Abstract
Hemoglobin A2, a tetramer of α- and δ-globin chains, comprises less than 3% of total hemoglobin in normal adults. In northern Europeans, single nucleotide polymorphisms (SNPs) in the HBS1L-MYB locus on chromosome 6q and the HBB cluster on chromosome 11p were associated with HbA2 levels. We examined the genetic basis of HbA2 variability in sickle cell anemia using genome-wide association studies (GWAS). HbA2 levels were associated with SNPs in the HBS1L-MYB interval that affect erythropoiesis and HbF expression and SNPs in BCL11A that regulate the γ-globin genes. These effects are mediated by the association of these loci with γ-globin gene expression and fetal hemoglobin (HbF) levels. The association of polymorphisms downstream of the β-globin gene (HBB) cluster on chromosome 11 with HbA2 was not mediated by HbF. In sickle cell anemia, levels of HbA2 appear to be modulated by trans-acting genes that affect HBG expression and perhaps also elements within the β-globin gene cluster. HbA2 is expressed pancellularly and can inhibit HbS polymerization. It remains to be seen if genetic regulators of HbA2 can be exploited for therapeutic purposes.
Introduction
Hemoglobin A2 (HbA2: α2δ2), a tetramer of α- and δ-globin chains, forms less than 3% of total hemoglobin in normal adults.[1] It has no known physiological function, but elevated levels are associated with β-thalassemia trait. Its levels are heritable. Genetic variation explains 42% of total HbA2 variability using a variance components model.[2] The genetic basis of HbA2 regulation has been studied in northern Europeans where single nucleotide polymorphisms (SNPs) in the HBS1L-MYB locus on chromosome 6q and the HBB cluster on chromosome 11p were associated with HbA2. This effect is presumably a result of modifying the kinetics of erythropoiesis and transcription within the HBB gene cluster.[2]
HbA2 can inhibit the polymerization of sickle hemoglobin (HbS) and it has a pancellular distribution.[3-6] High HbA2 might therefore be of benefit in sickle cell anemia.[7] Polymorphisms of the HBS1L-MYB interval that are associated with hematopoiesis and fetal hemoglobin (HbF) concentration are differentially distributed among populations,[8] and higher than normal HbF levels are characteristic of sickle cell anemia.[9] Stress erythropoiesis might also be partly responsible for increased HbF levels. A reciprocal relationship between HbA2 and HbF levels is present in acquired disorders where HbF levels are increased [10] and HbA2 levels are lowest in cells with increased HbF and in individuals with high HbF.[11, 12] Menzel found that females with more F-cells had lower HbA2 than males with fewer F-cells.[2] To confirm and extend the observations on the genetic regulation of HbA2 we examined the genetic basis of HbA2 variability in sickle cell anemia.
Materials & methods
Study subjects
The discovery cohort included 618 unrelated African American subjects from the Cooperative Study of Sickle Cell Disease (CSSCD; NCT00005277).[13] To replicate the associations with p<1E-5 we used 128 African American patients from the Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy (Walk-PHaSST;NCT00492531)[14], 45 African American cases from the Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease (PUSH; NCT 00495638) [15], and 580 Chinese subjects from the Hong Kong β-thalassemia trait study (Table 1).[16] The studies were approved by the Institutional Review Boards of all participating sites.
Table 1.
Replication of results from (Menzel et al., 2013) [2] in the CSSCD cohort.
| CHR | SNP | BP | Menzel P | P adj | CSSCD Effect* | P* | Effect adj** | P adj** | Effect HbF*** | P HbF*** | Effect Mediation+ | P Mediation+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome 6q | |||||||||||||
| 6 | rs1547247 | 135432529 | 3.02E-08 | 1.39E-05 | −0.17 | 2.17E-02 | −0.12 | 1.07E-01 | 1.72 | 2.22E-03 | − 0.05 | 6.30E-03 | |
| 6 | rs7775698 | 135460328 | 2.51E-09 | 3.30E-05 | −0.08 | 4.54E-02 | −0.06 | 1.54E-01 | 0.81 | 1.02E-02 | − 0.03 | 1.78E-02 | |
| 6 | rs9399137 | 135460711 | 5.19E-09 | 4.50E-05 | −0.17 | 1.98E-02 | −0.08 | 2.66E-01 | 2.97 | 9.22E-08 | − 0.09 | 8.16E-05 | |
| 6 | rs4895441 | 135468266 | 5.72E-09 | 5.50E-05 | −0.09 | 1.25E-01 | −0.03 | 5.46E-01 | 1.72 | 8.06E-05 | − 0.05 | 9.12E-04 | |
| 6 | rs9376092 | 135468837 | 8.69E-09 | 7.16E-05 | −0.03 | 4.71E-01 | −0.02 | 7.07E-01 | 0.46 | 1.42E-01 | − 0.01 | 1.52E-01 | |
| 6 | rs9494145 | 135474245 | 1.03E-08 | 1.42E-05 | −0.22 | 9.81E-04 | −0.13 | 4.10E-02 | 2.78 | 1.81E-08 | −0.08 | 6.33E-05 | |
| Chromosome 11p | |||||||||||||
| 11 | rs11036212 | 5178401 | 1.45E-09 | 1.03E-08 | −0.08 | 2.26E-02 | −0.07 | 4.50E-02 | 0.37 | 1.67E-01 | − 0.01 | 1.77E-01 | |
| 11 | rs7950726 | 5182023 | 1.23E-11 | 1.99E-10 | |||||||||
| 11 | rs12787404 | 5182342 | 6.82E-12 | 1.01E-10 | |||||||||
| 11 | rs10837582 | 5185284 | 3.12E-08 | 7.23E-08 | 0.03 | 6.62E-01 | 0.02 | 7.23E-01 | −0.21 | 7.01E-01 | 0.01 | 7.02E-01 | |
| 11 | rs12793110 | 5188141 | 5.11E-12 | 7.73E-11 | |||||||||
| 11 | rs10837628 | 5200980 | 1.53E-09 | 2.41E-09 | 0.03 | 6.60E-01 | 0.02 | 7.46E-01 | −0.28 | 6.05E-01 | 0.01 | 6.06E-01 | |
| 11 | rs11036364 | 5205580 | 2.80E-09 | 2.59E-08 | 0.35 | 1.37E-02 | 0.39 | 4.64E-03 | 1.22 | 2.52E-01 | − 0.04 | 2.60E-01 |
Results shown are regression effect sizes and p-values for
HbA2 adjusted for age and sex
HbA2 adjusted for age, sex, and HbF
HbF adjusted for age and sex
mediation analysis.
Italicized values in the mediation analysis are for an analysis where either no statistically significant adjusted effect on HbA2 or no statistically significant association with HbF was observed for that SNP. These values should not be interpreted for mediation, but are provided for completeness.
Genotyping
The DNA from the CSSCD, Walk-PHaSST, PUSH and Hong Kong samples were genotyped using the Illumina Human610-Quad array and processed and analyzed as described.[17] We used the genome-wide identity by descent analysis in PLINK to discover unknown relatedness. Pairs with identity by descent measurements greater than 0.2 were deemed to be related subjects and only one subject was included in the analysis. We analyzed only common SNPS (MAF>0.05), from individuals with call rates of at least 98%.
Phenotype
HbA2 was measured by DEAE Cellulose column chromatography [18, 19] (CSSCD) or high performance liquid chromatography (Walk-PHaSST, PUSH, β-thalassemia cohort). HbF was measured by alkali denaturation [20] (CSSCD) and HPLC (Walk-PHaSST, PUSH, β-thalassemia cohort). Measurements taken when a subject was under 5 years old were discarded as HbA2 values may not be stable before this age. HbA2 values of below 1.4 or above 7.9 were discarded in the sickle cell anemia cohorts, as values outside of these bounds are considered to be biologically unlikely and due to instrumentation or recording errors.
The CSSCD and Walk-PHaSST were both longitudinal studies, and some subjects had multiple HbA2 and HbF measurements and median HbA2, HbF and age measurements were used. In the CSSCD HbA2 and HbF were measured on the same blood sample.
Statistical analysis and genome-wide association study
Data are represented as mean and standard deviation or median and range. Relation between HbA2 and MCV was tested using linear regression, and the effect of α thalassemia was tested using an interaction between MCV and α thalassemia. Association between HbA2 and each SNP was tested using an additive genetic model in PLINK, [21] and a normal distribution for HbA2. The normal distribution assumption is shown by the QQ-plot (Figure S1). Two models were conducted, (1) adjusted for age and sex; (2) adjusted for age, sex, and HbF. SNPs that reached a significant association with HbA2 with p<1E-5 in the CSSCD samples were assessed in the remaining cohorts. At this significance level, if no associations were present and all SNPs were independent, we would expect to observe 5.5 false positives. The presence of gene deletion α thalassemia was adjusted for in the CSSCD cohort. A meta-analyses using Metal software assessed all studies simultaneously.[22]
For replication of the 9 available SNPs from the 13 previously found associated with HbA2 in normal individuals[2], we used a p-value threshold of p<0.05/9. To further examine previously reported associations, we conducted a mediation analysis to determine if there was mediation in the SNP/HbA2 association by HbF.[23] Mediation was assessed by performing a series of regressions adjusted for age and sex:
| (1) |
| (2) |
| (3) |
If, for a SNP, c and a are significantly different from zero, these regressions are then used to evaluate the effect of SNP on HbA2 through HbF by (c-c’) (Figure S2).[23] Values shown in Table 1 and Supplement Table 3 correspond to hypothesis tests of whether this mediated effect is nonzero.
Results
Patient Characteristics
CSSCD and PUSH patients were younger than Walk-PHaSST (Table S1).[24] The distribution of HbA 2 in all cohorts is shown in Figure S3 A-D. The mean value of HbA 2 was ~ 3% In the CSSCD, 3.8% in Walk-PHaSST and 3.5% in PUSH reflecting higher HbA2 measurement by HPLC in the presence of HbS. The Hong Kong cohort with β-thalassemia trait had a mean of 5.4%. In this cohort, HbA2 levels are also affected by the nature of the β-thalassemia mutation that also is associated with the numbers of F-cells.[16]
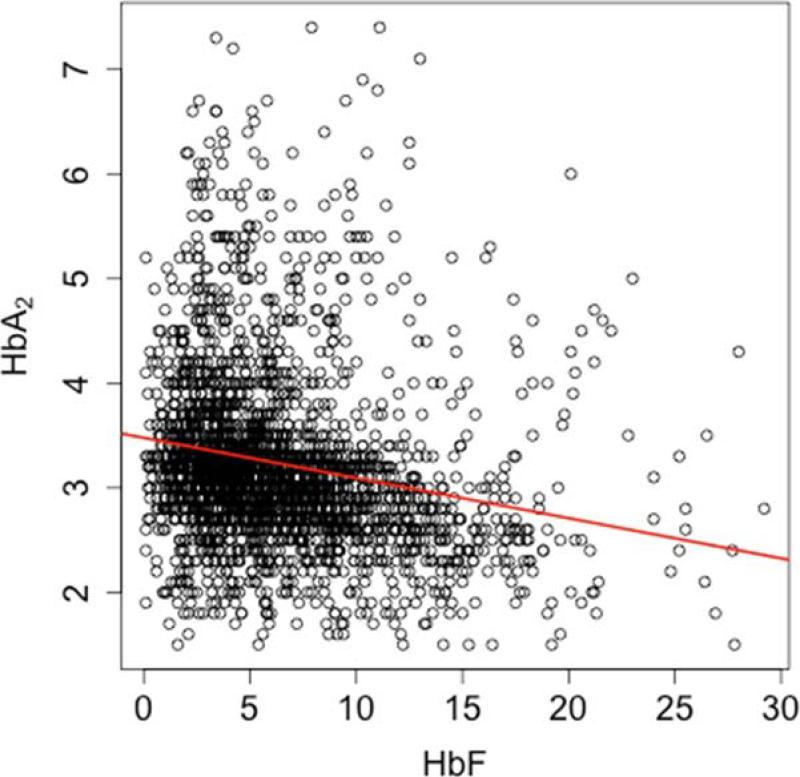
HbA2 and HbF were correlated (r=-0.20, nonzero with p=3.7E-24; Figure 1) as were HbA2 and MCV (r=0.58, p=1.8E-137) and to a lesser extent HbF and MCV (r=0.14, p=1.7E-08). The relationship between HbA2 and MCV remained significant after adjusting for HbF (p<2E-16). α Thalassemia was associated with a reduction of MCV; (Figure S4). Increased HbA2 in sickle cell anemia-α thalassemia is a result α/δ-globin dimerizing more readily than α/βS-globin.[18] α Thalassemia modified the relationship between HbA2 and MCV: without α thalassemia, a regression of HbA2 on MCV yielded a slope of -0.028; with α thalassemia, the slope was -0.038 (a -33% change, p= 0.0339).(Table S2.)
Figure 1.
Association between HbA2 and HbF. HbA2 and HbF are correlated (r=-0.20; nonzero with p=3.7E-24).
Replication of association in northern Europeans
In northern Europeans the HSB1L-MYB intergenic region on chromosome 6q and the HBB cluster on chromosome 11p were strongly associated with HbA2.[2] Of 13 reported associations, 9 SNPs were available for our analysis (Table 1). When adjusted for age and sex, nominal replication (p<0.05) was obtained for 4 SNPs in the HSB1L-MYB intergenic region (bold font in Table 1), and 1 SNP in the HBB gene cluster. Considering only SNPs with p<0.05 in the analysis adjusted for age, sex and HbF, only rs9494145 in HSB1L-MYB (p=4.5E-02), rs11036212 in HBB (p=4.5E-02), and rs11036364 in HBB (p=4.64E-03) were replicated. As reported, R2 between 0.01 and 0.02 for their top SNPs, we have power between 0.70 and 0.94 to nominally replicate (p=0.05) these results if such an association exists. For these SNPs in the CSSCD, we obtained R2 between 0.00017 and 0.0067.
The association between HbA2 and the SNPs identified in [2] were tested for mediation by HbF in CSSCD samples. Only rs9494145 in chromosome 6q was associated with HbA2 and this effect was mediated by HbF (p=6.33E-05). The effect of rs11036212 in chromosome 11p was not mediated by HbF. This is consistent with Menzel's results the results[2] as their associations in chromosome 11 remained strong even after adjustment for HbF.
GWAS results in CSSCD
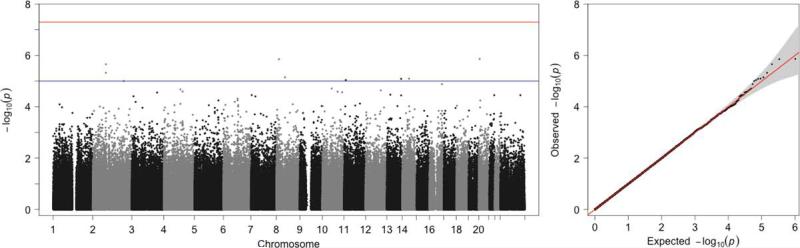
GWAS in the CSSCD are shown in Figure 2, Table 2 and Supplement Tables 3 and 4. Rs6038123 with the smallest p-value adjusted for age, sex, and HbF (p=1.37E-06) (20p12.3; position 5, 291,506), is located downstream of UBE2D3P1 (ubiquitin-conjugating enzyme E2D 3 pseudogene 1). This SNP also had the largest partial R2 (0.039) for any SNPs analyzed in the CSSCD samples. Uda et al found an association of rs6037828 (20p13; position 508,365 ) with HbF in Sardinians with β thalassemia.[25] In a meta-analysis of more than 2000 patients with sickle cell anemia we found no association of any SNP on chromosome 20 with HbF at p<1E-5.[26] The next-strongest association, adjusted for HbF, is at rs822278, located in the intergenic between MRPL49P2 (mitochondrial ribosomal protein L49 pseudogene 2) and FGF20 (fibroblast growth factor 20) on chromosome 8 (p=1.4E-06). These genes have not been implicated in hematological traits, and replication is required to ensure they are not false positives.
Figure 2.
Genome-wide association results for CSSCD adjusted for age, sex, and HbF. Left Panel: Manhattan plots of –log10(p-values) (y-axis) versus SNP positions (x-axis). No SNPs achieve genome-wide significance, but we find suggestive results on chromosomes 2, 6, and 11. Right Panel: QQ-plots for CSSCD GWAS results.
Table 2.
Top associations between SNPs and HbA2 in CSSCD GWAS.
| Adjusted for age and sex | Adjusted for age, sex, and HbF | Mediation by HbF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CHR | SNP | BP | MAF | A1 | Effect | P | Effect | P | P |
| 2 | rs766432 | 60573474 | 0.29 | C | −0.195 | 8.10E-08 | −0.1326 | 4.98E-04 | 3.50E-05 |
| 2 | rs10195871 | 60574093 | 0.33 | A | −0.166 | 1.69E-06 | −0.112 | 1.64E-03 | 2.35E-05 |
| 2 | rs17020489 | 81675692 | 0.3 | A | 0.1787 | 8.00E-07 | 0.1668 | 2.20E-06 | 1.73E-01 |
| 2 | rs17020632 | 81765823 | 0.31 | C | 0.1667 | 4.17E-06 | 0.161 | 4.75E-06 | 5.13E-01 |
| 2 | rs1113932 | 192760598 | 0.06 | G | 0.2881 | 2.00E-05 | 0.2895 | 9.95E-06 | 9.31E-01 |
| 8 | rs822278 | 16536220 | 0.47 | G | 0.1613 | 8.89E-07 | 0.1539 | 1.40E-06 | 3.49E-01 |
| 8 | rs1425912 | 54241746 | 0.36 | G | 0.1572 | 7.65E-06 | 0.1532 | 7.09E-06 | 6.37E-01 |
| 10 | rs10887055 | 123789677 | 0.26 | G | 0.1746 | 2.51E-06 | 0.1523 | 2.70E-05 | 1.55E-02 |
| 11 | rs11021763 | 11273929 | 0.37 | G | 0.1474 | 2.39E-05 | 0.1502 | 9.09E-06 | 7.45E-01 |
| 13 | rs1536807 | 40267790 | 0.44 | A | −0.1514 | 8.11E-06 | −0.1371 | 3.39E-05 | 8.19E-02 |
| 13 | rs2940695 | 107365156 | 0.25 | A | 0.175 | 5.24E-06 | 0.1665 | 8.19E-06 | 3.61E-01 |
| 14 | rs2296274 | 60986931 | 0.15 | A | −0.2204 | 3.49E-06 | −0.2063 | 8.00E-06 | 2.19E-01 |
| 16 | rs11150150 | 77897032 | 0.34 | G | −0.1627 | 4.21E-06 | −0.1501 | 1.31E-05 | 1.39E-01 |
| 20 | rs6038123 | 5220152 | 0.2 | A | −0.2138 | 4.69E-06 | −0.2193 | 1.37E-06 | 6.19E-01 |
SNPs significant at p<1E-05 in at least one analysis of CSSCD.
Replication in secondary cohorts was attempted for all CSSCD variants with p<1E-5 (Table S3, age and sex adjusted, and Table S4, age, sex, and HbF adjusted). Nominal replication (p<0.05) was achieved for rs766432 and rs10195871 in BCL11A. Both SNPs are associated with HbF levels.[17, 27-33] Rs766432 achieved genome-wide significance in meta-analysis (adjusted for age and sex only), with p=3.03E-10. All SNPs in Tables S3 and S4 reached statistical significance after Bonferroni correction (p < 0.05/14=0.004), and several SNPs had also consistent effects in the different studies. Mediation analysis suggested that the association of HbA2 with BCL11A is at least partially mediated by HbF.
Discussion
Normal newborns have less than 0.3% HbA2.[34] We studied the genetic regulation of HbA2 in sickle cell anemia and confirmed in part associations previously reported in normal northern Europeans.[2] A mediation analysis suggested, as did the studies of Menzel et al, that SNPs associated with HbA2 in the HBS1L-MYB locus on chromosome 6q affect HbA2 levels partially through their effects on HbF. An association of SNPs in BCL11A with HbA2, is also mediated by the effects of this gene on HbF expression. The enhancer of MYB within HMIP-2 of the HBS1LMYB locus [8, 35-37] is likely to modulate HbF by dual effects on erythropoiesis and the activation of other genes like KLF1 that modulate HbF expression.[37] Low MYB levels accelerate erythroid differentiation leading to release of early progenitors synthesizing predominantly HbF and also directly affect γ-globin gene expression.[38, 39]
The association of SNPs within the HBB cluster and HbA2 levels was not mediated by HbF. SNPs previously associated with HbA2 [2], and the SNP we replicated (rs11036212), are in the 3’ hypersensitive site (HS; downstream) of the HBB cluster, whereas SNPs associated with HbF are 5’ (upstream) of HBB. We could not replicate the associations found in Sardinians with β-thalassemia trait where the associations were in the 5’ β-globin gene locus control region.[26] Our results explain only a small amount of the variance of HbA2 that is likely to be affected by the complex regulation of globin gene expression.[40]
HbF is the major modulator of the phenotype of sickle cell anemia, but HbA2 has similar effects on HbS polymerization.[9, 41] Whether HBD expression can be increased, perhaps by targeting the mutated CACC binding site for KLF1 in the HBD promoter[7], is unknown. Other differences in HBD might thwart this approach.[42-49] Pancellular expression of HbA2 predicts that increased HbA2 levels could be especially beneficial.[50]
Gene expression within the HBB gene cluster is partially a function of competition among proximal gene promoters for transcription factors and the β-globin locus control region.[51] This is exemplified by increases in HbF and HbA2 when the promoter of the β-globin gene is deleted or mutated.[52-55] Substantial increases in HbA2 and HbF was found in HbS-β0 thalassemia when the β-globin gene promoter was deleted, and this was associated with mild disease.[56]
HbF was measured by two different methods our cohorts examined; within the range of HbF observed, both give similar results. Nevertheless, this could be a confounder.[57] HbA2 was also measured by differed means. When done by HPLC (Walk-PHaSST and PUSH trials) HbA2 levels are increased due to the co-elution of some HbS adducts.[58]
In sickle cell anemia, levels of HbA2 appear to be modulated by genes that directly and indirectly effect HBG expression and perhaps also by regulation within the β-globin gene cluster. It remains to be seen if these observations can be exploited for therapeutic purposes.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants R01 HL87681 (MHS), RC2 L101212 (MHS), 5T32 HL007501, T32GM074905 (PG), 2R25 HL003679-8 (VRG), R01 HL079912 (VRG), 2M01 RR10284-10 (VRG), R01HL098032 (MTG), R01HL096973 (MTG), P01HL103455 (MTG), the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (MTG)
Footnotes
Author contributions: PG, PS, HE and MHS analyzed the data, CTB performed laboratory analyses, VRG, MTG contributed patients to the studies, PG, PS, HE, MHS and DHKCHS analyzed and interpreted data and wrote and edited the manuscript.
Walk-PHAAST Investigators: DB. Badesch1, RJ Barst2, OL Castro3, JSR Gibbs4, RE Girgis5, MT Gladwin6, 7, JC Goldsmith8, VR Gordeuk3, KL Hassell1, GJ Kato9, L Krishnamurti10, S Lanzkron5, JA. Little11, R F Machado12, CR.Morris13, M Nouraie3, O Onyekwere3, EB Rosenzweig2, V Sachdev14, DE Schraufnagel12, MA Waclawiw15, R Woolson16, NA Yovetich16 1University of Colorado HSC, Denver, CO; 2Columbia University, New York, NY; 3Howard University, Washington, DC; 4National Heart & Lung Institute, Imperial College London, and Hammersmith Hospital, London; 5Johns Hopkins University, Baltimore, MD; 6Division of Pulmonary, Allergy and Critical Care Medicine and the 7Vascular Medicine Institute, at the University of Pittsburgh, Pittsburgh, PA; 8 National Heart Lung and Blood Institute/NIH, Bethesda, MD (personal views that do not represent the Government); 9 Cardiovascular and Pulmonary Branch, NHLBI, Bethesda, MD; 10 Children's Hospital of Pittsburgh, Pittsburgh, PA;11Albert Einstein College of Medicine, Bronx, NY; 12University of Illinois, Chicago, IL 13 Children's Hospital & Research Center Oakland, Oakland, CA; 14 Translational Medicine Branch, NHLBI, Bethesda, MD; 15 Office of Biostatistics Research, NHLBI, Bethesda, MD (personal views that do not represent the Government) 16Rho, Inc., Chapel Hill, NC
Walk-PHAAST Intramural NHLBI staff: Research nurses: C Seamon; A Chi; W Coles; Pulmonologists: S Alam; Hematologists: J Taylor; C Minniti; Protocol Management: MK. Hall;
Walk-PHAAST Children's Hospital & Research Center Oakland, Oakland, CA staff: L Lavrisha; W Hagar; H Rosenfeld; Echocardiography lab: C Brenneman; S Sidenko; C Birdsall; W Li, M St. Peter; C Brenneman
PUSH Investigators. M Arteta1, A Campbell1, OL Castro2, D Darbari3, N Dham3, G Ensing1, MT Gladwin4,5, VR Gordeuk2, GJ Kato6, L Luchtman-Jones,3 CP Minniti6, M Nouraie2, O Onyekwere2, S Rana2, C Sable3
1University of Michigan, Ann Arbor, MI; 2Howard University, Washington, DC; 3Children'p=s National Medical Center, Washington, DC; 4Division of Pulmonary, Allergy and Critical Care Medicine and 5Vascular Medicine Institute at the University of Pittsburgh, Pittsburgh, PA;
6Hematology Branch, National Heart, Lung and Blood Institute, Bethesda, MD; 5University of Pittsburgh, Pittsburgh, PA
Conflict-of Interest Disclosure: The authors declare no competing financial interests.
References
- 1.Steinberg MH, Adams JG., III. Hemoglobin A2 : Origin, evolution, and aftermath. Blood. 1991;78:2165–2177. [PubMed] [Google Scholar]
- 2.Menzel S, Garner C, Rooks H, et al. HbA2 levels in normal adults are influenced by two distinct genetic mechanisms. Br J Haematol. 2013;160:101–105. doi: 10.1111/bjh.12084. [DOI] [PubMed] [Google Scholar]
- 3.Heller P, Yakulis V. The distribution of hemoglobin A2. Ann NY Acad Sci. 1968;165:54. doi: 10.1111/j.1749-6632.1969.tb27776.x. [DOI] [PubMed] [Google Scholar]
- 4.Poillon WN, Kim BC, Rodgers GP, et al. Sparing effect of hemoglobin F and hemoglobin A 2 on the polymerization of hemoglobin S at physiologic ligand saturations. Proc Natl Acad Sci USA. 1993;90:5039–5043. doi: 10.1073/pnas.90.11.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel RL, Bookchin RM, Labie D, et al. Structural basis for the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci USA. 1979;76:670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waterman MR, Cottam GL, Shibata K. Inhibitory effect of deoxyhemoglobin A2 on the rate of deoxyhemoglobin S polymerization. J Mol Biol. 1979;129:337–341. doi: 10.1016/0022-2836(79)90287-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Chin K, Aerbajinai W, et al. Recombinant erythroid Kruppel-like factor fused to GATA1 up-regulates delta- and gamma-globin expression in erythroid cells. Blood. 2011;117:3045–3052. doi: 10.1182/blood-2010-07-294751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell JJ, Sherva RM, Chen ZY, et al. A 3-bp deletion in the HBS1L-MYB intergenic region on chromosome 6q23 is associated with HbF expression. Blood. 2011;117:4935–4945. doi: 10.1182/blood-2010-11-317081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinsheye I, Alsultan A, Solovieff N, et al. Fetal hemoglobin in sickle cell anemia. Blood. 2011;118:19–27. doi: 10.1182/blood-2011-03-325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dover GJ, Boyer SH, Zinkham WH, et al. Changing erythrocyte populations in juvenile chronic myelocytic leukemia: Evidence for disordered regulation. Blood. 1977;49:355–365. [PubMed] [Google Scholar]
- 11.Weatherall DJ, Clegg JB. The Thalassaemia Syndromes. Blackwell; Oxford: 1981. [Google Scholar]
- 12.Henri A, Testa U, Tonthat H, et al. Disappearance of Hb F and i antigen during the first year of life. Am J Hematol. 1980;9:161–170. doi: 10.1002/ajh.2830090204. [DOI] [PubMed] [Google Scholar]
- 13.Gaston M, Rosse WF. The cooperative study of sickle cell disease: review of study design and objectives. Am J Pediat Hematol-Oncol. 1982;4:197–201. [PubMed] [Google Scholar]
- 14.Machado RF, Barst RJ, Yovetich NA, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dham N, Ensing G, Minniti C, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104:713–720. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibney GT, Panhuysen CI, So JC, et al. Variation and heritability of Hb F and F-cells among beta-thalassemia heterozygotes in Hong Kong. Am J Hematol. 2008;83:458–464. doi: 10.1002/ajh.21150. [DOI] [PubMed] [Google Scholar]
- 17.Solovieff N, Milton JN, Hartley SW, et al. Fetal hemoglobin in sickle cell anemia: genome-wide association studies suggest a regulatory region in the 5′ olfactory receptor gene cluster. Blood. 2010;115:1815–1822. doi: 10.1182/blood-2009-08-239517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg MH, Rosenstock W, Coleman MB, et al. Effects of thalassemia and microcytosis upon the hematological and vaso- occlusive severity of sickle cell anemia. Blood. 1984;63:1353–1360. [PubMed] [Google Scholar]
- 19.Huisman THJ. Human hemoglobins. In: Yunis JJ, editor. Biochemical Methods in Red Cell Genetics. Academic Press; Orlando: 1969. pp. 391–504. [Google Scholar]
- 20.Betke K, Marti HR, Schlicht I. Estimation of small percentages of foetal haemoglobin. Nature. 1959;184:1887–1888. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- 21.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milton JN, Gordeuk VR, Taylor JGt, et al. Prediction of fetal hemoglobin in sickle cell anemia using an ensemble of genetic risk prediction models. Circ Cardiovasc Genet. 2014;7:110–115. doi: 10.1161/CIRCGENETICS.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci USA. 2008;105:1620–1625. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae H, Baldwin CT, Sebastiani P, et al. Meta-analysis of 2040 sickle cell anemia patients: BCL11A and HMIP are the major genetic modifiers of HbF in African Americans. Blood. 2012;120:1961–1962. doi: 10.1182/blood-2012-06-432849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menzel S, Garner C, Gut I, et al. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet. 2007;39:1197–1199. doi: 10.1038/ng2108. [DOI] [PubMed] [Google Scholar]
- 28.Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet. 2010;42:1049–1051. doi: 10.1038/ng.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U SA. 2008;105:11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sankaran VG, Menne TF, Xu J, et al. Human Fetal Hemoglobin Expression Is Regulated by the Developmental Stage-Specific Repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 31.Sankaran VG, Xu J, Byron R, et al. A functional element necessary for fetal hemoglobin silencing. N Engl J Med. 2011;365:807–814. doi: 10.1056/NEJMoa1103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer DE, Kamran SC, Lessard S, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedgewick A, Timofeev N, Sebastiani P, et al. BCL11A (2p16) is a major HbF quantitative trait locus in three different populations. Blood Cells, Mol Dis. 2008;41:255–258. doi: 10.1016/j.bcmd.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felicetti L, Novelletto A, Benincasa A, et al. The HbA/HbA2 ratio in newborns and its correlation with fetal maturity. Br J Haematol. 1984;56:465–471. doi: 10.1111/j.1365-2141.1984.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 35.Menzel S, Jiang J, Silver N, et al. The HBS1L-MYB intergenic region on chromosome 6q23.3 influences erythrocyte, platelet, and monocyte counts in humans. Blood. 2007;110:3624–3626. doi: 10.1182/blood-2007-05-093419. [DOI] [PubMed] [Google Scholar]
- 36.Thein SL, Menzel S, Peng X, et al. Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 influencing fetal hemoglobin levels in adults. Proc Natl Acad Sci USA. 2007;104:11346–11351. doi: 10.1073/pnas.0611393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stadhouders R, Aktuna S, Thongjuea S, et al. HBS1L-MYB intergenic variants modulate fetal hemoglobin via long-range MYB enhancers. JClin Invest. 2014;124:1699–1710. doi: 10.1172/JCI71520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tallack MR, Perkins AC. Three fingers on the switch: Kruppel-like factor 1 regulation of gamma-globin to beta-globin gene switching. Curr Opin Hematol. 2013;20:193–200. doi: 10.1097/MOH.0b013e32835f59ba. [DOI] [PubMed] [Google Scholar]
- 40.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. Am J Hematol. 2012;87:824–826. doi: 10.1002/ajh.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood WG, Old JM, Roberts AVS, et al. Human globin gene expression control of beta, delta and beta delta chain production. Cell. 1978;15:437–446. doi: 10.1016/0092-8674(78)90013-2. [DOI] [PubMed] [Google Scholar]
- 43.Ross J, Pizarro A. Human beta and delta globin messenger RNA's turn over at different rates. J Mol Biol. 1983;167:607–617. doi: 10.1016/s0022-2836(83)80101-6. [DOI] [PubMed] [Google Scholar]
- 44.Proudfoot NJ, Shander MHM, Manley JL, et al. Structure and in vitro transcription of human globin genes. Science. 1980;209:1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- 45.Humphries RK, Ley T, Turner P, et al. Differences in human alpha-, beta- and delta- globin gene expression in monkey kidney cells. Cell. 1982;30:173–183. doi: 10.1016/0092-8674(82)90023-x. [DOI] [PubMed] [Google Scholar]
- 46.Kosche KA, Dobkin C, Bank A. DNA sequences regulating human beta globin gene expression. Nucl Acids Res. 1985;13:7781–7793. doi: 10.1093/nar/13.21.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosveld GC, Rosenthal A, Flavell RA. Sequence requirements for the transcription of the rabbit beta- globin gene in vivo: The -80 region. Nuc Acids Res. 1982;10:4951–4971. doi: 10.1093/nar/10.16.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang DC, Ebb D, Hardison RC, et al. Restoration of the CCAAT box or insertion of the CACCC motif activates delta-globin gene expression. Blood. 1997;90:421–427. [PubMed] [Google Scholar]
- 49.Tang DC, Rodgers GP. Activation of the human delta-globin gene promoter in primary adult erythroid cells. Br J Haematol. 1998;103:835–838. doi: 10.1046/j.1365-2141.1998.01052.x. [DOI] [PubMed] [Google Scholar]
- 50.Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood. 2014;123:481–485. doi: 10.1182/blood-2013-09-528067. [DOI] [PubMed] [Google Scholar]
- 51.Hanscombe O, Whyatt D, Fraser P, et al. Importance of globin gene order for correct developmental expression. Genes & Devel. 1991;5:1387–1394. doi: 10.1101/gad.5.8.1387. [DOI] [PubMed] [Google Scholar]
- 52.Codrington JF, Li HW, Kutlar F, et al. Observations on the levels of Hb A 2 in patients with different á-thalassemia mutations and a ë chain variant. Blood. 1990;76:1246–1249. [PubMed] [Google Scholar]
- 53.Patterson M, Walker L, Eng B, et al. High Hb A2 beta-thalassemia due to a 468 bp deletion in a patient with Hb S/beta-thalassemia. Hemoglobin. 2005;29:293–295. doi: 10.1080/03630260500311651. [DOI] [PubMed] [Google Scholar]
- 54.Steinberg MH, Coleman MB, Adams JG, III, et al. High hemoglobin A2 β-thalassemia. J Lab & Clin Med. 1991;118:382–382. [PubMed] [Google Scholar]
- 55.Donaldson A, Thomas P, Serjeant BE, et al. Foetal haemoglobin in homozygous sickle cell disease: a study of patients with low HbF levels. Clin Lab Haematol. 2001;23:285–289. doi: 10.1046/j.1365-2257.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- 56.Waye JS, Chui DH, Eng B, et al. Hb S/beta zero-thalassemia due to the approximately 1 4-kb deletion is associated with a relatively mild phenotype. Am J Hematol. 1991;38:108–112. doi: 10.1002/ajh.2830380207. [DOI] [PubMed] [Google Scholar]
- 57.Fabry ME. Laboratory Diagnosis of hemoglobin Disorders and Animal Models for Their Study. In: Steinberg MH, Forget BG, Higgs DR, et al., editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press; Cambridge: 2001. pp. 910–940. [Google Scholar]
- 58.Suh DD, Krauss JS, Bures K. Influence of hemoglobin S adducts on hemoglobin A2 quantification by HPLC. Clin Chem. 1996;42:1113–1114. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.