Abstract
Mechanisms reducing inbreeding are thought to have evolved owing to fitness costs of breeding with close relatives. In small and isolated populations, or populations with skewed age- or sex distributions, mate choice becomes limited, and inbreeding avoidance mechanisms ineffective. We used a unique individual-based dataset on moose from a small island in Norway to assess whether inbreeding avoidance was related to population structure and size, expecting inbreeding avoidance to be greater in years with larger populations and even adult sex ratios. The probability that a potential mating event was realized was negatively related to the inbreeding coefficient of the potential offspring, with a stronger relationship in years with a higher proportion or number of males in the population. Thus, adult sex ratio and population size affect the degree of inbreeding avoidance. Consequently, conservation managers should aim for sex ratios that facilitate inbreeding avoidance, especially in small and isolated populations.
Keywords: Alces alces, adult sex ratio, population size
1. Introduction
The fitness costs of inbreeding [1] can lead to the evolution of inbreeding avoidance mechanisms [2–4]. Such mechanisms may involve active kin recognition and discrimination [2,5] or spatial displacement from relatives [2,5]. With habitat fragmentation and isolation, reduced dispersal may increase the cost of inbreeding avoidance [6]. Moreover, small populations and populations with skewed adult sex ratios offer less opportunity for mate choice, and individuals may have to choose between inbreeding or abstaining from reproduction [7]. Mate choice also depends on factors such as age, body size or ornamentation [8], so mating may also entail a trade-off between such factors and relatedness [9].
Small and isolated populations are subjected to increased risk of extinction, following reduced genetic variation and increased susceptibility to demographic stochasticity and inbreeding [10]. Specifically, conservation concern has been raised for the demographic consequences following lower recruitment rates [11,12]. Because population genetic processes that increase extinction risk are particularly relevant in such populations [13,14], understanding how demography and genetics interact in processes such as inbreeding and inbreeding avoidance is important [4].
To assess whether inbreeding avoidance is affected by mate choice opportunities, we estimated relatedness among individuals from a small and isolated moose (Alces alces) population in Norway based on a near complete pedigree [15]. Moose are a long-lived species that can start reproduction as yearlings, and later often give birth to twins [16]. Moose is sexually size-dimorphic and polygamous, and sexual selection on morphology and mating is likely influenced both by female mate choice and male–male competition [17]. When the population is large and has a high male–female ratio, females have more opportunities to choose among mates. We hypothesized that increased choice among potential male mates would increase the probability of inbreeding avoidance. Moreover, because reproductive effort tends to increase with age in males [18], and females prefer mating with older males in most ungulates [8], we expected more inbreeding avoidance in years with higher mean male age. Finally, we predicted increased inbreeding in years with low variation in relatedness and high mean relatedness, which provide fewer opportunities for inbreeding avoidance [4].
2. Material and methods
(a). Study area, data collection and construction of pedigree
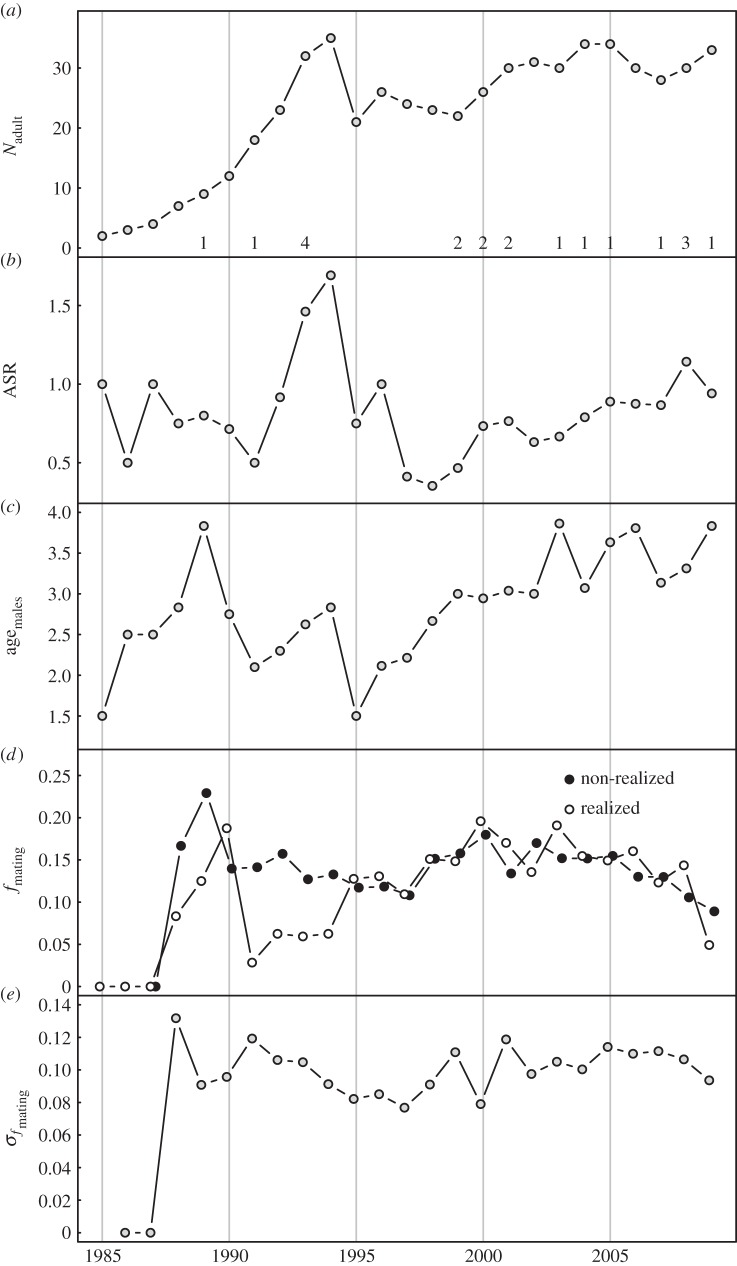
Situated 13 km off the coast of Norway, the island Vega (119 km2, 65°40′ N, 11°55′ E) provides excellent moose habitat [16]. The island was colonized by three moose in 1985 and immigration has been relatively low (figure 1a). Harvesting started in 1989 and has kept the number of breeding moose between 20 and 40. Variation in the proportion of adult males in the harvesting quotas [16] has caused large fluctuations in the age and sex-structure (figure 1a–c).
Figure 1.
Annual variation in properties of the moose population at Vega. (a) Absolute adult (≥1.5 years) population size (Nadult) with numbers of immigrants at their first potential mating year (unknown immigration year), (b) adult sex ratio (ASR), (c) mean age of adult males (agemales), (d) annual mean fmating of realized (gave offspring) and non-realized potential mating events, (e) standard deviation of fmating, σfmating.
Among 445 moose ever observed on Vega, we sampled and genotyped 388 individuals using 22 microsatellite loci (protocols described in reference [15]). We used parentage assignment and social information to identify both parents for 367 sampled individuals, where all twin pairs were identified as full-siblings. Twenty-five unsampled calves were assigned through the twin sibling (observed at marking), whereas 25 unsampled calves with known social mother were not assigned a father and were excluded from the analyses. Twenty immigrants were identified by lack of assigned parents. Following Wright [19], we calculated the identity-by-descent inbreeding coefficient, f, for all individuals (mean = 0.12, s.d. = 0.11, range = 0.00–0.47). Among the 392 calves, 284 belonged to twin pairs, giving 250 breeding events.
(b). Inbreeding avoidance and population characteristics
For all reproductively active females, we used pedigree data to calculate offspring f for every possible mating event, fmating, both realized and non-realized. We considered all yearling males or older to be able to mate. We assume that inbreeding level in realized offspring reflects relatedness between mating individuals during rut, which may not hold if abortion or neonatal mortality are related to inbreeding level. However, given the high recruitment rate in the population [16], we believe such a bias to be minor.
We analysed whether a potential mating was realized or not in generalized linear-mixed models (binomial family, logit link), with year and female as random factors. With any inbreeding avoidance, we expected the probability of realization, P(realization), to be negatively related to fmating. By using the interaction between fmating and the annual mean f-value for all possible mating events, fmean mating, we assessed whether inbreeding avoidance was stronger in years with overall higher f-values. Likewise, we calculated the annual variation in f for possible matings, σfmating, and tested the interaction between fmating and σfmating.
The number of possible mating events, and thereby the probability that a potential mating event was realized a given year, depends on population size, Nadult, and adult sex ratio, ASR = Nmales/Nfemales, and we therefore accounted for Nadult and ASR in all models. We included the interaction between fmating and Nadult, and fmating and ASR to assess whether inbreeding avoidance was higher in years with more potential mates (higher Nadult) or higher proportion of males (higher ASR). We also included a three-way interaction between fmating, ASR and Nadult. This was included because the absolute number of potential mating events increases multiplicatively with ASR and Nadult, and any effect of ASR on the relationship between fmating and P(realization) would therefore be expected to change if Nadult changes. Finally, we included the interaction between fmating and annual mean male age, agemales, as we predicted older males to be of higher quality. A higher proportion of old males in the population is expected to decrease the constraints put on females with regard to trade-off between mate quality and relatedness.
We used AICc [20] to rank candidate models (see the electronic supplementary material S2 for details regarding model selection). We excluded years prior to 1988 owing to no opportunity for females to choose among different levels of relatedness (σfmating = 0, figure 1d,e).
3. Results
Among the 250 realized breeding events, 67 involved outbreeding (f = 0) and 183 involved inbreeding (f > 0.01). In 29 cases, the female and male were close relatives (parent–offspring or full siblings). Among the 2454 non-realized mating events, 485 would have involved outbreeding and 214 inbreeding between close relatives.
The highest-ranked model explaining P(realization) included ASR, Nadult and fmating, as well as all their two-way and three-way interactions, and the main effect of agemales (see the electronic supplementary material S2 and table 1). An equally supported model (ΔAICc = 0.08) did not include agemales. The parameter estimate of agemales from model 1 was uncertain, and other parameter estimates were similar between the two models (table 1). We therefore present results from model 2. According to this model, P(realization) was related to fmating, but the relationship was affected by ASR and Nadult (figure 2). When Nadult and ASR were high (high male availability), there was a clear negative relationship between fmating and P(realization), indicating significant inbreeding avoidance. At low male availability (low ASR and/or NAdult), the relationship was considerably weaker suggesting less prominent inbreeding avoidance (figure 2 and the electronic supplementary material S3).
Table 1.
Parameter estimates (95% credible interval, CI) for the two highest-ranked models explaining the probability that a potential mating event was realized. See text for explanation of the variables. CIs are based 2.5% and 97.5% quantiles from 10 000 MCMC resampling from the posterior distribution of the parameter estimates.
| parameter name | model 1 (ΔAICc = 0.00) | model 2 (ΔAICc = 0.08) |
|---|---|---|
| intercept | 4.392 (1.037; 7.842) | 4.421 (0.994; 7.792) |
| fmating | –28.560 (–51.828; –5.211) | –27.980 (–51.051; –4.684) |
| ASR | –5.830 (–10.588; –1.094) | –5.717 (–10.421; –0.872) |
| Nadult | –0.253 (–0.375; –0.135) | –0.231 (–0.346; –0.115) |
| agemales | 0.190 (–0.061; 0.450) | — |
| fmating * ASR | 31.368 (–0.727; 63.317) | 31.538 (–0.729; 643.462) |
| fmating * Nadult | 1.122 (0.308; 1.929) | 1.096 (0.284; 1.895) |
| ASR * Nadult | 0.200 (0.048; 0.354) | 0.192 (0.037; 0.343) |
| fmating * ASR * Nadult | –1.241 (–2.311; –0.164) | –1.240 (–2.293; –0.159) |
Figure 2.
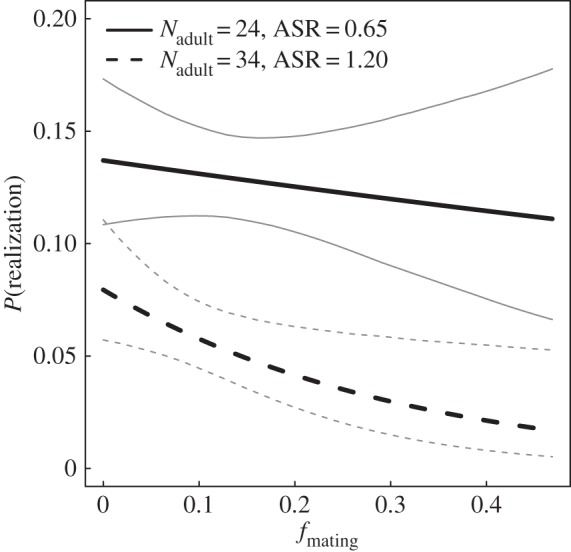
Predicted relationship between the probability that a potential mating was realized, P(realization), and inbreeding value of the resulting offspring, fmating (parameter estimates in table 1). Relationships are shown for 15% and 85% percentiles of adult population size (Nadult) and adult sex ratio (males/females, ASR) from the data used in the statistical model.
4. Discussion
Fitness of Vega moose is negatively related to parental relatedness [15], as is common in wild populations [3], suggesting that inbreeding avoidance is beneficial and expected to occur if allowed by the population structure. We found that the probability for realization of potential mating events was negatively associated with parental relatedness, but the strength of the relationship was affected by the number of males available for mating (figure 2).
In isolated populations, individuals cannot rely on emigration or immigration to minimize inbreeding. Inbreeding avoidance must therefore be based on recognition of related individuals [2,4]. However, rejecting a related mate can result in reproduction failure. Such a fitness cost becomes increasingly likely as mate availability decreases [9]. This can explain why female moose accepted higher levels of inbreeding in years with low availability of males.
Many populations experience fluctuations in size and age- or sex structure, particularly managed populations [21]. This may have demographic consequences, for example, if an inadequate number of males leads to lower recruitment [11,12], but will also affect the genetic structure through increased genetic drift [1,22]. While demographic consequences often can be restored relatively fast by changing the mortality pattern (e.g. altering harvesting quotas), high inbreeding levels and loss of genetic diversity are long-lasting and can only be compensated for by actions such as immigration or translocation [23], which raise other evolutionary and demographic concerns. To reduce loss of genetic diversity in small and isolated populations, managers should aim for sex ratios that facilitate inbreeding avoidance.
Supplementary Material
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.204bc.
Authors' contributions
H.H., I.H. and B.E.S. developed the concept and idea. H.H. performed genetic analyses and constructed the pedigree in collaboration with K.H.R. Statistical analyses were designed and performed by I.H. with input from H.H., E.J.S. and B.E.S., and M.H. was in charge of the fieldwork. I.H. and H.H. wrote the paper with input from all co-authors.
Funding statement
The study was funded by the Norwegian Environment Agency and the Norwegian Research Council (PRIBIO no. 19304/V40 and SFF III 223257/F50).
Conflict of interest
The authors have no conflict of interests.
References
- 1.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. ( 10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 2.Pusey A, Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. ( 10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 3.Crnokrak P, Roff DA. 1999. Inbreeding depression in the wild. Heredity 83, 260–270. ( 10.1038/sj.hdy.6885530) [DOI] [PubMed] [Google Scholar]
- 4.Szulkin M, Stopher KV, Pemberton JM, Reid JM. 2013. Inbreeding avoidance, tolerance, or preference in animals? Trends Ecol. Evol. 28, 205–211. ( 10.1016/j.tree.2012.10.016) [DOI] [PubMed] [Google Scholar]
- 5.Matthysen E. 2012. Multicausality of dispersal: a review. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM.), pp. 3–18. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Banks SC, Ward SJ, Lindenmayer DB, Finlayson GR, Lawson SJ, Taylor AC. 2005. The effects of habitat fragmentation on the social kin structure and mating system of the agile antechinus, Antechinus agilis . Mol. Ecol. 14, 1789–1801. ( 10.1111/j.1365-294X.2005.02535.x) [DOI] [PubMed] [Google Scholar]
- 7.Miller KA, Nelson NJ, Smith HG, Moore JA. 2009. How do reproductive skew and founder group size affect genetic diversity in reintroduced populations? Mol. Ecol. 18, 3792–3802. ( 10.1111/j.1365-294X.2009.04315.x) [DOI] [PubMed] [Google Scholar]
- 8.Clutton-Brock TH. 1988. Reproductive success: Studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Kokko H, Ots I. 2006. When to not avoid inbreeding. Evolution 60, 467 ( 10.1111/j.0014-3820.2006.tb01128.x) [DOI] [PubMed] [Google Scholar]
- 10.Lande R. 1998. Anthropogenic, ecological and genetic factors in extinction and conservation. Res. Popul. Ecol. 40, 259–269. ( 10.1007/BF02763457) [DOI] [Google Scholar]
- 11.Ginsberg J, Milner-Gulland E. 1994. Sex-biased harvesting and population dynamics in ungulates: implications for conservation and sustainable use. Conserv. Biol. 8, 157–166. ( 10.1046/j.1523-1739.1994.08010157.x) [DOI] [Google Scholar]
- 12.Mysterud A, Coulson T, Stenseth NC. 2002. The role of males in the dynamics of ungulate populations. J. Anim. Ecol. 71, 907–915. ( 10.1046/j.1365-2656.2002.00655.x) [DOI] [Google Scholar]
- 13.Berec L, Angulo E, Courchamp F. 2007. Multiple Allee effects and population management. Trends Ecol. Evol. 22, 185–191. ( 10.1016/j.tree.2006.12.002) [DOI] [PubMed] [Google Scholar]
- 14.Vercken E, Vincent F, Mailleret L, Ris N, Tabone E, Fauvergue X, Gurney W. 2013. Time-lag in extinction dynamics in experimental populations: evidence for a genetic Allee effect? J. Anim. Ecol. 82, 621–631. ( 10.1111/1365-2656.12051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haanes H, Markussen SS, Herfindal I, Røed KH, Solberg EJ, Heim M, Midthjell L, Sæther B-E. 2013. Effects of inbreeding on fitness-related traits in a small isolated moose population. Ecol. Evol. 3, 4230–4242. ( 10.1002/ece3.819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solberg EJ, Rolandsen CM, Heim M, Linnell JDC, Herfindal I, Sæther B-E. 2010. Age and sex-specific variation in detectability of moose (Alces alces) during the hunting season: implications for population monitoring. Eur. J. Wildl. Res. 56, 871–881. ( 10.1007/s10344-010-0385-x) [DOI] [Google Scholar]
- 17.Franzmann AW, Schwartz CC. 1997. Ecology and management of the North American moose. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 18.Mysterud A, Solberg EJ, Yoccoz NG. 2005. Ageing and reproductive effort in male moose under variable levels of intrasexual competition. J. Anim. Ecol. 74, 742–754. ( 10.1111/j.1365-2656.2005.00965.x) [DOI] [Google Scholar]
- 19.Wright S. 1922. Coefficients of inbreeding and relationship. Am. Nat. 56, 330–338. ( 10.1086/279872) [DOI] [Google Scholar]
- 20.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. A practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 21.Sæther B-E, Engen S, Solberg EJ. 2001. Optimal harvest of age-structured populations of moose Alces alces in a fluctuating environment. Wildl. Biol. 7, 171–179. [Google Scholar]
- 22.Sæther B-E, Engen S, Solberg EJ. 2009. Effective size of harvested ungulate populations. Anim. Conserv. 12, 488–495. ( 10.1111/j.1469-1795.2009.00278.x) [DOI] [Google Scholar]
- 23.Ebert D, Haag C, Kirkpatrick M, Riek M, Hottinger JW, Pajunen VI. 2002. A selective advantage to immigrant genes in a Daphnia metapopulation. Science 295, 485–488. ( 10.1126/science.1067485) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.204bc.