Abstract
Naked mole rats are the most hypoxia-tolerant mammals identified; however, the mechanisms underlying this tolerance are poorly understood. Using whole-animal plethysmography and open-flow respirometry, we examined the hypoxic metabolic response (HMR), hypoxic ventilatory response (HVR) and hypoxic thermal response in awake, freely behaving naked mole rats exposed to 7% O2 for 1 h. Metabolic rate and ventilation each reversibly decreased 70% in hypoxia (from 39.6 ± 2.9 to 12.1 ± 0.3 ml O2 min−1 kg−1, and 1412 ± 244 to 417 ± 62 ml min−1 kg−1, respectively; p < 0.05), whereas body temperature was unchanged and animals remained awake and active. Subcutaneous injection of the general adenosine receptor antagonist aminophylline (AMP; 100 mg kg−1, in saline), but not control saline injections, prevented the HVR but had no effect on the HMR. As a result, AMP-treated naked mole rats exhibited extreme hyperventilation in hypoxia. These animals were also less tolerant to hypoxia, and in some cases hypoxia was lethal following AMP injection. We conclude that in naked mole rats (i) hypoxia tolerance is partially dependent on profound hypoxic metabolic and ventilatory responses, which are equal in magnitude but occur independently of thermal changes in hypoxia, and (ii) adenosine receptors mediate the HVR but not the HMR.
Keywords: hypoxic ventilatory response, hypoxic metabolic response, plethysmography, respirometry, thermal response
1. Introduction
When O2 supply fails to meet O2 demand, cellular energy balance is disrupted, which can ultimately lead to cell death, organ failure and mortality. Therefore, matching O2 supply to O2 demand is critical to tolerating hypoxia [1]. Hypoxic environments are common on Earth, and exposure to periods of intermittent or prolonged hypoxia in the daily activities or annual life cycles of various animals has driven the evolution of diverse cellular, physiological and behavioural adaptations to low O2 stress [2]. These adaptations typically serve to increase O2 supply to cells and tissues, and/or to decrease cellular energy demand (i.e. metabolic rate depression) [2,3]. For example, when most mammals are exposed to acute hypoxia, their immediate compensatory response is to increase their ventilation [4], which is an energetically expensive means of increasing the delivery of O2 to cells. Conversely, some species decrease breathing in hypoxia, although this is not common in mammals. Such hypoxia-mediated changes in breathing are termed the hypoxic ventilatory response (HVR), which encompasses a suite of time domains depending on the duration of hypoxic exposure [4]. Within a few moments of the onset of the HVR, most mammals also exhibit a decrease in metabolic demand, termed the hypoxic metabolic response (HMR), which in turn decreases systemic O2 requirements. As metabolic demand decreases, ventilation (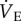 , expiratory minute ventilation) tends to decrease as well; however, in all mammals studied to date, the decrease in metabolism is greater than the decrease in
, expiratory minute ventilation) tends to decrease as well; however, in all mammals studied to date, the decrease in metabolism is greater than the decrease in 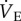 . Therefore, most mammals tend to hyperventilate in hypoxia because the HVR and HMR combine to affect a net increase in the animals' air convection requirement (ACR; i.e. the ratio of inspired O2 to metabolic rate).
. Therefore, most mammals tend to hyperventilate in hypoxia because the HVR and HMR combine to affect a net increase in the animals' air convection requirement (ACR; i.e. the ratio of inspired O2 to metabolic rate).
The magnitude of the HVR and HMR are key determinants of the duration and depth of hypoxia that an organism can tolerate. Certain air-breathing vertebrates, such as arctic ground squirrels (Urocitellus parryii); freshwater turtles (Chrysemys picta bellii) and crucian carp (Carassius carassius), can tolerate hypoxia for weeks to months without apparent detriment [2]. Typically, these animals are most hypoxia-tolerant while over-wintering. During this period, they exhibit metabolic rate depression mediated in part by a profound decrease in body temperature (Tb) and entry into a torpor-like state [5]. These responses drastically reduce systemic energy demands and extend survival time in hypoxia. The mechanisms that mediate these changes are poorly understood, but recent studies have demonstrated that in small mammals, adenosine receptors are key in initiating and maintaining (i) the HMR, (ii) decreases in Tb and (iii) the entry into torpor. In support of this, adenosine receptor agonism decreases metabolism and Tb, and induces entry into a torpor-like state in rats and arctic ground squirrels [6,7], whereas adenosine receptor antagonism prevents or reverses these changes in hibernating arctic ground squirrels [7,8], and during intra-day torpor or fasting-induced torpor in mice [9–11] and rats [6].
Naked mole rats (Heterocephalus glaber) experience lifelong hypoxia while living in large subterranean tunnel systems approximately 1–2 m underground in eastern Africa [12]. Naked mole rat burrows are poorly ventilated due to limited air diffusion through densely packed soil, which, in combination with group respiration-mediated O2 depletion, creates a challenging hypoxic environment [12,13]. Indeed, naked mole rats and their relatives experience burrow O2 levels as low as 6.2% without detriment [12–14] and tolerate hypoxia lower than 3% O2 for several hours in a laboratory setting [15]. Based on these observations, naked mole rats are considered to be the most hypoxia-tolerant mammal. Owing to the constancy of their exposure to environmental hypoxia and also the relatively high ambient temperature (Ta) in their burrows (approx. 30–34°C), naked mole rats cannot easily use behavioural and thermal means to alter their metabolic rate. Nonetheless, studies to date have described a handful of adaptations in naked mole rats that are likely to contribute to their tolerance of chronic hypoxia. These include the expression of a haemoglobin isomer with higher affinity for O2 relative to other rodents [16], a metabolic rate that is two-thirds of that predicted from allometry [17], and blunted neuronal Ca2+ influx during hypoxia [18–20], which is known to reduce excitotoxic cell death due to hypoxia or ischaemia [21]. However, even the most basic system-level responses, including ventilatory and metabolic responses to hypoxia, remain to be properly characterized in this species. The aims of this study were to examine (i) the steady-state metabolic, ventilatory and thermal responses of naked mole rats to acute hypoxia, and (ii) the role of adenosinergic signalling in mediating these responses.
2. Material and methods
(a). Animals
Naked mole rats were generous gifts from the University of Montana and the University of Texas at San Antonio. Naked mole rats were group-housed in interconnected multi-cage systems and held at 28°C in 50% humidity under a 12 L : 12 D cycle. Animals were fed fresh tubers, vegetables, fruit and Pronutro cereal supplement ad libitum. Animals were not fasted prior to the experimental trial.
(b). Experimental design
Fourteen male and female naked mole rats weighing 45.1 ± 1.8 g were individually placed, unrestrained, into an experimental chamber flushed with 21% O2, which was set inside a controlled environmental chamber to maintain Ta at approximately 28°C. Once placed within the experimental apparatus, naked mole rats were given at least 30 min to acclimate, followed by 60 min of baseline recordings of 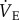 and metabolism. This period was necessary to stabilize the chamber temperature and to determine steady-state metabolism and
and metabolism. This period was necessary to stabilize the chamber temperature and to determine steady-state metabolism and 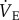 . After baseline recordings, the naked mole rat was quickly removed from the chamber and its Tb was measured rectally using a thermocouple. Naked mole rats were then randomly divided into two groups and treated with subcutaneous 500 µl injections of either (i) saline (n = 7) or (ii) the general adenosine receptor antagonist aminophylline (AMP) dissolved in saline (100 mg kg−1; n = 7). AMP was purchased from Sigma-Aldrich (St Louis, MO, USA). In the body, AMP is converted to theophylline with approximately 80% efficacy [22]. The pharmacokinetics of intravenous, intraperitoneal or subcutaneous AMP administration best fitted the two-compartment model, with rapid distribution and slow elimination. Titres of plasma theophylline peak within 8 min of injection and the normal elimination half-life of AMP ranges from 8 to 14 h, while the bioavailability of AMP is 80–95% in humans [23], dogs [24], cats [25], pigs [26] and chickens [25]. Although the pharmacokinetics and bioavailability of AMP have not been previously assessed in naked mole rats, these are likely to be similar to published values in other species. Furthermore, the pharmacological dose of AMP used in this study is consistent with that used to inhibit metabolic depression in studies conducted in small rodents in other laboratories (e.g. [11]). We observed no negative effects of AMP administration. Specifically, animals did not appear stressed, their behaviour was not altered, and their metabolic and ventilatory rates were unaffected. Immediately following AMP administration, animals were returned to the recording chamber, and ventilatory and metabolic measurements were collected in each gas mixture (21% O2, 7% O2 and recovery in 21% O2), under poikilocapnic conditions. The effects of AMP were determined by comparing data obtained from control animals (saline injection) relative to data collected from animals treated with AMP. Animals were held in each condition until metabolism and
. After baseline recordings, the naked mole rat was quickly removed from the chamber and its Tb was measured rectally using a thermocouple. Naked mole rats were then randomly divided into two groups and treated with subcutaneous 500 µl injections of either (i) saline (n = 7) or (ii) the general adenosine receptor antagonist aminophylline (AMP) dissolved in saline (100 mg kg−1; n = 7). AMP was purchased from Sigma-Aldrich (St Louis, MO, USA). In the body, AMP is converted to theophylline with approximately 80% efficacy [22]. The pharmacokinetics of intravenous, intraperitoneal or subcutaneous AMP administration best fitted the two-compartment model, with rapid distribution and slow elimination. Titres of plasma theophylline peak within 8 min of injection and the normal elimination half-life of AMP ranges from 8 to 14 h, while the bioavailability of AMP is 80–95% in humans [23], dogs [24], cats [25], pigs [26] and chickens [25]. Although the pharmacokinetics and bioavailability of AMP have not been previously assessed in naked mole rats, these are likely to be similar to published values in other species. Furthermore, the pharmacological dose of AMP used in this study is consistent with that used to inhibit metabolic depression in studies conducted in small rodents in other laboratories (e.g. [11]). We observed no negative effects of AMP administration. Specifically, animals did not appear stressed, their behaviour was not altered, and their metabolic and ventilatory rates were unaffected. Immediately following AMP administration, animals were returned to the recording chamber, and ventilatory and metabolic measurements were collected in each gas mixture (21% O2, 7% O2 and recovery in 21% O2), under poikilocapnic conditions. The effects of AMP were determined by comparing data obtained from control animals (saline injection) relative to data collected from animals treated with AMP. Animals were held in each condition until metabolism and 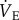 reached steady state (approx. 1 h), and Tb was measured following each exposure. The experimental trial took a total of approximately 4 h for each individual.
reached steady state (approx. 1 h), and Tb was measured following each exposure. The experimental trial took a total of approximately 4 h for each individual.
(c). Plethysmography and respirometry
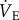 was measured using the barometric pressure method of plethysmography modified for continuous flow [27]. On the day of experimentation, individuals were placed into the experimental apparatus which consisted of two rectangular 450 ml Plexiglas chambers, one being the animal chamber and the other the reference chamber. The temperature of the animal chamber was recorded continuously throughout the experiment using an iButton (Maxim Integrated, Chandler, CA). Calibrated rotameters were used to supply naked mole rats with an inflowing gas mixture set at a flow rate of 110 ml min−1. The total airflow ensured that neither O2 nor CO2 were altered by more than 1.5% by the animal's metabolism. Fractional O2 and CO2 composition of inspired and expired gas were monitored using an O2 and CO2 analyser (Raytech, North Vancouver, BC, Canada). The gas analyser was calibrated for O2 and CO2 before each trial with a premixed gas (21% O2; 1.55% CO2 balanced with N2). O2 consumption was calculated from the product of the constant airflow through the chamber and the difference between the inflow and outflow in the fractional concentration of O2. CO2 production was calculated from the product of the constant airflow through the chamber and the difference between the outflow and inflow in the fractional concentration of CO2. The respiratory exchange ratio (RER) was calculated as the quotient of CO2 production and O2 consumption. The ACR was calculated as the quotient of
was measured using the barometric pressure method of plethysmography modified for continuous flow [27]. On the day of experimentation, individuals were placed into the experimental apparatus which consisted of two rectangular 450 ml Plexiglas chambers, one being the animal chamber and the other the reference chamber. The temperature of the animal chamber was recorded continuously throughout the experiment using an iButton (Maxim Integrated, Chandler, CA). Calibrated rotameters were used to supply naked mole rats with an inflowing gas mixture set at a flow rate of 110 ml min−1. The total airflow ensured that neither O2 nor CO2 were altered by more than 1.5% by the animal's metabolism. Fractional O2 and CO2 composition of inspired and expired gas were monitored using an O2 and CO2 analyser (Raytech, North Vancouver, BC, Canada). The gas analyser was calibrated for O2 and CO2 before each trial with a premixed gas (21% O2; 1.55% CO2 balanced with N2). O2 consumption was calculated from the product of the constant airflow through the chamber and the difference between the inflow and outflow in the fractional concentration of O2. CO2 production was calculated from the product of the constant airflow through the chamber and the difference between the outflow and inflow in the fractional concentration of CO2. The respiratory exchange ratio (RER) was calculated as the quotient of CO2 production and O2 consumption. The ACR was calculated as the quotient of 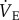 and O2 consumption. Inspiration humidifies and warms inspired air, resulting in pressure fluctuations that were compared with the pressure of the reference chamber and monitored with a differential pressure transducer (DP103-18, Validyne, Northridge, CA, USA) connected between the two chambers, amplified and electronically recorded by a computer. Respiratory frequency (fr) was calculated directly from the ventilation-induced pressure oscillations. Expiratory tidal volume (Vt) was determined by integrating negative periods of ventilatory flow and then calculated using the equation of Drorbaugh & Fenn [28] modified for flow-through plethysmography by Jacky [27]. To determine Vt, the system was calibrated dynamically with the animal present in the chamber, as described previously [29]. Briefly, prior to each experiment known volumes of air (0.2, 0.5 and 1.0 ml) were injected into the chamber at a rate similar to the naked mole rats' expiratory time to produce pressure deflections at least 10 times as great as that of the animal's breathing.
and O2 consumption. Inspiration humidifies and warms inspired air, resulting in pressure fluctuations that were compared with the pressure of the reference chamber and monitored with a differential pressure transducer (DP103-18, Validyne, Northridge, CA, USA) connected between the two chambers, amplified and electronically recorded by a computer. Respiratory frequency (fr) was calculated directly from the ventilation-induced pressure oscillations. Expiratory tidal volume (Vt) was determined by integrating negative periods of ventilatory flow and then calculated using the equation of Drorbaugh & Fenn [28] modified for flow-through plethysmography by Jacky [27]. To determine Vt, the system was calibrated dynamically with the animal present in the chamber, as described previously [29]. Briefly, prior to each experiment known volumes of air (0.2, 0.5 and 1.0 ml) were injected into the chamber at a rate similar to the naked mole rats' expiratory time to produce pressure deflections at least 10 times as great as that of the animal's breathing. 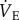 was measured with the system open and oscillations in recorded pressure were considered to be proportional to Vt. The advantage of an open-flow system is that the animal chamber is flushed constantly with air so that O2 depletion and CO2 accumulation are avoided, and metabolism and
was measured with the system open and oscillations in recorded pressure were considered to be proportional to Vt. The advantage of an open-flow system is that the animal chamber is flushed constantly with air so that O2 depletion and CO2 accumulation are avoided, and metabolism and 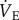 could be continuously and simultaneously recorded.
could be continuously and simultaneously recorded. 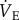 was calculated from the product of fr and Vt.
was calculated from the product of fr and Vt.
(d). Data collection and analysis
Ventilatory, metabolic and thermoregulatory variables were recorded on WinDaq acquisition software (Dataq Instruments, Akron, OH, USA). All data acquired in WinDaq were analysed in PowerLab. Average values were calculated for each variable during the last 5 min of steady state of each experimental treatment. For 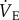 , Vt and fr, a series of at least 10 sets of 10 consecutive breaths were selected for analysis. Breaths were chosen from periods where the animal was awake but not active (i.e. not actively digging or exploring but also not asleep, as determined by visual examination).
, Vt and fr, a series of at least 10 sets of 10 consecutive breaths were selected for analysis. Breaths were chosen from periods where the animal was awake but not active (i.e. not actively digging or exploring but also not asleep, as determined by visual examination).
Statistical analysis was performed using commercial software (SPSS v. 15.0; SPSS Inc., Chicago, IL, USA). For all experiments, individual n values correspond to a single animal treated with either saline or AMP dissolved in saline as described above, before and after exposure to 21% O2 and then acute hypoxia (7% O2). Values are presented as mean ± s.e.m. p < 0.05 was considered to achieve statistical significance. All data were normally distributed with equal variance (p > 0.05). Significance was evaluated using two-way ANOVA to test for significant interactions between the two independent variables: (i) the acute inspired O2 level (7% versus 21%) and (ii) the treatment (saline versus AMP injection). Bonferoni post hoc multiple-comparison tests were run on each of the dependent variables to compare the single point means of interest. The dependent variables analysed were 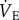 , fr, Vt, metabolic rate, RER, the ACR and Tb.
, fr, Vt, metabolic rate, RER, the ACR and Tb.
3. Results
(a). Naked mole rats exhibit robust metabolic depression with sustained Tb in acute hypoxia
Steady-state metabolism, 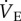 , and Tb were measured in naked mole rats breathing normoxic gas mixtures before and after subcutaneous saline or AMP injection. Neither saline nor AMP altered any of these variables in animals breathing normoxic gas mixtures (p > 0.05; n = 7 each; data not shown). Thus, for the purposes of data presentation and statistical analysis we used post-injection normoxic measurements as the control in all datasets. Relative to control levels in normoxia, saline-treated naked mole rats exposed to 7% O2 exhibited a 70% decrease in metabolic rate (from 39.6 ± 2.9 to 12.1 ± 0.3 ml O min−1 kg−1, and 31.3 ± 2.7 to 8.7 ± 1.1 ml CO2 min−1 kg−1, for O2 consumption and CO2 production, respectively; figure 1a,b), which was reversed following 30 min of recovery in normoxia (21% O2; to 45.4 ± 4.6 ml O2 min−1 kg−1 and 23.8 ± 3.4 ml CO2 min−1 kg−1). Similarly, AMP-treated naked mole rats breathing 7% O2 also exhibited metabolic rate depression of a similar magnitude (from 38.6 ± 2.3 to 15.1 ± 3.8 ml O2 min−1 kg−1, and 28.3 ± 1.6 to 17.2 ± 2.5 ml CO2 min−1 kg−1, for O2 consumption and CO2 production, respectively; figure 1a,b), although the hypoxic decrease in CO2 production in AMP-treated naked mole rats was significantly less than that observed in saline-treated animals (figure 1b). This difference may have been due to a secondary effect of AMP on the balance between the catabolism of lipids and carbohydrates as fuel substrates in hypoxic naked mole rats, although this requires further investigation [30,31]. These changes were also reversed following 30 min of recovery in normoxia (to 41.1 ± 3.3 ml O2 min−1 kg−1 and 22.5 ± 0.8 ml CO2 min−1 kg−1). Tb was not different between treatments or inspired O2 levels in any group (figure 1c). Visual observations revealed that naked mole rats remained alert and active throughout the experiment in all treatment groups.
, and Tb were measured in naked mole rats breathing normoxic gas mixtures before and after subcutaneous saline or AMP injection. Neither saline nor AMP altered any of these variables in animals breathing normoxic gas mixtures (p > 0.05; n = 7 each; data not shown). Thus, for the purposes of data presentation and statistical analysis we used post-injection normoxic measurements as the control in all datasets. Relative to control levels in normoxia, saline-treated naked mole rats exposed to 7% O2 exhibited a 70% decrease in metabolic rate (from 39.6 ± 2.9 to 12.1 ± 0.3 ml O min−1 kg−1, and 31.3 ± 2.7 to 8.7 ± 1.1 ml CO2 min−1 kg−1, for O2 consumption and CO2 production, respectively; figure 1a,b), which was reversed following 30 min of recovery in normoxia (21% O2; to 45.4 ± 4.6 ml O2 min−1 kg−1 and 23.8 ± 3.4 ml CO2 min−1 kg−1). Similarly, AMP-treated naked mole rats breathing 7% O2 also exhibited metabolic rate depression of a similar magnitude (from 38.6 ± 2.3 to 15.1 ± 3.8 ml O2 min−1 kg−1, and 28.3 ± 1.6 to 17.2 ± 2.5 ml CO2 min−1 kg−1, for O2 consumption and CO2 production, respectively; figure 1a,b), although the hypoxic decrease in CO2 production in AMP-treated naked mole rats was significantly less than that observed in saline-treated animals (figure 1b). This difference may have been due to a secondary effect of AMP on the balance between the catabolism of lipids and carbohydrates as fuel substrates in hypoxic naked mole rats, although this requires further investigation [30,31]. These changes were also reversed following 30 min of recovery in normoxia (to 41.1 ± 3.3 ml O2 min−1 kg−1 and 22.5 ± 0.8 ml CO2 min−1 kg−1). Tb was not different between treatments or inspired O2 levels in any group (figure 1c). Visual observations revealed that naked mole rats remained alert and active throughout the experiment in all treatment groups.
Figure 1.
Metabolism is markedly reduced during acute hypoxia independently of ADO receptor activation. (a,b) Average metabolic rate in naked mole rats breathing normoxic or acute hypoxic (7% O2) gas mixtures and treated with subcutaneous saline (white bars) or AMP (black bars) injection. (c) Average body temperature from the animals treated in (a,b). Data are mean ± s.e.m. from n = 7 naked mole rats per group. Asterisks (*) indicate significant differences between normoxia and acute hypoxia values; daggers (†) indicate significant differences between AMP- and saline-treated animals (p < 0.05).
(b). Naked mole rats exhibit ventilatory depression in acute hypoxia mediated by adenosine receptors
When breathing normoxic gas mixtures,  was 1412 ± 224 ml min−1 kg−1 in saline-treated animals (figure 2a). Following the switch to 7% O2,
was 1412 ± 224 ml min−1 kg−1 in saline-treated animals (figure 2a). Following the switch to 7% O2,  decreased by 70% to 417 ± 62 ml min−1 kg−1. This value reversed to 1071 ± 259 ml min−1 kg−1 after 30 min of recovery in normoxia, which was not significantly different from control values. The change in
decreased by 70% to 417 ± 62 ml min−1 kg−1. This value reversed to 1071 ± 259 ml min−1 kg−1 after 30 min of recovery in normoxia, which was not significantly different from control values. The change in 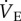 in 7% O2 was due to the combination of a 50% decrease in fr (from 120.1 ± 15.8 to 59.9 ± 4.5 breaths min−1; figure 2b) and a 47% decrease in Vt (from 13.6 ± 1.7 to 7.2 ± 1.3 ml kg−1; figure 2c), both of which were reversed by reoxygenation. In AMP-treated animals,
in 7% O2 was due to the combination of a 50% decrease in fr (from 120.1 ± 15.8 to 59.9 ± 4.5 breaths min−1; figure 2b) and a 47% decrease in Vt (from 13.6 ± 1.7 to 7.2 ± 1.3 ml kg−1; figure 2c), both of which were reversed by reoxygenation. In AMP-treated animals, 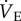 in normoxia was not different from saline-treated animals (1319 ± 260 ml min−1 kg−1; figure 2a); however, the hypoxia-mediated decrease in
in normoxia was not different from saline-treated animals (1319 ± 260 ml min−1 kg−1; figure 2a); however, the hypoxia-mediated decrease in 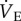 did not occur and
did not occur and 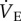 in AMP-treated animals breathing 7% O2 was 1344 ± 205 ml min−1 kg−1. In these naked mole rats, the hypoxia-mediated decrease in fr was not observed and fr tended to increase throughout the hypoxic exposure and recovery, although this trend did not reach significance (figure 2b). Conversely, the hypoxia-mediated decrease in Vt was only partially prevented by AMP treatment, and the observed Vt in these animals was statistically different from both pre-hypoxia control values in AMP-treated animals and also from hypoxic values in saline-treated animals breathing 7% O2 (figure 2c). In addition, the hypoxic decrease in Vt did not reverse upon reoxygenation in AMP-treated animals.
in AMP-treated animals breathing 7% O2 was 1344 ± 205 ml min−1 kg−1. In these naked mole rats, the hypoxia-mediated decrease in fr was not observed and fr tended to increase throughout the hypoxic exposure and recovery, although this trend did not reach significance (figure 2b). Conversely, the hypoxia-mediated decrease in Vt was only partially prevented by AMP treatment, and the observed Vt in these animals was statistically different from both pre-hypoxia control values in AMP-treated animals and also from hypoxic values in saline-treated animals breathing 7% O2 (figure 2c). In addition, the hypoxic decrease in Vt did not reverse upon reoxygenation in AMP-treated animals.
Figure 2.
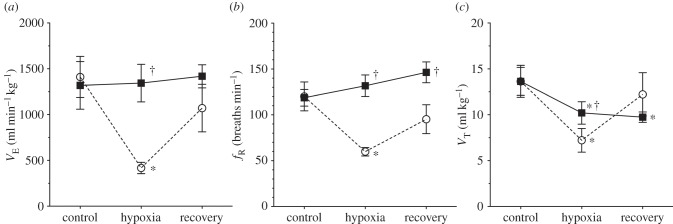
Ventilation is markedly reduced during acute hypoxia in an ADO receptor-dependent manner. Effects of acute hypoxia (7% O2), saline (white circles, dashed lines) and AMP (black squares, solid lines) on (a) total minute ventilation (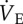 ), (b) breathing frequency (fr) and (c) tidal volume (Vt) in naked mole rats. Data are mean ± s.e.m. from n = 7 naked mole rats per group. Asterisks (*) indicate significant differences between normoxia and acute hypoxia values; daggers (†) indicate significant differences between AMP- and saline-treated animals (p < 0.05).
), (b) breathing frequency (fr) and (c) tidal volume (Vt) in naked mole rats. Data are mean ± s.e.m. from n = 7 naked mole rats per group. Asterisks (*) indicate significant differences between normoxia and acute hypoxia values; daggers (†) indicate significant differences between AMP- and saline-treated animals (p < 0.05).
(c). Hypoxia does not alter the air convection requirement in naked mole rats
The ACR for both O2 consumption and CO2 production indicated that saline-treated naked mole rats did not exhibit a change in breathing relative to metabolism when exposed to 7% O2 (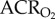 : 35.6 ± 4.6 in normoxia versus 34.5 ± 6.8 in hypoxia;
: 35.6 ± 4.6 in normoxia versus 34.5 ± 6.8 in hypoxia; 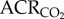 : 45.1 ± 3.2 in normoxia versus 47.9 ± 4.9 in hypoxia; figure 3a,b). This was expected, as the magnitudes of the HVR and HMR were equal. Similarly, the ACR of AMP-treated naked mole rats was not significantly different from saline-treated naked mole rats when both groups were breathing normoxic air (
: 45.1 ± 3.2 in normoxia versus 47.9 ± 4.9 in hypoxia; figure 3a,b). This was expected, as the magnitudes of the HVR and HMR were equal. Similarly, the ACR of AMP-treated naked mole rats was not significantly different from saline-treated naked mole rats when both groups were breathing normoxic air (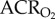 : 34.1 ± 10.8;
: 34.1 ± 10.8; 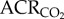 : 46.6 ± 7.8). Conversely, in AMP-treated naked mole rats, the
: 46.6 ± 7.8). Conversely, in AMP-treated naked mole rats, the 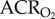 increased by 260% to 88.8 ± 6.2, whereas the
increased by 260% to 88.8 ± 6.2, whereas the 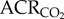 increased by 167% to 77.3 ± 9.3 when animals were exposed to 7% O2 because the HVR was prevented by AMP treatment, but the HMR was not. These hypoxia-induced changes were reversed upon reoxygenation and indicate that AMP-treated naked mole rats hyperventilated during acute hypoxia (i.e.
increased by 167% to 77.3 ± 9.3 when animals were exposed to 7% O2 because the HVR was prevented by AMP treatment, but the HMR was not. These hypoxia-induced changes were reversed upon reoxygenation and indicate that AMP-treated naked mole rats hyperventilated during acute hypoxia (i.e. 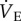 increased relative to metabolic demand because the metabolic rate decreased in hypoxia but
increased relative to metabolic demand because the metabolic rate decreased in hypoxia but 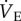 was unchanged when adenosine receptors were simultaneously blocked).
was unchanged when adenosine receptors were simultaneously blocked).
Figure 3.
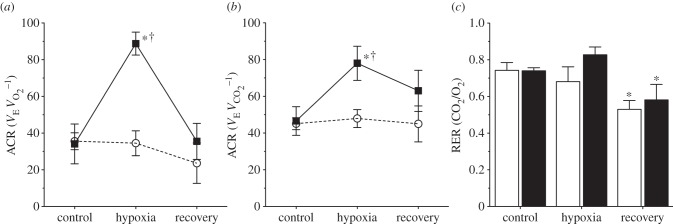
Naked mole rats do not exhibit a hypoxic change in their air convection requirement (ACR). (a,b) ACR of naked mole rats breathing normoxic or acute hypoxic (7% O2) gas mixtures and treated with subcutaneous saline (white circles, dashed lines) or AMP (black squares, solid lines) injection. (c) Average RER values from the animals treated in (a,b). Data are mean ± s.e.m. from n = 7 naked mole rats per group. Asterisks (*) indicate significant differences between normoxia and acute hypoxia values; daggers (†) indicate significant differences between AMP- and saline-treated animals (p < 0.05).
The RER was not significantly altered in animals breathing 7% O2 relative to normoxia, or by AMP treatment in either group (figure 3c). Respiratory exchange ratio values were slightly reduced upon recovery, indicating that the animals had not reached steady state, although their ventilatory and metabolic values were not statistically different from pre-hypoxia controls at this time point.
4. Discussion
Naked mole rats are the most hypoxia-tolerant mammals identified. In the present paper, we explore the metabolic, ventilatory and thermal responses of naked mole rats exposed to acute hypoxia, and the potential role for adenosine receptors in mediating these responses. Our study yielded three novel findings. First, naked mole rats undergo robust and reversible metabolic rate and ventilatory depression in acute hypoxia while maintaining Tb. Second; the 70% decrease in metabolic rate during acute hypoxia is matched equally by a 70% decrease in 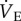 . Therefore,
. Therefore, 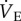 relative to metabolism (i.e. the ACR) is unchanged during acute hypoxia in naked mole rats, and thus, unique among mammalian responses to hypoxia; naked mole rats do not express a hypoxia-mediated increase in their ACR. Third, systemic antagonism of adenosine receptors prevents the HVR but does not affect the HMR.
relative to metabolism (i.e. the ACR) is unchanged during acute hypoxia in naked mole rats, and thus, unique among mammalian responses to hypoxia; naked mole rats do not express a hypoxia-mediated increase in their ACR. Third, systemic antagonism of adenosine receptors prevents the HVR but does not affect the HMR.
(a). Naked mole rats have a robust hypoxic metabolic response
Naked mole rats undergo a profound and reversible HMR when exposed to 7% O2 for 1 h. To our knowledge, only one other study has examined the effect of hypoxia on metabolic rate in naked mole rats. Nathaniel et al. [15] measured metabolism in awake, unrestrained naked mole rats exposed to 3% O2 for 1 h [15]. These authors reported a significant but very small (8%) hypoxic decrease in metabolic rate. Importantly, the resting metabolic rate reported in their study is approximately 1/10th that observed in our study, and both this difference and also the difference between our measurements of the extent of the HMR (8% and 70%, respectively) are likely to be due to problems with experimental design in the earlier study. Specifically, these authors used a chamber with a volume of approximately 3.5 l, a flow rate of 1 l min−1, and solely reported O2 consumption. Relative to the small body size of naked mole rats (approx. 45 g) and related low tidal volumes, this large chamber and high flow rate probably saturated the measurement of metabolism in normoxia and hypoxia, resulting in a significant underestimation of the resting metabolic rate and the HMR in the earlier study. Comparatively, our study used a chamber with a considerably smaller volume (approx. 450 ml) and a low flow rate of 110 ml min−1, which provided us greater sensitivity in our measurements of O2 consumption and CO2 production. In support of this, our normoxic metabolic rate measurements agree well with other measurements at a similar Ta in this species [32,33].
The cellular mechanism underlying the HMR of naked mole rats has not been examined previously; however, as described in the introduction, several recent studies have demonstrated a central role for adenosinergic signalling in regulating metabolic responses in other small rodents [6–11], although the mechanism via which adenosine receptors modulate metabolism have not been elucidated in any species. Therefore, we also asked whether or not adenosinergic signalling regulates systemic responses to hypoxia in this species. To our surprise, we observed no effect of a general adenosine receptor antagonist (i.e. AMP) on the HMR of the naked mole rat. This indicates that the role of adenosine in mediating the HMR is different in naked mole rats compared with other small mammals. The mechanism that induces and regulates the HMR in naked mole rats requires further study.
(b). Naked mole rats do not exhibit a thermal response to hypoxia
Naked mole rat Tb was approximately 32°C in our study and was not altered by saline or AMP injection, nor by exposure to hypoxia. Animals were held at a Ta of approximately 28°C and thus had only a small thermal range to accommodate hypoxia via metabolic rate depression associated with reductions in Tb. Our findings indicate that naked mole rats do not employ thermal strategies to tolerate hypoxia and that changes in Tb are probably not a component of their HMR. Our findings are in agreement with those of Nathaniel et al. [15]. These authors used surgically implanted emitters to record Tb throughout their experiment and reported an average Tb of 28.5 ± 0.2°C in normoxia, and no change in Tb in animals breathing 3% O2. Similar to our study, these animals were held at a Ta of 28°C, and thus had a minimal range to respond to hypoxia using thermal adaptations. We report a slightly higher Tb than the previous study, but in our experiments all animals remained active and alert, and dug and chewed at the chamber walls throughout the experiment, which may have resulted in a slightly increased Tb. Furthermore, we utilized a smaller chamber with a lower flow rate than the earlier study, which would reduce the rate of animal-generated heat loss from the chamber via thermal convection. To our knowledge, naked mole rats are the only mammals studied to date that exhibit such a robust metabolic depression in acute hypoxia without a concomitant decrease in Tb and entry into a torpor-like state [34]. These unique responses may be due to the warm and consistently hypoxic environment in which naked mole rats live. A high burrow Ta would restrict the scope for thermal adaptations to hypoxia; reducing physical activity in hypoxia would not be a sensible strategy as fresh air is typically only available to the colony through digging new tunnels and vents, which requires physical work. Given these environmental constraints, it appears that naked mole rats have evolved mechanisms of metabolic regulation that are independent of changes in Tb and physical activity.
(c). The naked mole rat hypoxic ventilatory response is mediated by adenosine receptors
Naked mole rats exhibit a robust decrease in 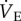 when exposed to hypoxia for 1 h, which is atypical of mammals [4,34]. Instead, a hypoxia-mediated increase in
when exposed to hypoxia for 1 h, which is atypical of mammals [4,34]. Instead, a hypoxia-mediated increase in 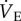 is consistently observed in other small mammals, and even in other fossorial species, which are typically also tolerant to prolonged hypoxia. For example, degus (Octodon degus), coruros (Spalacopus cyanus), plateau pikas (Ochotona curzoniae) and mole rats (Spalax ehrenbergi, a close cousin of naked mole rats) all exhibit increases in
is consistently observed in other small mammals, and even in other fossorial species, which are typically also tolerant to prolonged hypoxia. For example, degus (Octodon degus), coruros (Spalacopus cyanus), plateau pikas (Ochotona curzoniae) and mole rats (Spalax ehrenbergi, a close cousin of naked mole rats) all exhibit increases in 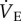 when in acute hypoxia [13,34–36]. The decrease in
when in acute hypoxia [13,34–36]. The decrease in 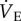 in hypoxia may represent a beneficial adaptation in naked mole rats since increased breathing, and indeed all other physiological mechanisms aimed at increasing O2 supply, are energetically expensive responses to hypoxia. In support of this, we observed that many naked mole rats struggled in hypoxia when adenosine receptors were blocked, and in one animal this combination of treatments was lethal, which led us to halt some experiments early in other cases where animals appeared to struggle in hypoxia following AMP injection. Given that naked mole rats are capable of tolerating hypoxia as low as 3% O2 in a laboratory setting, it is conceivable that they may still exhibit the more typical mammalian HVR of increasing
in hypoxia may represent a beneficial adaptation in naked mole rats since increased breathing, and indeed all other physiological mechanisms aimed at increasing O2 supply, are energetically expensive responses to hypoxia. In support of this, we observed that many naked mole rats struggled in hypoxia when adenosine receptors were blocked, and in one animal this combination of treatments was lethal, which led us to halt some experiments early in other cases where animals appeared to struggle in hypoxia following AMP injection. Given that naked mole rats are capable of tolerating hypoxia as low as 3% O2 in a laboratory setting, it is conceivable that they may still exhibit the more typical mammalian HVR of increasing 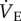 at lower inspired O2 levels; however, the level of hypoxia chosen for our study is similar to the most extreme level of environmental hypoxia experienced in nature by these organisms, and it is notable that other hypoxia-tolerant species exhibit a ‘normal’ HVR of increased breathing within the range of hypoxia they experience in their natural environment (e.g. plateau pika [35]). Nonetheless, further experiments examining the HMR and HVR in naked mole rats at deeper levels of hypoxia would be informative.
at lower inspired O2 levels; however, the level of hypoxia chosen for our study is similar to the most extreme level of environmental hypoxia experienced in nature by these organisms, and it is notable that other hypoxia-tolerant species exhibit a ‘normal’ HVR of increased breathing within the range of hypoxia they experience in their natural environment (e.g. plateau pika [35]). Nonetheless, further experiments examining the HMR and HVR in naked mole rats at deeper levels of hypoxia would be informative.
Unexpectedly, adenosine receptor antagonism with AMP entirely abolished the decrease in 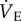 in naked mole rats breathing 7% O2. This inhibitory effect of adenosine on the HVR is consistent with previous studies in the literature, although the magnitude of its effect is unique to this organism. Adenosine is an inhibitory neurotransmitter that is known to accumulate in the blood during hypoxia and modulate the HVR [37]. Specifically, AMP treatment reduces (but does not abolish) the HVR in awake humans [38], cats [39], rats [40] and lambs [41], and in anaesthetized piglets [42], via modulation of the sensitivity of peripheral chemoreceptors. However, it is notable that in studies in which the effect of AMP on metabolism is examined along with its effects on
in naked mole rats breathing 7% O2. This inhibitory effect of adenosine on the HVR is consistent with previous studies in the literature, although the magnitude of its effect is unique to this organism. Adenosine is an inhibitory neurotransmitter that is known to accumulate in the blood during hypoxia and modulate the HVR [37]. Specifically, AMP treatment reduces (but does not abolish) the HVR in awake humans [38], cats [39], rats [40] and lambs [41], and in anaesthetized piglets [42], via modulation of the sensitivity of peripheral chemoreceptors. However, it is notable that in studies in which the effect of AMP on metabolism is examined along with its effects on 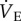 , AMP treatment always inhibits the HMR in addition to the HVR [41,43,44], indicating that the HVR and HMR are coupled in other mammals.
, AMP treatment always inhibits the HMR in addition to the HVR [41,43,44], indicating that the HVR and HMR are coupled in other mammals.
(d). The naked mole rat air convection requirement is not changed in hypoxia
In naked mole rats breathing hypoxic gas mixtures, the magnitudes of the HMR and HVR are equal. Therefore, the ACR is unaltered by hypoxia in this species. To our knowledge, this insensitivity of the ACR to hypoxia is unique among animals studied to date [4,34]. In addition, we report that AMP injection inhibits hypoxia-mediated ventilatory changes but has no effect on the hypoxia-mediated metabolic changes, indicating that the HMR and HVR are uncoupled by adenosine receptor antagonism in this species. As a result, AMP-treated naked mole rats hyperventilate in hypoxia, as indicated by an increase in their ACR.
Importantly, as the HMR is not altered when the HVR is blocked with AMP, it appears that the HMR is not regulated by O2 limitation (because AMP blocked the hypoxia-mediated decrease in breathing but not the change in metabolic rate, and thus AMP-treated animals hyperventilate, which should increase the amount of available O2 at the cellular level and enable a higher metabolic rate). As a result, the increase in metabolic demand associated with increased 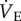 following AMP treatment in hypoxia is not matched by an increase in metabolic rate, leading to a mismatch between O2 supply and demand and deleterious effects to the organism. In support of this, AMP-treated naked mole rats struggled in hypoxia relative to saline-treated animals, and one naked mole rat died due to hypoxia exposure following AMP injection.
following AMP treatment in hypoxia is not matched by an increase in metabolic rate, leading to a mismatch between O2 supply and demand and deleterious effects to the organism. In support of this, AMP-treated naked mole rats struggled in hypoxia relative to saline-treated animals, and one naked mole rat died due to hypoxia exposure following AMP injection.
5. Conclusion
We report that naked mole rats, the most hypoxia-tolerant mammal presently identified, undergo a profound metabolic rate depression of 70% when breathing 7% O2, which is independent of changes in Tb and matched by an identical decrease in 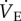 . As a result, breathing does not change in hypoxia relative to metabolism, and naked mole rats do not exhibit a hypoxia-mediated change in their ACR. This is a unique response to hypoxia among mammals studied to date. Furthermore, unlike in other mammals, the HMR is insensitive to adenosine receptor inhibition with AMP, whereas the HVR is entirely reversed by AMP. Our findings indicate that naked mole rats may have evolved unique adaptive responses to hypoxia that permit them to decrease metabolic rate independently of changes in Tb and without the benefit of increased O2 delivery via an energetically costly increase in their ACR. The specific mechanism of this HMR remains unknown, but probably involves cell-level adaptations that enable reduced metabolic rate through reductions in ion pumping, alternative fuel substrate usage or enhanced mitochondrial efficiency [2,3,45].
. As a result, breathing does not change in hypoxia relative to metabolism, and naked mole rats do not exhibit a hypoxia-mediated change in their ACR. This is a unique response to hypoxia among mammals studied to date. Furthermore, unlike in other mammals, the HMR is insensitive to adenosine receptor inhibition with AMP, whereas the HVR is entirely reversed by AMP. Our findings indicate that naked mole rats may have evolved unique adaptive responses to hypoxia that permit them to decrease metabolic rate independently of changes in Tb and without the benefit of increased O2 delivery via an energetically costly increase in their ACR. The specific mechanism of this HMR remains unknown, but probably involves cell-level adaptations that enable reduced metabolic rate through reductions in ion pumping, alternative fuel substrate usage or enhanced mitochondrial efficiency [2,3,45].
Ethics statement
All protocols were performed with the approval of the University of British Columbia Animal Care Program and in accordance with the relevant guidelines of the Canadian Council on Animal Care.
Data accessibility
Raw data used in this study can be found at doi:10.5061/dryad.q2h13.
Funding statement
This work was supported by an NSERC Discovery grant to W.K.M. and a Parker B. Francis Foundation PDF to M.E.P.
References
- 1.Buck LT, Pamenter ME. 2006. Adaptive responses of vertebrate neurons to anoxia: matching supply to demand. Respir. Physiol. Neurobiol. 154, 226–240. ( 10.1016/j.resp.2006.03.004) [DOI] [PubMed] [Google Scholar]
- 2.Bickler PE, Buck LT. 2007. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu. Rev. Physiol. 69, 145–170. ( 10.1146/annurev.physiol.69.031905.162529) [DOI] [PubMed] [Google Scholar]
- 3.Pamenter ME. 2014. Mitochondria: a multimodal hub of hypoxia tolerance. Can. J. Zool. 92, 569–589. ( 10.1139/cjz-2013-0247) [DOI] [Google Scholar]
- 4.Powell FL, Milsom WK, Mitchell GS. 1998. Time domains of the hypoxic ventilatory response. Respir. Physiol. 112, 123–134. ( 10.1016/S0034-5687(98)00026-7) [DOI] [PubMed] [Google Scholar]
- 5.Barnes BM. 1989. Freeze avoidance in a mammal—body temperatures below 0 degrees C in an Arctic hibernator. Science 244, 1593–1595. ( 10.1126/science.2740905) [DOI] [PubMed] [Google Scholar]
- 6.Tupone D, Madden CJ, Morrison SF. 2013. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J. Neurosci. 33, 14 512–14 525. ( 10.1523/JNEUROSCI.1980-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinka TR, Toien O, Drew KL. 2011. Season primes the brain in an Arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J. Neurosci. 31, 10 752–10 758. ( 10.1523/jneurosci.1240-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, Drew KL. 2013. Circannual rhythm in body temperature, torpor, and sensitivity to A(1) adenosine receptor agonist in Arctic ground squirrels. J. Biol. Rhythm. 28, 201–207. ( 10.1177/0748730413490667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iliff BW, Swoap SJ. 2012. Central adenosine receptor signaling is necessary for daily torpor in mice. Am. J. Physiol. 303, R477–R484. ( 10.1152/ajpregu.00081.2012) [DOI] [PubMed] [Google Scholar]
- 10.Iliff BW, Swoap SJ. 2010. Treatment with an adenosine receptor antagonist completely blocks fasting-induced torpor in mice. FASEB J. 24, 1032–1040. [Google Scholar]
- 11.Swoap SJ, Iliff BW, Le S. 2012. Adenosine, AMP, and daily torpor. In Living in a seasonal world (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 337–339. Vienna, Austria: Springer. [Google Scholar]
- 12.Sherman PW, Jarvis JU, Alexander RD. 1991. The biology of the naked mole-rat. Princeton, NJ: Princeton University Press. [Google Scholar]
- 13.Arieli R, Ar A. 1979. Ventilation of a fossorial mammal (Spalax ehrenbergi) in hypoxic and hypercapnic conditions. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 1011–1017. [DOI] [PubMed] [Google Scholar]
- 14.Shams I, Avivi A, Nevo E. 2005. Oxygen and carbon dioxide fluctuations in burrows of subterranean blind mole rats indicate tolerance to hypoxic-hypercapnic stresses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 142, 376–382. ( 10.1016/j.cbpa.2005.09.003) [DOI] [PubMed] [Google Scholar]
- 15.Nathaniel TI, Otukonyong E, Abdellatif A, Soyinka JO. 2012. Effect of hypoxia on metabolic rate, core body temperature, and c-fos expression in the naked mole rat. Int. J. Dev. Neurosci. 30, 539–544. ( 10.1016/j.ijdevneu.2012.04.004) [DOI] [PubMed] [Google Scholar]
- 16.Johansen K, Lykkeboe G, Weber RE, Maloiy GM. 1976. Blood respiratory properties in the naked mole rat Heterocephalus glaber, a mammal of low body temperature. Respir. Physiol. 28, 303–314. ( 10.1016/0034-5687(76)90025-6) [DOI] [PubMed] [Google Scholar]
- 17.Buffenstein R, Yahav S. 1991. Is the naked mole-rat, Heterocephalus glaber, a poikilothermic or poorly thermoregulating endothermic mammal? J. Therm. Biol. 16, 227–232. ( 10.1016/0306-4565(91)90030-6) [DOI] [Google Scholar]
- 18.Peterson BL, Larson J, Buffenstein R, Park TJ, Fall CP. 2012. Blunted neuronal calcium response to hypoxia in naked mole-rat hippocampus. PLoS ONE 7, e31568 ( 10.1371/journal.pone.0031568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson BL, Park TJ, Larson J. 2012. Adult naked mole-rat brain retains the NMDA receptor subunit GluN2D associated with hypoxia tolerance in neonatal mammals. Neurosci. Lett. 506, 342–345. ( 10.1016/j.neulet.2011.11.042) [DOI] [PubMed] [Google Scholar]
- 20.Nathaniel TI, Saras A, Umesiri FE, Olajuyigbe F. 2009. Tolerance to oxygen nutrient deprivation in the hippocampal slices of the naked mole rats. J. Integr. Neurosci. 8, 123–136. ( 10.1142/S0219635209002149) [DOI] [PubMed] [Google Scholar]
- 21.Bickler PE, Buck LT. 1998. Adaptations of vertebrate neurons to hypoxia and anoxia: maintaining critical Ca2+ concentrations. J. Exp. Biol. 201, 1141–1152. [DOI] [PubMed] [Google Scholar]
- 22.Aranda JV, Turmen T. 1979. Methylxanthines in apnea of prematurity. Clin. Perinatol. 6, 87–108. [PubMed] [Google Scholar]
- 23.Tanikawa K, Matsumoto Y, Matsuzaki T, Matsumoto M, Fukuoka M, Noguchi S, Goshima T. 1999. Population pharmacokinetic analysis of theophylline: relationship between serum concentrations and clinical effects in therapeutic drug monitoring. Yakugaku Zasshi 119, 861–867. [DOI] [PubMed] [Google Scholar]
- 24.Bach JE, Kukanich B, Papich MG, McKiernan BC. 2004. Evaluation of the bioavailability and pharmacokinetics of two extended-release theophylline formulations in dogs. J. Am. Vet. Med. Assoc. 224, 1113–1119. ( 10.2460/javma.2004.224.1113) [DOI] [PubMed] [Google Scholar]
- 25.Yin B, Li J, Wang SL, Yan SY, Sui C, Wan SJ, Liu F, Wan RZ. In press Studies on pharmacokinetics and bioavailability of aminophylline in partridge chickens. Eur. J. Drug Metab. Pharmacokinet. ( 10.1007/s13318-014-0225-6) [DOI] [PubMed] [Google Scholar]
- 26.Asheim P, Spigset O, Aasarod K, Walstad RA, Uggen PE, Zahlsen K, Aadahl P. 2008. Pharmacokinetics of intraperitoneally instilled aminophylline, terbutaline and tobramycin in pigs. Acta Anaesthesiol. Scand. 52, 243–248. ( 10.1111/j.1399-6576.2007.01535.x) [DOI] [PubMed] [Google Scholar]
- 27.Jacky JP. 1978. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J. Appl. Physiol. 45, 644–647. [DOI] [PubMed] [Google Scholar]
- 28.Drorbaugh JE, Fenn WO. 1955. A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- 29.Mcarthur MD, Milsom WK. 1991. Ventilation and respiratory sensitivity of euthermic Columbian and golden-mantled ground-squirrels (Spermophilus columbianus and Spermophilus lateralis) during the Summer and Winter. Physiol. Zool. 64, 921–939. [Google Scholar]
- 30.Frayn KN. 1983. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 55, 628–634. [DOI] [PubMed] [Google Scholar]
- 31.Koupenova M, Ravid K. In press. Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism. J. Cell Physiol. ( 10.1002/jcp.24352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman BD, Goldman SL, Lanz T, Magaurin A, Maurice A. 1999. Factors influencing metabolic rate in naked mole-rats (Heterocephalus glaber). Physiol. Behav. 66, 447–459. ( 10.1016/S0031-9384(98)00306-0) [DOI] [PubMed] [Google Scholar]
- 33.O'Connor TP, Lee A, Jarvis JU, Buffenstein R. 2002. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 835–842. ( 10.1016/S1095-6433(02)00198-8) [DOI] [PubMed] [Google Scholar]
- 34.Frappell P, Lanthier C, Baudinette RV, Mortola JP. 1992. Metabolism and ventilation in acute hypoxia—a comparative analysis in small mammalian species. Am. J. Physiol. 262, R1040–R1046. [DOI] [PubMed] [Google Scholar]
- 35.Pichon A, Zhenzhong B, Favret F, Jin G, Shufeng H, Marchant D, Richalet JP, Ge RL. 2009. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): role of nNOS and dopamine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R978–987. ( 10.1152/ajpregu.00108.2009) [DOI] [PubMed] [Google Scholar]
- 36.Tomasco IH, Del Rio R, Iturriaga R, Bozinovic F. 2010. Comparative respiratory strategies of subterranean and fossorial octodontid rodents to cope with hypoxic and hypercapnic atmospheres. J. Comp. Physiol. B. 180, 877–884. ( 10.1007/s00360-010-0465-y) [DOI] [PubMed] [Google Scholar]
- 37.Drumm D, Hoefer M, Juhasz J, Huszar E, Sybrecht GW. 2004. Plasma adenosine during investigation of hypoxic ventilatory response. Sleep Breath 8, 31–41. ( 10.1007/s11325-004-0031-5) [DOI] [PubMed] [Google Scholar]
- 38.Easton PA, Anthonisen NR. 1988. Ventilatory response to sustained hypoxia after pretreatment with aminophylline. J. Appl. Physiol. 64, 1445–1450. [DOI] [PubMed] [Google Scholar]
- 39.Long WQ, Anthonisen NR. 1994. Aminophylline partially blocks ventilatory depression with hypoxia in the awake cat. Can. J. Physiol. Pharmacol. 72, 673–678. ( 10.1139/y94-095) [DOI] [PubMed] [Google Scholar]
- 40.Maskrey M, Westwood KJ. 1994. Dual effect of aminophylline on the ventilatory response to hypoxia in the rat. J. Basic Clin. Physiol. Pharmacol. 5, 227–238. ( 10.1515/JBCPP.1994.5.3-4.227) [DOI] [PubMed] [Google Scholar]
- 41.Koos BJ, Kawasaki Y, Kim YH, Bohorquez F. 2005. Adenosine A2A-receptor blockade abolishes the roll-off respiratory response to hypoxia in awake lambs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1185–1194. ( 10.1152/ajpregu.00723.2004). [DOI] [PubMed] [Google Scholar]
- 42.Darnall RA., Jr 1985. Aminophylline reduces hypoxic ventilatory depression: possible role of adenosine. Pediatr. Res. 19, 706–710. ( 10.1203/00006450-198507000-00014) [DOI] [PubMed] [Google Scholar]
- 43.Barros RC, Branco LG, Carnio EC. 2006. Respiratory and body temperature modulation by adenosine A1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir. Physiol. Neurobiol. 153, 115–125. ( 10.1016/j.resp.2005.09.013) [DOI] [PubMed] [Google Scholar]
- 44.Maxwell DL, Fuller RW, Nolop KB, Dixon CM, Hughes JM. 1986. Effects of adenosine on ventilatory responses to hypoxia and hypercapnia in humans. J. Appl. Physiol. 61, 1762–1766. [DOI] [PubMed] [Google Scholar]
- 45.Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. 2012. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc. Natl Acad. Sci. USA 109, 8635–8640. ( 10.1073/pnas.1120523109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data used in this study can be found at doi:10.5061/dryad.q2h13.


