Abstract
Aims: Transfusion with stored red blood cells (RBCs) is associated with increased morbidity and mortality. Peroxiredoxin-2 (Prx-2) is a primary RBC antioxidant that limits hydrogen peroxide (H2O2)-mediated toxicity. Whether Prx-2 activity is altered during RBC storage is not known. Results: Basal and H2O2-induced Prx-2 activity was measured in RBCs (stored for 7–35 days). Basal Prx-2 thiol oxidation increased with RBC age, whereas H2O2-dependent formation of dimeric Prx-2 was similar. However, reduction of Prx-2 dimers to monomers became progressively slower with RBC storage, which was associated with increased H2O2-induced hemolysis. Surprisingly, no change in the NADPH-dependent thioredoxin (Trx)/Trx-reductase system, which recycles dimeric Prx-2, was observed in stored RBCs. Using mouse RBCs expressing human wild type (β93Cys) or hemoglobin (Hb) in which the conserved β93Cys residue is replaced by Ala (β93Ala), a role for this thiol in modulating Prx-2 reduction was demonstrated. Specifically, Prx-2 recycling was blunted in β93Ala RBC, which was reversed by carbon monoxide-treatment, suggesting that heme autoxidation-derived H2O2 maintains Prx-2 in the oxidized form in these cells. Moreover, assessment of the oxidative state of the β93Cys in RBCs during storage showed that while it remained reduced on intraerythrocytic Hb in stored RBC, it was oxidized to dehydroalanine on hemolyzed or extracellular Hb. Innovation: A novel mechanism for regulated Prx-2 activity in RBC via the β93Cys residue is suggested. Conclusion: These data highlight the potential for slower Prx-2 recycling and β93Cys oxidation in modulating storage-dependent damage of RBCs and in mediating post-transfusion toxicity. Antioxid. Redox Signal. 22, 294–307.
Introduction
Transfusion with packed red blood cell (pRBC) stored for up to 42 days in the blood bank is a currently approved therapeutic strategy to improve tissue oxygenation in multiple clinical settings. However, recent retrospective analyses have highlighted the potential for increased morbidity and mortality associated with transfusion of RBC stored for longer versus shorter periods of time (3, 25, 36, 56, 58, 61, 63). This perspective has been supported by preclinical studies demonstrating adverse effects of stored blood in providing a “second” hit that promotes toxicity compounded by the patient's underlying disease. Proposed mechanisms that constitute the “second” hit include oxidative stress, inhibition of nitric oxide (NO) signaling, iron overload and related toxicity, immune cell activation, and exacerbation of inflammation (2, 4, 8, 15, 27, 42, 48, 52–54, 64).
Storage causes multiple changes in the RBC, including oxidative damage, loss of ATP, and loss of membrane and volume with an associated transition from biconcave discs to echinocytes and less deformable cells (9, 20, 22, 48, 50, 56, 60). Recent studies are beginning to link these changes with mechanisms that lead to post-transfusion toxicity. For example, alterations in RBC shape, formation of microparticles, and hemolysis accelerate NO scavenging and promote oxidative stress; loss of chemokine binding capacity can promote inflammation; and released free iron can predispose transfusion recipients to infection (2, 4, 15, 27, 42, 54). This understanding has fuelled interest in preventing biochemical and morphological alterations in the RBC during storage to avoid post-transfusion toxicity. A potentially common mechanism in both these contexts is increased oxidative stress. Storage is associated with oxidative damage to the RBC indexed by accumulation of oxidized products and/or loss of endogenous antioxidants (20, 49, 50). Specific oxidative stress-derived biomarkers may help identify RBCs that are more susceptible to hemolysis during storage and/or cause injury in the transfusion recipient (20, 49). Moreover, prevention of morphologic and biochemical changes in RBCs stored under anaerobic conditions indicates a causative role of oxidative stress in the storage lesion (18, 62). Finally, RBCs are now appreciated as active participants in modulating redox homeostasis in vivo by both promoting (via hemoglobin [Hb] redox cycling-derived reactive species) and inhibiting (by detoxifying reactive species via erythrocytic antioxidant systems) oxidative injury in the systemic and pulmonary compartments (1, 6, 13, 19, 24, 31, 34, 46, 47, 51, 55, 57). Storage appears to perturb this balance, resulting in more pro-oxidative RBCs.
Innovation.
Red blood cell (RBC) hemolysis during storage is associated with adverse effects of transfusion with stored RBC. Mechanisms leading to RBC hemolysis during storage are not known. We show that peroxiredoxin-2 (Prx-2) recycling, a key element in its antioxidant activity, is compromised in stored RBC. We also show that the conserved β93Cys residue of hemoglobin is a novel regulator of Prx-2 recycling by controlling heme auto-oxidation, and that oxidation of this thiol to dehydroalanine during storage may lead to increased RBC hemolysis. These data provide novel mechanistic insights into how hemolysis occurs in stored RBCs and suggest that Prx-2 recycling and the β93Cys residue are novel targets to modulate this process.
RBCs are endowed with multiple antioxidant systems that limit peroxide (hydrogen peroxide [H2O2], lipid hydroperoxide) and peroxynitrite-dependent effects. Peroxiredoxin-2 (Prx-2) has emerged as the critical antioxidant protecting RBCs from H2O2 produced endogenously (by Hb autoxidation and subsequent superoxide dismutation) and exogenously (e.g., from activated neutrophils) at low (physiologic) concentrations (11, 32, 38, 40, 43, 45) and, therefore, may limit oxidative injury to other cells/tissues in the vasculature (6, 57). Prx-2 is a 2-Cys-Prx homodimer, by which the peroxidatic cysteine on one subunit is oxidized to a sulfenic acid by H2O2 (a two-electron oxidation). The second or resolving Cys on the other subunit reacts with the sulfenic acid to form a disulfide bridge to complete the reaction cycle (40). At higher fluxes of H2O2, the peroxidatic Cys may undergo further oxidation to sulfinic or sulfonic acid, which relies on sulfiredoxin for reduction (11). Under normal conditions, formation of disulfide-linked Prx-2 dimer is relatively rapid and is followed by reduction back to the monomer by the thioredoxin (Trx)–Trx reductase system, which is fuelled by NADPH (40). The reduction of oxidized Prx-2 is relatively slow compared with the initial oxidation, taking several minutes. While the slow recycling could limit the antioxidant efficacy of Prx-2, since this protein is present at relatively high concentrations (∼200–300 μM in pRBC) being the third most abundant protein in the RBC, its capacity to scavenge H2O2 remains high (11, 40). Thus, this noncatalytic, but high-capacity pathway has been suggested to limit oxidative damage to RBCs under physiological conditions.
Relatively, little is known on the role of Prx-2 in storage-dependent damage to RBCs nor whether its activity may be regulated by other factors beyond Trx-dependent reduction. Previous reports have documented the formation of oligomeric Prx-2 complexes, and shown increased association of Prx-2 with the RBC membrane, during storage (49, 50). However, whether Prx-2 activity is altered by storage is not known. This is important, as the slow reduction of Prx-2 becomes limiting under conditions of high H2O2 flux, as expected during inflammatory stress in transfusion recipients. Here, we show that reduction of disulfide-linked Prx-2 dimer is significantly inhibited in RBCs stored for >14 days, which occurs without changes in Trx reductase activity. We show an unexpected role for the β93Cys residue of Hb in affecting storage-dependent changes in Prx-2 recycling and, provide evidence for a novel function for this conserved RBC thiol as well as new insights into how RBC Prx-2 activity is regulated. These data are discussed in the context of a functional assay to assess RBC storage and potential mechanisms for post-transfusion toxicity.
Results
Prx-2 oxidation and recycling during RBC storage
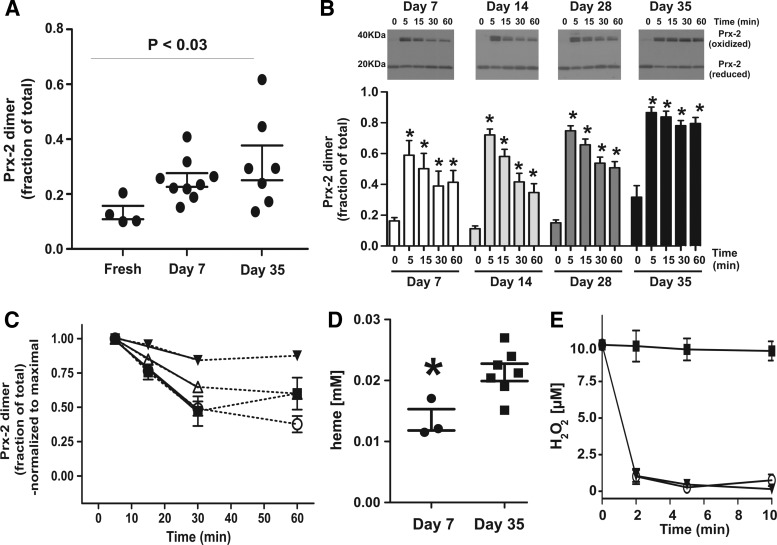
Figure 1A shows that basal Prx-2 oxidation by disulfide formation increased in RBCs stored for 7 or 35 days compared with freshly isolated cells. Notably, the variation in basal Prx-2 oxidation increased proportionally with storage age. An important aspect of Prx-2 activity is to metabolize peroxides and then be recycled back to the reduced form. H2O2-dependent formation of dimeric Prx-2 (oxidized Prx-2) in RBCs can be measured by Western blotting under nonreducing conditions. RBCs of different storage ages were exposed to H2O2, and time-dependent changes in Prx-2 oxidation were measured. Figure 1B presents these data and shows that (i) basal (time 0) Prx-2 oxidation was higher at day 35 relative to day 14 and 28 (p<0.05 by one-way analysis of variance (ANOVA) with Tukey post hoc test); (ii) H2O2 significantly increased Prx-2 oxidation, which was maximal at the first time point (5 min) measured; (iii) the magnitude of the maximal level of Prx-2 oxidation increased with RBC storage age (p<0.05 by one-way ANOVA with Tukey post hoc test for day 7 vs. 35 for 5 min data); and (iv) dimeric Prx-2 was slowly reduced back to the monomer over 60 min; however, this was not observed with day 35 RBC, where Prx-2 remained >75% oxidized. To determine how RBC storage affected the rate of Prx-2 reduction, Figure 1C replots these data after normalizing to the maximal Prx-2 oxidation. Interestingly, reduction was linear during the first 30 min, with no further reduction occurring thereafter. Linear regression analysis over 30 min showed that the rate of Prx-2 reduction progressively slowed by 30% and 70% for day 28 and 35 RBC, respectively, with no differences with RBC day 14 or younger. Finally, slower Prx-2 reduction correlated with increased H2O2 (10 μM)-induced hemolysis of day 35 RBC compared with day 7 RBC (Fig. 1D). Figure 1E shows that H2O2 is rapidly consumed (<5 min) after addition to RBC, with no differences between young and old RBC, suggesting that slower reduction of oxidized Prx-2 observed with day 35 RBC (Fig. 1C) is not due to slower metabolism of added H2O2.
FIG. 1.
Effect of storage time on Prx-2 oxidation and recycling kinetics. (A) Basal Prx-2 oxidation in RBC freshly isolated or after storage for 7 or 35 days. Each symbol represents an RBC preparation from a distinct donor. p-Value shown determined by one-way ANOVA (nonparametric assuming non-Gaussian distribution). (B) H2O2 (10 μM) was added to human RBCs stored for different times (7–35 days) in isotonic saline buffer (pH 7.4) and Prx-2 oxidation measured at 5, 15, 30, or 60 min as described in Materials and Methods section. Representative Western blots and quantitation of Prx-2 oxidation expressed as the fraction of the Prx-2 dimer relative to total Prx-2 are shown. Data are mean±SEM (n=6). *p<0.001 compared with respective basal (time 0) by one-way RM-ANOVA with Tukey's post hoc test. (C) Time-dependent changes in Prx-2 relative to the maximal amount formed (at 5 min). Solid lines show linear regression fits (p<0.001 for all slopes being not zero) for data between 5 and 30 min and were y = −0.021x+1.09 (day 7, ■); y = −0.02x+1.09 (day 14, ◯); y = −0.014x+1.07 (day 28, △); y = −0.0064x+1.04 (day 35, ▼). Slopes were significantly different between day 14 and 28 (p<0.01), between day 7 and 35 (p<0.001), and between day 14 and 35 (p<0.0001). (D) Hemolysis induced by addition of H2O2 (10 μM) to day 7 or 35 RBCs (after 60 min in isotonic saline buffer (pH 7.4). Each data point represents a distinct RBC preparation. *p<0.02 by t-test. (E) H2O2 (10 μM) was added to phosphate-buffered saline (■), day 7 RBC (▼), or day 35 RBC (◯) and H2O2 concentrations were measured at indicated times. Data shown are mean±SEM (n=3–4). No differences between day 7 and 35 RBC-dependent consumption of H2O2 were observed. H2O2, hydrogen peroxide; Prx-2, peroxiredoxin-2; RBC, red blood cell; RM-ANOVA, repeated measures-analysis of variance.
Effects of RBC storage on Trx reductase activity
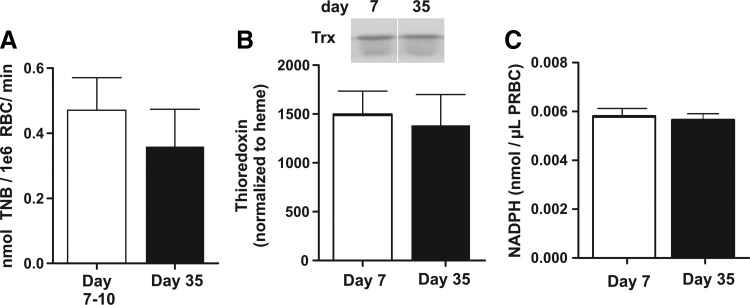
The Trx and NADPH-dependent Trx reductase system is rate limiting for reduction of oxidized Prx-2 in RBC. However, no differences in basal Trx-reductase activities, Trx protein levels, or NADPH levels were observed in day 7 versus 35 RBC (Fig. 2), suggesting that this was not the basis of differential Prx-2 reduction kinetics.
FIG. 2.
Effect of storage time on Trx reductase system in RBC. Thioredoxin reductase activity (A), thioredoxin levels (B), and NADPH levels (C) were measured in RBCs stored for 7 or 35 days as described in Materials and Methods section. Data are mean±SEM [n=8–9 for (A), and n=3 for (B, C)]. In all cases, differences were not significant by unpaired t-test. Trx, thioredoxin.
Effects of glucose on Prx-2 cycling in stored RBC
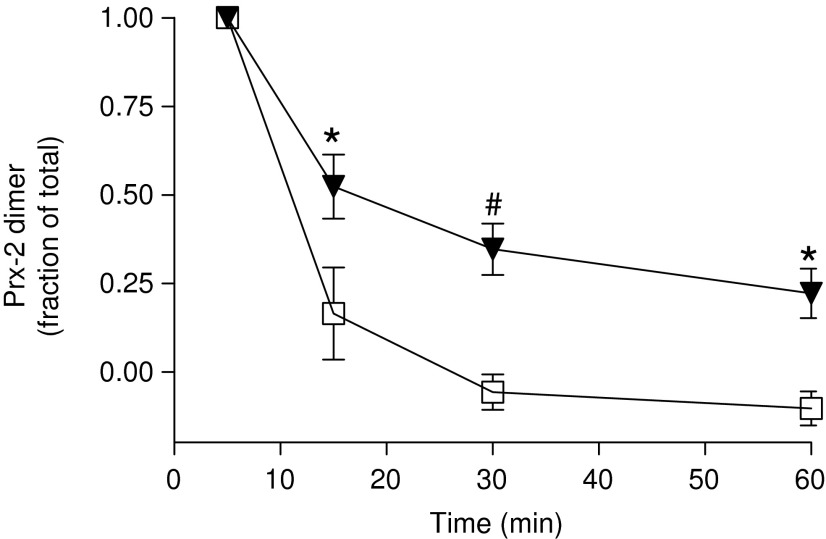
Since glucose levels decline during RBC storage (28), which could limit NADPH recycling, we next tested whether addition of glucose could rescue the defect in reduction of oxidized Prx-2 after H2O2 addition. Figure 3 shows time-dependent changes in Prx-2 oxidation normalized to maximal (at 5 min) after addition of H2O2. Inclusion of glucose increased the rate of Prx-2 reduction for both day 7 and 35 RBC compared with the reaction without glucose (compare to Fig. 1). For example, ∼85% reduction back to monomer was observed by 15 min for day 7 RBC compared with only ∼25% for day 7 RBC without glucose (Fig. 1). However, while glucose accelerated Prx-2 reduction for day 7 and 35 RBC, the latter was still slower compared with the former (Fig. 3), indicating that the inhibited rates of Prx-2 recycling in older RBC were not due to differences in glucose concentrations.
FIG. 3.
Effect of storage time on Prx-2 recovery after H2O2 exposure with added glucose. Human RBCs stored for 7 (□) or 35 days (▼) were exposed to 10 μM H2O2 for 5, 15, 30, or 60 min in the presence of glucose (5 mM) and Prx-2 oxidation was measured as described in Materials and Methods section. Data show values normalized to maximal levels of oxidized Prx-2 (at 5 min) and are mean±SEM (n=3). *p<0.05 and #p<0.01 by two-way ANOVA with Bonferroni post hoc test.
Effect of β93Cys on Prx-2 recycling in stored RBC
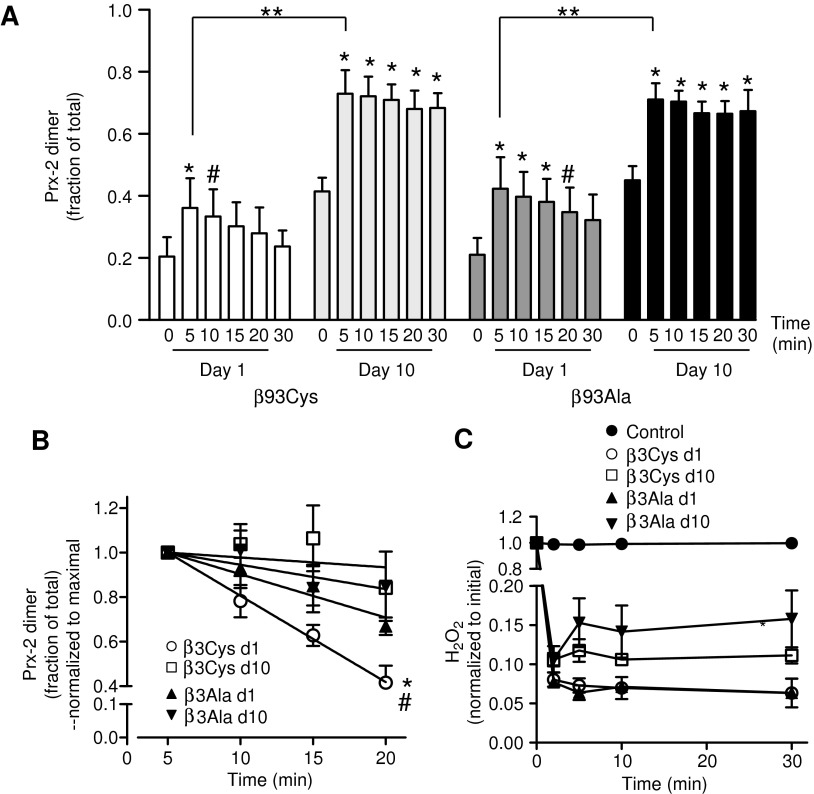
Our recent studies showed that H2O2-dependent oxidation of Prx-2 was greater in RBC expressing human Hb in which the β93Cys residue was replaced with Ala (57), suggesting a role for this conserved residue in erythrocyte redox homeostasis mechanisms. We, therefore, compared storage-dependent changes in Prx-2 oxidation and reduction in murine RBC expressing wild-type human Hb (β93Cys) or human Hb in which an Ala residue has been substituted in the β93 position (β93Ala). Our previous studies have confirmed the loss of Hb β93 thiol in β93Ala RBC without any compensatory changes in other Hb subunits or RBC thiols (30, 39, 57). RBCs were leukoreduced and stored for 1 or 10 days (a time frame that mimics storage of human RBCs for approximately 42 days) (7, 23, 42), and H2O2-dependent Prx-2 oxidation was measured. Figure 4A shows that there was no difference in basal Prx-2 oxidation between β93Cys and β93Ala RBCs consistent with our earlier study (57). H2O2 oxidized Prx-2 with kinetics similar to that observed in human RBCs. Maximal levels of oxidized Prx-2 were observed at 5 min followed by a time-dependent reduction to the monomer, which was linear over 20 min. The change in maximal increase in Prx-2 oxidation (i.e., 5 min Prx-2 dimer fraction minus basal) was greater for 10 days compared with 1 day for both RBC.
FIG. 4.
Effect of storage time and β93Cys on Prx-2 recovery after H2O2 exposure. RBCs from transgenic mice expressing human Hb with β93Cys or β93Ala were leukoreduced and stored as described in Materials and Methods section. After 1 or 10 days of storage, H2O2 (10 μM) was added and time-dependent (0–30 min) changes in Prx-2 oxidation were determined. (A) Prx-2 oxidation expressed as the fraction of the Prx-2 dimer relative to total Prx-2. Data are mean±SEM (n=4). *p<0.01 #p<0.05 compared with respective basal (time 0) by one-way RM-ANOVA with Tukey's post hoc test. **p<0.05 by t-test for comparison of maximal levels of Prx-2 oxidation at day 1 versus 10. (B) Time-dependent changes in Prx-2 relative to the maximal amount formed (at 5 min) with lines showing fits by linear regression. *p<0.01 between day 1 β93Cys and day 10 β93Cys RBC, #p<0.05 between day 1 β93Cys and day 1 β93Ala. (C) H2O2 concentrations (normalized to initial) after addition to β93Cys or β93Ala RBC stored for either 1 or 10 days. *p<0.05 relative to β93Ala RBC stored for 1 day by two-way RM-ANOVA with Bonferroni post hoc test (n=3–5). Hb, hemoglobin.
Figure 4B plots the time-dependent reduction of dimeric Prx-2 normalized to maximum (5 min). Linear regression analysis showed that reduction was linear over 20 min, and Prx-2 reduction was significantly slower in 10 days RBC compared with the respective 1 day RBC (by ∼10-fold) for β93Cys RBC. However, no storage-dependent effect on Prx-2 reduction rates were observed with β93Ala RBC. Notably, Prx-2 reduction was significantly slower (approximately twofold) in β93Ala RBC compared with β93Cys stored for only 1 day. Figure 4C shows ∼90% of added H2O2 was rapidly consumed (<2 min) by all RBC groups. However, a slight but significant increase in steady-state H2O2 levels after the initial consumption was observed with β93Ala RBC stored for 10 days compared with β93Ala RBC stored for 1 day; no differences between β93Cys RBC stored for 1 or 10 days were observed.
Carbon monoxide accelerates Prx-2 recycling
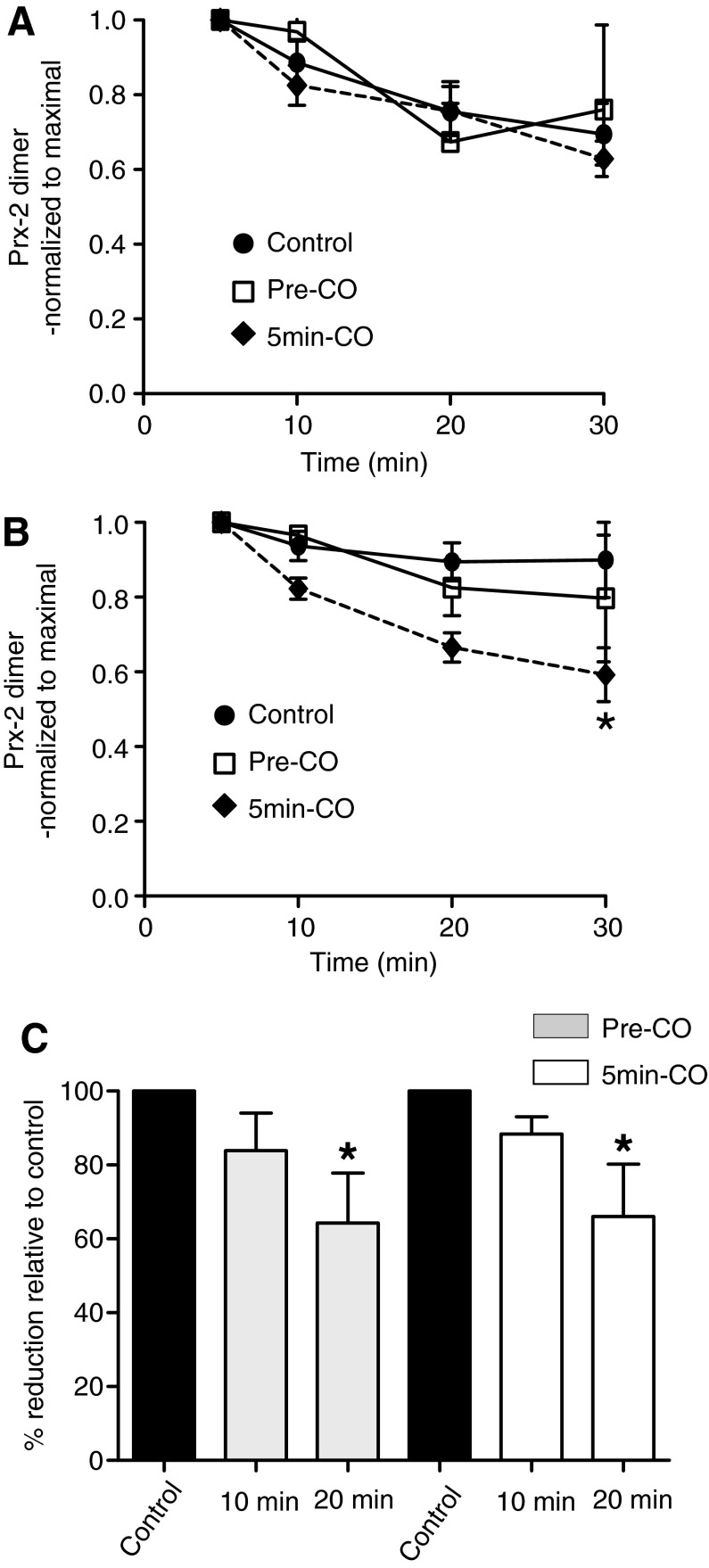
In the next series of experiments, the effects of carbon monoxide (CO) added before H2O2 or 5 min after H2O2 (i.e., after attaining maximum Prx-2 oxidation) on Prx-2 oxidation and reduction kinetics were tested. No differences in H2O2-dependent oxidation of Prx-2 with or without CO treatment in either cell were observed (not shown). Figure 5A and B show the effects of CO on the rate of Prx-2 reduction (after initial oxidation) in β93Cys and β93Ala RBC, respectively. Presaturation of RBC with CO affected neither initial H2O2-dependent Prx-2 oxidation nor Prx-2 reduction in either RBC, although a trend toward decreased Prx-2 oxidation over 5 min was noted in CO-treated cells (p=0.08 between with and without CO groups for β93Cys RBC). Notably, however, when administered 5 min post H2O2, CO significantly increased Prx-2 reduction rates in β93Ala RBC (Fig. 5B), with no effect of CO observed in β93Cys RBC (Fig. 5A). Figure 5C shows that pretreatment of human RBC stored for 35 days with CO, or CO-added 5 min after H2O2, significantly accelerated Prx-2 reduction from the dimer to monomer (by ∼35% relative to control at 20 min).
FIG. 5.
Effects of CO on Prx-2 recycling in β93Cys and β93Ala RBC. RBCs (stored for 1 day) were untreated, saturated with CO before, or 5 min after addition of H2O2 (10 μM), and time-dependent (0–30 min) changes in Prx-2 oxidation were determined as described in Materials and Methods section. (A, B) Time-dependent changes in Prx-2 relative to the maximal amount formed (at 5 min) for β93Cys and β93Ala RBC, respectively. Data are mean±SEM (n=3). *p<0.05 by two-way RM-ANOVA with Bonferroni post-test. (C) Effects of CO on H2O2-dependent Prx-2 oxidation relative to without CO in human RBC stored for 35 days. *p<0.05 by one-way RM-ANOVA relative to control. Data are mean±SEM, n=4. CO, carbon monoxide.
Oxidation of β93Cys during storage
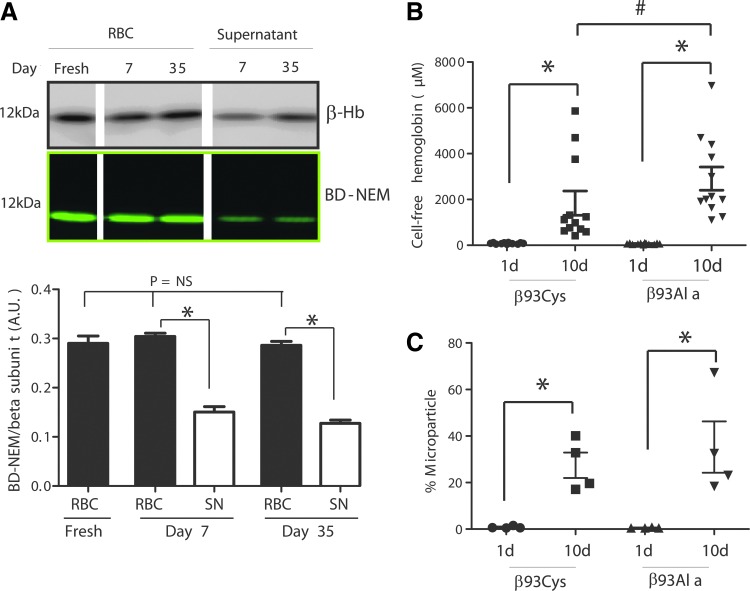
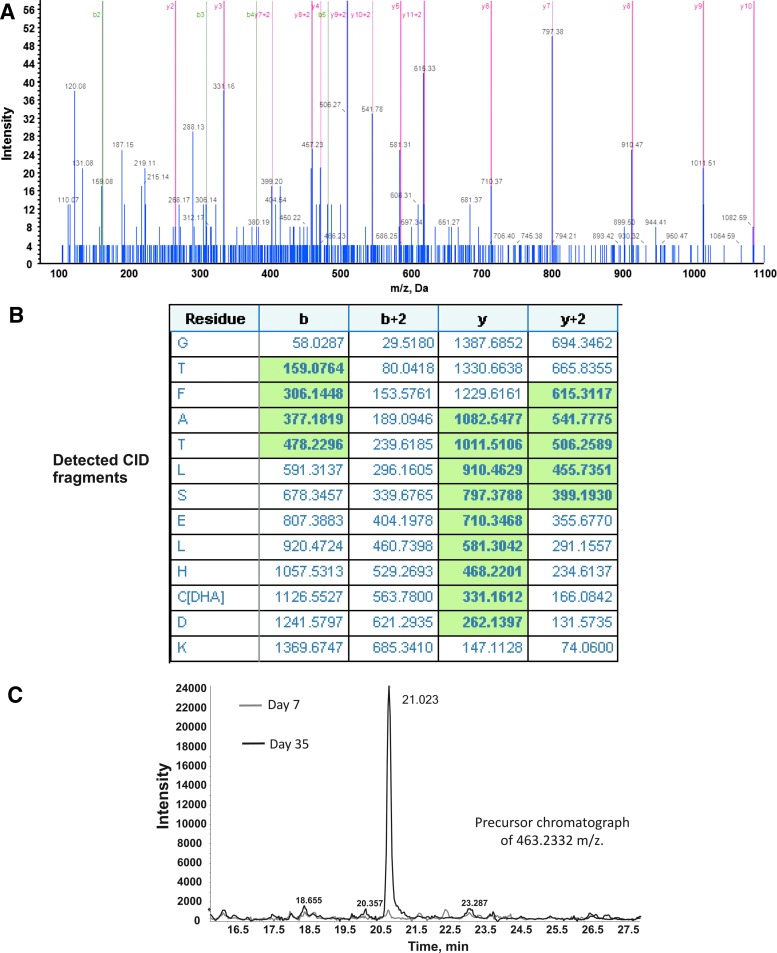
RBC storage is associated with oxidative stress characterized in part by glutathione and protein thiol oxidation (16, 20, 33). Whether the β93Cys residue is a target for oxidation during storage is not known. To evaluate this possibility, BODIPY N-ethylmaleimide (BD-NEM) labeling experiments were performed. Freshly isolated RBCs or cells stored for 7 or 35 days were collected and separated from the supernatant (SN) by centrifugation. Pelleted RBCs were then hemolysed by hypotonic lysis. BD-NEM was then added to the initial SN and hypotonically lysed RBC to label available cysteine residues. Each fraction was resolved by Western blotting, and degree of BD-NEM binding was determined and normalized to the Hb β-chain. Figure 6 shows that storage did not affect the relative binding of BD-NEM to erythrocytic Hb. However, BD-NEM binding to cell-free Hb arising from hemolysis during storage (i.e., SN fraction), was significantly decreased by ∼50–60% compared with Hb present inside the RBC. It should be noted that hemolysis was higher at 35 days indicated by higher levels of β-chain in day 35 SN. However, the relative loss of β93Cys reactivity to BD-NEM in SN Hb was similar between samples collected from RBCs stored for 7 or 35 days (Fig. 6A). To assess whether the β93Cys modulated storage-dependent RBC damage, hemolysis and formation of microparticles was compared in β93Cys and β93Ala RBC. Figure 6B shows that hemolysis increased after storage of either RBC, but the degree of hemolysis was significantly higher with β93Ala RBC, suggesting that this cysteine protects the RBC during storage. Figure 6C shows that storage also resulted in increased microparticle levels; however, no difference between the magnitude of this increase between β93Cys and β93Ala RBC was observed. Finally, to assess the fate of β93C on SN Hb LC-MS analyses of trypsin digested peptides were performed. Surprisingly, no evidence for formation of disulfides, sulfinic or sulfonic acids was observed. Figure 7 shows that, in fact, the β93Cys was oxidized to dehydroalanine (DHA) in 35 days SN Hb compared with 7 days SN Hb. No evidence of other thiol modifications was observed.
FIG. 6.
Role of β93Cys in RBC degradation during storage. (A) RBCs were collected from healthy volunteers, (designated fresh) or after 7 or 35 days of storage, and β93Cys levels were measured by BD-NEM labeling. For stored RBCs, BD-NEM labeling to Hb in cell-free fraction and inside RBCs is shown; no cell-free Hb was present in freshly prepared RBCs. Representative Western blot images show signals for β-chain Hb and matched BD-NEM labeling. Shown images are from the same gel (albeit not from adjacent lanes as indicated). Quantitation shows BD-NEM binding normalized to β-chain Hb and mean±SEM (n=3–6). *p<0.0001 by unpaired t-test. NS, not significant by one-way ANOVA. (B) Hemolysis was measured after storage of β93Cys or β93Ala RBC for 1 or 10 days. *p<0.001 by Wilcoxon-matched paired t-test, #p<0.03 by the Mann–Whitney t-test. (C) Microparticles were measured after storage of β93Cys or β93Ala RBC for 1 or 10 days. *p<0.05 by paired t-test. No difference in the magnitude of storage-dependent increases in microparticles between either RBC was observed. BD-NEM, BODIPY N-ethylmaleimide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 7.
Formation of DHA at the β93 position of extracellular Hb during storage. Extracellular Hb was collected by centrifugation from human RBC stored for 7 or 35 days, and fate of the β93Cys was determined by tandem mass spectrometry. Shown is a representative mass spectrum (A), b and y ions from detected trypsin fragments; highlighted values indicate ions detected and non-highlighted values are predicted ions (B) and chromatogram depicting relative intensities of DHA in day 7 versus 35 sample (C). DHA, dehydroalanine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Discussion
Numerous biochemical and morphologic changes occur in RBCs during cold storage, resulting in cells that contribute to transfusion-related toxicity. Increased oxidative stress is appreciated to play a key role in storage-dependent changes to the RBC. With regard to Prx-2, previous studies have shown that during storage, oligomeric complexes between Prx-2 and other RBC proteins are formed, and that there is an increased association between Prx-2 dimers and the RBC membrane. This is consistent with increased Prx-2 oxidation and a role for this enzyme in protecting RBC membrane constituents from storage-dependent oxidative stress (49, 50). Whether Prx-2 activity and specifically reduction after an initial oxidation is affected during storage is not clear. This is an important consideration, as the reduction step is rate limiting in the Prx-2 reaction cycle in RBCs. We show here that RBC storage results in increased basal Prx-2 oxidation, which did not affect the initial oxidation of Prx-2 mediated by exogenously added H2O2. However, oxidized (dimeric) Prx-2 is readily reduced back to the monomeric form in younger RBC, whereas this reduction process is significantly slowed in older RBC.
The potential significance of this finding is underscored by the fact that Prx-2 is considered the primary antioxidant system to negate H2O2-mediated oxidative damage in the RBC (41). In the model proposed, Prx-2 oxidation is rapid, but its reduction is slow (relative to Prx-2 reduction in other cell types). However, since Prx-2 is the third most abundant protein in the RBC, an efficient antioxidant function is evident. With RBC storage, reduction of oxidized Prx-2 is slowed even further, which could account for the progressive accumulation of oxidized Prx-2 in RBC during storage observed here, and potentially other oxidation epitopes reported by others (33). Moreover, RBCs with compromised Prx-2 recycling are less likely able to deal with oxidative stress encountered post-transfusion, indicated by increased H2O2-mediated hemolysis (Fig. 1D). In turn, this may be compounded by the fact that patients receiving transfusions are typically under heightened oxidative stresses (due to inflammatory cell activation, for example). We propose that attenuated Prx-2 activity is one mechanism that results in a more pro-oxidative environment in the vascular compartment and provides a second hit that underlies the pathology associated with transfusion of older RBCs.
Current FDA guidelines stipulate that storage procedures should result in RBCs that have ≥75% survival 24 h post-transfusion. Previous studies have noted variability in post-transfusion survival between distinct RBC units, leading to the concept that some donors are “good” storers versus some are “poor” storers (17). This has led to recent interest integrating current understanding of mechanisms of storage-related changes, with identifying donor-specific factors affecting the cells. Oxidative stress-dependent markers have been discussed in this context, and accumulation of Prx-2 in the RBC membrane has been proposed as one possible biomarker (20, 50). Our data (Fig. 1A) also show variance in basal Prx-2 oxidation status, which is amplified as units are stored for longer times. We speculate that assessment of basal Prx-2 oxidation and/or reduction kinetics after H2O2 challenge may provide an additional marker/activity to identify which RBCs are better candidates for storage and transfusion. Future studies will need to determine whether RBCs with higher basal Prx-2 oxidation correlate with poorer post-transfusion recovery and/or increased toxicity.
The mechanism through which Prx-2 reduction was slower in older RBC remains unclear. H2O2 was consumed equally rapidly (<5 min) after addition to day 7 or 35 RBCs, consistent with previous reports using freshly isolated cells (40), excluding variable H2O2 levels as mediating the observed differences in Prx-2 recycling kinetics. Surprisingly, no differences in Trx activity or Trx levels were observed. In addition, NADPH concentrations, which provide reducing equivalents to the Trx repair system, were not different between young and old RBCs, consistent with earlier reports that showed increases in NADPH during storage in saline, adenine, glucose, mannitol (SAGM) solutions (14). Interestingly, glucose supplementation increased the rate of Prx-2 recycling, presumably by increasing flux through NADPH formation pathways. However, significant differences between older and younger RBCs remained, suggesting that other mechanisms for Prx-2 recycling, beyond Trx reductase, are present in the RBC.
Our recent study indicated that the conserved Hb cysteine residue (β93Cys) is also a modulator of reactions between erythrocytes and H2O2 (57). Specifically, the reaction between oxyferrous heme (of oxyHb) and H2O2 was approximately twofold faster in RBCs lacking the β93Cys, which resulted in a greater degree of H2O2-mediated Prx-2 oxidation in β93Ala RBCs (57). Data presented here suggest that the β93Cys is also involved in Prx-2 reduction. In RBCs stored for 1 day, rates of Prx-2 reduction were approximately twofold slower in RBC lacking the β93Cys residue. Interestingly, Prx-2 reduction rates did not change on storage of β93Ala RBCs, but decreased in β93Cys RBCs to levels similar to those observed in β93Ala RBCs. In other words, RBC storage led to a loss of β93Cys-dependent control over Prx-2 reduction. With regard to the mechanism underlying this result, one consideration is the different reaction kinetics between β93Cys and β93Ala and H2O2. β93Ala Hb reacts faster, and thus the H2O2 concentration available to oxidize Prx-2 is predicted to be lower with β93Ala RBC, which should, in turn, be observed as faster reduction of Prx-2 dimer to monomer. However, the opposite was observed. Another possibility is that secondary generation of peroxides (H2O2 or lipid peroxides) derived from Hb redox cycling reactions will be greater with β93Ala; in this case, continued Prx-2 oxidation would be observed as slower rates of Prx-2 reduction, consistent with the observed data. To test this, we treated RBC with CO to ligate and prevented ferrous heme redox cycling and autoxidation. A recent study suggested that blocking ferrous heme prevented Prx-2 hyperoxidation induced by H2O2 generated by Hb autoxidation (12). While pre-CO-treatment had no effect on added H2O2-dependent Prx-2 oxidation or reduction, CO added 5 min after H2O2, a time point, when all added H2O2 has been consumed and Prx-2 oxidation is maximal, significantly accelerated Prx-2 reduction, but only in β93Ala RBC. This suggests that higher autoxidation rates of Hb lacking theβ93Cys, and/or inability of this Hb to react with superoxide/H2O2 generated from autoxidation, results in increased intracellular H2O2 that reacts with Prx-2, maintaining a higher steady state concentration of oxidized Prx-2. Blocking ferrous heme with CO prevents the secondary and endogenous generation of H2O2, resulting in the observed time-dependent reduction of Prx-2. These data also suggest that autoxidation of Hb lacking the β93Cys is stimulated by an initial challenge with H2O2, a possibility currently under investigation. CO also accelerated Prx-2 reduction in stored human RBC, suggesting increased autoxidation-derived H2O2 also sustains higher Prx-2 peroxidation levels in this setting. We also note that there remains a final possibility for explaining slower Prx-2 reduction in β93Ala RBC, such as direct protein–protein interactions between Hb and Prx-2. This premise has been suggested by reports of oligomeric Prx-2 binding to and stabilizing Hb, thereby preventing hemichrome formation (26).
Irrespective of the mechanism, the proposed role of the β93Cys residue in Prx-2 recycling prompted us to evaluate whether this residue is oxidized during RBC storage. No change in β93Cys oxidation on intraerythrocytic Hb was observed in stored RBC (Fig. 6). However, a significant decrease in β93Cys available for BD-NEM labeling for extracellular (hemolysed) Hb was noted. This result suggests two scenarios. First is that the β93Cys is oxidized only after hemolysis. However, the fact that the extent of β93Cys loss in cell-free Hb did not increase with storage time (∼50–60% oxidation was observed with day 7 and 35 samples), despite the fact that cell-free Hb increases with storage (due to ongoing hemolysis), suggests that little further oxidation of the β93Cys on extracellular Hb occurs during storage. The second possibility is that RBCs, in which significant β93Cys oxidation occurs, are more prone to hemolysis under storage conditions. This is further supported by increased storage-dependent hemolysis of β93Ala RBC compared with β93Cys RBC (Fig. 6B). While the precise mechanisms leading to hemolysis during RBC storage are not known, our data suggest that the β93Cys plays a role. We note that no differences in microparticle formation were observed, suggesting a selective effect of the β93Cys for controlling hemolysis. LC-MS analysis indicated that the β93Cys on extracellular Hb was oxidized to DHA. Formation of DHA on selenocysteine of glutathione peroxidase in RBC treated with H2O2 has been documented, suggesting an oxidative stress mechanism for DHA formation (11). Consistent with this finding, we also observed significant increases in carboxyethyllysine-modification, which is a product of increased oxidative stress in the presence of glucose (not shown). Several mechanisms for DHA formation from cysteine in model peptides have been discussed, albeit under relatively extreme conditions of heat, pH and involve β-elimination of HS• from Cys-disulfide radicals (10, 35, 44). That said, DHA has been detected on albumin in vivo (5). How formation of DHA occurs on the β93Cys during RBC storage remains to be elucidated. Of additional interest is the fact that DHA is electrophilic; in other words, a transition from a nucleophilic to electrophilic center occurs at the β93 site on the released Hb during storage. Whether this significantly alters the reactivity of Hb, and specifically alters its propensity to form adducts with nucleophiles after transfusion is an intriguing possibility that may have effects on transfusion toxicity. This possibility is under investigation.
Finally, we note that most of our studies were performed with human RBCs collected from segments of donor RBC units. Emerging data indicate that biomarkers for storage-dependent RBC damage (including hemolysis and complement fragments) can differ in magnitude between segments and matched bags. For example, hemolysis is higher in segments (37), but complement fragments are higher inside the bags (29), suggesting that currently no generalizable trend can be concluded. That said, we acknowledge this limitation in our study design, and note the need for future studies evaluating kinetics of Prx-2 oxidation in RBCs collected from the actual donor units rather than attached segments. An additional limitation includes using mouse RBCs stored in the presence of a gas head-space, which is not the case for human RBC storage, and which may result in an elevated oxidative stress. That said, we note that Prx-2 recycling differences were still observed between β93Cys and β93Ala RBC stored for only 1 day. A final consideration is that we only investigated Prx-2 in the cytosol. It is evident that membranes and their constituents play important roles in the RBC redox state. Recent studies, for example, demonstrate an active NADPH oxidase in RBC membranes that modulate RBC aging in sickle cell disease (21). In addition, oligomeric Prx-2 accumulates in the membrane during storage (50, 51). Whether membrane constituents affect slower Prx-2 recycling in stored RBC and the role of the β93Cys residue in this process remain to be determined.
Conclusions
In summary, we demonstrate that Prx-2 activity is compromised during storage. Given the critical role for this RBC protein in protecting against endogenous and exogenous H2O2-induced oxidative damage, we propose that loss of Prx-2 activity plays a role in storage-dependent damage to the RBC, and to post-transfusion redox imbalance and vascular oxidative stress in patients receiving older RBC units. Evidence is also provided to suggest that the Hb β93Cys residue maintains Prx-2 recycling, and we propose that its oxidation leads to storage-dependent hemolysis.
Materials and Methods
Materials
NEM was purchased from Pierce. Duo-Lux chemiluminescent/fluorescent substrate was purchased from Vector Laboratories, Inc. Sephadex G25 (fine) was purchased from GE Healthcare Life Science. Antibodies against peroxiredoxin, Trx, and Hb β-subunit were purchased from Abcam. Goat anti-rabbit IgG antibody conjugated to alkaline phosphatase was purchased from Millipore. BD-NEM (B-10250) was from Invitrogen. Nitrocellulose (162-0112, 0.2 μm pore size) and micro Bio-Spin 6 columns were purchased from Bio-Rad. All other materials were purchased from Sigma-Aldrich.
Animals
Hb β93Cys-positive (β93Cys) and -negative (β93Ala) mice were generated and maintained as previously described (30). Age-matched males between 12 and 18 weeks of age that do not contain detectable Hb gamma chain and display less Hb-reduced thiol content were used (30, 39, 57). Mice were anesthetized with isoflurane (5%) and maintained at 2–3% with lack of responsiveness assessed by loss of toe-pinch reflex. Blood was collected by cardiac puncture with 50 μl (trisodium citrate [22.0 g/L], citric acid [8.0 g/L], and dextrose [24.5 g/L]). As in earlier studies (30, 39, 57), no differences in reticulocyte counts were observed between β93Ala and β93Cys mice (10.9%±2.6% and 8.6%±2.5% [mean±SEM, n=4]). Leukoreduction was performed under sterile conditions by filtering whole blood through a Sephadex G25:Microcellulose column (1:3 w/w). Columns were then washed with 10 times the volume of phosphate-buffered saline (PBS). Preliminary studies demonstrated >99% loss of leukocytes by FACS analyses (not shown). Collected RBCs were centrifuged at 1500 g for 5 min at 4°C, washed thrice with cold Adsol, and concentrated to a hematocrit of 60% with Adsol in sterile Eppendorf tubes. RBCs (0.4 ml total volume at 60% Hct) were stored at 4°C for 1 or 10 days in 0.7 ml Eppendorf tubes. All experiments involving animals were conducted according to protocols approved by the University of Alabama at Birmingham (UAB) IACUC.
Human RBC collection and storage
Leukoreduced pRBCs of different storage ages (7–10, 14, 28, 35, and 42 days) were collected from segments (∼0.7 ml) attached to units from the UAB blood bank. Only pRBCs stored in Adsol were used for these studies. On the day of experiments, RBC were collected from segments, washed thrice with ice-cold isotonic phosphate buffer (80 mM NaPi, 40 mM NaCl, 10 mM KCl, and 100 μM DTPA), and centrifuged at 4°C (1500 g for 10 min per wash and the SN discarded). An RBC aliquot was taken and hemolysed in water (1:10). Hb concentration was determined by visible spectroscopy (450–700 nm) and measurement of oxyHb using the extinction coefficient 14.6 mM−1 cm−1 for absorbance at 577 nm. In all cases, oxyHb represented >99% of the total Hb as assessed by spectral deconvolution as described (54). All protocols were approved by the UAB Institutional Review Board.
CO-treatment or RBC
Where indicated, the effects of CO on Prx-2 oxidation kinetics were tested by gently bubbling CO gas (20 ml) through RBC (50 μM final heme, 5 ml) in isotonic phosphate buffer (as described next for Prx-2 oxidation) either immediately before addition of H2O2 or 5 min after H2O2 addition. Two microliters of pRBC were collected immediately after bubbling CO gas and at the end of the experiment, hemolysed in water (1:10) and the concentration of COHb was determined by visible spectroscopy. In every experiment, >95% of the Hb remained in the CO-bound ferrous (COHb) state (not shown). Prx-2 oxidation and hemolysis were determined as described next.
Prx-2 oxidation
RBCs were diluted to 50 μM heme using isotonic phosphate buffer (80 mM NaPi, 40 mM NaCl, 10 mM KCl, and 100 μM DTPA) and treated with H2O2 (10 μM) for approximately 60 min at 37°C. RBCs were mixed by inversion every 5–10 min to limit settling. Prx-2 oxidation was measured as previously described (57). Briefly, at each indicated time, RBCs were treated with 100 mM NEM for 20 min at 37°C to prevent further Prx-2 oxidation. RBC were then pelleted (2000 g, 30 s) and lysed in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer without β-mercaptoethanol (BME). Lysates were resolved by SDS-PAGE and transferred onto nitrocellulose. Membranes were blocked with 1–5% nonfat dried skim milk powder (w/v) in TBS-T (137 mM NaCl, 2.6 mM KCl, and 0.05% Tween-20 [v/v] in 25 mM Tris-HCl pH 7.4) for 1 h at room temperature and then washed with TBS-T for 30 min. Membranes were incubated with rabbit poly or monoclonal antibody against Prx-2 followed by secondary alkaline phosphatase-linked goat anti-rabbit IgG antibody (AP132A; Millipore). Blots were developed with Duo-Lux chemiluminescent/fluorescent substrate (Vector Laboratories, Inc.) and exposed to film. Prx-2 oxidation was assessed by the amount of dimeric Prx-2 (MWt ∼38 kDa) divided by the total Prx-2 (dimeric+monomeric [19 kDa] forms).
H2O2 measurement
RBCs (50 μM heme) were incubated with H2O2 (10 μM) in PBS containing 10 μM DTPA, at 37°C. Five hundred microliters aliquots were collected at indicated times, and RBC was rapidly pelleted (2000 g for 15 s). Four hundred microliters of the SN were collected, and H2O2 levels were determined using the FOX assay as described (40) with modifications to increase sensitivity. Specifically, 400 μl SN was added to 100 μl FOX reagent (excluding BHT) (9 ml methanol with 1.5 mM ferrous sulfate and 0.6 mM xylenol orange in 1 ml 125 mM sulfuric acid) for 30 min at room temperature in the dark, and absorbance at 560 nm was measured. H2O2 concentrations were calculated using ɛ560nm=43,970 M−1 cm−1. All experiments included buffer-alone blanks and H2O2-alone group (with no RBC added).
Trx reductase activity
Trx reductase activity was measured as previously described (40). Briefly, RBCs were washed with isotonic phosphate buffer and counted using a hemocytometer. 5×107 RBCs were treated with 60 μM 2,4-dinitrochlorobenzne (DNCB) or dimethyl sulfoxide vehicle alone for 30 min. Cells were pelleted, lysed in 5 mM phosphate buffer, pH 7.4+5 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and incubated at 37°C for 1 min. NADPH (200 μM final concentration) was then added and incubated for 5 min at 37°C. Lysates were then applied to Micro Bio-Spin 6 size-exclusion column (Bio Rad, Inc.), and eluted with 5 mM phosphate buffer, pH 7.4. The absorbance at 412 nm of the eluate was measured using a DU 800 Spectrophotometer (Beckman Coulter). Fold changes in activity were determined by a comparison of DNCB (Trx reductase inhibitor)-treated samples with vehicle control.
Trx measurement
RBCs were lysed in RIPA buffer (0.5% Na-deoxycholate [w/v], 0.1% SDS [w/v], 150 mM NaCl, EDTA 0.5–1 mM, and 1% NP-40 [v/v] in 50 mM Tris-HCl, pH 7.4) and 15 μg protein mixed 1:1 (by volume) with 2×reducing sample buffer (2% BME [v/v], 4% SDS [w/v], 10% glycerol [v/v], and 0.2% [v/v] bromophenol blue in 100 mM Tris HCl, pH 6.8) and resolved by Western blotting (SDS-PAGE) on an 18% gel. After transfer, membranes were blocked with 5% nonfat dried skim milk powder (w/v) in TBS-T (137 mM NaCl, 2.6 mM KCl, and 0.05% Tween-20 [v/v] in 25 mM Tris-HCl pH 7.4) for 1 h at room temperature and washed with TBS-T for 30 min. Membranes were incubated with rabbit polyclonal antibody against Trx (ab26320; Abcam) diluted 1:1000 in 5% nonfat dried skim milk (w/v) in TBS-T overnight at 4°C followed by secondary alkaline phosphatase-linked goat anti-rabbit IgG (AP132A; Millipore) diluted 1:1000 in 5% nonfat dried skimmed milk (w/v) in TBS-T at room temperature for 2 h. Membranes were then developed by chemiluminescence using Duo-Lux chemiluminescent/fluorescent substrate (Vector Laboratories, Inc.), and sequential images by AlphaEaseFC™ (Protein Simple, Inc.) were taken with quantification that was only performed on bands which had not reached saturation.
NADP(H) measurement
NADPH was measured as previously described (59). Twenty microliters pRBC were diluted in 1.98 ml extraction buffer (20 mM nicotinamide, 20 mM NaHCO3, and 100 mM Na2CO3), then subjected to freeze-thaw lysis, and centrifuged briefly to pellet cellular debris. Extracts were maintained on ice and shielded from light. An aliquot of each extract was heated for 30 min at 60°C. Fifty microliters of extract was mixed with 480 μl of cycling buffer (100 mM Tris-HCl pH 8.0, 0.5 mM MTT, 2 mM PES, 5 mM EDTA, and 1.3 U/ml G6P dehydrogenase) in quartz cuvettes and incubated at 37°C for 2 min. Fifty microliters of 10 mM glucose-6-phosphate was added, and the sample was mixed. The absorbance at 570 nm was measured at 20 s intervals over 5 min, and concentrations were calculated by a comparison to calculated rates from NADPH standards.
Measurement of the β93Cys using BD-NEM
Available thiol of the β93Cys residue was measured on Hb present in the RBC and Hb present after storage-dependent hemolysis. RBCs were centrifuged at 1500 g for 10 min at 4°C to separate the hemolyzed SN fraction from the intact RBCs. Intact RBCs were then lysed 1:5 in ice-cold distilled water containing DTPA (100 μM), that is, the intraerythrocytic fraction. To measure the reduced thiols, BD-NEM (500 μM) was added to both fractions (100 μM heme final) in PBS+DTPA (100 μM) and in the dark for 15 min at room temperature (20 mM BD-NEM stocks were dissolved in dimethylformide, and concentration was determined using ɛ504nm=76,000M−1cm−1). The reaction was terminated by addition of 5 mM L-Cysteine. Proteins were then resolved by SDS-PAGE on 10–18% gradient gels (under limited light exposure conditions and without bromophenol blue). After electrophoresis, BD-NEM-labeled proteins in the glass plates were developed on a Typhoon Imager (520BP 40, emission filter, blue laser [488 nm]). Proteins on the gel were transferred onto nitrocellulose membranes for 3 h at 80V in a wet transfer system. Membranes were blocked with 5% nonfat dried skimmed milk powder (w/v) in TBS-T (137 mM NaCl, 2.6 mM KCl, and 0.05% Tween-20 [v/v] in 25 mM Tris-HCl pH 7.4) for 1 h at room temperature and then washed with TBS-T for 30 min, followed by incubation with mouse monoclonal antibody against Hb β-subunit (ab55081; Abcam) diluted 1:1000 in 5% nonfat dried skim milk (w/v) in TBS-T (overnight, 4°C) followed by rabbit anti-mouse IgG alkaline phosphatase-conjugate (ab6729; Abcam) diluted 1:1000 in 5% nonfat dried skim milk (w/v) in TBS-T for 2 h. After washing with TBS-T for 30 min, membranes were developed by chemiluminescence using Duo-Lux chemiluminescent/fluorescent substrate (Vector Laboratories, Inc.), and sequential images by AlphaEaseFC (Protein Simple, Inc.) were taken with quantification that was only performed on bands which had not reached saturation. BD-NEM staining was normalized to anti-β-hemogloin antibody binding.
Assessment of β93C oxidation by MS analysis
In-gel digestion
SN fractions of RBC stored for 7 or 35 days were collected in urea (10 mM) and NEM (100 mM) dissolved in water. After 30 min incubation at room temperature, proteins were resolved by nonreducing SDS gel electrophoresis. Gels were stained with Coommassie blue to visualize protein bands. Bands corresponding to Hb were excised, and excess stains were removed by an overnight wash of 50% 100 mM ammonium bicarbonate/50% acetonitrile. After destaining, disulfide bonds were reduced by 25 mM dithiothreitol at 50°C for 30 min. Alkylation of any remaining free thiols groups was carried out with 55 mM iodoacetamide for 30 min in the dark. The excess alkylating agent was removed, and the gel pieces were washed twice with a 100 mM ammonium bicarbonate for 30 min. The gel pieces were evaporated to dryness in a SpeedVac (Savant) before the addition of trypsin, which was added at 12.5 ng/μl (Promega Gold Mass Spectrometry Grade), and incubated overnight at 37°C. Peptides were extracted from the gel pieces using a 1:1 mixture of 1% formic acid and acetonitrile twice for 15 min. Extracts were pooled and evaporated to dryness. The samples were then resuspended in 30 μl of 0.1% formic acid before mass spectrometry analysis.
Nano cHiPLC-tandem mass spectrometry
An aliquot (5 μl) of each digest was loaded onto a Nano cHiPLC 200 μm×0.5 mm ChromXP C18-CL 3 μm 120Å reverse-phase trap cartridge (Eksigent) at 2 μl/min using an Eksigent autosampler. After washing the cartridge for 4 min with 0.1% formic acid in ddH2O, the bound peptides were flushed onto a Nano cHiPLC column 200 μm×15 cm ChromXP C18-CL 3 μm 120Å (Eksigent) with a 45 min linear (5–50%) acetonitrile gradient in 0.1% formic acid at 1000 nl/min using an Eksigent Nano1D+LC. The column was washed with 90% acetonitrile-0.1% formic acid for 10 min and then re-equilibrated with 5% acetonitrile-0.1% formic acid for 10 min. The Applied Biosystems 5600 Triple-Tof mass spectrometer (AB-Sciex) was used to analyze the protein digest. The IonSpray voltage was 2300 V, and the declustering potential was 80 V. Ionspray and curtain gases were set at 10 and 25 psi, respectively. The interface heater temperature was 120°C.
Eluted peptides were subjected to a time-of-flight survey scan from 400 to 1250 m/z to determine the top 20 most intense ions for MSMS analysis. Product ion time-of-flight scans at 50 ms (over the range from m/z 100–1500) were carried out to obtain the tandem mass spectra of the selected parent ions. Spectra are centroided and de-isotoped by Analyst software, version TF (Applied Biosystems). A β-galactosidase trypsin digest was used to establish and confirm the mass accuracy of the mass spectrometer.
Protein Pilot 4.5 search queries
The tandem mass spectrometry data were processed to provide protein identifications using an in-house Protein Pilot 4.5 search engine (AB-Sciex) using the Homo sapiens Sprot protein database and using a trypsin digestion parameter. Possible modified peptides on the Hb-β subunit were evaluated by setting the software to analyze biological modifications with an emphasis on β93Cys modifications. De novo inspection of the data was then used to validate the potential modification sites.
Hemolysis measurement
At indicated times, RBCs were collected and pelleted by centrifugation (1500 g, 10 min, 5°C). Cell-free Hb in the SN was measured by visible spectroscopy (400–700 nm) and concentration and oxidation state were determined by deconvolution as previously described (54).
Microparticle measurement
Stored mouse RBCs were left unwashed (60% Hct) and incubated with anti-Ly76 antibody (TER-119)-FITC conjugated antibody (ab93587; Abcam at 0.1 μg/ml) for 60 min in PBS and in the dark at room temperature. Samples were then analyzed by flow cytometry using a Beckman Coulter 3500, and events were acquired using Beckman Coulter native software. Approximately 100,000 events were collected per measurement. All analyses were done with Kaluza software (Beckman Coulter, Inc.).
Statistical analysis
Storage time and H2O2 time-dependent changes in Prx-2 oxidation were analyzed by one-way or by two-way repeated measures (RM)-ANOVA. Rates of Prx-2 reduction were determined by linear regression as indicated. Changes in hemolysis were assessed by unpaired and paired t-test as appropriate. Data were also assessed for normal distribution by the Kolmogorov-Smirnov normality test or D'Agostino and Pearson normality test; if data were not normally distributed, Mann–Whitney (unpaired) or Wilcoxon (paired) t-tests were used. p-Values less than 0.05 were considered significant. All analyses used GraphPad Prism Software.
Abbreviations Used
- ANOVA
analysis of variance
- BD-NEM
BODIPY N-ethylmaleimide
- BME
β-mercaptoethanol
- CO
carbon monoxide
- DHA
dehydroalanine
- DNCB
2,4-dinitrochlorobenzene
- H2O2
hydrogen peroxide
- Hb
hemoglobin
- NO
nitric oxide
- PBS
phosphate-buffered saline
- pRBC
packed red blood cell
- Prx-2
peroxiredoxin-2
- RBCs
red blood cells
- RM-ANOVA
repeated measures-analysis of variance
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SN
supernatant
- Trx
thioredoxin
- UAB
University of Alabama at Birmingham
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL092624 and HL095468 to R.P.P.) and Cardiovascular T32 Pre-doctoral Training Grant to R.S. The AB Sciex 5600 Triple-Tof mass spectrometer used in this study was purchased with funds from a Shared Instrumentation grant from the National Center for Research Resources (S10 RR027822-01, S.B., Principal Investigator). The operation of the UAB Targeted Metabolomics and Proteomics Laboratory is additionally supported by federal grants P30 AR050948-10 Skin Disease Research Center; DK079337-06 O'Brien Acute Kidney Injury Center.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Al-Abed Y, VanPatten S, Li H, Lawson JA, FitzGerald GA, Manogue KR, and Bucala R. Characterization of a novel hemoglobin-glutathione adduct that is elevated in diabetic patients. Mol Med 7: 619–623, 2001 [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander JT, El-Ali AM, Newman JL, Karatela S, Predmore BL, Lefer DJ, Sutliff RL, and Roback JD. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion 53: 2619–2628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almac E. and Ince C. The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol 21: 195–208, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, and Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Or R, Rael LT, and Bar-Or D. Dehydroalanine derived from cysteine is a common post-translational modification in human serum albumin. Rapid Commun Mass Spectrom 22: 711–716, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bayer SB, Maghzal G, Stocker R, Hampton MB, and Winterbourn CC. Neutrophil-mediated oxidation of erythrocyte peroxiredoxin 2 as a potential marker of oxidative stress in inflammation. FASEB J 27: 3315–3322, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, Friend LA, Lentsch AB, and Pritts TA. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg 73: S128–S133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biffl WL, Moore EE, Offner PJ, Ciesla DJ, Gonzalez RJ, and Silliman CC. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: abrogation by poststorage washing but not prestorage leukoreduction. J Trauma 50: 426–431; discussion 432, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Blasi B, D'Alessandro A, Ramundo N, and Zolla L. Red blood cell storage and cell morphology. Transfusion Med 22: 90–96, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Chalker JM, Lercher L, Rose NR, Schofield CJ, and Davis BG. Conversion of cysteine into dehydroalanine enables access to synthetic histones bearing diverse post-translational modifications. Angew Chem Int Ed Engl 51: 1835–1839, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Cho C-S, Lee S, Lee GT, Woo HA, Choi E-J, and Rhee SG. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid Redox Signal 12: 1235–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho CS, Yoon HJ, Kim JY, Woo HA, and Rhee SG. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc Natl Acad Sci U S A 111: 12043–12048, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, and Patel RP. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood 104: 1375–1382, 2004 [DOI] [PubMed] [Google Scholar]
- 14.D'Alessandro A, D'Amici GM, Vaglio S, and Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica 97: 107–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, and Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124: 465–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumaswala UJ, Zhuo L, Mahajan S, Nair PN, Shertzer HG, Dibello P, and Jacobsen DW. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol 280: C867–C873, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Dumont LJ. and AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 48: 1053–1060, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Dumont LJ, Yoshida T, and AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion 49: 458–464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fibach E. and Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med 8: 609–619, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Francis RO, Jhang JS, Pham HP, Hod EA, Zimring JC, and Spitalnik SL. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang 105: 271–282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George A, Pushkaran S, Konstantinidis DG, Koochaki S, Malik P, Mohandas N, Zheng Y, Joiner CH, and Kalfa TA. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 121: 2099–2107, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gifford SC, Derganc J, Shevkoplyas SS, Yoshida T, and Bitensky MW. A detailed study of time-dependent changes in human red blood cells: from reticulocyte maturation to erythrocyte senescence. Br J Haematol 135: 395–404, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, and Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion 49: 1546–1553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giustarini D, Dalle-Donne I, Colombo R, Petralia S, Giampaoletti S, Milzani A, and Rossi R. Protein glutathionylation in erythrocytes. Clin Chem 49: 327–330, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion 50: 1164–1169, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Han YH, Kim SU, Kwon TH, Lee DS, Ha HL, Park DS, Woo EJ, Lee SH, Kim JM, Chae HB, Lee SY, Kim BY, Yoon do Y, Rhee SG, Fibach E, and Yu DY. Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem Biophys Res Commun 426: 427–432, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, and Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115: 4284–4292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holme S, Elfath MD, and Whitley P. Evaluation of in vivo and in vitro quality of apheresis-collected RBC stored for 42 days. Vox Sang 75: 212–217, 1998 [PubMed] [Google Scholar]
- 29.Hu X, Marques MB, Weinberg JA, Patel RP, and Barnum SR. The level of complement activation fragments is higher in red blood cell units than segments. Transfus Apher Sci 49: 692–693, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, and Townes TM. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med 14: 773–777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobasch G. and Rapoport SM. Hemolytic anemias due to erythrocyte enzyme deficiencies. Mol Aspects Med 17: 143–170, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Johnson RM, Ho YS, Yu DY, Kuypers FA, Ravindranath Y, and Goyette GW. The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free Radic Biol Med 48: 519–525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanias T. and Acker JP. Biopreservation of red blood cells—the struggle with hemoglobin oxidation. FEBS J 277: 343–356, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Kiefmann R, Rifkind JM, Nagababu E, and Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood 111: 5205–5214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Ha S, Lee HY, and Lee KJ. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom Rev 2014. [Epub ahead of print]; DOI 10.1002/mas.21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, and Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 358: 1229–1239, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kurach JD, Hansen AL, Turner TR, Jenkins C, and Acker JP. Segments from red blood cell units should not be used for quality testing. Transfusion 54: 451–455, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Lee TH, Kim SU, Yu SL, Kim SH, Park DS, Moon HB, Dho SH, Kwon KS, Kwon HJ, Han YH, Jeong S, Kang SW, Shin HS, Lee KK, Rhee SG, and Yu DY. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101: 5033–5038, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Sun CW, Honavar J, Townes T, and Patel RP. Role of the b93cys, ATP and adenosine in red cell dependent hypoxic vasorelaxation. Int J Physiol Pathophysiol Pharmacol 5: 21–31, 2013 [PMC free article] [PubMed] [Google Scholar]
- 40.Low FM, Hampton MB, Peskin AV, and Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 109: 2611–2617, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Low FM, Hampton MB, and Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal 10: 1621–1630, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, and Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood 113: 1158–1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohanty JG, Nagababu E, and Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 5: 84, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozziconacci O, Kerwin BA, and Schoneich C. Reversible hydrogen transfer reactions of cysteine thiyl radicals in peptides: the conversion of cysteine into dehydroalanine and alanine, and of alanine into dehydroalanine. J Phys Chem B 115: 12287–12305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagababu E, Mohanty JG, Friedman JS, and Rifkind JM. Role of peroxiredoxin-2 in protecting RBCs from hydrogen peroxide-induced oxidative stress. Free Radic Res 47: 164–171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, Szentmiklosi J, Mehes G, Csonka T, Smith A, Vercellotti GM, Balla G, and Balla J. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol 30: 1347–1353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman SF, Sultana R, Perluigi M, Coccia R, Cai J, Pierce WM, Klein JB, Turner DM, and Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer's disease inferior parietal lobule, a proteomics approach. J Neurosci Res 85: 1506–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, and Bar-Or D. The effect of storage on the accumulation of oxidative biomarkers in donated packed red blood cells. J Trauma 66: 76–81, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Rinalducci S, D'Amici GM, Blasi B, Vaglio S, Grazzini G, and Zolla L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 51: 1439–1449, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Rinalducci S, D'Amici GM, Blasi B, and Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie 93: 845–853, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Sampathkumar R, Balasubramanyam M, Sudarslal S, Rema M, Mohan V, and Balaram P. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem 38: 892–899, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus 8Suppl 3: S26–S30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinella PC, Sparrow RL, Hess JR, and Norris PJ. Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion 51: 894–900, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, Kerby JD, Barnum SR, Weinberg JA, Lancaster JR, Jr., and Patel RP. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J 446: 499–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takayama F, Tsutsui S, Horie M, Shimokata K, and Niwa T. Glutathionyl hemoglobin in uremic patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis. Kidney Int Suppl 78: S155–S158, 2001 [DOI] [PubMed] [Google Scholar]
- 56.van de Watering L. Red cell storage and prognosis. Vox Sang 100: 36–45, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Vitturi DA, Sun CW, Harper VM, Thrash-Williams B, Cantu-Medellin N, Chacko BK, Peng N, Dai Y, Wyss JM, Townes T, and Patel RP. Antioxidant functions for the hemoglobin beta93 cysteine residue in erythrocytes and in the vascular compartment in vivo. Free Radic Biol Med 55: 119–129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vlaar AP, Straat M, and Juffermans NP. The relation between aged blood products and onset of transfusion-related acute lung injury. A review of pre-clinical data. Clin Lab 57: 267–272, 2011 [PubMed] [Google Scholar]
- 59.Wagner TC. and Scott MD. Single extraction method for the spectrophotometric quantification of oxidized and reduced pyridine nucleotides in erythrocytes. Anal Biochem 222: 417–426, 1994 [DOI] [PubMed] [Google Scholar]
- 60.Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, and Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood 79: 1351–1358, 1992 [PubMed] [Google Scholar]
- 61.Weinberg JA, Barnum SR, and Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion 51: 867–873, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida T. and Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus 8: 220–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, and Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg 178: 570–572, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, and McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med 39: 2478–2486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]