Abstract
Chimpanzees are well known for their tool using abilities. Numerous studies have documented variability in tool use among chimpanzees and the role that social learning and other factors play in their development. There are also findings on hand use in both captive and wild chimpanzees; however, less understood are the potential roles of genetic and non-genetic mechanisms in determining individual differences in tool use skill and laterality. Here, we examined heritability in tool use skill and handedness for a probing task in a sample of 243 captive chimpanzees. Quantitative genetic analysis, based on the extant pedigrees, showed that overall both tool use skill and handedness were significantly heritable. Significant heritability in motor skill was evident in two genetically distinct populations of apes, and between two cohorts that received different early social rearing experiences. We further found that motor skill decreased with age and that males were more commonly left-handed than females. Collectively, these data suggest that though non-genetic factors do influence tool use performance and handedness in chimpanzees, genetic factors also play a significant role, as has been reported in humans.
Keywords: tool use, handedness, heritability, motor skill, chimpanzees, sex differences
1. Introduction
Tool use has been described in many species, but apart from humans, the complexity and variability found in chimpanzees is unmatched in the animal kingdom [1–6]. A variety of forms of tool use have been described in different great apes throughout Africa and Asia, and it has been suggested that social learning and local traditions have perpetuated within- and between-community variation in the acquisition of tool use in chimpanzees [6–8] and other great apes [9]. Many have suggested that the evolution of tool manufacture and use in humans had profound effects on a variety of complex cognitive functions such as language and speech, as well as increased brain size and complexity, including lateralization in structure and function in hand use [10–16]. Specifically, it has been hypothesized that the left hemisphere's specialization for praxic and speech functions evolved from adaptation in early hominids for tool manufacture and use [14,17,18]. In these evolutionary models, speech co-opted neural and biological systems involved in manual motor actions, such as prehensile grasping and tool use, with increasing selection for fine motor skill necessary for the articulation of speech in early humans, after the split from the common ancestor with chimpanzees.
Although there are now very good descriptions of various forms of tool use in many communities of chimpanzees, far less is known about the mechanisms that underlie individual and phylogenetic variability. Studies in wild and captive apes have demonstrated that learning to use tools can take many years [19–21]. Moreover, there is some evidence that females show a greater propensity and interest in tools and learn to use them at an earlier age than males [22,23]. Indeed, the claim that females have a greater propensity to use tools has also been reported in captive bonobos (Pan paniscus), a species for which evidence of tool use in the wild is relatively sparse [24,25]. One interpretation of the sex difference in tool use in chimpanzees is that female offspring spend more time in closer proximity to their mother when she is using tools compared with male offspring. The greater proximity of the female offspring affords them greater opportunity to learn the necessary actions and substrates needed for successful tool use.
Less studied but arguably equally important is tool use performance in both wild and captive chimpanzees and indeed, all primates [26–35]. As noted above, most studies of tool use in wild and captive chimpanzees have focused on the role that subject characteristics such as sex, age or rearing experience have on the usage frequency of different tools rather than their actual performance [36,37]. This is unfortunate, because presumably better performance or skill on a given tool-use task, particularly those that yield access to food resources, would likely be beneficial to the individual. In humans, there is a rich body of research on the effects of subject, experimental and genetic influences on motor skill. For instance, there is evidence of sex differences in tool use, with female humans reported to show better performance on fine motor tasks, whereas males perform better on tasks requiring more ballistic movements [38]. There is also a large body of data showing age-related changes in motor skill in humans [39], and there is evidence that tool-use learning and skill are both heritable [40,41].
In this report, we examined the role of both genetic and non-genetic factors on individual differences in motor performance and hand use for a probing tool-use task in chimpanzees using quantitative genetic analyses. At one level of analysis, we quantified motor skill on a precision tool-use task and assessed the relative contributions of genetic and non-genetic factors to individual variation in performance. Because no study like this has been previously conducted in chimpanzees (nor any non-human primates, as far as we know), we were essentially testing two heuristic explanations for how individual differences in motor skill might vary in the chimpanzees. From a purely operant conditioning perspective, individual performance would be primarily mediated by their reinforcement history on the task. Subjects that were better or worse would receive differential rates of reinforcement and therefore performance would improve at different rates across individuals, but the relatedness of the individuals would explain little variability in performance. This would lead to the prediction that heritability would essentially be zero and that other non-genetic factors would largely explain individual differences in performance. The alternative hypothesis would, in contrast, propose that inherent differences in motor skill for tool use would reflect potential genetic mechanisms. These initial genetic predispositions could interact with operant mechanisms, leading to differential rates of acquisition and performance, but unlike the operant explanation, inherent initial differences in skill would be attributable to genetic factors. This hypothesis would predict that at least some degree of heritability in motor skill would be found in the chimpanzees.
In addition to tool-use skill, we also assessed heritability in hand preference. Although historically many have argued that non-genetic mechanisms govern the expression of behavioural asymmetries in non-human vertebrates, including handedness [42], there is increasing evidence that this view is no longer tenable. For instance, fish can be selectively bred for lateralized eye preferences when viewing a predator [43]. In primates, there is evidence that handedness can run in families, as well as evidence in chimpanzees of small-to-moderate heritability in hand use for reaching, manual gestures and coordinated bimanual actions [44]. Because handedness in chimpanzees has been previously found to be heritable, we hypothesized that significant heritability in hand use would be found for the tool-use task employed in this study. Finally, we tested for genetic correlations between motor skill performance and hand preference measures. These analyses were performed to assess whether similar genes explain individual variation in motor skill and hand preference.
2. Methods
(a). Subjects
A total of 243 captive chimpanzees were observed, including 148 females and 95 males. The chimpanzees were housed at two facilities: the Yerkes National Primate Research Center (YNPRC) of Emory University (n = 90) and the Michale E. Keeling Center for Comparative Medicine and Research of the University of Texas MD Anderson Cancer Center (UTMDACC; n = 153). Between the two facilities, the chimpanzees ranged in age from 5 to 51 years (mean = 27.21 years, s.d. = 9.64). There were 133 mother-reared (MR), 57 human-reared (HR) and 53 wild-caught (WC) chimpanzees in the combined sample. All of the research was approved by the local Institutional Animal Care and Use Committees and followed the guidelines for the ethical use of chimpanzees in research outlined by the Institute of Medicine.
We defined a HR chimpanzee as an animal that was separated from his or her mother within the first 30 days of life, owing to unresponsive care, injury or illness [45,46]. These chimpanzees were placed in incubators, fed standard human infant formula and cared for by humans until they could sufficiently care for themselves, at which time they were placed with other infants of the same age until they were three years of age [45,46]. At three years of age, the HR chimpanzees were integrated into larger social groups of adult and subadult chimpanzees. MR chimpanzees were not separated from their mother for at least the first 30 days of life and were raised in ‘nuclear’ family groups of chimpanzees, with group sizes ranging from four to twenty individuals.
It should be noted that the subset of HR chimpanzees in this study were raised in this manner because their biological mothers did not exhibit adequate maternal care at birth, and this required intervention in order to protect the infants' well-being. Thus, the chimpanzees in this study were not HR with the goal of subsequently determining the effects of early-life experiences on development. The study we present here is opportunistic and retrospective; that is to say, we took advantage of the fact that some of the chimpanzees received different rearing experiences to determine whether this might have long-term consequences for tool-use behaviour. Finally, the WC individuals were generally older and were brought to captivity from the wild prior to 1974, when the Convention on International Trade in Endangered Species (CITES) banned all importation of chimpanzees.
Each chimpanzee colony was genetically isolated, in the sense that they both populated from different founder chimpanzees that were imported into the USA prior to the CITES ban in 1974. Indeed, many of the chimpanzees within the pedigrees had estimated birth dates in the 1940s, when some of these research colonies were originally formed [47]. Within each colony, pedigrees were well documented from the founder animals to the current populations. For all offspring, the mothers were known, but paternity was not always known or reported in the records. No paternity tests were conducted for the purposes of this study. The relatedness structure of the entire chimpanzee sample was derived from the pedigree analyses within the sequential oligogenic linkage analysis routine (SOLAR) and is shown in table 1.
Table 1.
Pedigree structure of the chimpanzee sample.
| relationship | n |
|---|---|
| parent–offspring | 380 |
| full-siblings | 24 |
| half-siblings | 375 |
| grandparent–grandchildren | 116 |
| avuncular | 12 |
| second degree | 6 |
| third degree | 191 |
| fourth degree | 24 |
(b). Materials
The device and general methods have been described in detail elsewhere [36]. Briefly, the task employed was designed to simulate the motor demands of termite fishing in wild chimpanzees [2]. The motor and cognitive requirements of termite fishing were simulated using threaded polyvinylchloride (PVC) pipes (approx. 4 cm in diameter and approx. 20 cm in length) attached to threaded PVC bases that were affixed to the subjects' home enclosures at multiple locations approximately 60–80 cm above the ground or floor. The pipes were fitted with a disc in one end with a 7 mm hole cut out to greatly reduce the size of the opening available for tool insertion, thus increasing the motor demands of the task. The other end was closed with a removable screw-on cap. The pipes were filled with a preferred food substance that would adhere to the tool, such as BBQ sauce, mustard, yogurt, syrup or apple sauce, before being screwed into the bases. The chimpanzees were provided with flexible, thin ‘lollipop’ sticks (approx. 11 cm in length and 4 mm in diameter, like those used to make large lollipops) made out of tightly rolled, thin paper. The animals used the lollipop sticks to dip into the small hole of the pipe and retrieve the food (figure 1). Identical materials and procedures were used at the two facilities.
Figure 1.

Picture of a chimpanzee using the simulated termite fishing device. (Online version in colour.)
(c). Procedure
Prior to testing, all of the chimpanzees had been exposed to a simulated termite fishing tool-use task similar to that described in this study. This form of tool use is used as an enrichment device for the apes at both facilities. Subjects were tested in their home enclosures. Each PVC pipe was filled with a preferred food substance and then screwed onto a base. After placing the device on the enclosure, each subject was supplied with a tool by either handing it directly to them or by dropping it near them. If the tool became unusable, a new tool was offered to the subject. The experimenter recorded the hand used by the subject (left or right) each time they successfully inserted the tool into the pipe. Occasionally, a subject would insert the stick with one hand and withdraw it with the other. In this circumstance, the hand used to insert the stick was recorded because it is the insertion that requires the greatest fine motor skill, motor planning and hand-to-eye coordination. In addition to hand use, the experimenter recorded the time required to insert the stick into the hole during each attempted probe. Time per dip was measured from the time the subject initiated an attempt to insert the tool with one hand and ended when the chimpanzee successfully inserted and removed the tool. If the subject stopped attempting to use the tool or switched the tool to their mouth or other hand, the response was not counted. A minimum of 50 successful tool insertions were recorded from each subject. Testing sessions were conducted on multiple days for each subject until a minimum of 50 successful responses were obtained for each chimpanzee.
(d). Data analysis
Hand preferences were characterized in several ways. First, for each subject, a handedness index was derived following the formula HI = [(#R − #L)/(#R + #L)]. Positive HI values reflected right-hand biases and negative values reflected left-hand biases. The absolute value of the handedness score reflected the magnitude of hand preference. For all analyses, α was set at p ≤ 0.05, and all post hoc tests were conducted using Tukey's honestly significance difference test. The average time per successful dip for each hand was calculated using the following formula: average left time = total left time/left frequency, and average right time = total right time/right frequency. To eliminate differences between chimpanzee colonies in their exposure to this tool-use device, the mean latency scores were converted to standardized z-scores within the YNPRC and UTMDACC cohorts.
(e). Heritability analysis
Heritability (h2) is the proportion of total phenotypic variance that is attributable to additive genetic sources. Total phenotypic variance is constrained to a value of 1; therefore, all non-genetic contributions to the phenotype are equal to 1 − h2. Many of the chimpanzees in each colony are related and this allowed for an analysis of heritability in tool use and asymmetry using quantitative genetics based on the entire pedigree (table 1). Consistent with approaches used by others [48–56], to estimate heritability, we used the software package SOLAR [57]. SOLAR uses a variance components approach to estimate the polygenic component of variance when considering the entire pedigree (see [48,51,54]). We included sex, rearing history and age as covariates in the estimates of heritability to account for their influence on phenotypic variation. To test for shared maternal effects (c2) on the phenotypes, we created a matrix identifying individuals that were born to the same mother and this variable was included as a covariate. We used the bivariate heritability estimate function within SOLAR to quantify genetic correlations between the different traits.
3. Results
(a). Quantitative genetic analysis
Prior to any analyses, we used box-plots to determine any outlier data points. For the latency scores, three subjects were removed (two males, one female) owing to extremely high scores (all had z-scores > 3.00). None of the subjects was removed from the handedness analyses. The results from the overall heritability analyses are shown in table 2. Both tool-use performance and hand use were found to be significantly heritable after adjustment for the covariates sex, age and rearing history. Additionally, for tool-use performance, age was a significant covariate, and subsequent analysis showed that latency increased with age (figure 2). For handedness, both age and sex were significant covariates. Males were found to be more left-handed than females (see table 3 for descriptive information on handedness). Using the bivariate function within SOLAR, we also found a significant genetic correlation between the HI and Z_latency scores (ρg = 0.865, s.e. = 0.345, p = 0.001) but no environmental correlation (ρe = 0.235, s.e. = 0.141, p = 0.100). Thus, common genes appear to underlie individual variation in both hand use and tool use performance.
Table 2.
Heritability coefficients (h2) for each measure.
| h2 | p | c2 | p | covariates | variance | |
|---|---|---|---|---|---|---|
| overall | ||||||
| Z_latency | 0.395 (0.129) | 0.000 | 0.040 (0.121) | 0.367 | age | (0.071) |
| HI | 0.213 (0.131) | 0.033 | 0.000 (—) | sex | (0.028) | |
| UTMDACC | ||||||
| Z_latency | 0.356 (0.155) | 0.006 | — | age | (0.165) | |
| HI | 0.206 (0.168) | 0.043 | — | sex | (0.020) | |
| YNPRC | ||||||
| Z_latency | 0.463 (0.190) | 0.006 | — | none | ||
| HI | 0.191 (0.218) | 0.081 | — | none | ||
| mother-reared | ||||||
| Z_latency | 0.548 (0.164) | 0.000 | — | age | (0.033) | |
| HI | 0.191 (0.208) | 0.081 | — | sex | (0.028) | |
| hand-reared | ||||||
| Z_latency | 0.617 (0.390) | 0.038 | — | age | (0.017) | |
| HI | 0.387 (0.298) | 0.045 | — | none | ||
Figure 2.
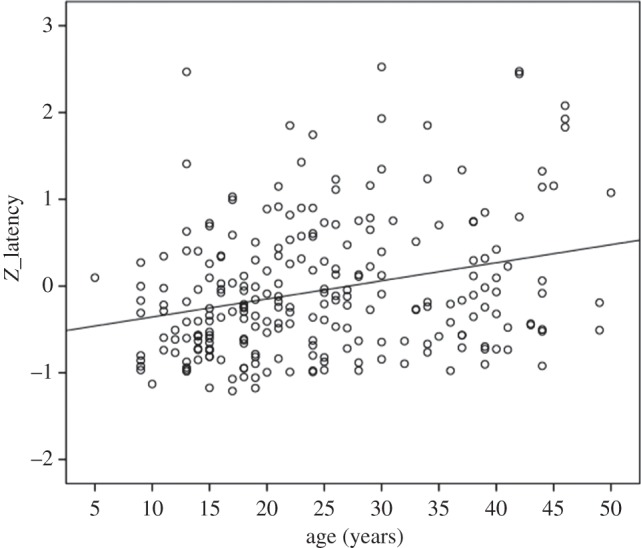
Scatterplot of the relationship between age and motor performance on the tool-use task.
Table 3.
Distribution of hand preference and mean HI values for male and female chimpanzees. Based on the total left- and right-hand frequencies, z-scores were used to evaluate whether the hand preferences of individual subjects deviated significantly from chance. This is the procedure most frequently used in the non-human primate literature (see [58,59]). Subjects with z-scores greater than 1.95 or less than –1.96 were classified as right- and left-handed, respectively. All other subjects were classified as ambiguously handed. Asterisk indicates p = 0.011 for a one-sample t-test on the HI scores, with zero being the estimated HI score for a normally distributed set of data.
| #L | #A | #R | mean HI | s.e. | t | |
|---|---|---|---|---|---|---|
| tool use | ||||||
| males | 43 | 23 | 29 | –0.144 | 0.055 | –2.59* |
| females | 55 | 35 | 58 | +0.014 | 0.045 | 0.31 |
| overall | 98 | 58 | 87 | –0.047 | 0.035 | –1.34 |
To explore both the consistency and magnitude of the observed heritability patterns, we performed several follow-up analyses. First, recall that the YNPRC and UTMDACC chimpanzees are genetically isolated groups of apes. That is to say, founder animals were unique to each colony, and no sires or dams were shared between the two facilities. Thus, to assess the consistency in the pattern of heritability, we performed separate analyses on each colony (table 2). For these and subsequent follow-up analyses, we included only those covariates that were significant in the full model analysis (age and sex). Similarly, we also eliminated the tests for maternal effects because this variable was not found to be significant for the full model analysis. Tool-use performance was significantly heritable in both colonies. In terms of hand preference, significant heritability was found in the UTMDACC colony, whereas the YNPRC heritability estimates approached conventional levels of statistical significance. Statistical comparisons demonstrated that the UTMDACC and YNPRC heritability estimates were not significantly different for either tool use performance or hand preference.
We next considered the potential mediating influence of early rearing experiences on heritability in tool use. For this analysis, we performed separate analyses on the MR and HR chimpanzees and these results are also shown in table 2. Significant heritability in tool-use performance and handedness was found within the MR and HR cohorts. Furthermore, age was a significant covariate for tool-use performance for both the MR and HR chimpanzees. Lastly, for hand preference, age and sex were significant covariates in the MR but not HR chimpanzees.
4. Discussion
Measuring tool-use performance and hand use in a relatively large sample of captive chimpanzees revealed three main findings. First, tool-use performance and hand preference were both significantly heritable. Importantly, heritability in tool performance and hand use was significant in two separate colonies of chimpanzees and was also evident independent of the early social rearing experiences of the chimpanzees. These collective data suggest a strong genetic component for both tool-use performance and hand preference in chimpanzees. Reinforcing this view were the findings of a significant genetic correlation between tool-use performance and hand preference, suggesting that overlapping genes are likely involved in individual variation in these two phenotypic components of motor function. Although there is evidence of heritability in hand preference in chimpanzees for other measures [44,60], we know of no other reports of heritability in motor skill in primates save humans. The collective heritability findings are consistent with the view that genetic mechanisms may underlie the expression of individual differences in motor skill and hand preference in humans and chimpanzees. Whether the same genes underlie human and chimpanzee motor skill and hand preference is not clear from this study, but warrants further investigation.
Second, older subjects performed this tool-use task more slowly than younger individuals. The evidence of age-related changes in motor performance in these subjects is consistent with at least one other report in chimpanzees. Lacreuse et al. [61] measured motor skill using the bent-wire task and, like the findings reported here, they found that older subjects performed more slowly than younger individuals. Moreover, age-related changes in motor skill were consistent between the UTMDACC and YNPRC apes, again suggesting that the age-related changes, though small, were consistent between the two cohorts.
Third, males were significantly more left-handed than females. These findings are also consistent with some reports on handedness in captive and wild chimpanzees. Sex differences in handedness for termite fishing have also been found in wild chimpanzees. When data from subjects residing at different field sites are combined [62], like the findings here, males were found to be more left-handed. Sex differences in hand preferences have also been found in wild chimpanzees for bimanual feeding [63]. It should be noted that many chimpanzees in this study did not exhibit a significant hand preference for the tool-use task, and this is a notable difference from findings in wild chimpanzees where the majority of individuals show a pronounced preference for the right or left hand, at least as it pertains to the use of tools [19,64–69]. What explains this discrepancy is not clear but we would speculate that factors related to the sensory and motor demands of the task, or perhaps length of experience in use of precision probing tools, may account for some of these differences.
One caveat to the findings of this study is that heritability was derived from tool-use performance in the chimpanzees on a well-learned the task. Thus, we estimated the degree of genetic contributions to motor skill for the tool-use task, not the heritability in learning the task. Both motor learning and motor skill have been found to be heritable in humans [41]. To obtain such measures in chimpanzees or other non-human animals would require measures of both the acquisition and terminal motor performance on a novel motor learning task.
A second caveat is the interpretation of the results reported here in the context of tool use in wild chimpanzees. It is increasingly clear that social learning plays an important role in the acquisition of the use of tools in wild chimpanzees [22] and this process may account for some heritability in tool use, because the mother serves such a critical role as the model for learning a specific tool-use task. Indeed, it may be possible that some of the significant heritability in handedness for tool use reported here might be attributable to social learning, particularly for the MR chimpanzees. However, in our view, it is difficult to imagine how social learning would account for the heritability in motor performance found in this study. The fact that significant heritability in tool-use performance was evident in both colonies and in MR and HR chimpanzees strongly suggests that social learning is not the likely mechanism underlying heritability but rather implies strong genetic control. Further support for this claim comes from the lack of significant shared maternal effect on either tool-use performance or hand preference.
Lastly, there has been some debate over the methods and statistics used in assessing handedness in non-human primates [58,70,71]. Specifically, some have suggested that recording each individual hand action when they are repeated and without some intervening event leads to a potential bias in handedness assessment [70,72]. Recall that we recorded each dipping response as an individual event in assessing motor performance and hand use; thus, we did not require intervening events but rather counted each response. We do not believe that collecting the data in this manner biased our heritability results in any meaningful way, because all the chimpanzees received the same number of trials and were tested using identical methods. Further, the assumption is that the inherent bias manifest by our recording method would add noise or error in the assessment of handedness. This source of error, in turn, would weaken or eliminate any significant heritability. The fact that we found significantly heritability in handedness, in the face of what some might argue is a procedure leading to biased results, leads us to conclude that whatever potential biases might exist are not strong enough to mask the genetic influences.
In summary, the findings presented here demonstrate that chimpanzee motor skill and handedness when assessed for tool use are significantly heritable. These results are fairly robust as manifested by the evidence that significant heritability was found in (i) two distinct, genetically isolated populations of chimpanzees and (ii) in both MR and HR chimpanzees. To what extent heritability in motor skill is evident in other species or for other motor tasks remains unknown but warrants further study. Notwithstanding, the findings presented here are consistent with the view that the genetic basis for tool use was present in the common ancestor of humans and chimpanzees, and in humans these genes may have been altered and elaborated upon in their functional control over more complex motor actions, such as speech, after their divergence from the genus Pan.
Data accessibility
The pedigree and behavioural data for this study are available at DRYAD (datadryad.org).
Funding statement
This work was supported in part by NIH grants RR-00165, U42-OD-011197, NS-42867, NS-73134 and HD-60563. The behavioural and genotype data for this study can be provided upon request to the corresponding author. Correspondence and reprint requests should be addressed to the corresponding author.
References
- 1.Candland D. 1987. Tool use. In Comparative primate biology; vol 2, Part B: behavior, cognition and motivation (eds Mitchell G, Erwin JM.), pp. 85–103. New York, NY: Alan R. Liss. [Google Scholar]
- 2.Van Lawick-Goodall J. 1970. Tool using in primates and other vertebrates. Adv. Study Behav. 3, 195–249. ( 10.1016/S0065-3454(08)60157-6) [DOI] [Google Scholar]
- 3.McGrew WC. 2013. Is primate tool use special? Chimpanzee and New Caledonian crow compared. Phil. Trans. R. Soc. B 368, 20120422 ( 10.1098/rstb.2012.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shumaker RW, Wallup KR, Beck BB. 2011. Animal tool behavior: the use and manufactre of tools by animals. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 5.Pruetz JD, Bertolani P. 2007. Savanna chimpanzees (Pan trogodytes verus) hunt with tools. Curr. Biol. 17, 412–417. ( 10.1016/j.cub.2006.12.042) [DOI] [PubMed] [Google Scholar]
- 6.Sanz CM, Morgan DB. 2007. Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol. 52, 420–433. ( 10.1016/j.jhevol.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 7.Whiten A, Goodall J, McGrew W, Nishida T, Reynolds V, Sugiyama Y, Tutin C, Wrangham R, Boesch C. 2001. Charting cultural variation in chimpanzees. Behaviour 138, 1489–1525. ( 10.1163/156853901317367717) [DOI] [Google Scholar]
- 8.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685. ( 10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 9.Van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. 2003. Orangutan cultures and the evolution of material cultures. Science 2, 102–105. ( 10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 10.Vaesen K. 2012. The cognitive bases of human tool use. Behav. Brain Sci. 35, 203–262. ( 10.1017/S0140525X11001452) [DOI] [PubMed] [Google Scholar]
- 11.Frey SH. 2008. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Phil. Trans. R. Soc. B 363, 1951–1957. ( 10.1098/rstb.2008.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw JL, Rogers LJ. 1993. The evolution of lateral asymmetries, language, tool use, and intellect. San Diego, CA: Academic Press. [Google Scholar]
- 13.Deaner RO, Isler K, Burkart J, Van Schaik CP. 2007. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav. Evol. 70, 115–124. ( 10.1159/000102973) [DOI] [PubMed] [Google Scholar]
- 14.Calvin WH. 1982. Did throwing stones shape hominid brain evolution. Ethol. Sociobiol. 3, 115–124. ( 10.1016/0162-3095(82)90010-3) [DOI] [Google Scholar]
- 15.Gibson KR, Ingold T. 1993. Tools, language and cognition in human evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Frost GT. 1980. Tool behavior and the origin of laterality. J. Hum. Evol. 9, 447–459. ( 10.1016/0047-2484(80)90002-0) [DOI] [Google Scholar]
- 17.Stout D, Chaminade T. 2012. Stone tools, language and the brain in human evolution. Phil. Trans. R. Soc. B 367, 75–87. ( 10.1098/rstb.2011.0099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roby-Brami A, Hermsdörfer J, Roy AC, Jacobs S. 2012. A neuropsychological perspective on the link between language and praxis in modern humans. Phil. Trans. R. Soc. B 367, 144–160. ( 10.1098/rstb.2011.0122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biro D, Sousa C, Matsuzawa T. 2006. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: case studies in nut cracking and leaf folding. In Cognitive development of chimpanzees (eds Matsuzawa T, Tomonaga T, Tanaka M.), pp. 476–507. New York, NY: Springer. [Google Scholar]
- 20.Inoue-Nakamura N, Matsuzawa T. 1997. Development of stone tool use by wild chimpanzees (Pan troglodytes). J. Comp. Psychol. 111, 159–173. ( 10.1037/0735-7036.111.2.159) [DOI] [PubMed] [Google Scholar]
- 21.Lonsdorf EV, Ross SR, Linick SA, Milstein MS, Melber TN. 2009. An experimental, comparative investigation of tool use in chimpanzees and gorillas. Anim. Behav. 77, 1119–1126. ( 10.1016/j.anbehav.2009.01.020) [DOI] [Google Scholar]
- 22.Lonsdorf EV, Eberly LE, Pusey AE. 2004. Sex differences in learning in chimpanzees. Nature 428, 715–716. ( 10.1038/428715a) [DOI] [PubMed] [Google Scholar]
- 23.McGrew WC. 1977. Socialization and object manipulation of wild chimpanzees. In Primate bio-social development (eds Chevalier-Skolnikoff S, Poirier FE.), pp. 261–288. New York, NY: Garland. [Google Scholar]
- 24.Gruber T, Clay Z, Zuberbuhler K. 2010. A comparison of bonobo and chimpanzee tool use: evidence for a female bias in the Pan lineage. Anim. Behav. 80, 1023–1033. ( 10.1016/j.anbehav.2010.09.005) [DOI] [Google Scholar]
- 25.Jordon C. 1982. Object manipulation and tool use in captive pygmy chimpanzees (Pan paniscus). J. Hum. Evol. 11, 35–39. ( 10.1016/S0047-2484(82)80029-8) [DOI] [Google Scholar]
- 26.Hopkins WD, Russell JL. 2004. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes). Neuropsychologia 42, 990–996. ( 10.1016/j.neuropsychologia.2003.11.017) [DOI] [PubMed] [Google Scholar]
- 27.Spinozzi G, Truppa V, Lagana T. 2004. Grasping behavior in tufted capuchin monkeys (Cebus apella): grip types and manual laterality for picking up a small food item. Am. J. Phys. Anthropol. 125, 30–41. ( 10.1002/ajpa.10362) [DOI] [PubMed] [Google Scholar]
- 28.Rigamonti MM, Previde EP, Poli MD, Marchant LF, McGrew WC. 1998. Methodology of motor skill and laterality: new test of hand preference in Macaca nemestrina. Cortex 34, 693–705. ( 10.1016/S0010-9452(08)70773-1) [DOI] [PubMed] [Google Scholar]
- 29.McGrew WC, Marchant LF. 1999. Laterality of hand use pays off in foraging success for wild chimpanzees. Primates 40, 509–513. ( 10.1007/BF02557586) [DOI] [Google Scholar]
- 30.Costello M, Fragaszy DM. 1988. Prehension in Cebus and Samiri: I. Grip type and hand preference. Am. J. Primatol. 15, 234–245. [DOI] [PubMed] [Google Scholar]
- 31.Christel MI. 1994. Catarrhine primates grasping small objects: techniques and hand preferences. In Current primatology, vol. III: behavioral neuroscience, physiology and reproduction (eds Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N.), pp. 37–49. Strasbourg, France: Universite Louis Pasteur. [Google Scholar]
- 32.Christel MI, Kitzel S, Niemitz C. 1998. How precisely do bonobos (Pan paniscus) grasp small objects? Int. J. Primatol. 19, 165–194. ( 10.1023/A:1020319313219) [DOI] [Google Scholar]
- 33.Tonooka R, Matsuzawa T. 1995. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. Int. J. Primatol. 16, 17–34. ( 10.1007/BF02700151) [DOI] [Google Scholar]
- 34.Pouydebat E, Reghem E, Borel A, Gorce P. 2011. Diversity of grip in adults and young humans and chimpanzees (Pan troglodytes). Behav. Brain Res. 218, 21–28. ( 10.1016/j.bbr.2010.11.021) [DOI] [PubMed] [Google Scholar]
- 35.Pouydebat W, Gorce P, Coppens Y, Bels V. 2009. Biomechanical study of grasping according to the volume of the object: human versus non-human primates. J. Biomech. 42, 266–272. ( 10.1016/j.jbiomech.2008.10.026) [DOI] [PubMed] [Google Scholar]
- 36.Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. 2009. Handedness for tool use in captive chimpanzees (Pan troglodytes): sex differences, performance, heritability and comparison to the wild. Behaviour 146, 1463 ( 10.1163/156853909X441005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menzel EW, Davenport RK, Rogers CM. 1970. The development of tool using in wild-born and restriction-reared chimpanzees. Folia Primatol. 12, 273–283. ( 10.1159/000155297) [DOI] [PubMed] [Google Scholar]
- 38.Kimura D. 1993. Neuromotor mechanisms in human communication. Oxford, UK: Oxford University Press. [Google Scholar]
- 39.Voelcker-Rehage C. 2008. Motor-skill learning in older adults—a review of studies on age-related differences. Eur. Rev. Aging Physiol. 5, 5–16. ( 10.1007/s11556-008-0030-9) [DOI] [Google Scholar]
- 40.Fox PW, Hershberger SL, Bouchard TJ. 1998. Genetic and environmental contributions to the acquisition of a motor sill. Nature 384, 355–358. [DOI] [PubMed] [Google Scholar]
- 41.Missitzi J, Gentner R, Misitzi A, Geladas N, Politis P, Klissouras V, Classen J. 2013. Heritability of motor control and motor learning. Physiol. Rep. 1, 1–10. ( 10.1002/phy2.188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warren JM. 1980. Handedness and laterality in humans and other animals. Physiol. Psychol. 8, 351–359. ( 10.3758/BF03337470) [DOI] [Google Scholar]
- 43.Bisazza A, Facchin L, Vallortigara G. 2000. Heritability of lateralization in fish: concordance of right–left asymmetry between parents and offspring. Neuropsychologia 38, 907–912. ( 10.1016/S0028-3932(00)00018-X) [DOI] [PubMed] [Google Scholar]
- 44.Hopkins WD, Adams M, Weiss A. 2013. Genetic and environmental contributions to the expression of handedness in chimpanzees (Pan troglodytes). Genes, Brain Behav. 12, 446–452. ( 10.1111/gbb.12044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bard KA, Platzman KA, Lester BM, Suomi SJ. 1992. Orientation to social and nonsocial stimuli in neonatal chimpanzees and humans. Infant Behav. Dev 15, 43–56. ( 10.1016/0163-6383(92)90005-q) [DOI] [Google Scholar]
- 46.Bard KA. 1994. Evolutionary roots of intuitive parenting: maternal competence in chimpanzees. Early Dev. Parent. 3, 19–28. ( 10.1002/edp.2430030104) [DOI] [Google Scholar]
- 47.Yerkes RM. 1943. Chimpanzees: a laboratory colony. New Haven, CT: Yale University Press. [Google Scholar]
- 48.Fears SC, et al. 2011. Anatomic brain asymmetry in vervet monkeys. PLoS ONE 6, e28243 ( 10.1371/journal.pone.0028243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers SE, Shelton SE, Shelledy W, Garcia R, Kalin NK. 2008. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes, Brain Behav. 7, 463–469. ( 10.1111/j.1601-183X.2007.00381.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairbanks LA, Bailey JN, Breidenthal SE, Laudenslager ML, Kaplan JR, Jorgensen MJ. 2011. Environmental stress alters genetic regulation of novelty seeking in vervet monkeys. Genes, Brain Behav. 10, 683–688. ( 10.1111/j.1601-183X.2011.00707.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fears SC, et al. 2009. Identifying heritable brain phenotypes in an extended pedigree of vervet monkeys. J. Neurosci. 29, 2867–2875. ( 10.1523/JNEUROSCI.5153-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochunov PV, et al. 2010. Genetics of primary cerebral gyrification: heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. Neuroimage 53, 1126–1134. ( 10.1016/j.neuroimage.2009.12.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips KA, Rogers J, Barrett EA, Glahn DC, Kochunov PV. 2012. Genetic contributions to the midsagittal area of the corpus callosum. Twin Res. Hum. Genet. 15, 315–323. ( 10.1017/thg.2012.10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers J, Kochunov PV, Lancaster JL, Sheeledy W, Glahn D, Blangero J, Fox PT. 2007. Heritability of brain volume, surface area and shape: an MRI study in an extended pedigree of baboons. Hum. Brain Mapp. 28, 576–583. ( 10.1002/hbm.20407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers J, et al. 2010. On the genetic architecture of cortical folding and brain volume in primates. Neuroimage 53, 1103–1108. ( 10.1016/j.neuroimage.2010.02.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins WD, Keebaugh AC, Reamer LA, Schaeffer J, Schapiro SJ, Young LJ. 2014. Genetic influences on receptive joint attention in chimpanzees (Pan troglodytes). Sci. Rep. 4, 1–7. ( 10.1038/srep03774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almasy L, Blangero J. 1998. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 62, 1198–1211. ( 10.1086/301844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hopkins WD. 2013. Independence of data points in the measurement of handedness: statistical problem or urban myth? Am. J. Phys. Anthropol. 151, 151–157. ( 10.1002/ajpa.22248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopkins WD. 1999. On the other hand: statistical issues in the assessment and interpretation of hand preference data in non-human primates. Int. J. Primatol. 20, 851–866. ( 10.1023/A:1020822401195) [DOI] [Google Scholar]
- 60.Hopkins WD. 1999. Heritability of hand preference in chimpanzees (Pan troglodytes): evidence from a partial interspecies cross-fostering study. J. Comp. Psychol. 113, 307–313. ( 10.1037/0735-7036.113.3.307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacreuse A, Russell JL, Hopkins WD, Herndon JG. 2014. Cognitive and motor aging in female chimpanzees. Neurobiol. Aging 35, 623–632. ( 10.1016/j.neurobiolaging.2013.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogart SL, Pruetz JD, Ormiston LK, Russell JL, Meguerditchian A, Hopkins WD. 2012. Termite fishing laterality in the Fongoli savanna chimpanzees (Pan troglodytes verus): further evidence of a left hand preference. Am. J. Phys. Anthropol. 149, 591–598. ( 10.1002/ajpa.22175) [DOI] [PubMed] [Google Scholar]
- 63.Corp N, Byrne RW. 2004. Sex difference in chimpanzee handedness. Am. J. Phys. Anthropol. 123, 62–68. ( 10.1002/ajpa.10218) [DOI] [PubMed] [Google Scholar]
- 64.McGrew WC, Marchant LF. 1992. Chimpanzees, tools, and termites: hand preference or handedness? Curr. Anthropol. 33, 114–119. ( 10.1086/204041) [DOI] [Google Scholar]
- 65.McGrew WC, Marchant LF. 1996. On which side of the apes? In Great ape societies (eds McGrew WC, Marchant LF, Nishida T.), pp. 255–272. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 66.Boesch C. 1991. Handedness in wild chimpanzees. Int. J. Primatol. 6, 541–558. ( 10.1007/BF02547669) [DOI] [Google Scholar]
- 67.Hopkins WD, Cantalupo C. 2005. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): a critical analysis and some alternative explanations. Laterality 10, 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. 1993. Hand preference and tool use in wild chimpanzees. Primates 34, 151–159. ( 10.1007/BF02381386) [DOI] [Google Scholar]
- 69.Humle T, Matsuzawa T. 2009. Laterality in hand use across four tool use behaviors among the wild chimpanzees of Bossou, Guinea, West Africa. Am. J. Primatol. 71, 40–48. ( 10.1002/ajp.20616) [DOI] [PubMed] [Google Scholar]
- 70.McGrew WC, Marchant LF. 1997. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearb. Phys. Anthropol. 40, 201–232. () [DOI] [Google Scholar]
- 71.Cashmore L, Uomini N, Chapelain A. 2008. The evolution of handedness in humans and great apes: a review and current issues. J. Anthropol. Sci. 86, 7–35. [PubMed] [Google Scholar]
- 72.Byrne RW, Byrne JM. 1991. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei). Cortex 27, 521–536. ( 10.1016/S0010-9452(13)80003-2) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The pedigree and behavioural data for this study are available at DRYAD (datadryad.org).


