Abstract
Plague, the causative agent of three devastating pandemics in history, is currently a re-emerging disease, probably due to climate change and other anthropogenic changes. Without understanding the response of plague systems to anthropogenic or climate changes in their trophic web, it is unfeasible to effectively predict years with high risks of plague outbreak, hampering our ability for effective prevention and control of the disease. Here, by using surveillance data, we apply structural equation modelling to reveal the drivers of plague prevalence in two very different rodent systems: those of the solitary Daurian ground squirrel and the social Mongolian gerbil. We show that plague prevalence in the Daurian ground squirrel is not detectably related to its trophic web, and that therefore surveillance efforts should focus on detecting plague directly in this ecosystem. On the other hand, plague in the Mongolian gerbil is strongly embedded in a complex, yet understandable trophic web of climate, vegetation, and rodent and flea densities, making the ecosystem suitable for more sophisticated low-cost surveillance practices, such as remote sensing. As for the trophic webs of the two rodent species, we find that increased vegetation is positively associated with higher temperatures and precipitation for both ecosystems. We furthermore find a positive association between vegetation and ground squirrel density, yet a negative association between vegetation and gerbil density. Our study thus shows how past surveillance records can be used to design and improve existing plague prevention and control measures, by tailoring them to individual plague foci. Such measures are indeed highly needed under present conditions with prevailing climate change.
Keywords: Yersinia pestis, climate change, structural equation model, trophic cascade model, bottom-up effect, top-down effect
1. Introduction
Climate change is accelerating, and, together with other anthropogenic changes, is an important factor in the currently increased risk of many infectious diseases to transmit and cause global outbreaks [1–3]. Worldwide public concerns have stimulated scientific studies on the effects of climate change on disease transmissions at multiple levels [4,5], dealing with topics such as the alteration of the immune defences of the host [6,7], the virulence of pathogens [8], and the geographical distribution of hosts and vectors [9,10]. It is clear that the occurrence of disease is a complex process involving many factors (climate, vegetation, food, host, vector, pathogen, etc.), and these factors are closely connected with each other. Yet, although the relationship between climatic factors and the dynamics of disease has been studied frequently [11–15], this is seldom done within the context of a full trophic web, whether via bottom-up (e.g. resource limitation) or top-down pathways (e.g. infection by pathogen or predation). By not studying these factors in an integrated way, we limit our capacity for surveillance and prevention of diseases.
Plague, caused by Yersinia pestis infection, is one of the most notorious infectious diseases in history, and has caused hundreds of millions deaths worldwide during the three plague pandemics (i.e. the Justinian, which started in AD 541, the Black Death in AD 1347 and the Third Pandemic in AD 1772) [15,16]. Plague is still active in many parts of the world. It can impose a serious threat to human health, both locally as a re-emerging disease and globally as a potential biological weapon [16–19]. Parmenter et al. [20] proposed a trophic cascade model based on ecological bottom-up effects in food webs to explain the pathways and mechanisms of climate-driven plague dynamics. The model assumes a positive cascade, in which high precipitation increases primary production, which in turn increases the population abundance of rodent hosts and flea vectors, resulting in an increase of the prevalence of plague (pathogen). A top-down pathway was added by Stapp [21], who took into account the fitness costs of fleas parasitizing on the rodents. But in these two models, the effects of temperature were not taken into account. The purpose of this study is to investigate the effects of both temperature and precipitation on rodent–vector–plague systems by using temporal–spatial surveillance data in two plague foci (i.e. Mongolian gerbil and Daurian ground squirrel plague foci) in Inner Mongolia, China. We used structural equation models (SEMs) to identify potential bottom-up and top-down pathways between climate and plague.
2. Material and methods
(a). Study sites
Inner Mongolia is the largest endemic region of plague persistence in China. The average monthly temperature ranges from −28.6 to 27.3°C, and the annual precipitation is 150–500 mm. It has experienced devastating human plague epidemics during the Third Plague Pandemic, which spread from southwestern China to Hong Kong, and from there across the world [11,16]. Plague is still active in the local natural plague foci of Inner Mongolia [22]. Precipitation and vegetation exhibit an environmental heterogeneity gradient from east to west in Inner Mongolia. The east has a larger amount of precipitation, more dense vegetation and lower temperature than the western regions of Inner Mongolia.
The plague surveillance system in Inner Mongolia is run by the Inner Mongolia Center for Endemic Diseases Control and Research, covering 32 sample sites from 1980 to 2006. It started in the 1980s with monitoring of rodent host population abundance, flea burden and the presence of Y. pestis infections, so as to prevent occurrence and transmission of plague. The surveillance dataset used in this study was used with permission by the Inner Mongolia Center for Endemic Disease Control and Research.
(b). Rodent host abundance, flea vector abundance and presence of plague in natural plague foci
Mongolian gerbils (Meriones unguiculatus; figure 1a) and Daurian ground squirrels (Spermophilus dauricus; figure 1b) are the main natural hosts and reservoirs, as well as predominant rodent species, in their respective plague foci. The Mongolian gerbil and Daurian ground squirrel plague foci are located adjacent to each other [23]. The Mongolian gerbil (weighing 45 to 67 g) is a social animal that does not hibernate, and its main habitat is the semi-arid deserts and steppes of western Inner Mongolia. The Mongolian gerbil population dynamics exhibit two seasonal peaks, usually in May and November. The surveillance was conducted twice a year, following the seasonality of the population dynamics, which was stored as annual average data. The Daurian ground squirrel (weighing 154–298 g) is a solitary animal that hibernates from September to next March; its main habitat is the arid grasslands and semi-arid deserts of eastern Inner Mongolia. Population dynamics of the Daurian ground squirrel exhibit only one seasonal peak during May and June, and thus the surveillance was done from May to June [23].
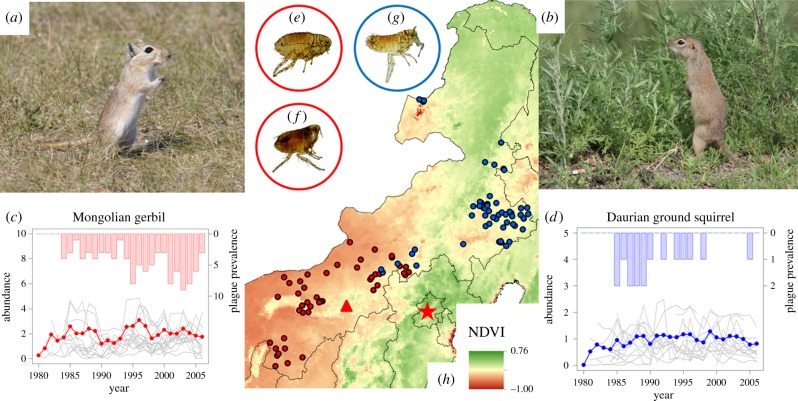
Figure 1.
(a) Photo of the Mongolian gerbil (Meriones unguiculatus Edwards, 1867). (b) Photo of the Daurian ground squirrel (Spermophilus dauricus Brandt, 1844). (c) The temporal dynamic of the Mongolian gerbil population density from 1980 to 2006, with red dots indicating the mean population density from all plague surveillance locations, and grey lines representing the Mongolian gerbil population density in each plague surveillance location. The barplot shows the temporal dynamic of plague infection incidence in gerbils, with the height of the rectangles indicating the number of locations where plague was diagnosed in local animal hosts (see y-axis plot on the right side). (d) As for panel (c), but for the temporal dynamic of the Daurian ground squirrel population density and the plague infection incidence in these squirrels. Please note that the y-axis scales differ between panels (c) and (d). There were fewer plague surveillance sites before 1984, explaining the lack of observed plague incidence prior to 1985, but their number has been quite stable since. (e) Photo of Xenopsylla conformis conformis. (f) Photo of Nosopsyllus laeviceps kuzenkovi. (g) Photo of Citellophilus tesquorum sungaris. Vector fleas are in general host-specific. The vectors in panel (e) and (f) mainly parasitize on the Mongolian gerbil, while the vector flea in panel (g) mainly parasitizes Daurian ground squirrels. (h) Plague surveillance locations for the Mongolian gerbil natural plague foci (red points) and the Daurian ground squirrel natural plague foci (blue points) in Inner Mongolia, with the background colour indicating the average vegetation that was measured by NDVI. Red pentagram marks the location of Beijing. Red triangle marks the location of Huhehot. Photos provided by (a) Lijun Chen and (b,e–g) Jianjun Wang.
The method of plague surveillance follows the plague manual of WHO [24] and China's national plague surveillance scheme. The surveillance area of each sample site (figure 1h) covered 25–100 km2, of which 0.5% was randomly selected for surveillance by stratified sampling, thus ensuring that representative samples were acquired from the whole plague foci area. The population density of both rodent species was estimated by using daytime live-trapping and was defined as the number of rodents captured per day per hm2. All captured rodents were identified in terms of species, length, weight, sex and age, and were inspected for fleas.
Fleas collected from each animal were placed in vials prior to identification and counting. The flea species for the Mongolian gerbil were mainly Xenopsylla conformis conformis (figure 1e), Nosopsyllus laeviceps kuzenkovi (figure 1f) and Neopsylla pleskei orientalis. The flea species of the Daurian ground squirrel were mainly Citellophilus tesquorum sungaris (figure 1g) [25]. Flea burden was estimated by using body flea index, which was defined as average number of fleas per host rodent [26]. Seasonality of flea density approximately synchronized with host population abundance [27]. Both the rodent host and flea vectors caught were tested for any presence of a plague infection.
Plague diagnosis of Y. pestis in the rodents was conducted both by serological and by aetiological methods [24]. Here we use only the results of the aetiological method, which depends on the successful culturing of Y. pestis from tissues such as the liver or spleen. This guarantees that plague was present in the year of sampling in a sample site, whereas the serological method cannot distinguish between the presence of plague and past exposure to the bacterium. The fleas were pooled and ground into a broth before being tested by culturing for the presence of Y. pestis. Only yearly averaged values of host abundance, flea index and plague intensity per year were stored, and are available for this study.
(c). Vegetation, temperature and precipitation data
The normalized difference vegetation index (NDVI) is often used to represent the primary production over large areas. Monthly NDVI data were obtained from the Environmental and Ecological Science Data Centre for West China, National Natural Science Foundation of China (http://westdc.westgis.ac.cn). Spring (March–May) and summer (June–August) NDVI of each plague surveillance location was calculated from the monthly geo-data by using the spatial analysis tool ‘zone statistics’ in ArcGIS (ESRI 1996; electronic supplementary material, figure S3).
Temperature and precipitation anomaly data were obtained from the China Meteorological Data Sharing Service System (http://cdc.cma.gov.cn/home.do). We used temperature and precipitation data from the meteorological station closest to each of the plague surveillance sites (electronic supplementary material, figure S1).
(d). Structural equation model
The SEM has been extensively applied in social science for identifying pathways of influential factors [28–30], but its use in ecology is still very limited [31–34]. The SEM modelling process estimates the structural correlation among the variables, which enables scientists to propose hypotheses about how systems work (e.g. the modelled interconnected relationships between variables) and to test these relationships using observational data [33,35]. In this study, the term SEM is equivalent to the pathway analysis because we did not include the measurement model or the latent variables; however, we still used the term SEM because we wanted to use the same terminology as in the software EQS or the potential extension of the modelling in the follow-up study. We formulated a SEM by following the trophic cascade model [20,21], but modified it to include temperature as another climate factor (electronic supplementary material, figure S2). Time lags for climate (temperature and precipitation) and for NDVI were set to 0 or 1 (i.e. the current and previous year); the time lag for host, vector and pathogen were all set at the current year. We constructed yearly and seasonal models by taking into account the yearly and seasonal climate/NDVI data, respectively (but in both models, we only have yearly data for rodents, fleas and plague). The yearly SEM models have three logical combinations of time lags: [0,0,0], [1,0,0] and [1,1,0] for climate, NDVI and the host–vector–pathogen system, respectively (electronic supplementary material, tables S1 and S2). The seasonal SEM models have 12 logical combinations of climate, NDVI and the host–vector–pathogen system if time lag of current and last years are considered (electronic supplementary material, figure S4).
The SEM models estimation was conducted by using EQS software (v. 6.1) [36]. The estimation of the models applied the maximum-likelihood method, and the convergence of model estimate was checked within 500 iterations. We chose the widely used χ2-test, root mean square error of approximation (RMSEA), comparative fit index (CFI) and Akaike information criterion (AIC) as model fit indices [30,31,35,36]. Normalized χ2-test (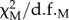 , i.e. model χ2 divided by the degrees of freedom) was used as a model test statistic [30] to verify whether the covariance matrix implied by conceptual model is close enough to the sample matrix that the differences may be reasonably considered as due to sampling error. Additionally, approximate fitting indices, such as the RMSEA [37] and CFI [38], were also used for model test statistic. AIC data are provided in electronic supplementary material, tables S2–S8 for the models tested, but we have not changed our model selection based on the AIC scores. We did not conduct further model selection between the model variants with different time lags because they present different model structures. We controlled the effects of variable location j to remove potential effects of spatial autocorrelation. We did not conduct data scaling and transformations before the SEM analysis, as the method does not require the data to be normally distributed. The robust method was used to run the SEM model in the EQS, which deals with abnormalities in the covariates. Candidate paths in electronic supplementary material, figure S2 were defined to be significant when significance level p < 0.05 and are shown in figures 2 and 3. Models with minimum AIC and CFI or largest RMSEA were defined as the best-fitting models.
, i.e. model χ2 divided by the degrees of freedom) was used as a model test statistic [30] to verify whether the covariance matrix implied by conceptual model is close enough to the sample matrix that the differences may be reasonably considered as due to sampling error. Additionally, approximate fitting indices, such as the RMSEA [37] and CFI [38], were also used for model test statistic. AIC data are provided in electronic supplementary material, tables S2–S8 for the models tested, but we have not changed our model selection based on the AIC scores. We did not conduct further model selection between the model variants with different time lags because they present different model structures. We controlled the effects of variable location j to remove potential effects of spatial autocorrelation. We did not conduct data scaling and transformations before the SEM analysis, as the method does not require the data to be normally distributed. The robust method was used to run the SEM model in the EQS, which deals with abnormalities in the covariates. Candidate paths in electronic supplementary material, figure S2 were defined to be significant when significance level p < 0.05 and are shown in figures 2 and 3. Models with minimum AIC and CFI or largest RMSEA were defined as the best-fitting models.
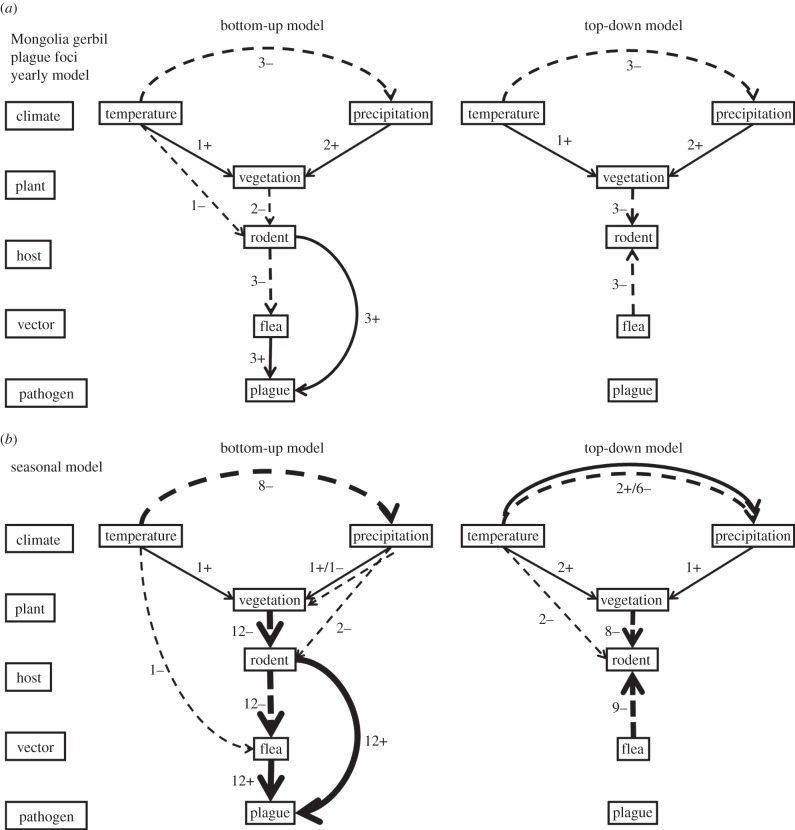
Figure 2.
Structure and results from our SEMs for Mongolian gerbil plague foci. (a) SEM models were built using the yearly climate and vegetation variables; a total of three yearly model variants were built for both the bottom-up and the top-down models based on combination of time lag of current and last years. (b) SEMs were built with seasonal climate and vegetation variables; a total of 12 seasonal model variants were built based on combination of time lag of current and last years. The numbers indicate the number of the models (also shown in line width) supporting a significant pathway between two components of the trophic web, with + and – indicating positive or negative associations. Arrows show the direction of the interaction that was assumed by the model.
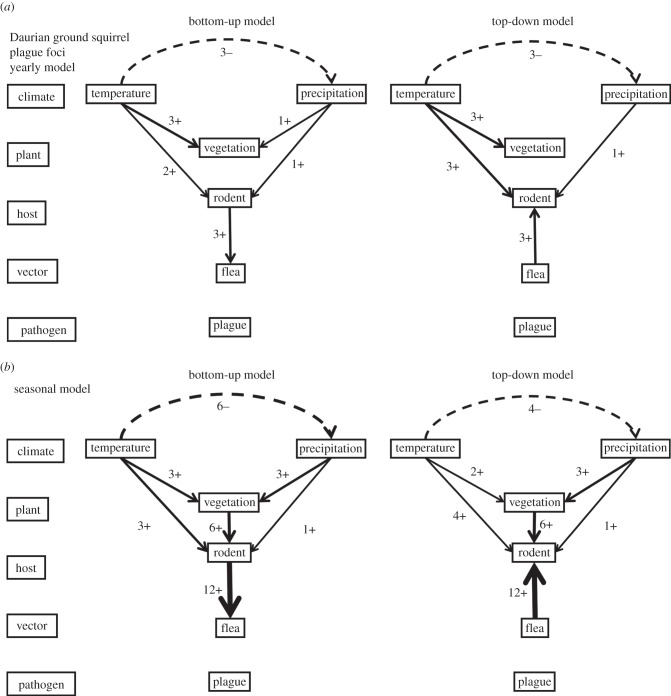
Figure 3.
Structure and results of SEMs for the Daurian ground squirrel plague foci. For detailed explanations, see figure 2.
3. Results
(a). Mongolian gerbil plague foci
(i). Bottom-up pathways
Yearly SEM analysis (figure 2a) indicated that temperature (T) and precipitation (PR) were significantly and negatively correlated with each other. Higher temperatures in the previous year (T−1) were associated positively with NDVI, as were high levels of precipitation (PR) in this year. Higher temperatures in the previous year showed a negative correlation on gerbil abundance in this year. Perhaps surprisingly, this was also the case for high NDVI scores in this and the previous year. Increased gerbil abundance was negatively correlated with the flea index (F) of the same year, and both increased gerbil abundance and flea index were positively associated with plague intensity (PL). Seasonal SEM analysis (figure 2b) confirmed most of these pathways (electronic supplementary material, table S3). Seasonal SEM analysis also revealed additional pathways where increased autumn temperature in the previous year (TA−1) was negatively associated with the flea index (F), and spring precipitation (which could be snow) in the previous year (Tsp−1) was negatively associated with rodent density (R). In summary, both yearly and seasonal SEM models consistently supported the existence of the following pathways: temperature/precipitation(pos) → NDVI(neg) → gerbil(pos) → plague, and gerbil(neg) → flea(pos) → plague, where (pos) and (neg) indicate a significant positive and negative coefficient, respectively.
(ii). Top-down pathways
The top-down SEM models are mixtures of top-down relationships where they are expected to be (predation, pathogenicity [21]), and bottom-up relationships otherwise (climate on vegetation, rodents and fleas, vegetation on rodent density). Yearly SEM analysis (figure 2c) showed a top-down negative association of flea index (i.e. the number of fleas per gerbil) on gerbil abundance, but no association between Y. pestis and flea index or gerbil abundance. The resource limitation relationships within the annual top-down SEM models are almost identical to those found in the annual bottom-up SEM modelling: a significant, but minor negative association between temperature in the previous year (T−1) and gerbil density (R) in the yearly bottom-up model (coefficient strength of −0.024, p < 0.05; electronic supplementary material, table S1) is not significant in the yearly top-down model (coefficient of −0.054, p > 0.05; electronic supplementary material, table S2). In the seasonal top-down models, the negative association is visible again as a minor association between the temperature in the previous spring (coefficient of −0.08, p < 0.05) or winter (coefficient of −0.019, p < 0.05; electronic supplementary material, table S3), and the gerbil density of the current year. The bottom-up seasonal SEM modelling and the bottom-up components of the top-down seasonal SEM modelling generally agree on the sign of the coefficients of the trophic web (electronic supplementary material, tables S3 and S4). In summary, both yearly and seasonal SEM models supported the existence of the follow ing pathway: temperature/precipitation(pos) → NDVI(neg) →gerbil ← (neg)flea, where (pos) and (neg) indicate a significant positive and negative coefficients, respectively.
These observations in both bottom-up and top-down models were also supported in the yearly and seasonally best-fitting models with minimum AIC and CFI or largest RMSEA (electronic supplementary material, tables S1–S6; figure 2).
(b). Daurian ground squirrel plague foci
(i). Bottom-up pathways
Yearly SEM analysis (figure 3a) showed negative associations between temperature (T) and precipitation (PR). Both temperature and precipitation were positively associated with NDVI, and with the abundance of ground squirrels (R). A positive association also existed between the abundance of ground squirrels and the flea index (F), and thus the trophic web of the Daurian ground squirrel seems to be in line with the trophic cascade hypothesis [20]. Seasonal SEM analysis mostly reconfirmed the pathways that were detected as significant in the yearly SEM models (figure 3), except that the seasonal SEM models indicate an additional association between spring vegetation and rodent density. Such an association is not found for summer vegetation, which might be the reason why the yearly models did not detect a direct correlation between vegetation and rodent density. Plague prevalence (PL) was neither in the annual nor the seasonal SEM models significantly associated with the trophic web of its reservoir host. In summary, both yearly and seasonal SEM models supported the existence of the following pathway: temperature/precipitation(pos) → squirrels(pos) → fleas. Nearly half of the seasonal models also indicated the existence of a positive association between NDVI and squirrel density.
(ii). Top-down pathways
Both yearly and seasonal SEM analysis showed a positive top-down association of the flea index (F) with the Daurian ground squirrel abundance (R), which is counterintuitive in terms of the fitness cost of higher parasitic loads on the host. No top-down associations of plague prevalence (PL) with R or F were detected. The bottom-up pathways in top-down yearly and seasonal SEM models were very similar to those of the bottom-up models, showing the same inclusion of a positive association between current spring NDVI and ground squirrel density when moving from annual to seasonal SEM models. In summary, both yearly and seasonal SEM models indicated the existence of the following pathway: temperature/precipitation(pos) → squirrels ← (pos)fleas. Nearly half of the seasonal models also indicated the existence of a positive association between NDVI and squirrel density.
These observations in both bottom-up and top-down models were also supported in the yearly best-fitting models with minimum AIC and CFI or largest RMSEA, but there were some differences in seasonal models (electronic supplementary material, tables S1–S6; figure 2).
4. Discussion
We studied two very different plague foci in Inner Mongolia, China, namely those of the solitary Daurian ground squirrel (Spermophilus dauricus) and the smaller, social Mongolian gerbil (Meriones unguiculatus), by analysing 26 years of Chinese plague surveillance data. We identified and compared the main ecological drivers of plague in their trophic webs using SEM, and showed the suitability of this type of analysis for rapidly assessing and possibly improving plague surveillance approaches in different plague foci, using existing surveillance data.
The trophic webs of these two plague foci were analysed both from a resource perspective (i.e. bottom-up trophic webs) and from a predation/pathogenicity perspective (i.e. top-down trophic webs). The trophic web of the Daurian ground squirrel largely confirmed the trophic cascade hypothesis for plague systems [20], in which an abundance of resources increases the abundance of higher trophic levels, especially when we take into account the seasonality of the system. However, we find no evidence for a causal link between rodent host density or flea index on plague prevalence in these ground squirrels. A straightforward conclusion for the Daurian plague focus is therefore that plague surveillance efforts in this area would best be targeted towards detecting the presence of Y. pestis, as the other components of the trophic web are not associated with plague prevalence in a substantial way (figure 3). The trophic web of the Mongolian gerbil supports the trophic cascade hypothesis for the gerbils and fleas, with significant interactions between different trophic levels. However, not all associations between the trophic levels of the Mongolian gerbil are positive: both the correlation between vegetation and rodent abundance, and the correlation between rodent abundance and flea density per rodent (flea index), have a negative coefficient. Nevertheless, the coefficient strength and significance of the associations between different levels of the trophic web in the Mongolian gerbil plague foci suggest that plague surveillance efforts in this area can benefit from low-cost surveillance practices, such as remote NDVI sensing, gerbil density estimates and predictive climate models.
In the Mongolian gerbil foci, the strongest linear effects from climate on gerbil, flea and plague appear to be functioning through the vegetation as an intermediate. The NDVI often has two significant aspects (i.e. food availability and vegetation cover). Unlike the Daurian ground squirrel, Mongolian gerbils prefer desert habitats and avoid habitats with dense and high grasses [39]. The negative association between NDVI and gerbil abundance reported earlier may therefore reflect changes in vegetation cover, rather than food availability. Such a complex response of small rodent species in desert or semi-arid regions to increased NDVI might explain earlier contrasting reports on the association between increased precipitation and rodent densities, even within the same species: increased precipitation was positively associated with Mongolian gerbil abundance in the typical Erdos desert [40], but negatively associated in the farming–pastoral zone of south-central Inner Mongolia [41]. Similar observations have been made for the Altai ground squirrel [42]. Owing to the dual role of vegetation as a food resource for rodents and as cover for both rodents and predators, the relationship between NDVI and rodent densities, as well as the relationship between precipitation and rodent densities (through NDVI) are likely to have nonlinear components [11].
That we did not find a significant association between plague prevalence and Daurian ground squirrel density is consistent with the findings in the black-tailed prairie dog (Cynomys ludovicianus) [43]. Two explanations are frequently given for this phenomenon. First, a secondary species might play an important role in plague cycle of the ecosystem. In the Daurian ground squirrel plague foci, Rattus norvegicus, Mus musculus and Cricetulus barabensis are present and were also found sometimes to carry plague infections [22]. Second, the solitary behaviour of the Daurian ground squirrels also might play a role, limiting the positive effects on plague transmissibility that a collapse of a high rodent density (and the subsequent rise in flea burden on the remaining rodents) can have in social rodent species [12]. In the solitary Daurian ground squirrels, fleas would have little opportunity to transfer themselves to other ground squirrels in the case of a population decrease. Besides these two explanations, we explored a third option (electronic supplementary material, tables S7 and S8) where we limited our use of the surveillance data of Daurian ground squirrels to 1985 or later in the annual SEM models (leaving out the period where no plague had yet been reported in the ground squirrel plague foci). While removing 7.8% of the data points changed the significance of four pathways in total, it did not alter the absence of an association between plague prevalence and the density of Daurian ground squirrels or their fleas.
In both the bottom-up and in the top-down models of the Daurian ground squirrel, we find a positive association between parasitic load and rodent abundance (figure 3). As the direction of causality is imposed by the researcher on the SEM model, and the method itself cannot determine the direction of causality, it is up to the researcher to interpret the results. Because a positive effect of increased parasitic load on ground squirrel densities seems unlikely, we assume that the direction of causality is that higher rodent abundances result in a higher parasitic load (i.e. a bottom-up pathway).
The negative association that we found between the Mongolian gerbil density and its flea burden (flea per individual) in the bottom-up models could be due to a time lag between the responses of the flea population to changes in rodent densities, something for which there is evidence in the great gerbils in Kazakhstan [44]. When the gerbil population is rapidly increasing in numbers, the flea population is diluted over a larger number of gerbils, decreasing the flea burden per gerbil. Vice versa, a decreasing gerbil population means that fleas will aggregate on the remaining hosts, thus causing a high flea index, and facilitating the rapid transmission of plague [45].
As we see no top-down associations between plague and fleas, or plague and rodent abundance in both the Mongolian gerbil plague foci and the Daurian ground squirrel plague foci, and furthermore, we find no negative association between flea index and Daurian ground squirrel densities, our results offer little support for the top-down model proposed by Stapp [21]. Only the top-down models for the Mongolian gerbils show a strong negative association between flea index and rodent densities, which could be interpreted as a strong parasitic relationship. This fits a top-down interpretation of the data, but could also be due to the interpretation given above, detailing the delayed response of flea populations to changes in the great gerbil population in Kazakhstan, which would also result in a negative relationship between gerbil density and flea burden [44]. A longer time lag between rodent densities and flea burden should be considered in future studies [44] to distinguish whether gerbil abundance is indeed depressed by flea burden.
In this study, we reported two major bottom-up pathways from temperature to plague in the gerbil plague foci—temperature → NDVI → gerbil → flea → plague, and temperature → NDVI → gerbil → plague—and one bottom-up pathway from temperature to plague in the ground squirrel plague foci: temperature → NDVI → squirrel → flea. Temperature showed positive effects on NDVI in both plague foci, which is consistent with some previous studies [46,47]. Temperature showed predominantly positive effects on population abundance of small rodents in the current year in Inner Mongolia [2,12]. Moderately high temperature increases the primary production of the dominant species of local vegetation, and thus promotes a higher population density of ground squirrels (Spermophilus spp. and Ammospermophilus spp.). However, conversely, temperature begins to negatively impact the survival or reproduction of rodents and fleas when it rises for a number of days above a particular temperature threshold [48]. We found temperature showed a predominantly direct positive effect on Daurian ground squirrels, consistent with the observation by Jiang et al. [46]. High temperature in winter and spring would benefit the survival and breeding success of hibernating animals like the Daurian ground squirrels. Temperature and precipitation are known to affect the reproduction, development, behaviour and population dynamics of arthropod vectors. High precipitation also decreases plague prevalence in wet regions at low latitudes, where climate affects plague dynamics directly through the flea vector, rather than mediated by the rodent population or vegetation [14,49].
Our results have significant application, suggesting strategies to reduce plague occurrence, as well as to anticipate the effects of climate change and human activities in the region. Accelerated global warming would significantly affect population dynamics of rodents [50,51]. During recent decades, the Inner Mongolia plateau has experienced a rapid increase in temperature and a heterogeneous change of precipitation in space [52]. We project that the continuation of this trend would in general decrease the risk of plague occurrences in the Mongolian gerbil plague foci, but may increase the risk in the Daurian ground squirrel plague foci. The abundance of vegetation (NDVI) is negatively associated with gerbil abundance, but positively with ground squirrel plague systems, indicating that livestock management can be used as a selective tool for plague prevention by making the environment less hospitable for these rodent species. Thus, implementing a grazing reduction policy for restoring degenerated grassland would increase the risk of plague outbreaks in the Daurian ground squirrel plague foci, but would decrease it in the Mongolian gerbil plague foci. Similarly, the accelerating development and industrialization of Inner Mongolia has in general a positive effect: the movement of people from rural areas to cities will reduce the contact rates between people and plague-infected vectors. However, as pointed out above, continued grazing activity in ground squirrel plague foci is important in reducing plague risk in these foci [39].
Altogether our results largely support the trophic cascade model proposed by Parmenter et al. [20], but we find little evidence for the top-down model suggested by Stapp [21]. Both models lack temperature as part of the trophic cascade of plague, yet our results indicate that it should be included. Our major novel finding is that the trophic response to climate variation of different plague ecosystems can vary significantly, with consequences for surveillance and control strategies.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Jie Zhou from Institute of Psychology, Chinese Academy of Sciences for helpful discussions and data analysis in this study.
Author contributions
Z.Z. and N.C.S. designed the study. L.X. and B.V.S. conducted the analysis. J.L. and X.S. provided data of plague, vector and host. All authors contributed to writing the study.
Funding statement
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB11050300) and grant no. 31370440) from the National Natural Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Karesh WB, et al. 2012. Ecology of zoonoses: natural and unnatural histories. Lancet 380, 1936–1945. ( 10.1016/S0140-6736(12)61678-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA. 2001. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ. Helath Perspect. 109, 223–233. ( 10.2307/3435012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilpatrick AM, Randolph SE. 2012. Zoonoses 2 drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380, 1946–1955. ( 10.1016/S0140-6736(12)61151-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 5.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162. ( 10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 6.Patz JA, Reisen WK. 2001. Immunology, climate change and vector-borne diseases. Trends Immunol. 22, 171–172. ( 10.1016/S1471-4906(01)01867-1) [DOI] [PubMed] [Google Scholar]
- 7.Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ. 2006. Negative effects of changing temperature on amphibian immunity under field conditions. Funct. Ecol. 20, 819–828. ( 10.1111/j.1365-2435.2006.01159.x) [DOI] [Google Scholar]
- 8.Kimes NE, et al. 2012. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J 6, 835–846. ( 10.1038/ismej.2011.154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monzon J, Moyer-Horner L, Palamar MB. 2011. Climate change and species range dynamics in protected areas. Bioscience 61, 752–761. ( 10.1525/bio.2011.61.10.5) [DOI] [Google Scholar]
- 10.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Liu Q, Stige LC, Ben Ari T, Fang X, Chan KS, Wang S, Stenseth NC, Zhang Z. 2011. Nonlinear effect of climate on plague during the third pandemic in China. Proc. Natl Acad. Sci. USA 108, 10 214–10 219. ( 10.1073/pnas.1019486108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenseth NC, et al. 2006. Plague dynamics are driven by climate variation. Proc. Natl Acad. Sci. USA 103, 13 110–13 115. ( 10.1073/pnas.0602447103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snall T, O'Hara R, Ray C, Collinge S. 2008. Climate-driven spatial dynamics of plague among prairie dog colonies. Am. Nat. 171, 238–248. ( 10.1086/525051) [DOI] [PubMed] [Google Scholar]
- 14.Cavanaugh DC, Marshall JD., Jr 1972. The influence of climate on the seasonal prevalence of plague in the Republic of Vietnam. J. Wildl. Dis. 8, 85–94. ( 10.7589/0090-3558-8.1.85) [DOI] [PubMed] [Google Scholar]
- 15.Xu L, et al. 2014. Wet climate and transportation routes accelerate spread of human plague. Proc. R. Soc. B 281, 20133159 ( 10.1098/rspb.2013.3159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, Gage KL, Leirs H, Rahalison L. 2008. Plague: past, present, and future. PLoS Med 5, 9–13. ( 10.1371/journal.pmed.0050003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. 1994. Plague still a killer disease in many countries, warns WHO. Press release WHO/18. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 18.Prentice MB, Rahalison L. 2007. Plague. Lancet 369, 1196–1207. ( 10.1016/S0140-6736(07)60566-2) [DOI] [PubMed] [Google Scholar]
- 19.Karimova TY, Neronov VM, Popov VP. 2010. Development of views on natural focality of plague. Biol. Bull. 37, 725–732. ( 10.1134/S1062359010070083) [DOI] [Google Scholar]
- 20.Parmenter R, Yadav E, Parmenter C, Ettestad P, Gage K. 1999. Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am. J. Trop. Med. Hyg. 61, 814–821. [DOI] [PubMed] [Google Scholar]
- 21.Stapp P. 2007. Trophic cascades and disease ecology. Ecohealth 4, 121–122. ( 10.1007/s10393-007-0099-z) [DOI] [Google Scholar]
- 22.Liu Y, Tan J. 2000. The atlas of plague and its environment in the People‘s Republic of China. Beijing, China: Science Press. [Google Scholar]
- 23.Fang XY. 1990. Natural plague foci in China. Beijing, China: People's Medical Publishing House. [Google Scholar]
- 24.WHO. 1999. Plague manual: epidemiology, distribution, surveillance and control. Wkly Epidemiol. Rec. 74, 447. See http://www.who.int/csr/resources/publications/plague/WHO_CDS_CSR_EDC_99_2_EN/en. [Google Scholar]
- 25.Gong ZD, et al. 2012. Ecological geographic landscapes of natural plague foci in China. VI. Biological characteristics of natural vectors of Y.pesties. Chin. J. Epidemiol. 33, 818–822. (In Chinese) [PubMed] [Google Scholar]
- 26.Smith CR, Tucker JR, Wilson BA, Clover JR. 2010. Plague studies in California: a review of long-term disease activity, flea-host relationships and plague ecology in the coniferous forests of the Southern Cascades and northern Sierra Nevada mountains. J. Vector Ecol. 35, 1–12. ( 10.1111/j.1948-7134.2010.00021.x) [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Shi G. 2009. Fleas in Inner Mongolia. Huhehot, China: Inner Mongolia People's Publishing House. [Google Scholar]
- 28.Vinokur AD, Pierce PF, Lewandowski-Romps L, Hobfoll SE, Galea S. 2011. Effects of war exposure on air force personnel's mental health, job burnout and other organizational related outcomes. J. Occup. Health Psychol. 16, 3–17. ( 10.1037/a0021617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Zomeren M, Spears R, Fischer AH, Leach CW. 2004. Put your money where your mouth is! Explaining collective action tendencies through group-based anger and group efficacy. J. Pers. Social Psychol. 87, 649–664. ( 10.1037/0022-3514.87.5.649) [DOI] [PubMed] [Google Scholar]
- 30.Kline RB. 2011. Methodology in the social sciences. New York, NY: Guilford Press. [Google Scholar]
- 31.Arhonditsis GB, Stow CA, Steinberg LJ, Kenney MA, Lathrop RC, McBride SJ, Reckhow KH. 2006. Exploring ecological patterns with structural equation modeling and Bayesian analysis. Ecol. Model. 192, 385–409. ( 10.1016/j.ecolmodel.2005.07.028) [DOI] [Google Scholar]
- 32.Veen GF, Olff H, Duyts H, van der Putten WH. 2010. Vertebrate herbivores influence soil nematodes by modifying plant communities. Ecology 91, 828–835. ( 10.1890/09-0134.1) [DOI] [PubMed] [Google Scholar]
- 33.Sutton-Grier AE, Kenney MA, Richardson CJ. 2010. Examining the relationship between ecosystem structure and function using structural equation modelling: a case study examining denitrification potential in restored wetland soils. Ecol. Model. 221, 761–768. ( 10.1016/j.ecolmodel.2009.11.015) [DOI] [Google Scholar]
- 34.Guan P, Huang DS, He M, Shen TF, Guo JQ, Zhou BS. 2009. Investigating the effects of climatic variables and reservoir on the incidence of hemorrhagic fever with renal syndrome in Huludao City, China: a 17-year data analysis based on structure equation model. BMC Infect. Dis. 9, 109 ( 10.1186/1471-2334-9-109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grace JB, Kelley JE. 2006. A structural equation model analysis of postfire plant diversity in California shrublands. Ecol. Appl. 16, 503–514. ( 10.1890/1051-0761(2006)016[0503:ASEMAO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 36.Bentler PM. 2006. EQS 6 structural equations program manual, pp. 86–102. Los Angeles, CA: BMDP Statistic Software. [Google Scholar]
- 37.Steiger JH. 1990. Structural model evaluation and modification: an interval estimation approach. Multivariate Behav. Res. 25, 173–180. ( 10.1207/s15327906mbr2502_4) [DOI] [PubMed] [Google Scholar]
- 38.Bentler PM. 1990. Comparative fit indexes in structural models. Psychol. Bull. 107, 238–246. ( 10.1037/0033-2909.107.2.238) [DOI] [PubMed] [Google Scholar]
- 39.Zhong WQ, Wang MJ, Wan XR. 1999. Ecologically-based management of rodent pests (eds Singleton GR, Leirs H, Zhang ZB.), pp. 199–214. Bruce, Australia: ACIAR. [Google Scholar]
- 40.Li ZL, Zhang WR. 1993. Association of climate factors and Mongolia gerbils population abundance. Acta Theriol. Sin. 13, 131–135. [Google Scholar]
- 41.Liu W, Wan X, Zhong W. 2007. Population dynamics of the Mongolian gerbils: seasonal patterns and interactions among density, reproduction and climate. J. Arid. Environ. 68, 383–397. ( 10.1016/j.jaridenv.2006.07.002) [DOI] [Google Scholar]
- 42.Ricankova V, et al. 2006. Habitat requirements of the long-tailed ground squirrel (Spermophilus undulatus) in the southern Altai. J. Zool. 270, 1–8. ( 10.1111/j.1469-7998.2006.00136.x) [DOI] [Google Scholar]
- 43.Webb CT, Brooks CP, Gage KL, Antolin MF. 2006. Classic flea-borne transmission does not drive plague epizootics in prairie dogs. Proc. Natl Acad. Sci. USA 103, 6236–6241. ( 10.1073/pnas.0510090103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reijniers J, Begon M, Ageyev VS, Leirs H. 2014. Plague epizootic cycles in Central Asia. Biol. Lett. 10, 20140302 ( 10.1098/rsbl.2014.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reijniers J, et al. 2012. A curve of thresholds governs plague epizootics in Central Asia. Ecol. Lett. 15, 554–560. ( 10.1111/j.1461-0248.2012.01767.x) [DOI] [PubMed] [Google Scholar]
- 46.Jiang G, et al. 2011. Effects of ENSO-linked climate and vegetation on population dynamics of sympatric rodent species in semiarid grasslands of Inner Mongolia, China. Can. J. Zool. 89, 678–691. ( 10.1139/z11-048) [DOI] [Google Scholar]
- 47.Jiang GS, et al. 2013. Climate warming increases biodiversity of small rodents by favoring rare or less abundant species in a grassland ecosystem. Integr. Zool. 8, 162–174. ( 10.1111/1749-4877.12027) [DOI] [PubMed] [Google Scholar]
- 48.Enscore R, et al. 2002. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960–1997. Am. J. Trop. Med. Hyg. 66, 186–196. [DOI] [PubMed] [Google Scholar]
- 49.Pham H, Dang D, Minh N, Nguyen D, Nguyen T. 2009. Correlates of environmental factors and human plague: an ecological study in Vietnam. Int. J. Epidemiol. 38, 1634–1641. ( 10.1093/ije/dyp244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krebs C. 2014. Rodent biology and management. Integr. Zool. 9, 229–230. ( 10.1111/1749-4877.12090) [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z. 2013. Biological consequences of global change: past and future. Integr. Zool. 8, 123 ( 10.1111/1749-4877.12043) [DOI] [PubMed] [Google Scholar]
- 52.Lu N, Wilske B, Ni J, John R, Chen J. 2009. Climate change in Inner Mongolia from 1955 to 2005-trends at regional, biome and local scales. Environ. Res. Lett. 4, 045006 ( 10.1088/1748-9326/4/4/045006) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.