Abstract
Objective
The purpose of this study was to evaluate the effects of different laser dose and force levels on the stability of orthodontic mini screws used for anchorage, by histomorphometric analyses.
Background data
Low-level laser therapy speeds up blood flow, improves the mechanism of the revitalization processes, reduces the risk of infection, boosts metabolic activities, and accelerates the healing of the damaged tissue. Although there are many research studies about low-level laser therapy applications in a variety of areas, no investigations were found concerning mini screw stability using various laser dose levels with different force level applications.
Methods
Seventeen New Zealand white rabbits were used. A total of 68 cylindrical, self-drilling orthodontic mini screws were threaded at the fibula. Experimental subjects were divided into six groups; force application was not performed in the first three groups, whereas 150g of force was applied via nickel-titanium closed-coil springs placed between two mini screws in the other three groups. Measurements of the initial torque values (10 Ncm) were manipulated by a digital portable torque gauge. Various low-level laser doses were applied to the groups during the postoperative 10 days. After 4 weeks, bone-to-implant contact and cortical bone thickness were histomorphometrically analyzed.
Results
In the 150g force plus 20 J/cm2 dosage group, the highest bone-to-implant contact values were observed. (p<0.05) There were no statistically significant correlations between cortical bone thickness and bone-to-implant contact values; on the other hand, no significant difference was found among the same groups in terms of cortical bone thickness values (p>0.05).
Conclusions
Low-level laser therapy was noticed to induce the mini screw–bone contact area. Low-level laser therapy may be a supplementary treatment method to increase the stability of the orthodontic mini screw.
Introduction
Titanium based dental implants, which were introduced in 1969 by Branemark, have shown great improvements since then. Recently, implants are used as reliable instruments for dental rehabilitation as well as for anchoring tooth and bone in clinical orthodontics.
The osseointegrated implants used for this purpose have many disadvantages, such as requirement for operation, expensiveness, and the long duration need for osseointegration.1 Kanomi, in 1997, designed the mini-implant for orthodontics.2 Factors that may increase the need for a mini screw are uncooperative patients, undesirable extraoral appliances, and the inadequacy of the dental elements and the surrounding bone.3 In addition, these screws have some advantages such as easy applicability, immediate loading, and time saving.4,5 However, the most common clinical challenge is the early loss of the mini screw. Primary stability is essential to be able to apply force to the mini screws.
It has been noted that the majority of the losses of mini screws result from primary stability failure.6 This problem can be solved by longer and thicker implant usage in prosthodontic treatment;7 however, this method is not conducive to the use of orthodontic mini screws, because of the placement regions. Therefore, clinicians are trying to develop alternative choices to increase the stability.
Many processes that have been shown to be stimulated by low-level laser therapy (LLLT) are cell proliferation,8,9 collagen and protein syntheses,10 wound healing,11–13 differentiation of bone and cartilage cells,14,15 and cell regeneration.16
LLLT speeds up the blood flow, improves the mechanism of the revitalization processes, reduces the risk of infection, boosts the metabolic activities, and accelerates the healing of the damaged tissue.16 Although there are many research studies of LLLT applications in a variety of areas, no investigations were found concerning mini screw stability using various laser dose levels with different force level applications. Therefore, the aim of this study was to evaluate the effects of different laser doses and force levels on the stability of orthodontic mini screws used for anchorage, by histomorphometric analyses, including the mini screw and the bone tissue intact together. It was hypothesized that LLLT and force application would not significantly affect the orthodontic mini screw's stability.
Materials and Methods
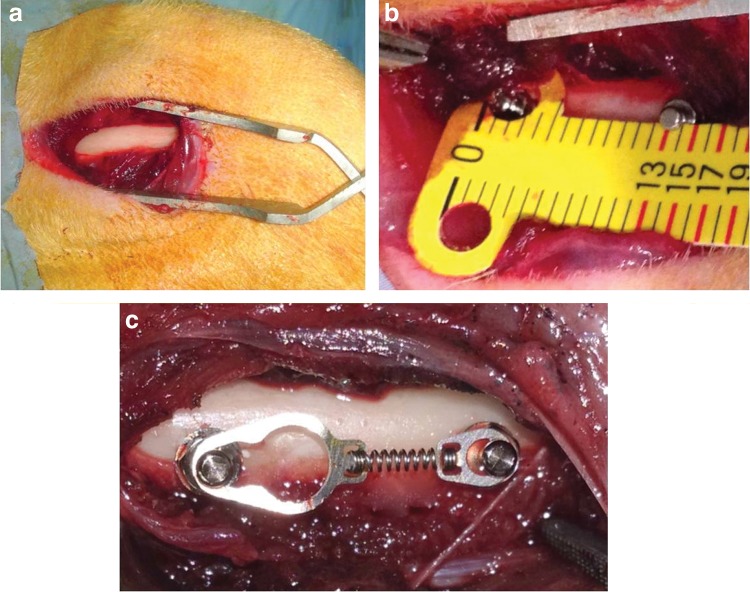
The protocol for this study was approved by the Experimental Animal Committee of Gaziantep University (30.11.2012/353). Seventeen 6-month-old male New Zealand white rabbits, weighing 3.0–3.5 kg, were used. A total of 68 cylindrical, self-drilling orthodontic mini screws (JeilMed, Seoul, Korea) made of Ti6Al4V alloy with a diameter of 1.4 mm and length of 8 mm were included. All surgeries were performed under sterile conditions in a veterinary operating room. Rabbits were anesthetized using an intramuscular injection of ketamine hydrochloride (100 mg/kg) and xylazine (5 mg/kg). After that, the hair on the medial surfaces of the right and left fibulas was clipped, and the skin was cleaned with iodinate surgical soap. A 50 mm incision was made parallel to the longitudinal axis of the fibula, and the periosteum was stripped (Fig. 1a). Mini screws were placed into the first cortex of the fibula and their longitudinal axes were adjusted parallel to each other and perpendicular to the external cortical fibula without interfering with the secondary cortex (Fig. 1b). TAD coil spring gauge was used for determining the distance between the mini screws on each fibula (Fig. 1b). Two mini screws were placed in the randomly selected fibulas of each rabbit, and 150g of force was immediately applied using a nickel-titanium (Ni-Ti) closed-coil spring (TAD, GH Wire Company, Hanover, Germany; C2 size: medium, 15 mm) (Fig. 1c). The identification of the groups were as follows: in group 1 (n=8) no force and no laser were used; in group 2 (n=12) no force was used and laser dosage was 10 J/cm2; in group 3 (n=12) no force was used and laser dosage was 20 J/cm2); in group 4 (n=12) the force amount was 150g and no laser was used; in group 5 (n=12) the force amount was 150g and laser dosage was 10 J/cm2; and in group 6 (n=12) the force amount was 150g and laser dosage was 20 J/cm2.
FIG. 1.
(a) Image of the bone, after dissection. (b) Measurement of the distance between mini screws. (c) Application of Ni-Ti coil spring to mini screws.
All mini screws were inserted using an electronic torque meter (JeilMedical Corporation, ORTHONIA 111-ED-010, Seoul, Korea) (10 Ncm) by the same operator (M.G.). The tissues were then closed with absorbable sutures, and carprofen (4 mg/kg) was given for 3 days after surgery to minimize infection risks.
A GaAlAs diode laser device (Cheese dental laser; Wuhan Gigaa Optronics Technology Co. Ltd., Wuhan, China) was used in this study. This system operates in the near-infrared spectrum at a continuous wavelength of 810 nm and an output power of 0.3 W, and produces a spot size of ∼5.85 cm2. Treatment was initiated immediately after surgery and performed daily for 10 consecutive days. The application period per point was 195 or 390 sec, releasing an energy density of 10 or 20 J/cm2.
Four weeks post-surgery, all rabbits were euthanized with an intravenous overdose of sodium pentothal. The fibulas were dissected, and 68 bone blocks containing one mini screw were prepared, each with at least 2 mm of surrounding bone. Mini screws were prepared for histomorphometrical analyses. The specimens were fixed in 10% buffered formalin, dehydrated in increasing concentrations of ethanol (70–99%) over a period of 10 days, and embedded in methyl methacrylate (Technovit 7200 VLC; HeraeusKulzer, South Bend, IN). Fifty micrometer thick, undecalcified sections were prepared by use of a diamond-coated saw cutting and grinding system (Exakt, Norderstedt, Germany). Sections were stained with toluidine blue, and digital images were obtained with a digital camera attached to a light microscope (Olympus DP 70; Olympus, Tokyo, Japan) at a magnification rate of ×40. The percentages of bone to implant contact (BIC) at the lateral sides of the implants and cortical bone thickness (CBT) were calculated by image analysis software (ImageJ 1.33u; National Institutes of Health, Bethesda, MD). Because the surface at the bottom of the mini screws was a machined surface (implants were cut to a height of 6 mm during the manufacturing process), the apical surfaces were not included in the BIC calculations.
BIC values were calculated using the following equation17 (Fig. 2):
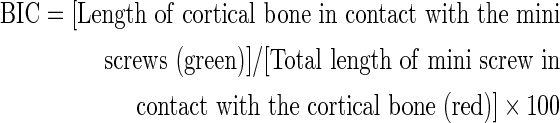 |
FIG. 2.
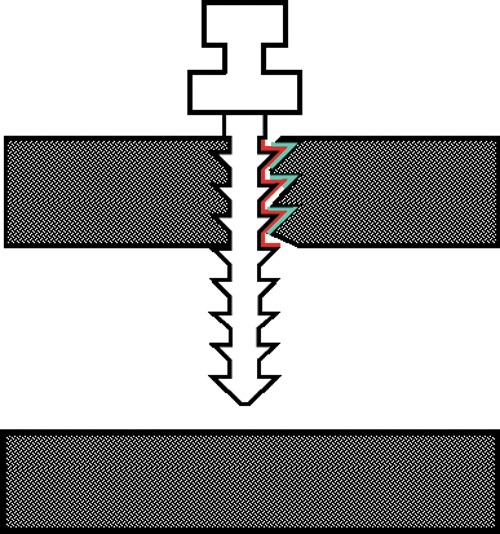
Measurement of bone to implant contact (BIC) and cortical bone thickness (CBT) values.
CBT values were calculated by taking the average of measurements contacting the mini screw on both sides18 (Fig. 2).
SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analyses. One way ANOVA and Tukey's honest significant difference (HSD) multiple comparison tests were used for independent samples to compare quantitative measurements (p<0.05). Pearson correlation coefficient was used to evaluate the associations between the BIC and CBT values.
Results
After 4 weeks of the experimental period, the clinical observation results of all mini screws in the force-applied and non-applied groups were all successful and there was no evidence of mobility.
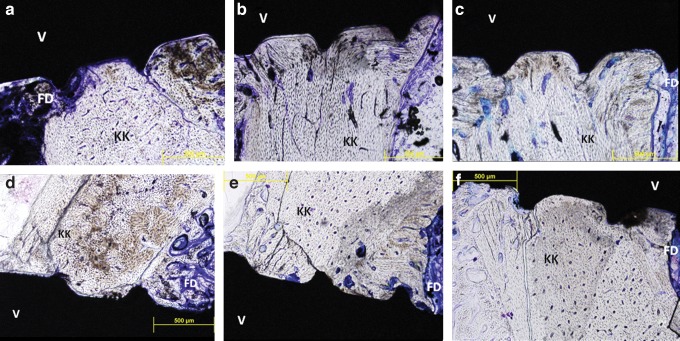
Images of the histological sections were used for histomorphometrical analyses (Fig. 3). More cortical bone tissues were detected throughout the insertion areas of the mini screw, and in some cortical regions, connective tissue was observed to be intertwined within the mini screw grooves.
FIG. 3.
Image of the histological sections according to Groups (a) 1, (b) 2, (c) 3, (d) 4, (e) 5, and (f) 6.
The descriptive statistical results of groups and results of ANOVA are shown in Table 1. The highest BIC value was observed in group 6 (83.11±1.75). This was followed by, respectively, group 5 (72.70±2.04), group 3 (64.87±1.78), group 4 (57.18±1.42), group 2 (53.99±1.82), and group 1 (36.15±2.45).
Table 1.
Descriptive Statistics Results of BIC and CBT Values According to Groups
| 95% Confidence interval for mean | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Standard deviation | Lower bound | Upper bound | Minimum | Maximum | F | p | ||
| BIC | Group 1 | 8 | 36.15 | 2.45 | 30.34 | 41.97 | 29 | 48.70 | 67.51 | 0.001 |
| Group 2 | 12 | 53.99 | 1.82 | 49.99 | 58.01 | 44 | 68.20 | |||
| Group 3 | 12 | 64.87 | 1.78 | 60.94 | 68.80 | 55.52 | 78.20 | |||
| Group 4 | 12 | 57.18 | 1.42 | 54.04 | 60.32 | 50.33 | 68.20 | |||
| Group 5 | 12 | 72.70 | 2.04 | 68.21 | 77.19 | 54.20 | 83.70 | |||
| Group 6 | 12 | 83.11 | 1.75 | 79.27 | 86.95 | 73 | 89.20 | |||
| Total | 68 | 61.33 | 1.87 | 30.34 | 86.95 | 29 | 89.20 | |||
| CBT | Group 1 | 8 | 1.93 | 0.31 | 1.21 | 2.67 | 0.89 | 3.51 | 0.14 | 0.982 |
| Group 2 | 12 | 2.01 | 0.16 | 1.65 | 2.37 | 1.32 | 3 | |||
| Group 3 | 12 | 2.16 | 0.20 | 1.72 | 2.61 | 0.96 | 3.22 | |||
| Group 4 | 12 | 2.03 | 0.25 | 1.47 | 2.60 | 0.67 | 3.44 | |||
| Group 5 | 12 | 1.95 | 0.17 | 1.57 | 2.34 | 0.74 | 3.00 | |||
| Group 6 | 12 | 1.99 | 0.22 | 1.52 | 2.48 | 0.74 | 3.42 | |||
| Total | 68 | 2.01 | 0.22 | 1.21 | 2.67 | 0.67 | 3.51 | |||
BIC, bone to implant contact; CBT, cortical bone thickness.
There were significant differences in BIC values among all groups (F=67.51, p=0.001<0.05) (Table 1). The multiple comparison results of CBT values are shown in Table 2. There were significant differences between groups 1 and 2 (p=0.001<0.05), groups 1 and 3 (p=0.001<0.05), groups 1 and 4 (p=0.001<0.05), and group 1 and groups 5 and 6 (p=0.001<0.05). The values of group 1 were lower than those of the other groups.
Table 2.
Statistical Evaluation of BIC Values Between Groups
| Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|
| Group 1 | 0.001a | 0.001a | 0.001a | 0.001a | 0.001a |
| Group 2 | 0.001a | 0.811 | 0.001a | 0.001a | |
| Group 3 | 0.041a | 0.036a | 0.001a | ||
| Group 4 | 0.001a | 0.001a | |||
| Group 5 | 0.002a |
p<0.001.
BIC, bone to implant contact.
The statistical evaluation of CBT values was determined according to the groups in Table 1. The highest CBT value was observed in group 3 (2.16±0.20). This was followed by, respectively, group 4 (2.03±0.25), group 2 (2.01±0.16), group 6 (1.99±0.22), group 5 (1.95±0.17) and group 1 (1.93±0.31). There were no significant differences in CBT values for any of the groups (p=0.982>0.05) (Table 1).
There were no statistically significant correlations between CBT and BIC values (r = −0.012, p=0.922).
Discussion
The results obtained in the present study demonstrate that LLLT of bone with a diode laser significantly improved the BIC values of orthodontics mini screws. Therefore, the first null hypothesis, which stated that diode laser application would not significantly affect the connection amount of mini screw to bone, was rejected.
When the groups, which had a similar laser application procedure and different force amounts, were compared, it was obvious that the force application positively affected the quantity of attachment. Therefore, because of the bone remodeling process, the second null hypothesis was also rejected.
In the present study, it was found that there was no significant difference in the CBT values for any of the groups. In addition, no substantial correlations were detected between CBT and BIC values. Therefore, the third null hypothesis, which declared that the CBT would not affect the osseointegration of the mini screw, was accepted within the conditions of this study.
In the literature, there are many different opinions about timing of animal euthanasia. Eighteen weeks of human bone metabolic process corresponds to 6 weeks of rabbit bone turnover.19 This means that the rabbit metabolism is three times faster than the human metabolism. Previously, adequate time for osseointegration of the placed mini screws was reported to be 8 weeks.20,21 Although it was reported that the lamellar bone formation and secondary remodeling events occurred in this period of time,21 recently, it has been accepted that the critical time for osseointegration is 4 weeks, and that a longer waiting period for proper connection is pointless.22,23 Therefore, in present study the time of euthanasia was determined to be 4 weeks.
A pilot study was performed just before the experimental stage of this research, in which the mini screws were placed in the tibia of the rabbits;24–26 however, unfortunately it was detected that the legs of the animals were broken because of the weak bone structure. It was thought that length of the mini screw could be the cause of the fracture, but as the most commonly used length of the mini screws is 8 mm in orthodontic practice, it was decided to change the experimental bone to the fibula, rather than changing the dimension of the mini screw.27
It has been reported that the most suitable wavelength for biostimulation is 550–950 nm.28 The laser types in this range are He-Ne and diode laser systems. For this study, the selected diode laser was in the infrared spectrum, having a high level of tissue penetration depth.28
The force amount to be applied to mini screws is a controversial issue. There are many investigators reporting that it should be between 100 and 200g29 and that it would not be successful if it was >200g.3,30–34 For this reason, the quantity of force was determined to be 150g.
When the previous studies were analyzed for determining the dose of LLLT, it was noticed that a consensus was absent concerning this issue. However, it is a fact beyond doubt that the dose of laser that is required for influencing the hard tissue should be more than the dose of laser that is required for stimulation of the soft tissue.23 It is described as ranging from 4 to 10 J/cm2 for soft tissues.35–39 In light of this information, the laser doses selected were 10 and 20 J/cm2 in this investigation.
Although some researchers reported that there is no relationship between the cortical bone thickness and the stability of the mini screw,40–42 others reported an opposing argument.43,44 According to the results of the present study, there was no statistically significant difference among the groups. This outcome may be associated with providing adequate stability of the mini screws for maintaining stability until the end of the experiment. The limitation of this study is that initial cortical bone thickness cannot be standardized. The limitation of this study is that the initial cortical bone thickness cannot be standardized since measuring cortical bone thickness on live animals is an extremely difficult issue. For the future, there is a need for research that compares clinically successful and unsuccessful mini screw groups, and, in addition, better standardization procedures are greatly needed for determining reliable impacts of these variables. Another limitation of this study is that the condition of experiment cannot be simulated in the mouth. The mini screws are placed in humans in an open oral environment. More accurate results can be obtained with human trials.
Conclusions
In this present research, clinical and histomorphometrical findings were evaluated, and the following results were obtained.
1. Utilizing orthodontic mini screws as anchorage devices is a reliable, effective, and easy method.
2. No mobility of the mini screws was noticed during the experimental period, which can be assumed as a success indicator.
3. The BIC values of groups receiving 20 J/cm2 laser doses were higher than those of the other groups. Therefore, LLLT may be an alternative method for increasing the stability of mini screws.
4. There was no correlation between CBT values and the BIC values for the stability of the orthodontic mini screw.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Deguchi T, Takano-Yamamoto T, Kanomi R, Hartsfield J, Roberts W, Garetto L. The use of small titanium screws for orthodontic anchorage. J Dent Res 2003;82:377–381 [DOI] [PubMed] [Google Scholar]
- 2.Kanomi R. Mini implant for orthodontic anchorage. J Clin Orthod 1997;31:763–767 [PubMed] [Google Scholar]
- 3.Park HS, Bae SM, Kyung HM, Sung JH. Micro-implant anchorage for treatment of skeletal Class I bialveolar protrusion. J Clin Orthod 2001;35:417–422 [PubMed] [Google Scholar]
- 4.Moon CH, Lee DG, Lee HS, Im JS, Baek SH. Factors associated with the success rate of orthodontic miniscrews placed in the upper and lower posterior buccal region. Angle Orthod 2008;78:101–106 [DOI] [PubMed] [Google Scholar]
- 5.Lim SA, Cha JY, Hwang CJ. Insertion torque of orthodontic miniscrews according to changes in shape, diameter, and length. Angle Orthod 2008;78:234–240 [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Ahn SJ, Lee JW, Kim SH, Kim TW. Survival analysis of orthodontic mini-implants. Am J Orthod Dentofacial Orthop 2010;137:194–199 [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Baek SH, Kim TW, Chang YI. Comparison of stability between cylindrical and conical type mini-implants: mechanical and histologic properties. Angle Orthod 2008;78:692–698 [DOI] [PubMed] [Google Scholar]
- 8.Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD. Effect of wavelength on low intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg Med 2005:36:8–12 [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 2009;16:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC. Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 2003;18:95–99 [DOI] [PubMed] [Google Scholar]
- 11.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 2005;31:334–340 [DOI] [PubMed] [Google Scholar]
- 12.Hopkins JT, McLoda TA, Seegmiller JG, Baxter GD. Low-level laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled study. J Athl Train 2004;39:223. [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins D, Houreld N, Abrahamse H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann NY Acad Sci 2005;1056:486–493 [DOI] [PubMed] [Google Scholar]
- 14.Arisu HD, Türköz E, Bala O. Effects of Nd: Yag laser irradiation on osteoblast cell cultures. Lasers Med Sci 2006;21:175–180 [DOI] [PubMed] [Google Scholar]
- 15.Ueda Y, Shimizu N. Pulse irradiation of low-power laser stimulates bone nodule formation. J Oral Sci. 2001;43:55. [DOI] [PubMed] [Google Scholar]
- 16.King PR. Low level laser therapy: a review. Laser Med Sci 1989;4:141–150 [Google Scholar]
- 17.Yano S, Motoyoshi M, Uemura M, Ono A, Shimizu N. Tapered orthodontic miniscrews induce bone-screw cohesion following immediate loading. Eur J Orthod 2006;28:541–546 [DOI] [PubMed] [Google Scholar]
- 18.Jung YR, Kim SC, Kang KH, et al. Placement angle effects on the success rate oforthodontic microimplants and other factorswith cone-beam computed tomography. Am J Orthod Dentofacial Orthop 2013;143:173–181 [DOI] [PubMed] [Google Scholar]
- 19.Roberts WE, Smith RK, Zilberman Y, Mozsary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod Dentofacial Orthop 1984;86:95–111 [DOI] [PubMed] [Google Scholar]
- 20.Mori H, Manabe M, Kurachi Y, Nagumo M. Osseointegration of dental implants in rabbit bone with low mineral density. J Oral Maxillofac Surg 1997;55:351–361 [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Bai YX, Wang BK. Biomechanical and histomorphometric characterizations of osseointegration during mini-screw healing in rabbit tibiae. Angle Orthod 2009;79: 558–563 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann O, Angelov N, Zafiropoulos GG, Andreana S. Osseointegration of zirconia implants with different surface characteristics: an evaluation in rabbits. Int J Oral Maxillofac Implants 2012;27:352. [PubMed] [Google Scholar]
- 23.Rodrigues Pinto M, dos Santos RL, Pithon MM, de Souza Araujo MT, Braga JPV, Nojima LI. Influence of low-intensity laser therapy on the stability of orthodontic mini-implants: a study in rabbits. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:e26-e30 [DOI] [PubMed] [Google Scholar]
- 24.Uysal T, Ekizer A, Akcay H, Etoz O, Guray E. Resonance frequency analysis of orthodontic miniscrews subjected to light-emitting diode photobiomodulation therapy. Eur J Orthod 2010;34:44–51 [DOI] [PubMed] [Google Scholar]
- 25.Prodanov L, Lamers E, Domanski M, Luttge R, Jansen JA, Walboomers XF. The effect of nanometric surface texture on bone contact to titanium implants in rabbit tibia. Biomaterials 2013;34:2920–2927 [DOI] [PubMed] [Google Scholar]
- 26.Khadra M, Ronold HJ, Lyngstadaas SP, Ellingsen JE, Haanaes HR. Low level laser therapy stimulates bone-implant interaction: an experimental study in rabbits. Clin Oral Implant Res 2004;15:325–332 [DOI] [PubMed] [Google Scholar]
- 27.Topcuoglu T, Bicakci AA, Avunduk MC, Sahin Inan ZD. Evaluation of the effects of different surface configurations on stability of miniscrews. Sci World J 2013;2013:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation: a review. J Clin Periodontol 1996;23:492–496 [DOI] [PubMed] [Google Scholar]
- 29.Cheng SJ, Tseng IY, Lee JJ, Kok SH. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants 2004;19:100. [PubMed] [Google Scholar]
- 30.Park HS, Jeong SH, Kwon OW. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofacial Orthop 2006;130:18–25 [DOI] [PubMed] [Google Scholar]
- 31.Kuroda S, Sugawara Y, Deguchi T, Kyung HM, Takano–Yamamoto T. Clinical use of miniscrew implants as orthodontic anchorage: success rates and postoperative discomfort. Am J Orthod Dentofacial Orthop 2007;131:9–15 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Kyung HM, Zhao WT, Yu WJ. Critical factors for the success of orthodontic mini-implants: a systematic review. Am J Orthod Dentofacial Orthop 2009;135:284–291 [DOI] [PubMed] [Google Scholar]
- 33.Motoyoshi M, Yano S, Tsuruoka T, Shimizu N. (2005). Biomechanical effect of abutment on stability of orthodontic mini-implant. Clin Oral Implants Res 2009;16:480–485 [DOI] [PubMed] [Google Scholar]
- 34.Chaddad K, Ferreira AFH, Geurs N, Reddy MS. Influence of surface characteristics on survival rates of mini-implants. Angle Orthod 2008;78:107–113 [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira Guirro EC, de Lima Montebelo MI, de Almeida Bortot B, da Costa Betito Torres MA, Polacow MLO. Effect of laser (670 nm) on healing of wounds covered with occlusive dressing: a histologic and biomechanical analysis. Photomed Laser Surg 2010;28:629–634 [DOI] [PubMed] [Google Scholar]
- 36.Ferreira MC, Gameiro J, Nagib PRA, Brito VN, Vasconcellos ECC, Verinaud L. Effect of low intensity Helium–Neon (HeNe) Laser irradiation on experimental paracoccidioidomycotic wound healing dynamics. J Photochem Photobiol 2009;85:227–233 [DOI] [PubMed] [Google Scholar]
- 37.Usumez A, Cengiz B, Oztuzcu S, Demir T, Aras MH, Gutknecht N. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci 2014;29:1807–1813 [DOI] [PubMed] [Google Scholar]
- 38.Bensadoun RJ, Nair RG. Efficacy of low-level laser therapy (LLLT) in oral mucositis: what have we learned from randomized studies and meta-analyses? Photomed Laser Surg 2012;30:191–192 [DOI] [PubMed] [Google Scholar]
- 39.Houreld N, Abrahamse H. In vitro exposure of wounded diabetic fibroblast cells to a helium-neon laser at 5 and 16 J/cm2. Photomed Laser Surg 2007;225:78–84 [DOI] [PubMed] [Google Scholar]
- 40.Marco Miglioratiemail SB, Alessio S, Sara D, Fabrizio B, Henry T, Armando SB. Miniscrew design and bone characteristics: an experimental study of primary stability. Am J Orthod Dentofacial Orthop 2012;142:228–234 [DOI] [PubMed] [Google Scholar]
- 41.Shank SB, Beck FM, D'Atri AM, Hujaemail SS. Bone damage associated with orthodontic placement of miniscrew implants in an animal model. Am J Orthod Dentofacial Orthop 2012;141:412–418 [DOI] [PubMed] [Google Scholar]
- 42.Gracco A, Lombardo L, Cozzani M, Siciliani G. Quantitative cone-beam computed tomography evaluation of palatal bone thickness for orthodontic miniscrew placement. Am J Orthod Dentofacial Orthop 2008;134:361–369 [DOI] [PubMed] [Google Scholar]
- 43.Mina KI, Kim SC, Kang KH, et al. Root proximity and cortical bone thickness effects on the success rate of orthodontic micro-implants using cone beam computed tomography. Angle Orthod 2012:82:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duaibis R, Kusnoto B, Natarajan R, Zhao L, Evans C. Factors affecting stresses in cortical bone around miniscrew implants. Angle Orthod 2012;82:875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]