Abstract
Using an affinity resin and photoaffinity label based on phospholipid analogs of inositol 1,3,4,5-tetrakisphosphate (InsP4), we have isolated, characterized, and cloned a 46-kDa protein from rat brain, which we have named centaurin-α. Binding specificity was determined using displacement of 1-O-[3H](3-[4-benzoyldihydrocinnamidyl]propyl)-InsP4 photoaffinity labeling. Centaurin-α displayed highest affinity for phosphatidylinositol 3,4,5-trisphosphate (PtdInsP3) (IC50 = 120 nm), whereas InsP4, PtdInsP2, and InsP3 bound with 5-, 12-, and >50-fold lower affinity, respectively. Screening a rat brain cDNA library with a polymerase chain reaction product, generated using partial amino acid sequence from tryptic peptides, yielded a full-length clone. The 2,450-base pair cDNA contained an open reading frame (ORF) encoding a novel protein of 419 amino acids. Northern analysis revealed a 2.5-kilobase transcript that is highly expressed in brain. The deduced sequence contains a novel putative zinc finger motif, 10 ankyrin-like repeats, and shows homology to recently identified yeast and mammalian Arf GTPase-activating proteins. Given the specificity of binding and enrichment in brain, centaurin-α is a candidate PtdInsP3 receptor that may link the activation of phosphoinositide 3-kinase to downstream responses in the brain.
Receptor-stimulated phosphoinositide (PI)1 metabolism generates numerous inositol polyphosphates (InsPns) and inositol phospholipids, many of which may function as potential second messengers (1). Of the possible PI metabolites, Ins(1,4,5)P3 (InsP3) and diacylglycerol (DAG) are the best characterized second messengers. Generated by receptor-stimulated phospholipase C, hydrolysis of PtdIns(4,5)P2 (2), Ins(1,4,5)P3 binds to and gates an InsP3 receptor calcium channel on the endoplasmic reticulum (2, 3). The lipid DAG remains in the membrane where it activates several protein kinase C isoforms and may regulate other targets (4, 5). In the membrane, DAG is metabolized rapidly to monoacylglycerol and to several phospholipids. In the cytoplasm, Ins(1,4,5)P3 can be phosphorylated to Ins(1,3,4,5)P4 by an InsP3 3-kinase. Other isomers of InsP4, InsP5, and InsP6, some of which are synthesized independently of Ins(1,4,5)P3, have been identified (for review, see Ref. 6), and their production may also be regulated by receptors or during cell growth. Information from receptor binding studies, using radioactive InsP4 and InsP6, have demonstrated that a number of important regulatory proteins contain high affinity InsPn binding sites. InsPns have been implicated in the regulation of clathrin assembly proteins AP-2 (7, 8), AP-3 (9), the non-clathrin-associated coatomer proteins (10), synaptotagmin (11), and the regulation of the small GTPases ras and/or rap via a specific GTPase-activating protein (GAP) activity (12).
Inositol phospholipids have also been postulated as messenger molecules. From in vivo, genetic, and permeabilized cell studies, evidence for critical roles for the inositol phospholipids PtdIns(3)P, PtdIns(4)P, and PtdIns(4,5)P2 as regulators of membrane vesicle trafficking and cytoskeletal rearrangements is accumulating rapidly (5, 13–15). One inositol phospholipid, PtdIns(3,4,5)P3, has emerged as a potential messenger molecule in receptor-stimulated cells (16–18). Synthesized by receptor-stimulated PI 3-kinase phosphorylation of PtdIns(4,5)P2 (18), PtdInsP3 is not a substrate for PI-specific phospholipase Cs (19). PtdInsP3 production is stimulated by numerous inflammatory, growth, and trophic factors, including insulin, epidermal growth factor, platelet-derived growth factor, nerve growth factor, fMLP, platelet-activating factor, and the inter-leukins IL-2, IL-3, and IL-4, via activation of receptor-regulated PI 3-kinase (for review, see Ref. 20). Numerous studies have implicated receptor-stimulated PI 3-kinase in regulating a variety of cellular activities, including mitogenesis (21), membrane ruffling, and actin cytoskeletal reorganization (22–24) particularly via the small GTPase rac (25), glucose transporter translocation (26–28), membrane trafficking (29), stimulation of p70 ribosomal S6-kinase (28, 30), regulation of Akt kinase (protein kinase B) (31–33), and neurite outgrowth (34, 35).
Although the molecular basis by which PI 3-kinase regulates these cellular activities is still unclear, the existence of specific receptor targets for PtdInsP3, analogous to the receptors for InsP3 and DAG, has been proposed (35, 36). In vitro, PtdInsP3 competes with phosphotyrosine for binding to the SH2 domain of the p85 subunit of PI 3-kinase (37). PtdInsP3 has been shown to stimulate pleckstrin phosphorylation in permeabilized platelets (38), and there is evidence that 3-phosphorylated inositol phospholipids may interact with Akt kinase (protein kinase B) (32), various protein kinase C isoforms (39–41), and PRK, a protein kinase C-related kinase (42). The diversity of activities regulated by PtdInsP3 predicts that numerous cellular effectors for PtdInsP3 may exist. To isolate and identify specific, high affinity PtdInsP3 receptors from mammalian brain, we have used affinity chromatography with a tethered aminopropyl-InsP4 linked to Affi-Gel 10 and a hydrophobic InsP4 photoaffinity probe. Here, we report the identification, characterization, and cloning of a novel candidate PtdInsP3 receptor that is highly enriched in rat brain.
EXPERIMENTAL PROCEDURES
Materials
Fine chemicals were purchased, if not specified, from Sigma. The [α-32P]dCTP and 5′-[α-35S]dA(thio)TP were obtained from DuPont NEN and Amersham, respectively. Restriction enzymes and DNA/RNA-modifying enzymes were obtained from Promega, Stratragene, and Clontech. The InsP4 affinity resin (aminopropyl-InsP4 linked to Affi-Gel 10 (Bio-Rad)) was synthesized as described previously (43, 44).
Synthesis of 1-O-[3H](3-[4-Benzoyldihydrocinnamidyl]propyl)-InsP4
The precursor 1-O-(3-aminopropyl)-d-myo-Ins(1,3,4,5)P4 was synthesized in 12 steps from α-d-glucose (43) and was coupled with tritium-labeled 4-benzoyldihydrocinnamoyl N-hydroxysuccinimide ester (BZDC-NHS) (45, 46). An ethyl acetate solution of 2.76 mCi (80 nmol) of this reagent was evaporated to dryness under N2 and dissolved in 200 μl of N,N-dimethylformamide. To this solution, 100 μl of 0.25 m triethylammonium bicarbonate (TEAB) buffer was added, followed by the addition of 100 μl of a 0.56 mg/ml solution of aminopropyl-InsP4 (0.81 μmol) in 0.25 m TEAB buffer. The reaction was kept in the dark and stirred overnight at room temperature. The mixture was then concentrated in vacuo; the residue was dissolved in 500 μl of deionized water and applied to a Pasteur pipette (4 × 0.5 cm) column of DEAE-cellulose (HCO −3 form). The column was washed with 1 ml of deionized or distilled water and eluted sequentially with the following: 1 ml of 0.1 m TEAB, 1 ml of 0.2 m TEAB, 1 ml of 0.3 m TEAB, 1 ml of 0.35 m TEAB, 1 ml of 0.4 m TEAB, 1 ml of 0.4 m TEAB, 1 ml of 0.5 m TEAB, and 1 ml of 0.6 m TEAB. Fractions were analyzed by reverse phase HPLC (15% acetonitrile in 0.05 m KH2PO4 buffer) and monitored with a radiochemical detector. The highly radioactive fractions (generally, the 0.35, 0.4, and 0.5 m TEAB buffer fractions) had a retention time of 8.30 min, which corresponded to the retention time of nonradiolabeled BZDCInsP4 detected by UV absorption. The radiochemical yield was 40%.
Protein Purification
Centaurin2-α was purified from whole rat brain of 150–300-g male Sprague-Dawley rats (Charles River) using methods described previously with all procedures at 0–4 °C (44, 47). Briefly, whole brains were homogenized in 50 mm Tris/HCl, pH 7.7, containing 1 mm EDTA; 1 mm EGTA; 1 mm β-mercaptoethanol; 100 mg/liter phenylmethylsulfonyl fluoride; 5 mg/liter each of chymostatin, antipain, and pepstatin; 10 mg/liter aprotinin and leupeptin; and 250 mg/liter CBZ-phenylalanine (PB). After centrifugation at 45,000 × g for 15 min, the soluble fraction was diluted 3–5-fold with PB containing 250 mm NaCl. Resuspended membranes were solubilized for 45 min with PB plus 1% CHAPS and 250 mm NaCl and centrifuged for 30 min at 45,000 × g. All buffers for membrane-associated fractions contained 1% CHAPS. CHAPS-solubilized membranes and soluble proteins each were incubated with heparin agarose for 1 h. The resin was washed with PB plus 250 mm NaCl and eluted with PB containing 1 m NaCl. The eluates were concentrated in Amicon Centripreps to a final volume of 1–2 ml for each preparation of 10–15 rat brains for photolabeling experiments. For further purification by InsP4 affinity chromatography, the concentrated heparin-agarose eluates were diluted with 50 mm Tris/HCl, pH 7.4, containing 1 mm EDTA (ACB) and loaded onto an InsP4 affinity column (44) adapted to an FPLC (Pharmacia, column dimensions, 10 × 3 cm) at a rate of 0.2 ml/min. The column was washed with 10 ml of 200 mm NaCl in ACB and eluted with a gradient of 0.2 m to 1.5 m NaCl at 0.2 ml/min. Fractions (2 ml) were collected and assessed for protein or [3H]BZDC-InsP4 photoaffinity labeling. InsP4 column fractions from the soluble fraction of 100 whole rat brains were concentrated for tryptic digestion and peptide sequencing.
Photoaffinity Labeling of Native Centaurin-α with [3H]BZDC-InsP4
Concentrated heparin-agarose column eluate (5–30 μl) was incubated with 40–60 μl of 25 mm Tris, pH 7.4, containing 1 mm EDTA, 0.5 mm potassium phosphate, 0.5 mm potassium pyrophosphate, and 20–70 nm [3H]BZDC-InsP4. To determine the affinity and specificity of photolabeled centaurin-α, 10 nm to 3 μm concentrations of the indicated inositol phosphate or phospholipid were included with the [3H]BZDCInsP4. Samples were equilibrated for 10 min at 0 °C before exposure to UV light (360 nm) for 1 h at 0 °C. SDS sample buffer was then added to the samples and the proteins separated by SDS-PAGE on 10% Laemmli gels. Gels were fixed and fluorographed with the Entensify System (Amersham Corp.), dried, autoradiographed, and digitized. All of the photolabeling studies were performed in at least three independent experiments.
Peptide Sequence Analysis
Approximately 50 μg of concentrated centaurin-α purified by InsP4 affinity column chromatography was subjected to SDS-PAGE and transferred to Immobilon-P membrane. The membrane was stained with Coomassie to localize the protein band with an apparent molecular size of 46 kDa, which was then excised and digested with trypsin. The resultant tryptic peptides were fractionated via reverse phase HPLC with a linear gradient of acetonitrile, 0.1% trifluoroacetic acid (90 min). Individual tryptic peptides were then subjected to automated Edman degradation on a Procise 492 protein sequencer (Perkin-Elmer, ABD division). The nine unique peptide sequences that were obtained were compared with sequences in the GenBank data base using the TFASTA and BLAST DNA analysis software package of the University of Wisconsin Genetics Computer Group.
cDNA Cloning
Rat brain template cDNA was prepared by reverse transcription of rat brain poly(A)+ RNA (Clontech). Degenerate oligo-nucleotide primers were designed based on two tryptic peptides having 100% homology to putative peptide sequences at the ends of a human-expressed sequence tag (GenBank accession number T09325). The sequences of the primers synthesized (DNA International) were as follows: 5′-TT(T/C)CA(T/C)TA(T/C)(T/C)TICA(A/G)GTIGCITT(T/C)CC-3′ (26-mer, 64-fold degeneracy) and 5′-C(C/T)TGIGGIAGCATIGG(C/ T)CT(G/A)TCIAC-3′ (25-mer, 8-fold degeneracy). Using these primers in PCR on the rat brain template cDNA, we were able to obtain a product corresponding to a predicted size of approximately 400 bp. The PCR product was subsequently cloned using the TA cloning system (Invitrogen). A total of 1.6 × 106 independent recombinants of a λZap II postnatal day 7 rat brain cDNA library (kindly provided by Dr. Craig Garner, University of Alabama at Birmingham) were screened with a [α-32P]dCTP-labeled 400-bp PCR product as a probe (Promega Prime-a-Gene System). Twenty-five strongly positive plaques were picked and excised with the Stratagene Solr System. The clone containing the longest insert of 2,450 bp was sequenced and called centaurin-α. Using a nested deletion based strategy, both strands of the centaurin-α clone were sequenced as double-stranded plasmids with M13 forward and reverse primers by the dideoxynucleotide chain termination method using deoxyadenosine 5′-[α-35S]thiotriphosphate and Sequenase 7.0 (U. S. Biochemical Corp.).
Northern Analysis
The multiple tissue Northern blot containing poly(A)+ RNA from the indicated rat tissues was obtained from Clontech. According to the company's catalog, “Multiple tissue Northern blots are premade Northern blots of quality poly(A)+ RNA from different (rat) tissues. The poly(A)+ RNA is purified by several passages through oligo(dT)-cellulose columns, then run on a denaturing formal-dehyde, 1.2% agarose gel, and blotted onto a positively charged nylon membrane. The lanes of the blots each contain approximately 2 μg of pure poly(A)+ RNA from specific tissues. The RNA loaded in each lane is adjusted so that a visible β-actin signal is present in every lane.” Prehybridization was performed at 42 °C in 6 × SSPE (1.08 m NaCl, 60 mm sodium phosphate, 6 mm EDTA, pH 8.0, 5 × Denhardt's solution, 50% formamide, 100 μg/ml sheared salmon sperm DNA, and 0.1% SDS for 4 h. An [α-32P]dCTP-labeled AvaI/BamHI fragment (4.5 × 109 cpm/ μg, 1,240 bp) from the 3′ end of centaurin-α was hybridized to the membrane for 24 h at 42 °C (protocol recommended by Clontech). The membrane was washed four times in 1 liter of 2 × SSC (0.9 m NaCl, 99 mm sodium citrate), 0.1% SDS for 15 min at 50 °C, followed by two 15-min washes in 1 liter of 1 × SSC, of 0.5 × SSC, and of distilled deionized water at 50 °C. Autoradiography was performed at −80 °C with Kodak X-Omat-AR film with intensifying screens for 24 h.
Expression of Recombinant Centaurin-α in Esherichia coli
A COOH-terminal fusion protein construct (containing ankyrin repeats 7–10) was made by digesting the 2.5-kb pair centaurin-α cDNA with BglII and KpnI to generate a 1.6-kb pair fragment that was cloned into the Pinpoint Xa2 expression vector (Promega). The expression of recombinant centaurin-α in the bacterial strain DH5α was induced by inositol-1-thio-β-d-galactopyranoside. The COOH-terminal fusion protein was extracted with 8 m urea from insoluble bacterial pellets. The extract was clarified by centrifugation. Then, the supernatant was dialyzed to remove the urea. The COOH-terminal fusion protein was then purified using the Soft-link avidin resin (Promega). Approximately 1 mg of purified fusion protein was obtained from 1 liter of bacterial culture.
Generation of Polyclonal Antisera
A peptide containing the NH2-terminal amino acids 2–19 of centaurin-α was synthesized and conjugated to PPDT (purified protein derivative of tuberculin). The bacteri-ally expressed COOH-terminal Pinpoint centaurin-α fusion protein and the PPDT-conjugated NH2-terminal peptide were injected into rabbits following a standard booster immunization protocol. The NH2-terminal antipeptide antiserum was designated J49 and the COOH-terminal fusion protein antiserum, J4.
Expression of Recombinant Centaurin-α in COS-7 Cells
COS-7 cells were routinely cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. The EagI/SphI fragment of the centaurin-α cDNA was subcloned into the p63d2 vector downstream of the cytomegalovirus promoter (22) to give the centaurin-α vector named p63d.2.13. Transient transfections of COS-7 cells (plated at 50–80% confluence) were performed using 10–20 μg of recombinant centaurin-α DNA and 50 μl of lipofectamine (Life Technologies, Inc.)/10-cm dish, according to the manufacturer's protocol. Following transfection, the cells were cultured for an additional 48–60 h. The expressed recombinant centaurin-α protein was isolated and partially purified by heparin-agarose and InsP4 affinity column chromatography under the same conditions as for the native rat brain centaurin-α protein. Approximately 20 μg of purified recombinant centaurin-α protein was obtained from one 10-cm dish of cells.
Immunoblot Analysis
Fractions from rat brain homogenate and cell lysates were separated by SDS-PAGE and transferred to Protean nitrocellulose membranes (Schleicher & Schuell). Membranes were probed with either the rabbit polyclonal NH2-terminal J49 antipep-tide or the J4 antiserum at 1:500 dilution. Horseradish peroxidaseconjugated anti-rabbit secondary antibodies were detected using 3,3′-diaminobenzidine.
RESULTS
Identification of a PtdInsP3 Receptor-binding Protein
Previously, we have used an InsP4 affinity column to isolate specific, high affinity InsPn-binding proteins from the particulate fraction of rat cerebella (44, 47). This strategy led to the identification of one high affinity InsPn-binding protein, the clathrin-associated protein AP-2 (7). In addition to the proteins characterized for [3H]InsP4 binding and photolabeling, we noted the presence of a 46-kDa protein (47). We predicted that this 46-kDa protein would have the highest affinity for InsP4, since the other InsPn-binding proteins eluted from the resin in rank order of their affinity for InsP4, and the 46-kDa protein eluted from the column last. However, under the conditions used for reversible [3H]InsP4 binding and photoaffinity labeling with an radioiodinated arylazido probe, [125I]ASA-InsP4 (43), we could not detect binding to this protein (47). Thus, we were unable to characterize its specificity and affinity or determine its localization. As shown in Fig. 1, A and B, a similar heparin-agarose and InsP4 affinity chromatographic protocol has enabled us to purify this 46-kDa protein (named centaurin-α) from the soluble fraction of whole rat brain. Because of the presence of multiple high affinity InsPn-binding proteins and the presence of endogenous inhibitors in whole brain supernatant, we could not estimate the specific activity of binding in the crude fraction. Approximately 0.5 mg of 90% homogeneous protein could be purified from the supernatant fraction from 50 whole rat brains. Assuming a recovery of 25% (since this is a two-step, rapid purification procedure), this predicts an abundance of centaurin-α protein of about 0.01% of the total brain protein, well within the range of expression of several other PI signaling proteins, such as the InsP3 receptor (3).
Fig. 1. Purification and photoaffinity labeling of centaurin-α.
Panel A, samples of the crude soluble fraction (S), column flow-through (F), and column eluate (E) obtained during heparin-agarose column chromatography of rat brain as described under “Experimental Procedures” were separated by SDS-PAGE and the gel stained with Coomassie Blue. Panel B, early (E) and late (L) eluting fractions from the aminopropyl-InsP4 affinity column purification. Proteins were separated by SDS-PAGE and stained with Coomassie Blue. The 46-kDa protein is marked with an arrowhead. For panels A and B, the molecular mass standards, 205, 116, 97.4, 66, and 45 kDa, are indicated with dashes. Panel C, photoaffinity labeling of the partially purified (heparin-agarose column eluate) fraction from rat brain supernatant. Molecular mass markers, 112, 84, and 52.5 kDa, are indicated with dashes. Panel D, heparin-agarose purified fractions of soluble and detergent-extracted membranes from rat brain and heart, with the 42–48-kDa region of the gel shown. For panels C and D, total photolabeling (−) with 70 nm [3H]BZDC-InsP4 and nonspecific photolabeling (+) were determined with 70 nm [3H]BZDC-InsP4 plus 10 μm unlabeled InsP4. Typical purification and photolabeling profiles are shown and were repeated five times with similar results.
Although in earlier studies we determined that centaurin-α did not bind [3H]InsP4 or [125I]ASA-InsP4, we have used a more hydrophobic and more chemically stable photoprobe, [3H]BZDC-InsP4, to assess the relative binding affinities of centaurin-α. Previously, a related [3H]BZDC-InsP3 label was used to identify a specific InsP3 binding amino acid sequence in the InsP3 receptor calcium channel (48). The BZDC moiety, which is considerably more hydrophobic than the ASA moiety, is especially useful because of the high efficiency of photocoupling (49). Because the label is covalently attached to the binding protein, this allows for separation by SDS-PAGE and visualization in partially purified fractions as well. Fig. 1C shows that the 46-kDa centaurin-α protein (arrowhead) was labeled in the partially purified fractions from rat brain supernatant. Specific photolabeling was blocked by including 10 μm unlabeled InsP4 in the reaction. Photolabeling also displayed a broad pH optimum, which peaked between pH 7.0 and 7.5 (data not shown). Centaurin-α partitions in a 2:1 ratio into the soluble and membrane-associated fractions from rat brain. Both fractions behave identically in the InsP4 affinity column purification and can be specifically photolabeled (Fig. 1D). In addition to brain, a 46-kDa protein is also specifically photolabeled in heparin-agarose fractions from the soluble fraction of rat heart (Fig. 1D), indicating that centaurin-α, or a related protein, is also expressed in non-neuronal tissues. These results demonstrate that under the conditions used, centaurin-α binds to the InsP4 affinity resin with the highest affinity of the soluble and membrane-associated brain and heart proteins and can be specifically photolabeled by [3H]BZDC-InsP4.
Photoaffinity labeling with the [3H]BZDC-InsP4 was then used to determine the specificity and affinity of centaurin-α for several InsPns and inositol phospholipids. As shown in Fig. 2, increasing concentrations of these unlabeled ligands in the binding reaction causes incremental displacement of [3H]BZDCInsP4 from centaurin-α. The most effective ligand at displacing the label was a synthetic dipalmitoyl PtdIns(3,4,5)P3, which had an IC50 of 120 nm. The next most potent ligand was the deacylated PtdInsP3, glycerophosphoinositol 3,4,5-trisphosphate (gPInsP3), which had an IC50 of 430 nm. The rank order of affinity of the other compounds tested and their IC50 values are diacetyl-PtdInsP3 (daPtdInsP3) [550 nm] > InsP4 [620 nm] > PtdIns(4,5)P2 [1.5 μm] > gPInsP2 [2.2 μm] > InsP3 [>3 μm]. Given an observed IC50 for InsP4 of 620 nm, it is not surprising that in previous reversible binding studies using 5–10 nm [3H]InsP4, binding was not observed (47). These data show that several groups are important for binding to centaurin-α. The long chain fatty acid on the glycerol backbone led to an approximate 4-fold increase in binding affinity. The glycerol group esterified to the inositol head group led to a 1.4-fold increase in binding. The 3-phosphate on the d-myo-inositol increased binding between 5- and 13-fold. These results demonstrate that centaurin-α has a preference for binding InsPn analogs that are P-1 phosphodiesters and which contain a 3-phosphate on the inositol ring. Importantly, the InsP4 affinity resin also contains both of these crucial substructures. Together with the data showing that it has extremely high affinity for the InsP4 resin, this specificity is consistent with the suggestion that centaurin-α is a high affinity PtdInsP3-binding protein.
Fig. 2. Specificity of photoaffinity labeling of centaurin-α.
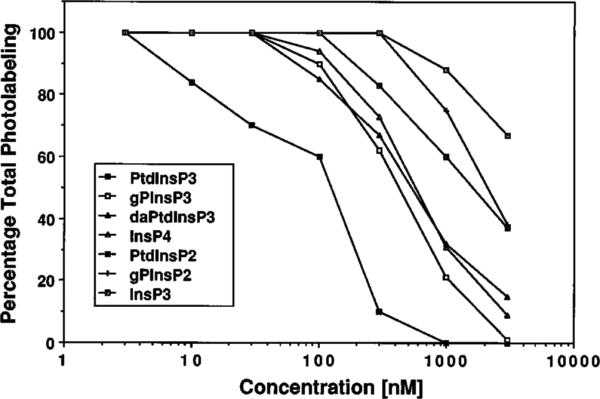
Competition of photolabeling was done by including increasing concentrations of unlabeled ligand (indicated). The heparin eluate fraction from the supernatant of rat whole brain was used for photolabeling with 20–50 nm [3H]BZDC-InsP4, and the 46-kDa region of the fluorograph was scanned using a densitometer. Results are presented as a percentage of total photolabel (in the absence of unlabeled competitor), and each value represents the average of at least four independent determinations. The abbreviations are defined in Footnote 1.
Cloning of Full-length Centaurin-α cDNA
Centaurin-α was purified from whole rat brain supernatant, separated on SDS-polyacrylamide gels, transferred to an Immobilon-P membrane, and digested on the membrane with trypsin. The resultant tryptic peptides were fractionated by reverse phase HPLC. The amino acid sequence of 11 peaks (9 unique) was determined by automated Edman degradation. A GenBank data base search revealed that five of the tryptic fragments of centaurin-α had 100% identity to the predicted amino acid sequence of a 375-bp human expressed sequence tag from a human infant brain cDNA library. This identity allowed us to design degenerate oligonucleotide primers and synthesize a PCR product of predicted size (400 bp) from rat brain cDNA template. The nucleotide sequence of the PCR product was 79% identical to the human-expressed sequence tag. The predicted amino acid sequence of the rat brain PCR product and the tryptic fragments of centaurin-α revealed that the human-expressed sequence tag contained a reading error that resulted in a frameshift. The 400-bp cDNA was used to screen a λZap II postnatal day 7 rat brain cDNA library. Of 25 positive clones identified, the largest, of 2,450 bp in length, was sequenced in both directions using a nested deletion strategy. The entire nucleotide sequence of this clone, designated centaurin-α, is presented in Fig. 3.
Fig. 3. Nucleotide and deduced amino acid sequence of cloned cDNA of centaurin-α.
The predicted amino acid sequence is expressed in the single-letter system. The nucleotide residue number is indicated along the left, and the amino acid residue is along the right. Amino acid sequences identical to microsequenced tryptic peptides are underlined.
Sequence Analysis of Centaurin-α cDNA and Proposed Amino Acid Sequence
Centaurin-α contains a 112-bp 5′-untranslated region lacking any in-frame stop codons, with a putative Kozak's consensus sequence upstream of the first ATG, an open reading frame of approximately 1.4 kb pairs, and a large 3′-untranslated region with a 3′-poly(A)+ tract. The predicted amino acid sequence of centaurin-α (Fig. 3) encodes a novel protein of 419 amino acids with a calculated molecular mass of 48 kDa, which also contains all of the tryptic peptides (underlined). The calculated molecular mass of centaurin-α protein is close to the molecular mass of 46 kDa estimated from SDS-PAGE of the native protein. Information from the calculated isoelectric point (8.82) predicted that overall, centaurin-α is likely to be a basic protein, which would be consistent with its ability to bind to the highly negatively charged inositol phospholipid. In addition, information from hydropathy analysis indicated that centaurin-α is a soluble protein lacking any putative membrane-spanning regions. However, a region at the NH2 terminus (see below) shows a stretch of 30 amino acids with considerable hydrophobic character.
Centaurin-α Contains a Putative Zinc Coordination Site and Ankyrin-like Repeats
Searching protein and nucleic acid sequence data bases indicated that centaurin-α shows significant homology to a number of other proteins, putative ORFs, and expressed sequence tags (more than 30 distinct sequences) found in animals, plants, and fungi. The most highly conserved region across all of these putative proteins lies in a region of approximately 50 residues, which may form a novel zinc coordination site around two pairs of cysteine residues with the consensus CX2CX16–17CX2C (Fig. 4, A and B). Within this region, centaurin-α shows greatest identity (56%) with the putative YIE4 protein of Saccharomyces cerevisiae and next (52%) to both a human protein RIP1 (a cofactor for retroviral Rev proteins) and a human ORF, KIAA0041, expressed in myeloid cells. Further, this region of centaurin-α has 42% identity with an Arf-GAP purified recently from rat liver (50). Outside of this region, centaurin-α shows considerably less homology to these proteins with overall only 25.9% conservation of identity with S. cerevisiae YIE4, 25.3% identity with the human ORF KIAA0041, and only 20% identity with the rat Arf-GAP (Fig. 4). However, the human EST (EST07218, Gen-Bank accession number T09325), the sequence that aided in the design of PCR primers, shows 91% identity to centaurin-α in a region outside of the putative zinc binding site. This suggests that none of these proteins is a direct homolog of the rat brain centaurin-α protein, but instead that each represents a different member of a large family of centaurin-α-related proteins.
Fig. 4.
Panel A, schematic presentation of conserved domains in centaurin-α and related proteins. Black bars indicate the positions of the potential Zn2+ coordinating cysteine residues; shaded boxes represent ankyrin repeat domains. In the S. cerevisiae GTS1/LSR1 protein, the region of homology with the Drosophila melanogaster Per proteins (61) is marked, as are the conserved nucleoporin-like FP repeats in the human RIP1/RabCCF protein (68, 69). The minimal domain having full Arf-GAP activity (50) is marked by a broken line under RnArf-GAP. A line beneath centaurin-α indicates a region of homology with TERM proteins (see Fig. 5C). The percentage identity between each protein and centaurin-α (produced by pairwise alignment using the GAP program of GCG8) is presented on the right side of the figure. Panel B, comparison of sequences in putative Zn2+ coordination region of centaurin-α and related proteins. The amino acid residue number marking the beginning of the sequence in each protein is given on the left side of the alignment. The percentage identity between each protein and centaurin-α in this region is presented on the right side of the alignment. Residues conforming to a consensus for the members of this family are marked above the sequence and within the alignment are indicated by bold type. In addition to rat centaurin-α, the sequences represented are: SpAC26a3 10, a putative S. pombe ORF (GenBank accession number Z69240); ScYIE4, the putative product of the S. cerevisiae YIE4 gene (accession number P40529); HsKIAA0041 and HsKIAA0050, two human putative ORFs (accession numbers D26069 and D30758); ScGCS1, the product of the S. cerevisiae GCS1 gene (59); ScGTS1/LSR1, the predicted product of the S. cerevisiae gene cloned both as GTS1 (61) and LSR1 (accession number X91489); ScSPS18, the putative product of the S. cerevisiae SPS18 sporulation-specific gene (60); HsRIP1/RabCCF, the cellular cofactor of retroviral Rev and Rex RNA regulatory proteins (68, 69); RnArf-GAP, the rat liver Arf-GAP (50); ScD971727, the predicted product of the S. cerevisiae putative ORF D71727 (accession number U33057); ScGLO3, the product of the S. cerevisiae GLO3 gene (59).
Our initial searches also indicated that centaurin-α showed homology to ankyrin and ankyrin-related proteins from a wide variety of species (for review, see Ref. 51). Subsequent analysis has indicated that centaurin-α itself contains 10 divergent ankyrin-like repeats (Fig. 5A). A variable number of such repeats, ranging from 4 to 12 repeats, is found in each of the other centaurin-α-related proteins. The sequences of the first and second repeats are the best conserved between different centaurin family members; this may reflect a conservation of function. Notably in centaurin-α, as well as in a number of the other proteins, the second ankyrin repeat (Fig. 5B) contains a sequence quence similar to one present in two InsPn-binding proteins, GAPInsP4-BP (12) and synaptotagmin II (11), including the presence of a conserved positive residue (asterisk) in the position of a lysine known to be critical for InsP4 binding to synaptotagmin.
Fig. 5.
Panel A, alignment of repeat sequences in centaurin-α with repeats 15–24 of human erythrocyte ankyrin. Residues in upper case show conservation of identity between the two proteins. Boxes indicate residues belonging to the same Dayhoff groups (GPAST, KRH, NQED, MILV, FWY, C) conserved between the two proteins. Consensus sequences for centaurin repeats and ankyrin repeats (54) are presented above and below the sequences, respectively. Asterisks indicate the positions of residues that are conserved in both consensus sequences. Panel B, comparison of sequences in second ankyrin repeat of centaurin-α with the inositol polyphosphate binding regions. The second ankyrin repeat of centaurin-α was compared with the C2B domains of mouse synaptotagmin II (MmSynaptotagminII; 11) and human InsP4-regulated ras-GAP (HsInsP4-BP; 12). The suggested consensus sequence for inositol polyphosphate binding is marked; + indicates a conserved positively charged residue, and residues conforming to this sequence are printed in bold in the alignment. The lysine residue demonstrated to be essential for InsP4 binding to synaptotagmin is marked with an * above the sequence. The sequence of repeat 16 of human ankyrin (54) and the consensus sequence for centaurin repeats derived in panel A are also presented. Panel C, alignment of a region including centaurin repeat 6 with homologous sequences in members of the TERM protein family. The repeat 6 region of centaurin-α was compared with some of the TERM family proteins (53): MmMerlin, L28176; HsEzrin, P15311; and HsMoesin, P26038. The consensus sequence for the centaurin-α repeats is shown above the alignment. The residues showing conservation within Dayhoff groups in all proteins are in bold, and a consensus sequence for this homology is presented below the alignment.
In the centaurin-α sequence, there is a region of homology with some of the TERM family proteins (talin, ezrin, radixin, moesin, and merlin), of which band 4.1, identified in erythrocytes, is a prototype (for review, see Ref. 53). The region of homology with the TERMs, present in the sixth repeat region of centaurin-α, is shown in Fig. 5C. The highest degree of similarity is with merlin, a tumor suppressor protein identified in neurofibromatosis type 2. The TERM family proteins have been proposed to link the actin cytoskeleton to membrane proteins in a variety of substructures, including microvilli, filopodia, and cell-cell junctions (53).
The Centaurin-α mRNA Transcript Is Highly Expressed in Rat Brain
A 3′ fragment (1,244 bp) was used to probe a multiple-tissue Northern blot to determine the tissue-specific distribution of centaurin-α. The Northern blot analysis revealed that a 2.5-kb mRNA is highly enriched in brain, with substantially lower but detectable levels in lung, kidney, and spleen (Fig. 6). The 2.5-kb transcript size on the Northern is in good agreement with the size of the isolated centaurin-α clone of 2,450 bp. In rat brain, other minor transcripts, one of which was 4.0 kb, were also detected with the centaurin-α probe, but at a considerably lower level of expression than the 2.5-kb transcript.
Fig. 6. Northern analysis of centaurin-α mRNA in rat tissues.
Each lane of the multiple tissue Northern contained 2 μg of poly(A)+ RNA. H, heart; B, brain; S, spleen; G, lung; V, liver; M, skeletal muscle; K, kidney; and T, testis. Gel locations of size markers of 9.2, 7.5, 4.4, 2.4, and 1.35 kb are indicated with dashes.
COS-7 Cell-expressed Centaurin-α Binds to InsP4 Affinity Resin
To ascertain whether the centaurin-α cDNA encodes for the PtdInsP3-binding protein that we purified, the coding sequence of the centaurin-α was subcloned into a mammalian expression vector and expressed in transfected COS-7 cells. The COS-7 cell-expressed centaurin-α protein was isolated from lysed cells by both heparin-agarose (not shown) and InsP4 affinity resin (Fig. 7A). The Coomassie-stained SDS-polyacryl-amide gel revealed the specific enrichment of a protein of 46 kDa (arrowhead), which corresponds, in molecular size, to centaurin-α from rat brain. The binding of the COS-7-expressed protein to the affinity column demonstrates that centaurin-α encodes a protein that contains a high affinity InsP4 resin binding site, similar to the endogenous 46-kDa protein. A bacterial fusion protein also bound to the heparin-agarose column under conditions used for the brain centaurin-α (not shown), which suggests that auxiliary proteins are probably not required to form the PtdInsP3 binding site in centaurin-α, and further, that this binding site is determined by the primary amino acid sequence.
Fig. 7. Characterization of native and recombinant centaurin-α using InsP4 affinity resin and anti-centaurin-α antibodies.
Panel A, lysates of COS-7 cells, mock-transfected or centaurin-α-transfected, were purified with InsP4 affinity resin. T, whole cell lysates; F, InsP4 affinity column flow-through; W, InsP4 affinity column wash; E, InsP4 affinity column eluate. A typical purification scheme is shown. The purification was repeated three times with similar results. The arrowhead highlights the purification of recombinant centaurin-α in InsP4 column eluate. Gel locations of molecular mass standards, 205, 116, 97.4, 66, and 45 kDa, are indicated with dashes. Panel B, fractions from rat brain and COS-7 cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-centaurin-α COOH-terminal fusion protein antibodies (J4) and anti-peptide NH2-terminal antibodies (J49). Lane 1, postnatal day 14 rat brain homogenate; lane 2, heparin-agarose column eluate from rat brain; lane 3, InsP4 affinity column eluate from rat brain; lane 4, mock-transfected COS-7 cell lysate; lane 5, p63d.2.13 (centaurin-α)-transfected COS-7 cell lysate. Molecular mass standards of 108, 84, 67, 55, and 39.5 kDa are indicated with dashes.
Centaurin-α Antibodies Recognize the Rat Brain 46-kDa Protein
Antibodies generated against either a synthetic NH2− terminal peptide of centaurin-α (J49) or a COOH-terminal fusion protein of centaurin-α (J4) recognized a 46-kDa protein band in immunoblot analysis (Fig. 7B) of fractions from rat brain homogenate, heparin-agarose column eluate, or InsP4 affinity column eluate (lanes 1–3). The J49 and J4 antibodies were also immunoreactive to the expressed centaurin-α protein in transfected COS-7 cell lysate (lane 5), but not in mock-transfected cell lysate (lane 4). Thus, antibodies raised against sequences generated from the cloned centaurin-α protein recognized the authentic 46-kDa rat brain centaurin-α protein and COS-7-expressed protein.
DISCUSSION
We have shown here the isolation and cloning of a 46-kDa protein, which we have named centaurin-α. Centaurin-α appeared to bind to the InsP4 resin with the highest affinity of any InsP4 binding protein identified. Paradoxically, although it interacted very tightly with the InsP4 affinity resin, initial binding studies with [3H]InsP4 and photolabeling with [125I]ASA-InsP4 indicated that centaurin-α did not bind InsP4 with high affinity (44, 47). We have demonstrated here that a more hydrophobic photoprobe, [3H]BZDC-InsP4, can efficiently label the centaurin-α in fractionated rat brain extracts. Important to this study is the observation that the hydrophobic benzophenone photophore, appended to an aminopropyl chain and attached via a phosphodiester linkage to Ins(1,3,4,5)P4, makes this photoaffinity label a good structural surrogate for the inositol phospholipid PtdInsP3 (Fig. 8). Both the InsP4 affinity column and the BZDC-InsP4 photolabel have three key substructures that mimic PtdIns(3,4,5)P3: (i) the correct 3,4,5-trisphosphate on a d-myo-inositol ring; (ii) linkage to a three-carbon chain through a P-1 phosphodiester linkage; and (iii) substantial hydrophobic character in the acyl or amidyl groups appended to the three-carbon chain. Thus, these two materials resemble PtdInsP3 more closely than they mimic the water-soluble InsP4. In an analogous fashion, [3H]BZDC-InsP3 was used as a mimic of PtdIns(4,5)P2 to accomplish the isozymeand domain-selective photocovalent modification of the pleckstrin homology binding domain of phospholipase Cδ1.3
Fig. 8. Comparison of [3H]BZDC-InsP4 and PtdInsP3 structures.
Both molecules have the 3,4,5-trisphosphate recognition element of d-myo-Ins(1,3,4,5)-P4, as well as a P-1 phosphodiester linked to a 3-carbon chain with a hydrophobic appendage.
The benzophenone group has largely replaced the arylazide as the photoprobe of choice for many biochemical studies (49). It is chemically more stable, does not decompose in ambient light, and selectively modifies hydrophobic binding regions in target macromolecules. Thus, [3H]BZDC-InsP3 was used to map the InsP3 binding site in the rat brain InsP3 receptor, but insufficient labeled protein was obtained with an [125I]ASA-modified photoprobe (48). An entire family of phosphoinositide photoaffinity probes (55) has been developed based on the efficacy of the BZDC-InsPn probes for selective covalent modification of target proteins in signaling pathways.
Displacement of the [3H]BZDC-InsP4 photoprobe with PI pathway intermediates indicated that centaurin-α had the highest affinity for synthetic dipalmitoyl PtdIns(3,4,5)P3 with an apparent IC50 of 120 nm. Since the photolabeling assay occurs under nonequilibrium conditions, the absolute values for binding constants are only approximate. Nonetheless, the rank order of displacement accurately reflects the relative affinity. The other inositol-containing compounds tested were less potent, with dipalmitoyl PtdInsP3 > gPInsP3> daPtdInsP3 > InsP4 > PtdInsP2 > gPInsP2 > InsP3, indicating that the 3-phosphate group on the inositol ring and the long chain fatty acids on the glycerol backbone were both important in ligand binding. Based on this specificity comparison, we propose that centaurin-α is an excellent candidate PtdIns(3,4,5)P3 receptor. As arachidonate is normally esterified at the C-2 position in endogenous PtdInsP3 (Fig. 8), it is possible that centaurin-α may bind the authentic PtdInsP3 with even higher affinity than synthetic PtdInsP3.
Both the bacterial and mammalian cell-expressed centaurin-α protein could be partially purified by heparin-agarose chromatography under the same conditions used to isolate the native rat brain protein. Moreover, the COS-7 cell expressed protein bound to the InsP4 affinity resin. Polyclonal antisera against a bacterial COOH-terminal fusion protein and an NH2-terminal peptide recognize both the native rat brain protein and COS-7 cell-expressed protein. Together with the tryptic peptide data and similar molecular size of the cloned protein, this expression and immunological data are evidence that the centaurin-α clone is identical to or very highly homologous to the 46-kDa PtdInsP3-binding protein isolated by InsP4 affinity chromatography.
The observation that both soluble and membrane-associated centaurin-α isolated from rat brain behave similarly in binding to the InsP4 affinity resin and the [3H]BZDC-InsP4 photoprobe suggests that centaurin-α can partition to the lipid bilayer. Receptor-stimulated membrane association is a common theme for many signaling proteins, including protein kinase C, PI 3-kinase, phospholipase A2, and phospholipase C (for review, see Ref. 20). Centaurin-α may be related to a 42-kDa InsP4-binding protein recently isolated from pig cerebella (56, 57) since two peptides from the 42-kDa protein show 100% identity to centaurin-α. However, the porcine 42-kDa protein is smaller than the rat 46-kDa protein described here, and a third peptide is only 70% identical to an NH2-terminal sequence in centaurin-α (56). Antibodies to the 42-kDa pig cerebellar protein recognize proteins in soluble and particulate fractions from the brain of several animal species, including rat (57). This may, therefore, represent a very closely related isoform of centaurin-α, perhaps arising through the use of alternative mRNA splicing. Consistent with this, we have observed that in addition to the major 2.5-kb centaurin-α transcript, brain also contains several minor mRNA species that could represent alternatively spliced centaurin mRNAs.
Data base searches revealed that centaurin-α is one of a family of related proteins represented in fungi, plants, and animals. The region of highest homology between each of the centaurin relatives is in the putative zinc coordination site. A wide variety of signaling proteins, including protein kinase Cs, chimaerins, rafs, and DAG kinases, contain zinc coordination sites. In these proteins, a pair of zinc binding sites composed of both cysteine and histidine residues cooperate to form an individual zinc “butterfly” domain. In protein kinase C, this zinc butterfly domain has been shown to form a hydrophobic cleft that provides the site of phorbol ester and DAG binding (58). In centaurin-α and its relatives, there is only a single potential zinc coordination site composed of four cysteine residues, which is distinct from that found in these other signaling proteins. However, hydropathy plots of centaurin(s) predict that this zinc finger domain comprises a highly hydrophobic region that could provide a surface for interaction with membrane lipids.
One allele of the centaurin homolog, GCS1, has a point mutation in one of these putative zinc-binding cysteines which gives rise to a mutant phenotype, similar to a null GCS1 mutant, which causes failure to resume growth from stationary phase at restrictive temperatures, indicating an important function for this region in cell regulation (59). Another centaurin relative has been identified recently as a GAP for the small molecular weight GTPase Arf (50). Again, the zinc coordination site appears to be critical for this activity as an equivalent cysteine substitution abolishes Arf-GAP activity in this protein in vitro. GCS1 has also been reported to show Arf-GAP activity. Less is known about the biological activities of other centaurin relatives. In yeast, SPS 18 has been implicated in the process of sporulation (60), whereas mutations affecting the GTS1/LSR1 gene lead to alterations in timing of budding and cell size determination (61). Although the zinc coordination region is highly conserved in centaurin-α and its relatives, it remains to be seen whether they also possess GAP activity toward Arf or other small GTPases. Further, it is not yet clear whether other members of the centaurin family are capable of binding PtdInsP3 or what may be the consequences of this binding for their function.
Recently, potential PtdIns(4,5)P2 binding motifs have been identified in numerous proteins. In the pleckstrin homology domain of α-actinin, phospholipase Cδ, β-spectrin, and Grb-7, a subdomain RX7(H/R/K)X2–3W(R/K) has been identified as a PtdInsP2 binding site consensus sequence (62). Centaurin-α does not have homology with the pleckstrin homology domain or this pleckstrin homology subdomain, nor does it have homology with a distinct PtdInsP2 binding region identified in cofilin (63). We have, however, located a basic sequence, PX13–14 (R/K)X4 (R/K)XKX5FX6E, in centaurin-α which is also found in the C2B domains of the InsP4-binding protein GAPInsP4-BP (12) and InsP4/InsP6-binding protein synaptotagmin II (11), which may be involved in the binding of the 3,4,5-trisphosphate moiety of PtdInsP3. This region is located within the second ankyrin repeat in centaurin-α and is partially conserved in other family members.
All the members of the centaurin family also contain a variable number of repetitive domains which show homology to those found in the cytoskeletal protein ankyrin and a wide variety of other proteins (51). The potential importance of these repeats in the centaurin family is provided by the observation that truncations of GCS1, in which the protein terminates within the fourth or fifth ankyrin repeats, produce phenotypes similar to that in the mutant cysteine (zinc coordination) allele. The fact that these sequences are divergent in centaurin family members may indicate that these regions are responsible for providing specific interaction information that contributes distinct activities and specialization to each member. Specific cytoskeletal association may be predicted as centaurin-α also has a short region of homology with the TERM proteins (53), which have been shown to be involved in linking membrane proteins to the actin cytoskeleton.
Data from many reports have led to the prediction that PtdInsP3 is involved in regulating actin cytoskeletal rearrangements and/or vesicle trafficking events (15, 22–24, 27–30). The finding here that a PtdInsP3-binding protein has homology to a family of proteins which may regulate Arf-GTPase activity supports the suggestion that PI 3-kinase-coupled receptors might act via centaurin-α to regulate Arf-dependent postendosomal membrane trafficking events (64) which transport lipid and protein components to the plasma membrane during processes such as ruffling, glucose transporter translocation, or neurite outgrowth. Further, centaurin(s) may provide a link between PtdInsP3 production and receptor regulation of phospholipase D activation, a process shown to be sensitive to inhibition of PI 3-kinase (65) and to involve the regulation of Arf proteins (66, 67).
In conclusion, we propose that centaurin-α is a member of a new class of PtdInsP3-binding proteins which may have a role in mediating downstream events stimulated by growth/trophic factor activation of PI 3-kinase in the brain. The predicted homologies of centaurin-α with other proteins, its affinity for PtdInsP3, and its partitioning between soluble and membrane compartments are consistent with it playing a pivotal role linking regulation of the actin cytoskeleton and/or specific vesicle movements to changes in PtdInsP3 production in the lipid bilayer. Whether a cell signaling pathway linking PtdInsP3 production to centaurin-α and the regulation of Arf-GTPase activities is important in the brain awaits future investigation.
Acknowledgments
We thank Dr. Craig Garner for providing the cDNA library and for valuable discussion, Dr. Roy Gigg for synthesis of the dipalmitoyl PtdInsP3, Dr. Peter Cullen and Dr. Phillip Hawkins for the gPInsP3, and Christina Burga for technical support. We also thank Dr. D. Ahern (DuPont NEN) and Dr. J. Olszewski for providing [3H]BZDC-NHS ester.
Footnotes
This work was supported in part by National Institutes of Mental Health Grants R29MH50102 and DDRC P50HD32901 (to A. B. T.). Work at Stony Brook was supported by National Institutes of Health Grant NS29632 (to G. D. P.). The first two authors contributed equally to this study. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) U51013.
The abbreviations used are: PI, phosphoinositide; InsPn, inositol polyphosphate; Ins(1,4,5)P3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; InsP4, inositol 1,3,4,5-tetrakisphosphate; InsP5, inositol pentakisphosphate; InsP6, inositol hexakisphosphate; PtdIns(3)P, phosphatidylinositol 3-phosphate; PtdIns(4)P, phosphatidylinositol 4-phosphate; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PtdInsP3, phosphatidylinositol 3,4,5-trisphosphate; TEAB, triethyl-ammonium bicarbonate; [125I]ASA-InsP4, 1-O-[125I](3-[2-iodo-4-azidosalicylamidyl]propyl)-inositol tetrakisphosphate; [3H]BZDC-InsP4, 1-O-[3H](3-[4-benzoyldihydrocinnamidyl]propyl)-inositol tetrakisphosphate; BZDC-NHS, 4-benzoyldihydrocinnamoyl N-hydroxysuccinimide ester; HPLC, high performance liquid chromatography; gPInsP3, glycerophosphoinositol 3,4,5-trisphosphate; daPtdInsP3, diacetylphosphatidylinositol 3,4,5-trisphosphate; gPInsP2, glycerophosphoinositol 4,5-bisphosphate; GAP, GTPase-activating protein; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; PAGE, polyacryl-amide gel electrophoresis; PCR, polymerase chain reaction; bp, base pair(s); kb, kilobase(s); ORF, open reading frame.
The chimaeric nature of centaurin-α and its homology to a family of GTPase-activating proteins is reminiscent of another distinct family of molecules named the chimaerins (5), and we have therefore named our protein after another chimaera, the centaur (half-man, half-horse) of Greek mythology.
E. Tall, G. Dorman, P. Garcia, S. Shah, G. D. Prestwich, and M. J. Rebecchi, submitted for publication.
REFERENCES
- 1.Michell RH. Trends Biochem. Sci. 1992;17:274–276. doi: 10.1016/0968-0004(92)90433-a. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 3.Ferris CD, Snyder SH. Annu. Rev. Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Nishizuka Y. J. Biochem. 1994;115:1029–1034. doi: 10.1093/oxfordjournals.jbchem.a124451. [DOI] [PubMed] [Google Scholar]
- 5.Liscovitch M, Cantley LC. Cell. 1994;77:329–334. doi: 10.1016/0092-8674(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 6.Menniti FS, Oliver KG, Putney JW, Shears SB. Trends Biochem. Sci. 1993;18:53–56. doi: 10.1016/0968-0004(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 7.Voglmaier SM, Keen JH, Murphy JE, Ferris CD, Prestwich GD, Snyder SH, Theibert AB. Biochem. Biophys. Res. Commun. 1992;187:158–163. doi: 10.1016/s0006-291x(05)81473-1. [DOI] [PubMed] [Google Scholar]
- 8.Timerman AP, Mayrleitner MM, Lukas TJ, Chadwich CC, Saito A, Watterson DM, Schindler H, Fleischer S. Proc. Natl. Acad. Sci. U. S. A. 1992;89:12655–12662. doi: 10.1073/pnas.89.19.8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye W, Ali N, Bembenek ME, Shears SB, Lafer EM. J. Biol. Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- 10.Fleischer B, Xie J, Mayrleitner M, Shears SB, Palmer DJ, Fleischer S. J. Biol. Chem. 1994;269:17826–17832. [PubMed] [Google Scholar]
- 11.Fukuda M, Aruga M, Niinobe M, Aimoto S, Mikoshiba K. J. Biol. Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 12.Cullen PJ, Hsuan JJ, Troung O, Letcher AJ, Jackson TR, Dawson AP, Irvine RF. Nature. 1995;376:527–530. doi: 10.1038/376527a0. [DOI] [PubMed] [Google Scholar]
- 13.Janmey PA. Annu. Rev. Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 14.Liscovitch M, Cantley LC. Cell. 1995;81:659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 15.De Camilli P, Emr SD, McPherson PS, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 16.Traynor-Kaplan AE, Harris AL, Thompson BL, Taylor P, Sklar LA. Nature. 1988;334:353–356. doi: 10.1038/334353a0. [DOI] [PubMed] [Google Scholar]
- 17.Stephens LR, Hughes KT, Irvine RF. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins PT, Jackson TR, Stephens LR. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- 19.Serunian LA, Haber MT, Fukui T, Kim JW, Rhee SG, Lowenstein JM, Cantley LC. J. Biol. Chem. 1989;264:17809–17815. [PubMed] [Google Scholar]
- 20.Stephens LR, Jackson TR, Hawkins PT. Biochim. Biophys. Acta. 1993;1179:27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 21.Fantl WJ, Escobedo JA, Martin GA, Turck CW, del Rosario M, McCormick F, Willians LT. Cell. 1992;69:413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- 22.Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Curr. Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 23.Wymann M, Acaro A. Biochem. J. 1994;298:517–520. doi: 10.1042/bj2980517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotani K, Yanezawa K, Hara K, Ueda H, Kitamura Y. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins PT, Equinoa A, Qiu R-G, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, Stephens L. Curr. Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. J. Biol. Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 27.Hara K, Yonezawa K, Sakaue H, Ando A, Kotani K, Kitamura T, Kitamura Y, Ueda H, Stephens L, Jackson TR, Hawkins PT, Dhand R, Clark AE, Holman GD, Waterfield MD, Kasuga M. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Mol. Cell. Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joly M, Kazlauskas A, Corvera S. J. Biol. Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- 30.Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 31.Burgering BMT, Coffer PJ. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 32.Franke TF, Yang S-I, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan D, Tsichlis PN. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 33.Kohn AD, Kovacina KS, Roth R. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. J. Biol. Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 35.Jackson TR, Blader IJ, Hammonds-Odie LP, Burga CR, Cooke F, Hawkins PT, Wolf AG, Heldman KA, Theibert AB. J. Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa W, Roth RA. Biochim. Biophys. Acta. 1994;1224:533–540. doi: 10.1016/0167-4889(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 37.Rameh LE, Chen C-S, Cantley LC. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Falck JR, Reddy KK, Abrams CS, Zhao W, Rittenhouse SE. J. Biol. Chem. 1995;270:22807–22810. doi: 10.1074/jbc.270.39.22807. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi H, Brewer KA, Exton JH. J. Biol. Chem. 1993;268:13–18. [PubMed] [Google Scholar]
- 40.Singh SS, Chauhan A, Brockerhoff H, Chauhan VP. Biochem. Biophys. Res. Commun. 1993;195:104–112. doi: 10.1006/bbrc.1993.2016. [DOI] [PubMed] [Google Scholar]
- 41.Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC. J. Biol. Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 42.Palmer RH, Dekker LV, Woscholski R, Le Good JA, Gigg R, Parker PJ. J. Biol. Chem. 1995;270:22412–22416. doi: 10.1074/jbc.270.38.22412. [DOI] [PubMed] [Google Scholar]
- 43.Estevez VA, Prestwich GD. J. Am. Chem. Soc. 1991;113:9885–9887. [Google Scholar]
- 44.Theibert AB, Estevez VA, Ferris CD, Danoff SK, Barrow RK, Prestwich GD, Snyder SH. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3165–3169. doi: 10.1073/pnas.88.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olszewski JD, Dorman G, Elliot JT, Hong Y, Ahern DG, Prestwich GD. Bioconjugate Chem. 1995;6:395–400. doi: 10.1021/bc00034a009. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhary A, Dorman G, Prestwich GD. Tetrahedron Lett. 1994;35:7521–7524. [Google Scholar]
- 47.Theibert AB, Estevez VA, Mourey RJ, Marecek JF, Barrow RK, Prestwich GD, Snyder SH. J. Biol. Chem. 1992;267:9071–9079. [PubMed] [Google Scholar]
- 48.Mourey RJ, Estevez VA, Marecek JF, Barrow RK, Prestwich GD, Snyder SH. Biochemistry. 1993;32:1719–1726. doi: 10.1021/bi00058a004. [DOI] [PubMed] [Google Scholar]
- 49.Dorman G, Prestwich GD. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 50.Cukierman E, Huber I, Rotman M, Cassel D. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- 51.Bork P. Proteins. 1993;17:363–374. doi: 10.1002/prot.340170405. [DOI] [PubMed] [Google Scholar]
- 52.Deleted in proof [Google Scholar]
- 53.Arpin M, Algrain M, Louvard D. Curr. Opin. Cell Biol. 1994;6:136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 54.Lambert S, Yu H-I, Prchal JT, Lawler J, Ruff P, Speicher D, Cheung MC, Kan YW, Palek J. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prestwich GD, Dorman G, Elliott JT, Marecak DM, Chaudhary A. Photochem. Photobiol. 1996 doi: 10.1111/j.1751-1097.1997.tb08548.x. in press. [DOI] [PubMed] [Google Scholar]
- 56.Reiser G, Kunzelmann U, Hulser E, Stricker R, Hoppe J, Lottspeich F, Kalbacher H. Biochem. Biophys. Res. Commun. 1994;214:20–27. doi: 10.1006/bbrc.1995.2251. [DOI] [PubMed] [Google Scholar]
- 57.Stricker R, Kalbacher H, Lottspeich F, Reiser G. FEBS Lett. 1995;370:236–240. doi: 10.1016/0014-5793(95)00822-q. [DOI] [PubMed] [Google Scholar]
- 58.Zhang G, Kazanietz MG, Blumberg PM, Hurley JH. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 59.Ireland LS, Johnston GC, Drebot MD, Dhillon N, De Maggio AJ, Hoekstra MF, Singer RA. EMBO J. 1994;13:3812–3821. doi: 10.1002/j.1460-2075.1994.tb06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coe JG, Murray LE, Dawes IW. Mol. & Gen. Genet. 1994;244:661–672. doi: 10.1007/BF00282757. [DOI] [PubMed] [Google Scholar]
- 61.Mitsui K, Yaguchi S, Tsurugi K. Mol. Cell. Biol. 1994;14:5569–5578. doi: 10.1128/mcb.14.8.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fukami K, Sawada N, Endo T, Takenawa T. J. Biol. Chem. 1996;271:2646–2650. doi: 10.1074/jbc.271.5.2646. [DOI] [PubMed] [Google Scholar]
- 63.Yonezawa N, Homma Y, Yahara I, Sakai H, Nishida E. J. Biol. Chem. 1991;266:17218–17221. [PubMed] [Google Scholar]
- 64.Whitney JA, Gomez M, Sheff D, Kreiss TE, Mellman I. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 65.Standaert ML, Avignon A, Yamada K, Bandyopadhyay G, Farese RV. Biochem. J. 1996;313:1039–1046. doi: 10.1042/bj3131039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 67.Hammond SM, Altshuller YM, Sung T-C, Rudge SA, Rose K, Engebrecht J, Morris AJ, Frohman MA. J. Biol. Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 68.Fritz CC, Zapp ML, Green MR. Nature. 1995;376:530–533. doi: 10.1038/376530a0. [DOI] [PubMed] [Google Scholar]
- 69.Bogerd HP, Fridell RA, Madore S, Cullen BR. Cell. 1995;82:485–494. doi: 10.1016/0092-8674(95)90437-9. [DOI] [PubMed] [Google Scholar]









