg/ml mIL-9, and 1 ng/ml mIL-3 (PeproTech), as previously described (19). Peritoneal cell–derived mast cells (PCMCs) were generated by cultivating peritoneal cells in Opti-MEM (Life Technologies) supplemented with 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin, and 4% SCF containing hybridoma supernatant harvested from CHO-KL cell cultures or 50 ng/ml recombinant mSCF (PeproTech), as previously described (20). Maturity and purity of CTLMCs, MLMCs, and PCMCs were examined by toluidine blue staining or flow cytometric analysis using anti-CD117 and anti-FcεRI Abs.
Mast cell activation ex vivo and β-hexosaminidase release
CTLMCs and MLMCs were sensitized with IgE by incubation with 15% hybridoma supernatant containing 1 μg/ml monoclonal mouse anti–2,4,6-trinitrophenol (TNP) IgE Ab (IgEl-b4; American Type Culture Collection) for 90 min. The cells were washed and resuspended in PBS with 0.2% BSA or normal media without cytokines before the addition of 100 ng/ml TNP-BSA (coupling ratio of 15:1; Sigma-Aldrich). Alternatively, mast cells were stimulated with 0.5 μg/ml ionomycin or 0.5 μM A23187 (both from Sigma-Aldrich) for 24 h, as previously described (21). The activation was measured by a β-hexosaminidase–release assay (19). In experiments in which activation-induced survival after FcεRI cross-linking was measured, mast cells were deprived of cytokines postactivation for 24 h and analyzed by enumeration using trypan blue and a Countess automatic cell counter (Invitrogen).
IgE-dependent passive systemic anaphylaxis
Mice were sensitized passively with IgE by i.p. injection of 10 μg DNP-specific IgE Ab (clone SPE-7; Sigma-Aldrich) in 500 μl PBS and then challenged i.p. 24 h later with 200 μg DNP (Sigma-Aldrich) in 500 μl PBS. Body temperature was measured immediately before and at 5-min intervals after Ag challenge for up to 1 h (Physitemp model BAT-12).
IgE-dependent passive cutaneous anaphylaxis
IgE-dependent passive cutaneous anaphylaxis (PCA) was induced in the ear pinna as described previously (22). Briefly, mice under anesthesia and analgesia were sensitized passively with IgE by intradermal injection of 20 ng DNP-specific IgE Ab (clone SPE-7; Sigma-Aldrich) in 20 μl PBS and were challenged 24 h later by i.v. injection with 200 μg DNP (Sigma-Aldrich) in 100 μl PBS. Ear swelling was measured immediately before and at 30-min intervals after Ag challenge for 6 h using a micrometer (Mitutoyo Digimatic Micrometer 0–25 mm).
Analysis of histamine, mast cell protease-1, and mall cell protease-2 release
Blood was collected from the submandibular vein 10 min after DNP injection. Serum was isolated by centrifugation using microvette 500 Z-Gel columns (Sarstedt). Levels of histamine were quantified by histamine-release assay (RefLab, Tagensvej, Denmark). Mouse mast cell protease (MCP)-1 (eBioscience) or MCP-2 (R&D Systems) serum level was quantified by ELISA using a 1:10 dilution of sera.
qRT-PCR analysis of mRNA expression levels
Quantitative RT-PCR (qRT-PCR) on mRNA was performed with an Omniscript RT Kit (QIAGEN) using 1 μg total RNA pretreated with 1 U/μl RQ1 DNase (Promega). PCR conditions were 95°C for 7 min and 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. qRT-PCR was performed using Platinum SYBR Green qPCR SuperMix-UDG reagent (Invitrogen). Primers used were A1 fwd 5′-AGAGCAGATTGCCCTGGATGTA-3′; A1 rev 5′-GATAACCATTCTCGTGGGAG-3′; GAPDH fwd 5′-CATCACCATCTTCCAGGAGCG-3′; and GAPDH rev 5′-GTCTTCTGGGTGGCAGTGATGG-3′. Reactions were performed in triplicates. Relative quantification was performed using the ΔΔCT method, using the formula 2-ΔΔCt where ΔΔCt = ΔCt experimental − ΔCt control sample and ΔCt = Ct gene of interest − Ct GAPDH.
Statistics
All data are presented as the mean ± SEM, unless otherwise stated. The differences among different groups were determined by two-way ANOVA with the Bonferroni correction for multiple comparisons or the Student t test. The p values < 0.05 were considered significant.
Results
The Vav gene promoter drives mi-short hairpin RNA-A1 expression in mast cells
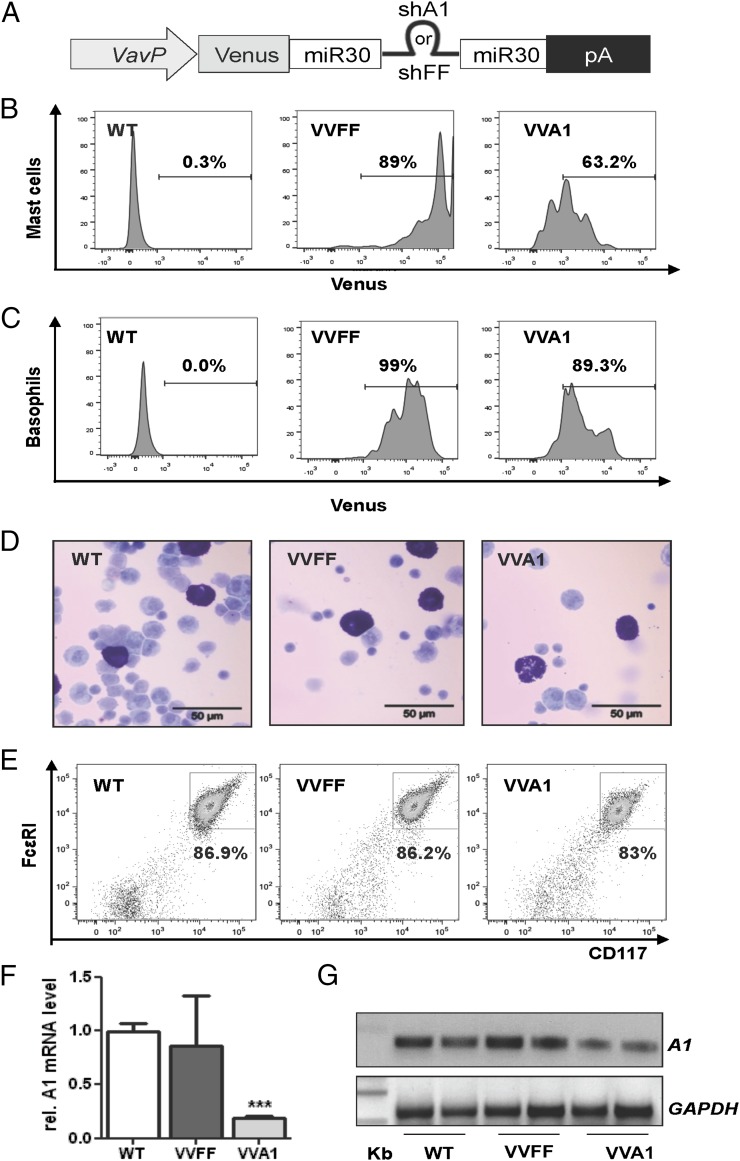
VVA1 mice (18) express a microRNA (miRNA)-30–based precursor harboring a short hairpin RNA (shRNA) sequence (mi-shRNA) that is processed into a small interfering RNA that targets all A1 isoforms. This sequence is embedded in the 3′ untranslated region of the cDNA encoding the fluorescent protein Venus that allows detection and isolation of cells expressing the mi-shRNA, under control of the VavP promoter (23), limiting A1 knockdown to hematopoietic cells (Fig. 1A). As a control, we used mice expressing a mi-shRNA targeting firefly luciferase (VVFF mice). To examine the extent of mi-shRNA expression, we monitored the levels of the Venus reporter in FcεRI+CD117+ mast cells and FcεRI+CD49b+ basophils in the peritoneal lavage fluid from these mice by flow cytometric analysis (Fig. 1B, 1C). Notably, although we detected high expression levels of Venus in VVFF mice, VVA1 mice displayed a fraction of mast cells and basophils that no longer expressed the reporter gene, suggesting variegated transgene expression (Fig. 1B, 1C; see Supplemental Fig. 1 for gating strategies), a phenomenon that we noted previously when using the VavP promoter to drive Venus expression alone (24). Peritoneal mast cells from aged VVA1 mice, as well as VVFF mice, presented normal morphology, with abundant cytoplasmic secretory granules (Fig. 1D). We also examined the knockdown efficiency by quantifying A1 mRNA levels by qRT-PCR, because reliable commercial Abs are still lacking, in PCMCs from wild-type (WT), VVFF, and VVA1 mice. PCMCs were enriched by cultivating peritoneal cells in the presence of SCF for 1 wk until >80% of the cells stained positive for CD117 and FcεRI (Fig. 1E). Consistent with our previously published data on splenocytes (18), we observed an ∼80% reduction in total A1 mRNA expression in VVA1-derived mast cells compared with WT and VVFF controls (Fig. 1F, 1G).
FIGURE 1.
The Vav gene promoter drives mi-shRNA expression in mast cells. (A) Schematic representation of plasmids used for mouse transgenesis. Venus transgene expression in CD117+FcεRI+ peritoneal mast cells (B) and basophils (C) from VVA1 and VVFF mice compared with nontransgenic WT littermate controls. Graphs are representative examples obtained in at least three mice/genotype. Percentages refer to Venus+ cells. (D) May-Grünwald-Giemsa–stained cytospins of cells derived from peritoneal lavage fluid from WT, VVFF, and VVA1 mice. Mast cells are stained purple. (E) Representative plots show comparable expression of CD117 and FcεRI on PCMCs expanded for 1 wk in culture. Knockdown efficiency was evaluated by qRT-PCR (F) and conventional RT-PCR (G) on cDNA generated from PCMCs using primers amplifying all A1 isoforms and GAPDH for normalization. Images have been gray scale inverted for easier visualization of PCR products. Data from three individual PCMC cultures are shown as means in (F). ***p < 0.001, versus WT and VVFF PCMCs, Student t test.
A1 knockdown impairs CTMC homeostasis
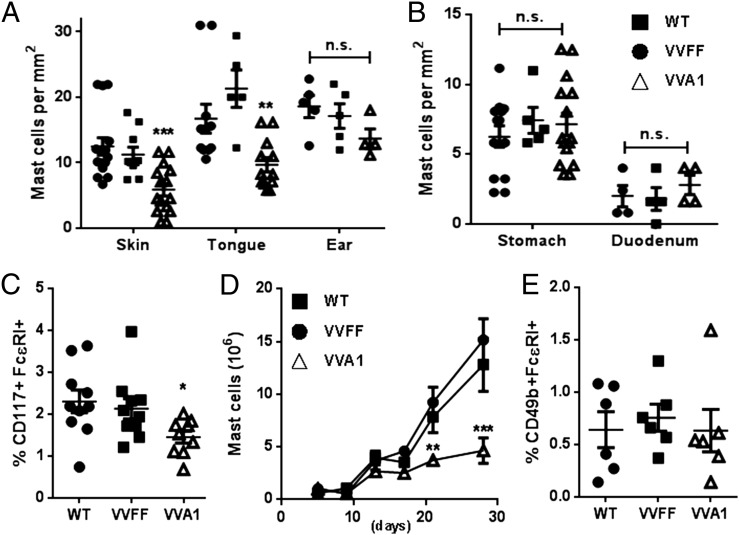
Metachromatic staining of skin or tongue sections revealed an ∼50% reduction in the CTMC number in VVA1 mice compared with WT or VVFF control mice (Fig. 2A, Supplemental Fig. 2). Ear pinna sections showed a trend toward lower mast cell numbers in VVA1 mice, but a statistically significant difference was not achieved (Fig. 2A). Interestingly, MMC numbers in the glandular stomach wall, as well as in the duodenum, were not reduced in A1-knockdown mice (Fig. 2B, Supplemental Fig. 2B). These results suggest that A1 may be a more crucial survival factor for CTMCs than for MMCs or that the knockdown of A1 in these cells is inefficient in the latter. Consistent with a reduction in the number of CD117+ (c-Kit) cells in the peritoneal cavity of VVA1 mice (Fig. 2C), PCMCs from A1-knockdown mice showed reduced outgrowth ex vivo (Fig. 2D), suggesting a survival deficit. Nonetheless, the mast cells derived from all genotypes had normal morphology and were activated effectively by IgER cross-linking, as assessed by β-hexosaminidase–release assay (data not shown). The percentages of basophiles were comparable in the peritoneum of mice from all genotypes (Fig. 2E).
FIGURE 2.
Loss of CTMCs in A1-knockdown mice. Enumeration of mast cells/mm2 in skin, tongue, and ear pinna (connective tissue) (A) and in the glandular stomach wall and duodenum (mucosal tissues) (B) was performed using ImageJ (n = 5–15 animals/genotype). **p < 0.01, ***p < 0.001, versus WT and VVFF mice, Student t test. (C) Percentage of mast cells (FcεRI+ CD117+) in the peritoneal cavity. *p < 0.05, versus WT mice, Student t test. (D) Proliferation of PCMCs in culture was monitored over 4 wk. **p < 0.01, ***p < 0.001, versus WT and VVFF cells, two-way ANOVA with Bonferroni correction for multiple comparisons. (E) Percentage of basophils (FcεRI+ CD49b+) in the peritoneal cavity. n.s., not significant.
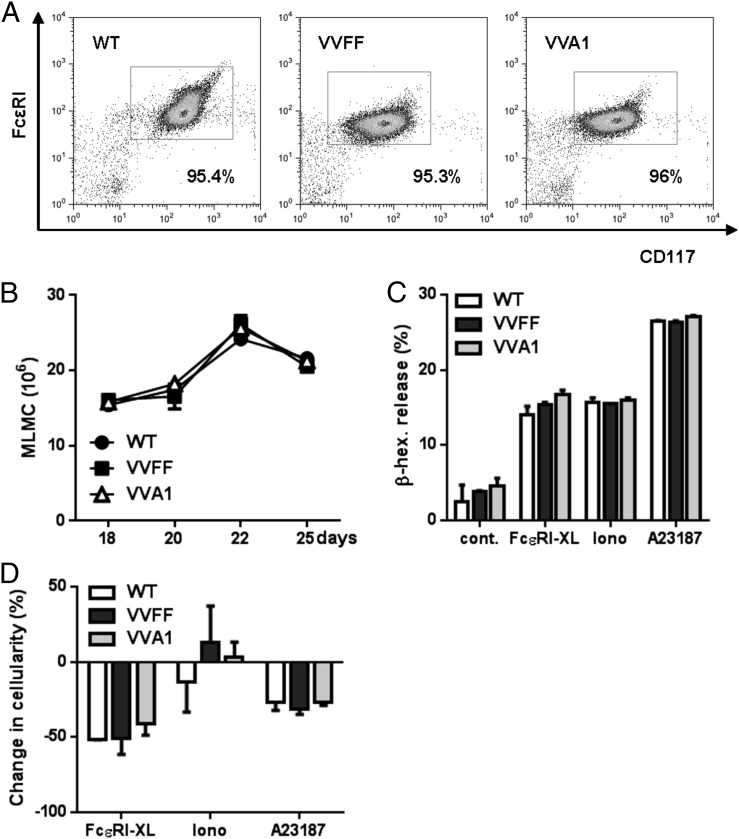
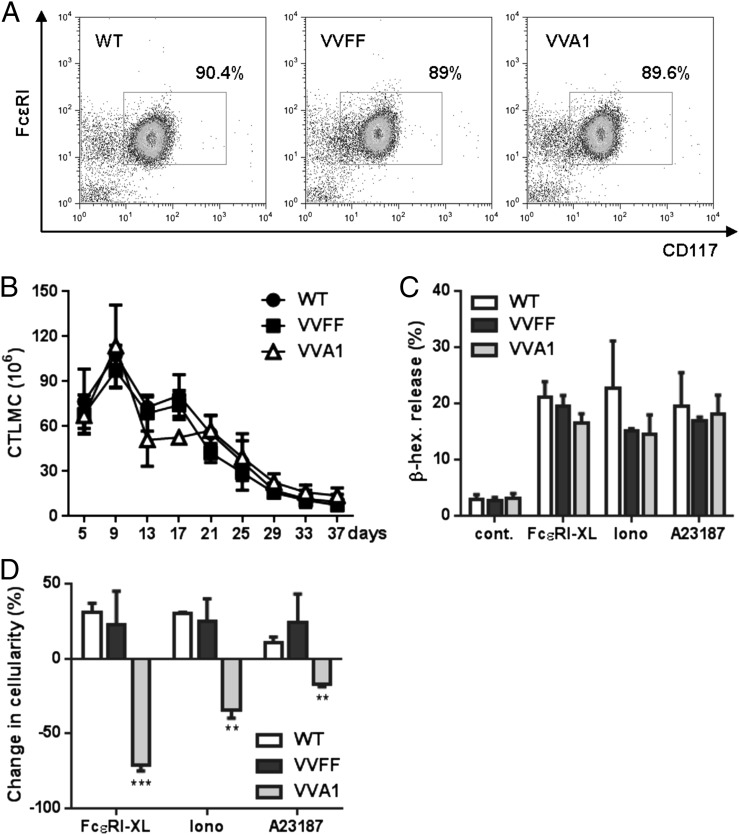
To explore the role of A1 in CTMCs versus MMCs, we compared the in vitro development and phenotype of CTLMCs and MLMCs derived from bone marrow. Mast cells from VVA1-derived bone marrow cultivated in the presence of SCF/IL-3/IL-9/TGF-β1, conditions that promote development into MLMCs, proliferated at rates similar to cells derived from WT and VVFF mice and showed normal CD117 and FcεRI expression levels and morphology (Fig. 3A, 3B, data not shown). In addition, MLMCs were activated by IgER cross-linking or the calcium ionophores, ionomycin or A23187, to the same extent as were the relevant controls and did not show differences in survival (Fig. 3C, 3D). These findings are in line with published data suggesting that A1 plays only a minor role in the survival of MLMCs (19). In contrast, VVA1 mast cells cultured in SCF plus IL-4, promoting differentiation into CTLMCs, showed normal differentiation and activation (Fig. 4A–C) but impaired activation-induced survival after FcεRI cross-linking or ionophore-mediated activation (Fig. 4D), again in line with our previous data reporting increased survival of CTLMCs by induction of A1 expression (19, 25). Collectively, our data indicate that A1 knockdown mainly affects CTMC survival upon activation ex vivo, consistent with previous findings that A1 mRNA is significantly induced upon activation in CTLMCs but not in MLMCs (19).
FIGURE 3.
A1 knockdown does not affect MLMCs. (A) Representative dot plots confirming expression of CD117 and FcεRI in MLMCs after 4 wk in culture. (B) Number of MLMCs in culture over time. Cells were sensitized with 1 μg/ml of IgE–anti-TNP for 90 min and left treated or subsequently challenged with 100 ng/ml TNP to induce FcεRI cross-linking (FcεRI-XL) or activated with the ionophores ionomycin (0.5 μg/ml) or A23187 (0.5 μM). As a control, the cells were kept in media without cytokines. Release of β-hexosaminidase (C) and change in cellularity (D) were measured 24 h posttreatment. Symbols represent means ± SEM of six MLMC cultures from individual mice/genotype.
FIGURE 4.
A1 knockdown impaired CTLMC survival upon FcεRI cross-linking. (A) Representative dot plots confirm expression of CD117 and FcεRI in CTLMCs after 4 wk in culture. (B) Number of CTLMCs in culture over time. Symbols represent mean ± SEM of three CTLMC cultures/genotype. (C) Activation of CTLMCs upon FcεRI cross-linking (FcεRI-XL) or stimulation with the ionophore ionomycin or A23187 was measured by quantifying the release of β-hexosaminidase after stimulation, as described in Fig. 3C. Data are mean ± SEM of six CTLMC cultures/genotype. (D) Change in cell numbers measured at 24 h postactivation, as in Fig. 3D. Data are results from CTLMC cultures derived from six mice/genotype. **p < 0.01, ***p < 0.001, versus WT and VVFF mice, Student t test.
A1 knockdown impairs passive systemic and cutaneous anaphylaxis
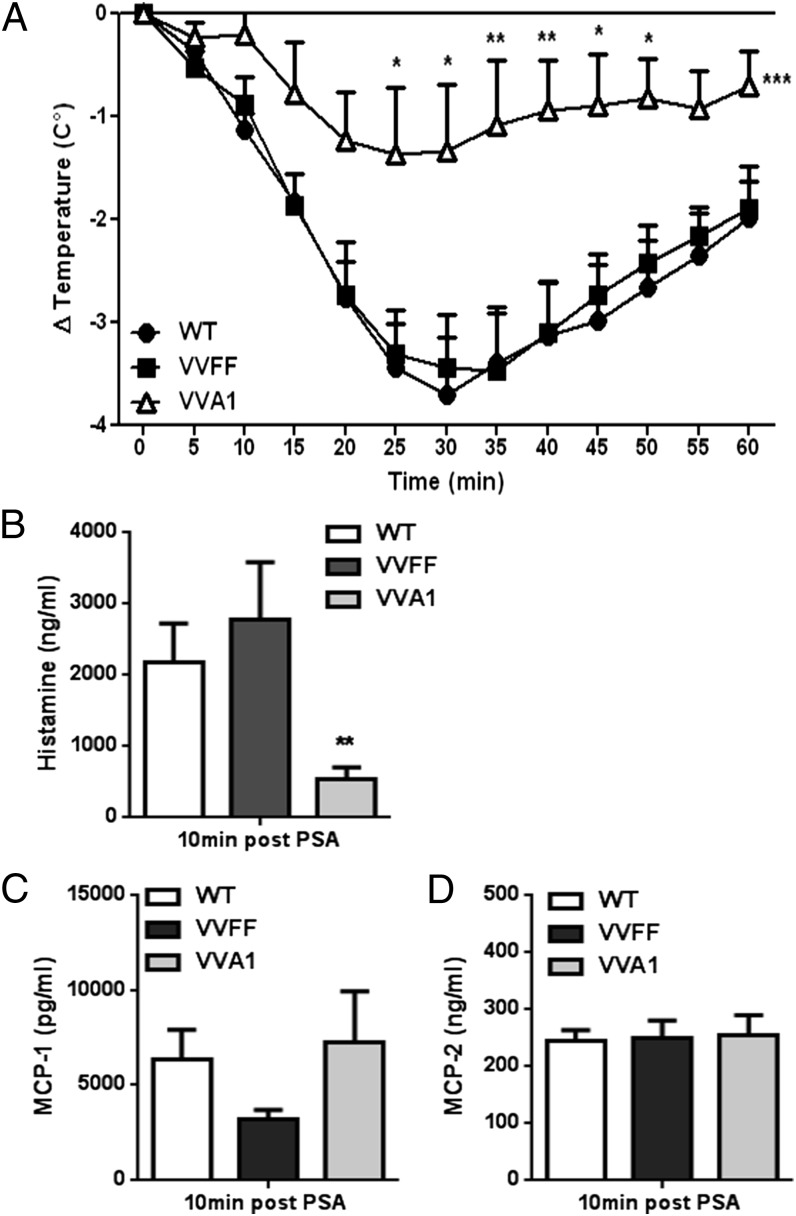
To determine whether the noted reduction in CTMCs in VVA1 mice also resulted in decreased systemic responses in vivo, we performed an experiment of mast cell–driven IgE-dependent passive systemic anaphylaxis (PSA). Mice were sensitized with Ag-specific IgE and subsequently challenged with DNP-Ag. This induces the cross-linking of IgE bound to FcεRI on mast cells, triggering systemic and acute activation resulting in marked hypothermia. Consistently, we noted a median drop in body temperature of 3.5°C in WT and VVFF mice. In contrast, IgE-sensitized VVA1 mice were significantly less responsive, with a median drop in body temperature of only 1.5°C (Fig. 5A). Assessment of serum histamine levels 10 min postactivation revealed strongly reduced plasma histamine levels in VVA1 mice compared with WT and VVFF controls (Fig. 5B). To investigate whether the impaired response of VVA1 mice to PSA was due to the reduction in CTMCs or impaired activation of MMCs in vivo, we also measured the levels of MCP-1 and MCP-2, proteases found exclusively in MMCs (26). In line with a minor role for A1 in MMC survival (Fig. 3), the levels of MCP-1 and MCP-2 in the sera from VVA1 mice were comparable to the ones observed in WT and VVFF mice (Fig. 5C, 5D), indicating that the reduced severity of anaphylaxis in VVA1 mice was caused by the reduction in CTMCs and impaired survival upon activation, matching our ex vivo data.
FIGURE 5.
IgE-dependent systemic PSA is markedly reduced in A1-knockdown mice. (A) WT mice (n = 12), VVFF mice (n = 8), and VVA1 mice (n = 8) were sensitized passively by i.p. injection of 10 ng of DNP-specific IgE (SPE-7) and then challenged by i.p. injection the next day with 200 μg of DNP. Body temperature was measured immediately before and in 5-min intervals after Ag challenge as a read-out of the allergic response. Symbols represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, versus WT and VVFF mice, two-way ANOVA with Bonferroni correction for multiple comparisons. The data shown were pooled from four independent experiments performed, each of which gave similar results. Serum histamine (B) and MCP-1 (C) and MCP-2 (D) concentrations were evaluated in blood samples drawn 10 min post-DNP injection. **p < 0.01, versus WT and VVFF mice, Student t test.
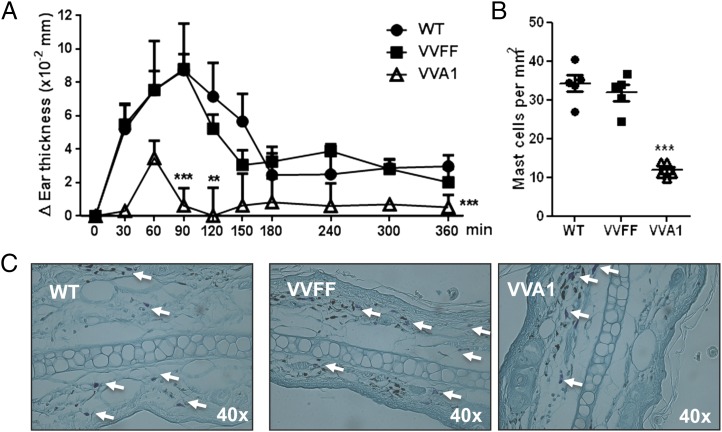
To determine whether the deficiency in CTMCs in A1-knockdown mice and their decreased activation in vivo also prevented cutaneous allergic reaction, we performed an experiment of IgE-dependent PCA. Mice were sensitized with Ag-specific IgE intradermally in the ear pinna and subsequently challenged by i.v. injection of DNP-Ag. This induces activation of the dermal mast cells and a robust local inflammatory response, with leukocyte infiltration and tissue swelling. Inflammation peaked between 60 and 90 min after Ag challenge in control mice but was markedly reduced in VVA1 mice (Fig. 6A). Interestingly, VVA1 mice also exhibited a significant reduction in mast cell numbers in ear pinnae at sites of PCA reactions (Fig. 6B, 6C), suggesting that cutaneous tissue mast cells suffer from impaired activation-induced survival in vivo, as well as ex vivo. Collectively, these data indicate that the reduced mast cell number and their impaired survival upon activation, due to A1 knockdown, also inhibit cutaneous allergic reactions.
FIGURE 6.
A1-knockdown mice exhibit markedly reduced IgE-dependent cutaneous PCA. (A) WT mice (n = 6), VVFF mice (n = 7), and VVA1 mice (n = 5) were sensitized passively by intradermal injection of 20 ng of DNP-specific IgE (SPE-7) and then challenged by i.v. injection the next day with 200 μg of DNP. Ear swelling was measured immediately before and at 30-min intervals after Ag challenge for 6 h. **p < 0.01, ***p < 0.001, versus WT and VVFF mice, two-way ANOVA with Bonferroni correction for multiple comparisons. The data shown were pooled from three independent experiments, each of which gave similar results. (B) Numbers of mast cells in the dermis of ear pinnae at sites of PCA 6 h after DNP challenge. ***p < 0.001, versus WT and VVFF mice, Student t test. (C) Representative toluidine blue staining of activated mast cells in ear pinnae 6 h after induction of a PCA reaction. Arrows indicate mast cells.
Discussion
Recently, A1/Bfl-1 was reported to be an important regulator of leukocyte survival and differentiation in mice (18). A1/Bfl-1 also was suggested to be the major effector in activation-induced survival of mast cells in mice and humans (7, 15, 27). Human mast cells activated by IgER cross-linking show increased viability that is diminished by inhibition of A1/Bfl-1 (15). In line with these findings, A1/Bfl-1 seems to be an important player in some forms of hypersensitivity reactions in humans. A1/Bfl-1 expression was increased significantly in challenged skin of birch pollen–allergic patients, as well as in skin lesion biopsies from patients with atopic dermatitis or psoriasis, compared with nonlesion skin (15). In this article, we present data that indicate that A1 is indeed required for the normal development and survival of CTMCs. This is supported by histological analysis that shows reduced mast cell numbers in the skin and tongue from A1-knockdown mice, whereas the numbers of mast cells present in the mucosa of the gastrointestinal system or in the duodenum were not altered significantly (Fig. 2). Furthermore, in vitro bone marrow–derived CTLMCs from VVA1 mice showed impaired activation-induced survival when stimulated by IgER cross-linking (Fig. 4), which is comparable to what was shown using mast cells from A1-a–knockout mice (7). Our results are in accordance with previous findings that showed that A1 is differentially expressed in in vitro–derived MLMCs and CTLMCs. Indeed, A1 mRNA appears to be present in both mast cell types, but only a minor upregulation was observed in MLMCs after FcεRI cross-linking. In contrast, FcεRI cross-linking caused a profound upregulation of A1 in CTLMCs (19). The molecular basis for this difference is unknown. However, consistent is the observation that MLMCs do not respond with increased survival after activation, whereas CTLMCs activated by either Ca++ ionophores or FcεRI cross-linking Abs showed impaired survival (Figs. 3, 4) (19). Although it remains possible that insufficient knockdown in MLMCs accounts for a lack of phenotype, we propose that the repeated activation of CTMCs in vivo causes their gradual depletion over time.
In line with the marked reduction in the number of CTMCs (Fig. 2), A1-knockdown mice also exhibited a significant reduction in the severity of features of IgE-dependent PSA (Fig. 5). The impaired response to the allergic reaction correlated with reduced levels of histamine in the sera, but not of MCP-1 or MCP-2, which are secreted selectively by MLMCs (26). Accordingly, A1-knockdown mice exhibit a strong reduction in mast cell numbers in ear pinnae at sites of PCA reactions, indicating that CTMCs suffer from impaired activation-induced survival and are lost in cutaneous allergic reactions in vivo (Fig. 6). These results indicate that A1 knockdown mainly affects CTMC homeostasis but not that of MMCs, suggesting differences in expression patterns within the Bcl2 family between those two mast cell types. Consistently, A1 is not the only antiapoptotic Bcl-2 family member that plays a role in mast cell survival. Of note, deletion of Mcl-1 in the mast cell linage using Cpa3-driven Cre recombinase expression leads to a more severe systemic mast cell deficiency compared with our A1-knockdown mice (13). Not unexpectedly, these mast cell–deficient Cpa3-Mcl-1f/f mice are essentially unreactive to PSA. In contrast to the study by Lilla et al. (13), who also noted a severe loss of basophils in these mice, their numbers appeared to be unaffected in A1-knockdown mice (Fig. 2D, data not shown). Because mast cells and basophils share a similar developmental pathway, Mcl-1 may play a more important role during the early stages of mast cell and basophil differentiation and maturation, consistent with a critical role for Mcl-1 in hematopoietic stem and progenitor cells (28). As such, the role of Mcl-1 in the activation and survival of mature mast cells cannot be assessed, because Cpa3-Mcl-1f/f mice do not develop mast cells. Hence, this issue requires additional experimental input (e.g., by the analysis of Mcl-1 heterozygote mice). Subsequently, it also remains unclear whether Mcl-1 plays different roles in MMC and CTMC homeostasis or development. Regardless, based on our findings, A1 function seems to be restricted to CTMCs and does not affect MMCs homeostasis. Of note, in addition to their involvement in allergic reactions, MMCs in the gastrointestinal tract are involved in many inflammatory responses to bacterial pathogens and parasite infections. Therefore, pharmacological targeting of A1 currently developed for cancer treatment might allow selective treatment of cutaneous allergy reactions without impairing mast cell responses to foreign pathogens or parasites.
Supplementary Material
Acknowledgments
We thank K. Rossi, C. Soratroi, and I. Gaggl for technical assistance and animal care, as well as D. Mairhofer and L. Peintner for help with intradermal injections.
A.V. and G.P.N. are cosenior authors.
This work was supported by grants from the Swedish Research Council (Medicine and Health), the Swedish Cancer Society, and the Karolinska Institute (to K.L. and G.P.N.). E.O. and A.V. were supported by Doctoral College W1101 “Molecular Cell Biology and Oncology” and D-A-CH initiative FOR2036 (I1298), both financed by the Austrian Science Fund.
The online version of this article contains supplemental material.
- CTLMC
- connective tissue–like mast cell
- CTMC
- connective tissue mast cell
- m
- murine
- MCP
- mast cell protease
- miRNA
- microRNA
- mi-shRNA
- miR-30–based precursor harboring an shRNA sequence
- MLMC
- mucosal-like mast cell
- MMC
- mucosal mast cell
- PCA
- passive cutaneous anaphylaxis
- PCMC
- peritoneal cell–derived mast cell, PSA, passive systemic anaphylaxis
- qRT-PCR
- quantitative RT-PCR
- shRNA
- short hairpin RNA
- TNP
- 2,4,6-trinitrophenol
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Urb M., Sheppard D. C. 2012. The role of mast cells in the defence against pathogens. PLoS Pathog. 8: e1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAlpine S. M., Enoksson M., Lunderius-Andersson C., Nilsson G. 2011. The effect of bacterial, viral and fungal infection on mast cell reactivity in the allergic setting. J. Innate Immun. 3: 120–130. [DOI] [PubMed] [Google Scholar]
- 3.St John A. L., Abraham S. N. 2013. Innate immunity and its regulation by mast cells. J. Immunol. 190: 4458–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalesnikoff J., Galli S. J. 2008. New developments in mast cell biology. Nat. Immunol. 9: 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valent P. 2013. Mast cell activation syndromes: definition and classification. Allergy 68: 417–424. [DOI] [PubMed] [Google Scholar]
- 6.Ekoff M., Nilsson G. 2011. Mast cell apoptosis and survival. Adv. Exp. Med. Biol. 716: 47–60. [DOI] [PubMed] [Google Scholar]
- 7.Xiang Z., Ahmed A. A., Möller C., Nakayama K., Hatakeyama S., Nilsson G. 2001. Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J. Exp. Med. 194: 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallgren J., Gurish M. F. 2011. Mast cell progenitor trafficking and maturation. Adv. Exp. Med. Biol. 716: 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura Y., Oboki K., Ito A. 2007. Development of mast cells. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 83: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon T. C., St Laurent C. D., Morris K. E., Marcet C., Yoshimura T., Sekar Y., Befus A. D. 2010. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 3: 111–128. [DOI] [PubMed] [Google Scholar]
- 11.Puxeddu I., Piliponsky A. M., Bachelet I., Levi-Schaffer F. 2003. Mast cells in allergy and beyond. Int. J. Biochem. Cell Biol. 35: 1601–1607. [DOI] [PubMed] [Google Scholar]
- 12.Fukuzumi T., Waki N., Kanakura Y., Nagoshi J., Hirota S., Yoshikawa K., Kitamura Y. 1990. Differences in irradiation susceptibility and turnover between mucosal and connective tissue-type mast cells of mice. Exp. Hematol. 18: 843–847. [PubMed] [Google Scholar]
- 13.Lilla J. N., Chen C. C., Mukai K., BenBarak M. J., Franco C. B., Kalesnikoff J., Yu M., Tsai M., Piliponsky A. M., Galli S. J. 2011. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 118: 6930–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlberg M., Ekoff M., Labi V., Strasser A., Huang D., Nilsson G. 2010. Pro-apoptotic Bax is the major and Bak an auxiliary effector in cytokine deprivation-induced mast cell apoptosis. Cell Death Dis. 1: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekoff M., Lyberg K., Krajewska M., Arvidsson M., Rak S., Reed J. C., Harvima I., Nilsson G. 2012. Anti-apoptotic BFL-1 is the major effector in activation-induced human mast cell survival. PLoS ONE 7: e39117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatakeyama S., Hamasaki A., Negishi I., Loh D. Y., Sendo F., Nakayama K., Nakayama K. 1998. Multiple gene duplication and expression of mouse bcl-2-related genes, A1. Int. Immunol. 10: 631–637. [DOI] [PubMed] [Google Scholar]
- 17.Hamasaki A., Sendo F., Nakayama K., Ishida N., Negishi I., Nakayama K., Hatakeyama S. 1998. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J. Exp. Med. 188: 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottina E., Grespi F., Tischner D., Soratroi C., Geley S., Ploner A., Reichardt H. M., Villunger A., Herold M. J. 2012. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood 119: 6032–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekoff M., Strasser A., Nilsson G. 2007. FcepsilonRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells. J. Immunol. 178: 4177–4183. [DOI] [PubMed] [Google Scholar]
- 20.Malbec O., Roget K., Schiffer C., Iannascoli B., Dumas A. R., Arock M., Daëron M. 2007. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J. Immunol. 178: 6465–6475. [DOI] [PubMed] [Google Scholar]
- 21.Gulliksson M., Carvalho R. F., Ullerås E., Nilsson G. 2010. Mast cell survival and mediator secretion in response to hypoxia. PLoS ONE 5: e12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer, B., A. M. Piliponsky, T. Oka, C. H. Song, N. P. Gerard, C. Gerard, M. Tsai, J. Kalesnikoff, and S. J. Galli. 2013. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J. Allergy Clin. Immunol. 131: 541–548.e1–9. [DOI] [PMC free article] [PubMed]
- 23.Ogilvy S., Metcalf D., Gibson L., Bath M. L., Harris A. W., Adams J. M. 1999. Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood 94: 1855–1863. [PubMed] [Google Scholar]
- 24.Grespi F., Ottina E., Yannoutsos N., Geley S., Villunger A. 2011. Generation and evaluation of an IPTG-regulated version of Vav-gene promoter for mouse transgenesis. PLoS ONE 6: e18051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekoff M., Möller C., Xiang Z., Nilsson G. 2006. Coaggregation of FcepsilonRI with FcgammaRIIB inhibits degranulation but not induction of Bcl-2 family members A1 and Bim in mast cells. Allergy Asthma Clin Immunol 2: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pejler G., Rönnberg E., Waern I., Wernersson S. 2010. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 115: 4981–4990. [DOI] [PubMed] [Google Scholar]
- 27.Xiang Z., Möller C., Nilsson G. 2006. IgE-receptor activation induces survival and Bfl-1 expression in human mast cells but not basophils. Allergy 61: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 28.Opferman J. T., Iwasaki H., Ong C. C., Suh H., Mizuno S., Akashi K., Korsmeyer S. J. 2005. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307: 1101–1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.