Abstract
Objective: The purpose of this study was to determine the clinical benefits of intense-pulsed-light therapy for the treatment of dry-eye disease caused by meibomian gland dysfunction (MGD). Background data: MGD is the leading cause of evaporative dry eye disease. It is currently treated with a range of methods that have been shown to be only somewhat effective, leading to the need for advanced treatment options. Methods: A retrospective noncomparative interventional case series was conducted with 91 patients presenting with severe dry eye syndrome. Treatment included intense-pulsed-light therapy and gland expression at a single outpatient clinic over a 30-month study. Pre/post tear breakup time data were available for a subset of 78 patients. For all patients, a specially developed technique for the treatment of dry eye syndrome was applied as a series of monthly treatments until there was adequate improvement in dry eye syndrome symptoms by physician judgment, or until patient discontinuation. Results: Primary outcomes included change in tear breakup time, self-reported patient satisfaction, and adverse events. Physician-judged improvement in dry eye tear breakup time was found for 68 of 78 patients (87%) with seven treatment visits and four maintenance visits on average (medians), and 93% of patients reported post-treatment satisfaction with degree of dry eye syndrome symptoms. Adverse events, most typically redness or swelling, were found for 13% of patients. No serious adverse events were found. Conclusions: Although preliminary, study results of intense-pulsed-light therapy treatment for dry eye syndrome caused by meibomian gland dysfunction are promising. A multisite clinical trial with a larger sample, treatment comparison groups, and randomized controlled trials is currently underway.
Introduction
Meibomian gland dysfunction (MGD) is the leading cause of evaporative dry eye disease (DED).1–4 Patients with this disease produce an abnormal meibum that is more viscous than the usual olive-oil-like secretion.5,6 These patients can have severe inflammation and bacterial overgrowth that exacerbates the problem. Most standard treatments, such as anti-inflammatory drops or oral antibiotics,7,8 aim at decreasing the inflammation associated with this disease.9 Another treatment has been to use warm compresses in an effort to melt the thick meibum produced by these secretions.10 Finally, doctors have recommended lid scrubs to lower the bacterial load and cleanse the lid margin.11 Such treatments have been only somewhat effective for patients with MGD, leading some to suggest the need for a multifaceted treatment approach.12,13
Intense pulse light (IPL) has been used in dermatology practices for several years as a treatment for rosacea and acne.14 IPL uses Xenon flashlamp to emit wavelengths of light from 400 to 1200 nm. When placed on the light, a filter restricts the wavelength to the visible light range of ∼500 nm. When applied to the skin, this 500 nm light causes the blood cells in the abnormal telangiectasias to absorb the light, coagulate, and, finally, to close the blood vessels.
In the case of rosacea, these abnormal blood vessels secrete inflammatory mediators over time that damage the skin. Closing of the telangiectasias and the inflammatory mediators they secrete is one of the mechanisms proposed to explain how IPL improves the skin in rosacea patients.15 In the case of acne, the 500 nm wavelength is also proposed to eradicate the bacteria, which affect acne patients; hence they also improve.
Normal meibum contains antimicrobial properties that keep the lid margin clear from overgrowth.16 Abnormal blood vessel growth from chronic inflammation called telangiectasias surround the meibomian glands and secrete inflammatory mediators that cause malfunction of the glands.17 This dysfunction leads to formation of an abnormal meibum. Potentially, IPL near the lid should cause closing of the abnormal blood vessels secreting inflammatory mediators and decrease bacterial overgrowth; an eventuality we began to observe early in our practice when some of our patients treated with IPL showed improvement in their MGD and DED.
In 2002, we began to observe that some of our patients treated with IPL showed improvement in MGD and DED.18 Based on these observations, the Toyos Clinic continued to develop and refine the treatment. Since that time, we have presented study results at several meetings showing how IPL improves MGD and DED.19,20 Over the years, we have perfected the technique and technology to maximize results and minimize complications. In 2007–2008, an IPL treatment technology–the Diamond Q4 by DermaMed Solutions – was specifically configured to our specifications with the goal of stimulating secretion of normal meibum via skin treatment effects on the meibomian glands. Importantly, we also discovered that dry eye patients are better positioned for gland expression, as IPL seems to liquefy the abnormal viscous meibum and dilate the glands. Patients report that gland expression relieves their dry eye symptoms more effectively than IPL alone, with little of the usual discomfort.
The objective of this retrospective noncomparative interventional case series study is to describe clinical data concerning effectiveness and safety of IPL skin treatment using the Toyos Technique, as described and refined over 6 years for patients with evaporative dry eye caused by meibomian gland dysfunction.
Materials and Methods
Beginning in 2002, and culminating in 2007–2008 with the development of the IPL Diamond Series Q4 and preferred apparatus settings as established by the Toyos Clinic, (now proprietary to DermaMed Solutions), the following technique and technology for the treatment of DED evolved over 6 years.
This study was conducted in accordance with the guidelines of the Declarations of Helsinki with informed consent for IPL treatment obtained for each patient.
Eligibility for treatment
Candidates eligible for IPL must have Fitzpatrick Skin Types 1, 2, and 3 (and sometimes 4).21 Darker skins cannot tolerate IPL and are relatively prone to side effects such as depigmentation. Parameters in the Q4 are chosen by skin type with specific power chosen by the physician.
Treatment procedure
This protocol was first described in a case report by Toyos et al. in EyeWorld September, 2005.19 IPL treatment intensity ranges from a low power of 8 J/cm2, and increases sequentially to a high power of 20 J/cm2, with higher power levels indicated as age and lid margin disease severity increase. Once the physician selects the skin-appropriate power setting, the patient is ready for treatment as described here.
1. Patented disposable IPL eye pads (Sperian Inc.) are placed over closed eyes to cover the area completely.
2. Ultrasound gel (Parker Laboratories) is placed on the patient's face from tragus to tragus including the nose. The patient's skin area receives one full pass with overlapping flashes to ensure treatment of the entire area.
3. Following the initial pass, the patient receives more ultrasound gel, and a second pass is performed.
4. With completion of the second pass, the gel is removed from the face, and the patient is brought to the slit lamp where a drop of 1% proparacaine is administered and a gland expression is performed (using a sterile cotton tip applicator).
5. The cotton tip is placed on the palpebral conjunctiva in the area of the meibomian gland and the physician places a finger on the skin next to the same gland.
6. The patient is told to look up, and while applying gentle continuous pressure with both the cotton tip and the finger, the gland is expressed for 30 sec.
7. The procedure is repeated over the length of the lower lid on both sides.
8. Upper lid expression follows using finger pressure, and is performed with the patient looking down. If upper lid glands are unresponsive to this technique, a sterilized cotton tip is used as described. Once the glands have been expressed, the patient is given either a drop of topical steroid or a nonsteroidal anti-inflammatory drug (NSAID).
This procedure is repeated approximately every 30 days. During the study period, treatment protocols called for four visits as a target for improving DED.
Study procedure
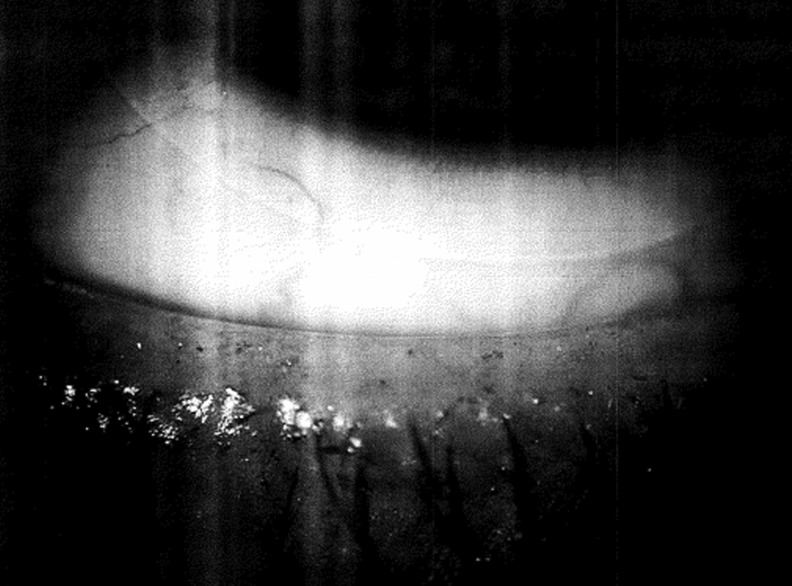
A chart review was conducted for 123 patients, with those targeted who presented with signs and symptoms of severe dry eye as determined by tear breakup time (TBUT) of ≤5 sec (International Dry Eye Workshop [DEWS], 2007), and who also had abnormal meibum secretions and abnormal lid margins, including telangiectasias (see Figs. 1 and 2).
FIG. 1.
A pretreatment view from one patient presenting with abnormal lid margin and dry eye disease (DED) symptoms.
FIG. 2.

A post-treatment view for the same patient as depicted in Fig. 1.
In all cases, study patients were persons who had reportedly tried or exhausted conventional DED treatments, and who actively sought out the Toyos Clinic. These patients were driven to seek alternatives based on their subjective feelings of discomfort, and did not in all cases have TBUT of ≤5 sec. TBUT ranged from a low of 1 sec to a high of 16 sec. All told, 10% of patients had TBUTs>5 sec.
All study patients were among those visiting our single outpatient clinic in Memphis, Tennessee at least once over the 30 months of this study (May 2009 through October 2011).
Sufficient data were available for analysis on 91 patients with 182 eyes who completed IPL and gland expression treatment for DED caused by meibomian gland dysfunction.
Results
Study patients were predominantly female (74%), Caucasian (99%, 1% Asian) with a median age of 54 (ranging from 21 to 84 years of age). Patients made median of seven total treatment visits and four maintenance visits (post-treatment).
Ninety-one patients with 182 eyes presented with severe DED based, in most cases, on a combination of TBUT, abnormal meibum, abnormal lid margins, and patient discomfort.
TBUT
TBUT was measured using either of two methods: oculus tear break up time (OTBUT) or standard tear break up time (STBUT). Table 1 shows the mean TBUTs at initial and end-of-treatment for 79 patients (excludes maintenance sessions) along with statistical testing for corresponding change in TBUT as determined by paired t test. All analyses were conducted using SPSS 16.0 (Chicago, IL).
Table 1.
Comparison of Pre/Post TBUT (Mean Seconds) for Treatment of DED Using the Study Treatment Technique
| TBUT (sec) | Initial treatment mean TBUT | End-of-treatment mean TBUT | Mean difference TBUT | t Valuea | |
|---|---|---|---|---|---|
| Oculus method (OD) | 23 | 3.2 | 6.3 | 3.1 | 4.7 |
| Oculus method (OS) | 23 | 2.2 | 6.0 | 3.8 | 4.3 |
| Standard method (OD) | 34 | 2.9 | 7.8 | 4.9 | 12.8 |
| Standard method (OS) | 34 | 2.7 | 7.6 | 4.9 | 11.7 |
| Standard initial oculus end of treatment (OD) | 21 | 2.1 | 7.6 | 5.5 | 8.3 |
| Standard initial oculus end of treatment (OS) | 21 | 2.0 | 7.5 | 5.5 | 8.8 |
| Overall (OD)b | 78 | 2.8 | 7.2 | 4.4 | 13.0 |
| Overall (OS)b | 78 | 2.4 | 7.2 | 4.8 | 14.2 |
Includes Bonferroni adjustment; p=0.006. All results were significant at p=0.000.
Includes combined results for the oculus or standard methods for determining TBUT.
TBUT, tear breakup time; DED dry eye disease.
To help determine the extent to which any differences in sensitivity of the oculus and standard methods might confound efforts to evaluate pre/post changes in TBUT, a means test compared the initial TBUT times for the oculus to standard method. Results for both left and right eye revealed no significant differences in mean TBUT time at initial treatment (F=0.135, p=0.714 [OD], F=0.106, p=0.746 [OS]). Accordingly, we treat both methods as equivalent for the remainder of this article.
Overall, a statistically significant mean improvement was found (paired t test; p=0.000) in TBUT from initial to end of treatment (4.4 OD, 4.8 OS). Using the TBUT dry eye severity parameters originally developed by Behrens et al.22 (2006) and reported in DEWS,23 the average patient was categorized as “severe” (≤5 sec) at start of treatment (mean TBUT=2.8 OD, 2.4 OS) improving to “moderate” (≤10 sec) by end-of-treatment (mean TBUT=7.2 OD, 7.2 OS).
As shown in Table 2, the mean differences in pre/post TBUT by paired t test were evaluated further by gender and by age quartiles. Statistically significant differences in overall TBUT were found for both genders and across age quartiles.
Table 2.
Comparison of Pre/Post TBUT for Treatment of DED By Age and Gender
| Age (n=77) | Number of cases | OD (right) | OS (left) |
|---|---|---|---|
| 21–39 | 21 | 3.7 | 4.5 |
| 40–54 | 17 | 5.2 | 5.5 |
| 55–64 | 21 | 4.2 | 4.8 |
| 65–84 | 18 | 5.1 | 4.5 |
| Sex (n=89) | |||
| Male | 23 | 4.5 | 4.4 |
| Female | 57 | 4.5 | 5.0 |
Statistically significant changes are in bold.
TBUT, tear breakup time; DED, dry eye disease.
The average number of total treatments was seven (median). The average number of maintenance treatments was four (median). A small significant Pearson correlation was found for the number of treatment visits and the number of maintenance visits (r=0.371, p=0.000).
Considering individual differences from start to end of treatment, 86% of the 78 patients with pre/post TBUT times improved in both eyes, 9% remained the same in one or both eyes, and 5% worsened in one. No patient worsened in both eyes.
Other metrics
Other metrics were available for physician-judged improvements in meibum and lid margins, and self-reported improvement and patient satisfaction (asked at end of treatment only). These were obtained for all patients initially presenting to the Toyos Clinic, and were posted to the clinical record. Post-treatment clinical assessments were captured with comparative physician subjective evaluations of improvement, stabilization, or worsening reported here. Quantitative metrics were not recorded, and were not available for study purposes.
More than 90% of all respondents appeared to improve across all three measures (94% meibum, 98% lid margin, 93% satisfaction). No patient failed to improve for at least one of the metrics.
Considering individual differences from start to end of treatment, 86% of the 78 patients with pre/post TBUT times improved in both eyes, 9% remained the same in one or both eyes, and 5% worsened in one. No patient worsened in both eyes.
Several other metrics reported as dichotomous (yes/no) data at end of treatment further suggest the effectiveness of IPL treatment for DED caused by MGD: changes in meibum, lid margin, and patient satisfaction.
Adverse effects
Of 91 patients, 13 (14%) experienced an adverse event with only 15% (2 of 13) terminating treatment. Adverse events included blistering (typically a red spot lasting <1 week), cheek swelling, conjunctival cyst, floaters, hair loss at brow and forehead, light sensitivity, and redness of face. In most cases, adverse effects such as swelling self-resolved within 1 week.
As defined by the United States Food and Drug Administration (FDA), no serious adverse events were reported and no statistically significant difference was found in the number of follow-up or maintenance visits for the 13 patients.
Discussion
As shown in this study, >90% of respondents to the IPL treatment improved over all three metrics used to evaluate the MGD. The leading cause of MGD is evaporative DED: a disease in which meibum production is more viscous than usual and from which patients can experience severe inflammation and bacterial overgrowth that exacerbates abnormal meibum production.5–7 Usual treatments have ranged from warm compresses to lid scrubs, with mild cases proving easier to treat than moderate and especially severe cases.24,25
Partly serendipitously, the value of IPL for treatment of DED was first identified by Dr. Toyos in 2002 when patients with DED who were being treated for rosacea, acne or other skin problems reported improvements in their dry eye symptoms. Following these early observations, a grant14 was obtained in which a sample of 100 patients was provided treatment per the evolving study treatment technique. Although not significant, the data were favorable and informed additional modifications to the study treatment technique including the eventual development of an IPL device (the DermaMed Diamond Series Q4) specifically aimed at the treatment of DED caused by MGD (and adjustable to differences in individual skin types). Following these efforts, patients learned of and actively sought out the Toyos Clinic for treatment.
TBUT: Pre/post effectiveness of treatment
Overall, pre/post changes in TBUT were available for only 78 patients. Of these, 23 were assessed using the oculus method and 34 were assessed using the standard method; 21 were assessed using the standard method at the initial visit and the oculus method at end of treatment. Comparisons between the oculus and standard methods at the initial visit found no significant differences, allowing us to combine the TBUT data from the two methods for analysis purposes.
Of 78 patients for whom pre/post measures of TBUT were available, statistically significant gains were found from 3.0 to 7.2 (mean difference 4.2 OD) and from 2.6 to 7.0 (mean difference 4.4 OS). In effect, these changes reflect a shift from severe to moderate TBUT (DEWS, 2007).16 This shift is key.
Moderate, and, especially, mild DED have been shown to be more amenable to the usual treatments,24,25 whereas severe DED has proven more difficult to treat. That the study treatment technique appears to be effective for those with severe dry eye is most promising. Because the majority of patients presented with initial TBUT of ≤5 sec, the effectiveness of the Toyos method for moderate or mild cases of DED was not formally evaluated, but should be in the future.
TBUT: Number of treatments
When patients were trichotomized by number of treatments, statistically significant differences in TBUT were found for percent of patients improving in both eyes (χ2=11.8, p=0.019 OS; χ2=17.6, p=0.001 OD). Using OD as an example, with one to three treatments, 69% improved; with four treatments, 89% improved, and with five or more treatments, 95% improved. Odds ratios were calculated and showed that those who had five or more treatments were 17.5 times more likely than those who had one to three treatments to exhibit statistically significant improvement in TBUT; p=0.000. Those with four treatments were no more likely to show statistically significant improvements in TBUT than those with one to three treatments (p=0.279; although nearly 9 in 10 experienced improvement in TBUT, meibum, lid margins, and patient satisfaction).
(Please note that the study treatment technique has been developed over time since 2002, and continues to be refined as experience and data accrue.)
Other metrics
Only post-treatment metrics were available for self-reported patient satisfaction (patients were asked whether they felt that their symptoms had improved). These were obtained for all patients initially presenting to the Toyos Clinic, and posted to the clinical record with physician determination of “abnormal meibum” as a prerequisite for treatment. Post-treatment clinical assessments were also captured with comparative subjective evaluations of improvement, stabilization, or worsening reported here.
Results were most favorable, with >93% of all respondents indicating satisfaction with treatment.
Demographics
Demographic differences in responsiveness to treatment were null, whereas differences in the range of demographic groups should be considered. Use of a larger study population for all demographic groups should be considered.
Limitations
Limitations of the study relate primarily to efficacy testing. As no comparison group exists in which DED patients were directly evaluated for their responsiveness to alternative treatments, we cannot generate comparison statistics.
Although offset partly by the availability of a more objective pre/post measure for TBUT, pre/post determination of change in meibum and lid margins was based on physician judgement of pretreatment versus post-treatment condition. In other words, no objective pre/post-treatment metrics were available for study analysis of meibum or lid margins. However, only those with abnormal meibum and lid margins were included for study purposes, and only two patients terminated treatment prematurely.
Similarly, the availability of only post-study measures for patient satisfaction and “yes/no” measurement further limits the value of this study.
Other limitations relate to the potential subjectivity of the physician, who is both the developer of the study treatment technique and the evaluator of its effectiveness. Independent evaluation is warranted.
Conclusions
All told, the results suggest that IPL holds promise as an option for treatment of evaporative DED caused by MGD, with a limited adverse event profile. A larger sample size with a comparison group and random assignment to treatment would be helpful for better assessing both effectiveness of the study treatment technique as well as for determining the range and frequency of adverse events. A rigorous multisite prospective study is currently under development.
Author Disclosure Statement
Rolando Toyos discovered the IPL treatment technique and was provided a consulting fee by DermaMed Solutions for consulting and medical-technical input into the article. Patient study data were collected by Dr. Toyos and Toyos Clinic staff prior to involvement with DermaMed, and this collection not compensated in any way by DermaMed Solutions. William McGill served as an independent statistician for the project and as a primary contributor to article preparation. He was involved with the project only after data were collected, and was financially compensated by DermaMed Solutions. Dustin Briscoe is an optometric resident with Toyos Clinic and served as a contributor to article preparation. He was not compensated by DermaMed Solutions.
References
- 1.Nichols K, Foulks G, Bron A, et al. . The International Workshop on Meibomian Gland Dysfunction: Executive Summary. Investigat Ophthalmol Vis Sci 2011;52:1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemp M, Crews L, Bron A, Foulks G, Sullivan B. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort. Cornea 2012;31:472–478 [DOI] [PubMed] [Google Scholar]
- 3.Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea 2008;27:1142–1147 [DOI] [PubMed] [Google Scholar]
- 4.Korb DR, Blackie CA. Restoration of meibomian gland functionality with novel thermodynamic treatment device–A case report. Cornea 2010;29:930–933 [DOI] [PubMed] [Google Scholar]
- 5.Korb DR, Blackie CA. Meibomian gland therapeutic expression: quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens 2011;37:298–301 [DOI] [PubMed] [Google Scholar]
- 6.Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens 2003;29:96–99 [DOI] [PubMed] [Google Scholar]
- 7.Facts About Dry Eye. Dry Eye, Facts About [NEI Health Information]. (n.d.). Available at: http://www.nei.nih.gov/health/dryeye/dryeye.asp (Last accessed July22, 2014)
- 8.Tu E. Dry Eye. Ophthalmology 2008. Available at: http://www.mdconsult.com/das/book/body/1994000895/0/1869/0.html (Last accessed May10, 2010)
- 9.Ehlers JP, Shah CP. Dry-eye syndrome. In: The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 5th ed., Philadelphia: Lippincott Williams & Wilkins, 2008, pp. 52–54 [Google Scholar]
- 10.Dry Eye Disease. American Academy of Ophthalmology, n.d.. Available at: http://one.aao.org/asset.axd?id=be593214-34af-4073-ab93-2bccbdf62aae (Last accessed May10, 2010)
- 11.Care of the patient with ocular surface disorders. American Optometric Association, 2010. Available at: http://www.aoa.org/documents/CPG-10.pdf (Last accessed March26, 2010) [Google Scholar]
- 12.Caceres V. Ocular surface treating dry eye. Eye World, 2011. Available at: http://www.eyeworld.org/printarticle.php?id=5835 (Last accessed June5, 2012)
- 13.Bowling E, Russell G. Topical steroids and the treatment of dry eye. Review of Cornea and Contact Lenses, 2011. Available at: http://www.reviewofcontactlenses.com/content/d/dry_eye/c/27245/ (Last accessed July22, 2014)
- 14.Papageorgiou P, Clayton W, Norwood S, Chopra S, Rustin M. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol 2008;159:628–632 [DOI] [PubMed] [Google Scholar]
- 15.Toyos R. Intense pulsed broadband light: a novel treatment for dry eye disease. ASCRS 2005 Research Grant Winner, 2005. Available at http://www.eyeworld.org/article.php?sid=2638&strict=&morphologic=&query=ophthalmology (Last accessed December18, 2014)
- 16.Holland E. Management of meibomian gland disease and ocular surface inflammation. Healio Ophthalmology, 2009. Available at: http://www.osnsupersite.com/view.aspx?rid=41658 (Last accessed July22, 2014)
- 17.Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'brien T, et al. . The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Management and Treatment of Meibomian Gland Dysfunction. Invest Ophthalmol Vis Sci 2011;52:2050–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent C. Intense pulsed light: for treating dry eye. Review of Ophthalmology®, 2010. Available at: http://www.revophth.com/content/d/technology_update/c/25857/ (Last accessed July22, 2014)
- 19.Toyos R, Buffa C, Youngerman S. Case report: Dry–eye symptoms improve with intense pulsed light treatment. EyeWorld News Magazine, 2005. Availablea t: http://www.eyeworld.org/article.php?sid=2698 (Last accessed July22, 2014)
- 20.Toyos R. IPL therapy aids in dry eye. Ophthalmology Times Europe, 2010. Available at: http://www.oteurope.com/ophthalmologytimeseurope/Cornea/IPL-therapy-aids-in-dry-eye/ArticleStandard/Article/detail/695640 (Last accessed July22, 2014)
- 21.Fitzpatrick T. The validity and practicality of sun-reactive skin Types I through VI. Arch Dermatol 1988; 124:869–871 [DOI] [PubMed] [Google Scholar]
- 22.Behrens A, Doyle JJ, Stern L, et al. . Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 2006;25:900–907 [DOI] [PubMed] [Google Scholar]
- 23.Foulks G, ed. 2007Report of the International Dry Eye Workshop (DEWS). Ocul Surf 2007;5(2) [DOI] [PubMed] [Google Scholar]
- 24.Nelson JD, Helms H, Fiscella R, Southwell Y, Hirsch JD. A new look at dry eye disease and its treatment. Adv Ther 2000;17:84–93 [DOI] [PubMed] [Google Scholar]
- 25.Perry HD, Solomon R, Donnenfeld ED, et al. . Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol 2008;126;1046–1050 [DOI] [PubMed] [Google Scholar]