Abstract
It has been suggested that tropical defaunation may unleash community-wide cascading effects, leading to reductions in plant diversity. However, experimental evidence establishing cause–effect relationships thereof is poor. Through a 5 year exclosure experiment, we tested the hypothesis that mammalian defaunation affects tree seedling/sapling community dynamics leading to reductions in understorey plant diversity. We established plot triplets (n = 25) representing three defaunation contexts: terrestrial-mammal exclosure (TE), medium/large mammal exclosure (PE) and open access controls (C). Seedlings/saplings 30–100 cm tall were marked and identified within each of these plots and re-censused three times to record survival and recruitment. In the periods 2010–2011 and 2011–2013, survival was greater in PE than in C plots and recruitment was higher in TE plots than in C plots. Overall, seedling density increased by 61% in TE plots and 23% in PE plots, whereas it decreased by 5% in C plots. Common species highly consumed by mammals (e.g. Brosimum alicastrum and Ampelocera hottlei) increased in their abundance in TE plots. Rarefaction curves showed that species diversity decreased in TE plots from 2008 to 2013, whereas it remained similar for C plots. Given the prevalence of tropical defaunation, we posit this is an anthropogenic effect threatening the maintenance of tropical forest diversity.
Keywords: anthropogenic impact, tropical diversity, plant–mammal interactions, tropical rainforest mammals
1. Introduction
Unsustainable vertebrate overexploitation (hunting, illegal trading and poaching), combined with reductions in their natural habitat extent and connectivity have led to the proliferation of the so-called ‘empty forest syndrome’ throughout the tropics [1,2]. Increasing evidence is unveiling the magnitude of the anthropogenic extirpation of vertebrates (primarily top predators and large-bodied species) from otherwise seemingly undisturbed tropical forests [2–4] and the myriad of cascading effects such defaunation can have on tropical ecosystems [5–8]. For example, it is known that the loss of medium and large body-sized vertebrates reduces seed dispersal distances, especially of large-seeded plants [9,10]. Moreover, extirpation of vertebrate seed predators and herbivores favours escape of large seeds and their seedlings from vertebrate attack [9,11–13]. In addition, defaunation affects the role of animal trampling as a source of seedling damage and mortality [14,15]. Some evidence, based on a reduced number of sites, plant species, and life-forms (e.g. large-seeded trees and palms) [13,16,17] suggests that altered patterns of seed dispersal, predation or herbivory can alter plant recruitment. For example, mammalian herbivores can disrupt plant establishment patterns in forest understories by preferentially consuming more abundant or dominant species [13,18], or by reducing dispersal limitation [19,20]. Therefore, herbivore defaunation has the potential to alter understorey plant species diversity and composition [12,21,22]. However, recent reviews show that not only are studies assessing those effects still scant, but also that resulting effects are not consistent [6,23]. This seems to be related, at least partly, to different methodological approaches used in the studies. In particular, a variety of settings have been used as proxies of defaunation, including forest fragments [24], islands [25], forests hunted at different intensities [12] and experimentally excluded plots [26]. However, in some instances, for example when using small forest fragments or islands, effects of abiotic variables can operate as confounding factors [27]. Furthermore, available studies are of variable duration, ranging from a few months [28] to several years [29].
Very recently a few long-term (more than or equal to 3 years) studies have been undertaken using a more directly comparative methodological approach: exclosure devices, to evaluate the impact of mammalian defaunation on understorey plant communities [22,26]. These studies have provided valuable insights on the impact of mammal defaunation on understorey plant communities; yet, the picture emerging from them is still far from complete or may even be biased, because larger herbivores (more than 8 kg) were in low abundance or missing from reference sites owing to biogeographic or anthropogenic factors [22,26]. Moreover, the relative importance of different herbivores (e.g. rodents versus medium and larger animals) is not explored in these studies. Another important complication in studies assessing the impact of mammal defaunation on plant communities is the limited resolution regarding identification of seedlings and saplings (a significant problem in rich tropical forests) [30]. Therefore, while collectively these studies offer very valuable insights on the consequences of defaunation on plant communities, there remains an urgent need to conduct controlled long-term, experimental studies with high plant-identification resolution, to garner a more comprehensive view of the potential of herbivore mammal defaunation to impact tropical understorey plant diversity and structure.
Here, we present results of a 5 year experimental study in a tropical rainforest site in southeast Mexico, aimed at mimicking herbivore mammal defaunation by establishing exclosures in which we distinguish between the role of large-bodied mammals and small mammals (i.e. rodents). Differential defaunation (sensu [8]) is typically observed in tropical forests, whereby human impact negatively affects medium and large species, while small mammals are not impacted or are even benefited [5,9]. Specifically, we assess the effects of excluding terrestrial herbivore/granivore mammals on the underlying dynamics of the regenerative tree community (i.e. rates of individual seedling/sapling survival and recruitment) and the resulting impact on the structure (plant density and species diversity) of such communities. We test the predictions that: (i) exclusion of mammals will increase the survival and recruitment of tree seedlings/saplings; (ii) species that are naturally abundant in the understorey and constitute an important feeding resource for herbivore/granivore mammals (e.g. large-seeded plants and their seedlings/saplings) will be benefitted by the exclusion of mammals leading to greater plant density but lower species diversity in the understorey; (iii) terrestrial-mammal exclosure (TE) plots will have greater effects than medium/large mammal exclosure (PE) plots on plant dynamics owing to the fact that TE plots combine the effect of the absence of small rodents (mainly granivores) and medium and large mammals (granivores and also browsers), and as a consequence, we expect to see a greater change in species richness and diversity in TE plots than in PE plots. Moreover, we examined whether changes in species richness and diversity among treatments were related to the increase of rare species or release of already abundant species. Given the current prevalence of anthropogenic vertebrate defaunation [8] and the characteristics of our experimental manipulations (as discussed above), our results should advance the understanding of the ecology of defaunation and be of broad significance.
2. Material and methods
(a). Study site
The study site is the Montes Azules Biosphere Reserve (MABR) in southern Mexico. MABR covers 331 200 ha of tropical rainforest and harbours 112 mammalian species including the entire guild of understorey herbivores and granivores [31–34] (electronic supplementary material, figure S1).
(b). Experimental design and seedling/sapling censuses
In January 2008, we established 25 clusters of three 6 × 3 m plots (triplets) with a minimum separation of 60 m and a maximum of 4 km (mean = 1.6 km). All triplets were placed away from trails and located on alluvial terraces of flat fertile soil, along the southern limit of the MABR (electronic supplementary material, figure S1). Within each triplet, we randomly assigned the position of the following treatments: open access to all mammals (controls = C), exclosure of large but not small non-volant mammals (partial exclosure = PE) and exclosure of small and large non-volant mammals (total exclosure = TE) (electronic supplementary material, text S1). We identified and marked, with aluminium tags, all tree seedling/saplings between 30 and 100 cm, located in 4 m2 central subplots within each plot, to avoid or minimize edge effects. We re-censused all subplots in December 2010, November 2011 and January 2013, when we recorded survival of tagged individuals and identified and tagged new recruits (individuals reaching 30 cm in height). We restricted our analyses to 21 triplets that did not suffer any damage due to tree falls or floods during the course of the study.
(c). Mammal presence in the study area
We recorded presence and relative abundance of mammalian fauna in our study area through (i) track detection in sand quadrats established adjacently to the plots, (ii) camera trapping, and (iii) compilation of information resulting from independent on-site studies (electronic supplementary material, text S2).
(d). Statistical analyses
We examined baseline plant density, species richness and diversity differences among treatments at the onset of the experiment, using a one-way ANOVA. To examine changes in observed species richness and diversity (Shannon index) over time, we applied a repeated measurements linear mixed effects model with treatments and time as fixed effects and plot clusters nested within treatments as a random effect. To analyse the impact of treatments on plant survival, recruitment and density, we divided the corresponding data in three inter-census periods and fitted a Jolly–Seber open population model. This approach, successfully used in studies on seedling dynamics, accounts for tag loss, which in our case was estimated at 16% overall [30]. Spatial autocorrelation among each triplet's plant dynamics was assessed via a Mantel test, using physical distances and plant survival rates of control plots as variables. To analyse changes in plant richness and diversity within treatments between the onset and the end of the experiment, we calculated rarefaction curves with 95% confidence intervals for the years 2008 and 2013 based on raw plant abundance.
We conducted an analysis of covariance to examine if observed changes in plant abundance were proportional to initial abundances across treatments, or if species with an initial higher abundance were more benefited by mammal exclosure. For this analysis, we only included plant species that were present at the onset and end of the experiment in the three treatments. Additionally, we performed a contingency analysis to examine whether the relative frequency of rare and abundant species (Importance Value Index less than 1.30 and more than 2.9, respectively) changed with time. A full description of statistical analyses is given in the electronic supplementary material, text S3.
3. Results
(a). Overall characteristics of the plant community
We tagged and followed the fate of 502 tree seedlings/saplings, 97% of which we were able to identify to species level (n = 71) representing 33 families. The most common recorded species at the onset of the experiment were Brosimum alicastrum and Ampelocera hottlei, which accounted for 23% and 10% of the initially tagged plants, respectively.
Plant density, species richness and Shannon's diversity index did not differ among treatments at the onset of the experiment (F2,60 = 1.4, p = 0.254; F2,52 = 2.3, p = 0.106; and F2,52 = 2.7, p = 0.079; respectively). Species abundance in each treatment showed similar lognormal distributions (electronic supplementary material, figure S2).
(b). Mammal presence in the study area
We recorded evidence of the activity of 23 mammalian species, including the most important herbivores and granivores historically known for the region: tapir, peccaries, paca, agouti and deer (electronic supplementary material, table S1). Paca and tapir were consistently the most frequent understorey mammalian herbivores across plot clusters (electronic supplementary material, figure S3).
(c). Effects of experimental defaunation on plant dynamics: survival, recruitment and density
We did not detect evidence of spatial autocorrelation in the plant dynamics of cluster plots using the control's plant survival as an analysis variable (r = −0.05, p = 0.923). Analyses based on Jolly–Seber estimates showed that plant survival did not differ among treatments during the 2008–2010 period. However, in the 2010–2011 period, survival was significantly higher in PE (t317 = 3.3, p < 0.001) and TE plots (t315 = 2.9, p = 0.002) than in C plots. For the 2011–2013 period, only plant survival in PE plots was significantly higher than in C plots (t317 = 2.0, p = 0.023; figure 1a). By contrast, plant recruitment was higher in PE plots than in C plots (t317 = 2.2, p = 0.015) during the 2008–2010 period. In the 2010–2011 and 2011–2013 periods, only recruitment in TE plots was higher than in C plots (2010–2011: t315 = 2.4, p = 0.009; 2011–2013: t315 = 2.1, p = 0.018; figure 1b).
Figure 1.
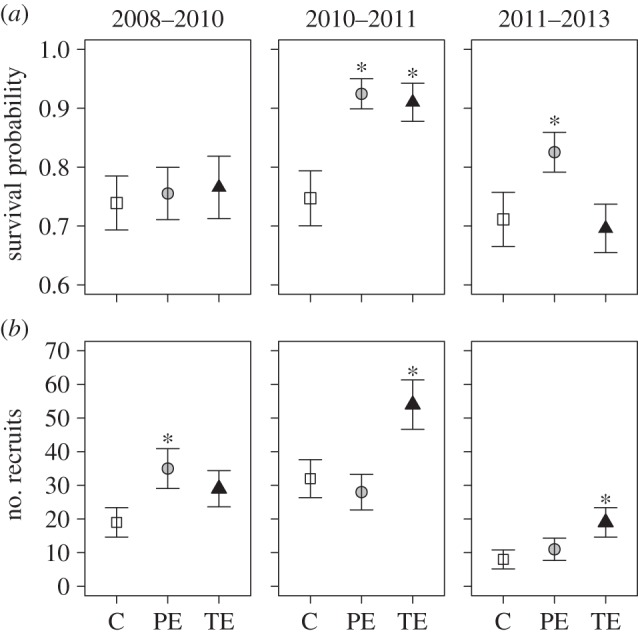
Temporal changes in (a) survival probability and (b) tree seedling/sapling recruitment among treatments for the three periods of study (values come from Jolly–Seber open population models ± 1 s.e.). C, control plot; PE, partial exclosure; TE, total exclosure. Asterisks (*) indicate significant differences compared with control (p < 0.05).
Massive production of Vatairea lundellii fruits occurred in the year 2011 affecting one experimental triplet but ensuring that more than 60% of all new recruits belonged to this species. To assess the influence of this event on plant survival and recruitment patterns, we repeated the above described analyses excluding this species. We found that survival was no longer different between control and exclosure plots in the 2011–2013 period and we did not find any effect in terms of recruitment patterns (see the electronic supplementary material, figure S4).
Observed patterns of plant survival and recruitment caused density to increase during the course of the experiment by 23% in PE plots and by 61% in TE plots. By contrast, plant density was slightly reduced (by 5%) in C plots. Therefore, final density in PE and TE was, respectively, 1.5 (t317 = 61.3, p < 0.001) and 1.4 (t315 = 273.2, p < 0.001) times greater than that of C plots (figure 2). These results were not affected when excluding V. lundellii seedlings (electronic supplementary material, figure S4).
Figure 2.
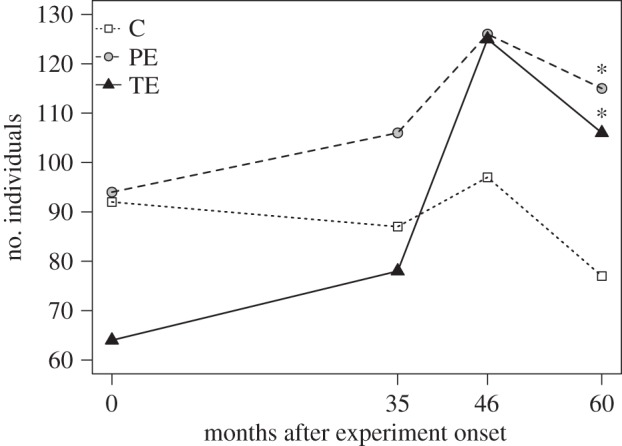
Plant density for each treatment estimated using Jolly–Seber open population models for 0, 35, 46 and 60 months after the experiment was initiated in MABR southern Mexico. C, control plot; PE, partial exclosure; TE, total exclosure. Asterisks (*) indicate significant differences compared with control (p < 0.05).
(d). Effect of treatments on seedling/sapling species richness and diversity
There was a significant interaction effect of time and treatment on species richness and Shannon diversity (F2,180 = 6.6, p < 0.001; F2,168 = 7.3, p < 0.001, respectively). Species richness and diversity showed an increasing trend in the excluded plots, whereas in the control plots they remained the same (electronic supplementary material, figure S5). However, for a fixed number of tree seedlings/saplings, rarefied species richness (figure 3c) in TE plots was significantly lower in 2013 than in 2008 (no overlap in 95% confidence intervals). In PE plots, overlap in 95% confidence intervals was partial for rarefied species richness (figure 3b). Finally, there was a complete overlap in 95% confidence intervals in C plots (figure 3a). Diversity (Shannon Index) exhibited the same pattern (electronic supplementary material, figure S6). These results were similar when Fisher's α was used.
Figure 3.
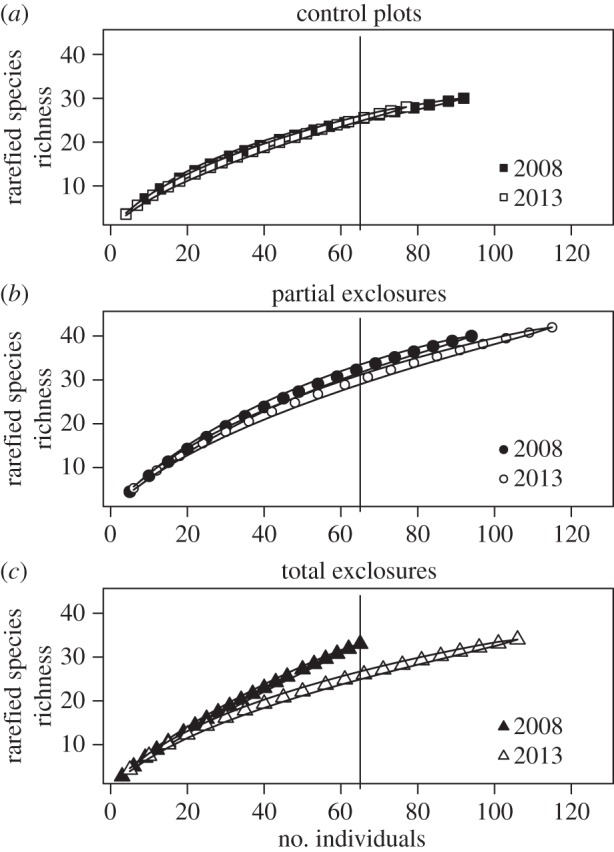
Rarefaction curves showing changes in species richness between 2008 and 2013 in each of the experimental treatments. Continuous lines represent 95% confidence intervals. Vertical lines show the minimum number of individuals found in any treatment.
(e). Effects of experimental defaunation on dominant and rare tree species
The increase in abundance of relatively common species was greater in TE than in PE and C plots (slope of TE plots was significantly greater than that observed in PE plots, t27 = −2.4, p = 0.023 and in C plots, t27 = −2.1, p = 0.047, and also marginally greater than 1, t27 = 1.9, p = 0.062; figure 4). We did not observe changes in the frequency of dominant or rare species but the number of species that recruited exclusively during the course of the study was higher in C plots (10) than in PE (3) or TE plots (4).
Figure 4.
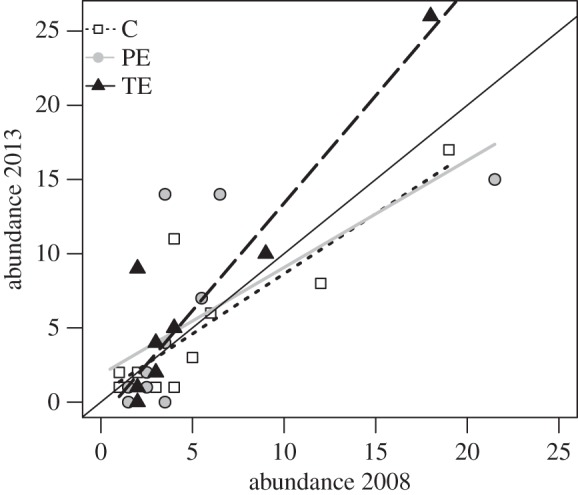
Initial (2008) and final (2013) abundances of 11 plant species present in all treatments of the experiment of mammalian defaunation conducted in MABR. C, control plot; PE, partial exclosure; TE, total exclosure. Continuous black line corresponds to changes directly proportional to initial abundance (slope = 1). Only the slope of TE plots is significantly different from that corresponding to C plots. Points are slightly shifted to avoid complete overlap among them. A full list of species used for this analysis is provided in the electronic supplementary material, table S2.
4. Discussion
Our findings show that activity of mammalian herbivores has a clear impact on the patterns of seedling/sapling survival and recruitment, which translates into changes in plant density, species richness and diversity in the understorey plant community. Evidence was particularly strong when all mammals were excluded, indicating the importance of different groups of herbivores in maintaining plant diversity in the understorey. Indeed, our results show that in TE plots, density increased by 61% and diversity (controlling for plant density) decreased by 22%.
We observed a variation in the effects of treatments on seedling survival and recruitment over the course of the experiment. In the 2010–2011 period, survival in PE and TE plots was similar but greater than in the observed in C plots, suggesting that medium/large mammals were the main animals responsible for killing seedlings and saplings by consuming them or as a consequence of trampling. In the 2011–2013 period, only survival in PE plots was significantly higher than in C plots. However, when V. lundellii seedlings were excluded in this analysis, we found that differences among treatments were in the same direction as those observed in the 2010–2011 period (i.e. higher survival in PE and TE plots than in C plots), although they were not significant (see the electronic supplementary material, figure S4). An explanation for this lack of significance might be related to a reduction in medium/large mammalian herbivore abundance during the 2011–2013 period. This is supported by the camera-trapping data, which indicates that capture frequency (picture records/100 camera-trapping days) of species such as paca, collared peccary and white-lipped peccary decreased in that period by 29%, 39% and 100%, respectively, when compared with data from a study conducted 1 year before [33,34].
Effects of treatments on seedling recruitment also varied among periods. In 2008–2010, seedling recruitment was higher only when medium/large mammals were absent. However, in the period 2010–2011, only TE plots underwent increased seedling recruitment. In the last period (2011–2013), results were similar (i.e. greater recruitment only in TE plots) but less contrasting. These results are consistent with evidence of small rodents representing an important source of mortality for seeds and young seedlings [35–37]. Again, as in the case of survival, changes in the magnitude of effect of the exclosures might be related to mammal fluctuations (in this case small rodents), a commonly documented feature of tropical forests [36,38].
Observed effects at the plant population level (survival, recruitment and density) altered the structure and composition of seedling/sapling communities. When we rarefied species richness to control for contrasts in plant density among treatments, we detected that mammal absence negatively affected plant richness. By contrast, the impact of mammal exclosure on non-rarefied species richness was positive. These contrasting results might be related to the activity of canopy vertebrate seed dispersers. Arrival of seeds through primary dispersal was not interrupted in our exclosure treatments, something that probably helped the accumulation of plant species in excluded plots protected from the activity of herbivore mammals. However, in a more realistic defaunation scenario, primary seed dispersal is expected to also be affected by the absence of medium and large arboreal animals (e.g. primates, large birds, etc.), reducing the amplitude of seed rain shadows and increasing the abundance of locally produced seeds [39,40]. Therefore, real defaunation effects are probably more similar to the effects we observed when rarefied species richness is used.
We observed high variability in the interspecific response to exclosure treatments. However, as we expected, relatively common species (e.g. B. alicastrum and A. hottlei) had greater increases in abundance than expected given their initial abundance in TE compared with PE and C plots. This result explains in part the decrease in rarefied species diversity observed when all terrestrial mammals are excluded. On the other hand, a set of species with low to intermediate abundances at the onset of the experiment showed a slight to marked increase in both types of exclosures, especially in TE plots. The rank-abundance curve for TE plots in 2013 demonstrates the rise of species such as Virola koschnyi, Inga pavoniana, Inga punctata and Castilla elastica (see the electronic supplementary material, figure S2). Finally, we did not find any pattern in relation to the frequency of rare species per treatment, however we detected more exclusive species in C plots than in exclosure plots. Differential response of plant species to defaunation is probably associated with contrasts in life-history traits, including reproductive rates, plant longevity and growth rate. Therefore, these results highlight the need to consider the multiplicity of traits and mechanisms underlying plant responses to defaunation, instead of an approach focused on single traits (e.g. seed size).
Animal surveys conducted during this experiment show that our study site supports a diverse mammalian community (electronic supplementary material, table S1). However, rodents in the genus Proechimys, which play an important role as seed predators in other neotropical forest [41,42], were absent because they are not naturally distributed in Mexico. On the other hand, an ongoing study documenting animal visits to fruiting trees using camera-traps, has found that medium/large body-sized mammals such as tapirs, peccaries and pacas are among the most active in the MABR understorey (A.A.C-S. 2014, unpublished data; electronic supplementary material, figure S3). Therefore, it seems safe to assume our study site is representative of a Neotropical forest with a healthy mammalian community and that seeds and seedlings/saplings in our control plots were exposed to the potential effects of consumption and trampling of these animals. Moreover, the diversity and level of conservation of the mammalian fauna in our study site, particularly large-bodied species such as tapirs and peccaries (white-lipped and collared), contrasts with that reported in other studies in which a similar approach has been undertaken to assess the impacts of defaunation. Those studies, performed in Australia [22] and Brazil [26], found weak or non-existent, respectively, impacts of experimental exclosure of ground-dwelling mammals on seedling diversity but those sites can be regarded as comparatively depauperated or subjected to a long-term defaunation history.
The extent to which the impacts of defaunation we observed in the seedling/sapling community would translate into changes in the composition of mature tree communities remains an open question. Over the long-term, we can expect defaunated forests to undergo reduced diversity if the species that experience an ecological release in the absence of mammal herbivores and granivores become dominant at the understorey. A recent study in Lambir, Borneo, provides some insights into this issue: a large-scale census of a 52 ha plot found that historic vertebrate defaunation correlated with low plant species diversity at the sapling level [43]. Such long-term correlative studies, coupled with experimental manipulations like the ones reported here, support the hitherto largely anecdotal contention that mammalian defaunation has the potential to impact the structure and diversity of understorey plant communities. Also, these studies underscore the urgency of protecting the few forests still retaining a healthy vertebrate community.
Supplementary Material
Acknowledgements
We thank R. Méndez, B. Chagala, G. Jamangapé, A. Jamangapé, An. Jamangapé, A. Garmendia and J.P. Carbajal for field assistance. The staff of the Chajul Field Station provided invaluable logistical support. Use of facilities during the beginning of this study was facilitated by support from PEMEX to the Chajul Field Station. F. Espinosa and D. Valenzuela provided valuable input, and E. Brenner read and improved a previous draft. Two anonymous reviewers and T. Theimer provided valuable comments on an earlier draft of this paper. This contribution is a partial requirement for the PhD degree of A.A.C.S within the Graduate Program of Biological Sciences, UNAM.
Data accessibility
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.28v87.
Funding statement
A.A.C.S. was supported by a scholarship provided by the Mexican National Council of Science and Technology (CONACyT). This study was funded by grants FE005 from CONABIO and CN-09-347 from UC Mexus-CONACyT.
References
- 1.Redford KH. 1992. The empty forest. Bioscience 42, 412–422. ( 10.2307/1311860) [DOI] [Google Scholar]
- 2.Wilkie DS, Bennett EL, Peres CA, Cunningham AA. 2011. The empty forest revisited. Ann. NY Acad. Sci. 1223, 120–128. ( 10.1111/j.1749-6632.2010.05908.x) [DOI] [PubMed] [Google Scholar]
- 3.Fa JE, Peres CA, Meeuwig J. 2002. Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv. Biol. 16, 232–237. ( 10.1046/j.1523-1739.2002.00275.x) [DOI] [PubMed] [Google Scholar]
- 4.Peres CA, Palacios E. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39, 304–315. ( 10.1111/j.1744-7429.2007.00272.x) [DOI] [Google Scholar]
- 5.Galetti M, Dirzo R. 2013. Ecological and evolutionary consequences of living in a defaunated world. Biol. Conserv. 163, 1–6. ( 10.1016/j.biocon.2013.04.020) [DOI] [Google Scholar]
- 6.Kurten EL. 2013. Cascading effects of contemporaneous defaunation on tropical forest communities. Biol. Conserv. 163, 22–32. ( 10.1016/j.biocon.2013.04.025) [DOI] [Google Scholar]
- 7.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 8.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 9.Wright SJ. 2003. The myriad consequences of hunting for vertebrates and plants in tropical forests. Perspect. Plant. Ecol. 6, 73–86. ( 10.1078/1433-8319-00043) [DOI] [Google Scholar]
- 10.Muller-Landau HC. 2007. Predicting the long-term effects of hunting on plant species composition and diversity in tropical forests. Biotropica 39, 372–384. ( 10.1111/j.1744-7429.2007.00290.x) [DOI] [Google Scholar]
- 11.Dirzo R, Mendoza E, Ortíz P. 2007. Size related differential seed predation in a heavily defaunated neotropical rain forest. Biotropica 39, 355–362. ( 10.1111/j.1744-7429.2007.00274.x) [DOI] [Google Scholar]
- 12.Dirzo R, Miranda A. 1991. Altered patterns of herbivory and diversity in the forest understory: a case study of the possible consequences of contemporary defaunation. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price PW, Lewinsohn TM, Fernandes GW, Benson WW.), pp. 273–287. New York, NY: John Wiley and Sons. [Google Scholar]
- 13.Silman MR, Terborgh JW, Kiltie RA. 2003. Population regulation of a dominant rain forest tree by a major seed predator. Ecology 84, 431–438. ( 10.1890/0012-9658(2003)084[0431:PROADR]2.0.CO;2) [DOI] [Google Scholar]
- 14.Uusimaa H. 2004. Consequences of defaunation: variation in the intensity of trampling in the understorey of two Mexican rain forests of contrasting conservation. Master's thesis, University of Helsinki, Helsinki, Finland. [Google Scholar]
- 15.Roldán AI, Simonetti JA. 2001. Plant–mammal interactions in tropical Bolivian forests with different hunting pressures. Conserv. Biol. 15, 617–623. ( 10.1046/j.1523-1739.2001.015003617.x) [DOI] [Google Scholar]
- 16.Wright SJ, Zeballos H, Domínguez I, Gallardo MM, Moreno MC, Ibáñez R. 2000. Poachers alter mammal abundance, seed dispersal, and seed predation in a neotropical forest. Conserv. Biol. 14, 227–239. ( 10.1046/j.1523-1739.2000.98333.x) [DOI] [Google Scholar]
- 17.Wright SJ, Duber HC. 2001. Poachers and forest fragmentation alter seed dispersal, seed survival, and seedling recruitment in the palm Attalea butyraceae, with implications for tropical tree diversity. Biotropica 33, 583–595. ( 10.1111/j.1744-7429.2001.tb00217.x) [DOI] [Google Scholar]
- 18.Royo AA, Carson WP. 2005. The herb community of a tropical forest in central Panamá: dynamics and impact of mammalian herbivores. Oecologia 145, 66–75. ( 10.1007/s00442-005-0079-3) [DOI] [PubMed] [Google Scholar]
- 19.Forget P, Jansen PA. 2007. Hunting increases dispersal limitation in the tree Carapa procera, a nontimber forest product. Conserv. Biol. 21, 106–113. ( 10.1111/j.1523-1739.2006.00590.x) [DOI] [PubMed] [Google Scholar]
- 20.Wang BC, Sork VL, Leong MT, Smith TB. 2007. Hunting of mammals reduces seed removal and dispersal of the Afrotropical tree Antrocaryon klaineanum (Anacardiaceae). Biotropica 39, 340–347. ( 10.1111/j.1744-7429.2007.00275.x) [DOI] [Google Scholar]
- 21.Ickes K, Dewalt SJ, Appanah S. 2001. Effects of native pigs (Sus scrofa) on woody understorey vegetation in a Malaysian lowland rain forest. J. Trop. Ecol. 17, 191–206. ( 10.1017/S0266467401001134) [DOI] [Google Scholar]
- 22.Theimer TC, Gehring CA, Green PT, Connell JH. 2011. Terrestrial vertebrates alter seedling composition and richness but not diversity in an Australian tropical rain forest. Ecology 92, 1637–1647. ( 10.1890/10-2231.1) [DOI] [PubMed] [Google Scholar]
- 23.Beckman NG, Muller-Landau HC. 2007. Differential effects of hunting on pre dispersal seed predation and primary and secondary seed removal of two neotropical tree species. Biotropica 39, 328–339. ( 10.1111/j.1744-7429.2007.00273.x) [DOI] [Google Scholar]
- 24.Galetti M, Donatti CI, Pires AS, Guimarães PR, Jr, Jordano P. 2006. Seed survival and dispersal of an endemic Atlantic forest palm: the combined effects of defaunation and forest fragmentation. Bot. J. Linn. Soc. 151, 141–149. ( 10.1111/j.1095-8339.2006.00529.x) [DOI] [Google Scholar]
- 25.Asquith NM, Terborgh J, Elizabeth Arnold A, Riveros M. 1999. The fruits the agouti ate: Hymenaea courbaril seed fate when its disperser is absent. J. Trop. Ecol. 15, 229–235. ( 10.1017/S0266467499000772) [DOI] [Google Scholar]
- 26.Brocardo CR, Zipparro VB, de Lima RAF, Guevara R, Galetti M. 2013. No changes in seedling recruitment when terrestrial mammals are excluded in a partially defaunated Atlantic rainforest. Biol. Conserv. 163, 107–114. ( 10.1016/j.biocon.2013.04.024) [DOI] [Google Scholar]
- 27.Asquith NM, Mejía-Chang M. 2005. Mammals, edge effects, and the loss of tropical forest diversity. Ecology 86, 379–390. ( 10.1890/03-0575) [DOI] [Google Scholar]
- 28.DeMattia EA, Rathcke BJ, Curran LM, Reinaldo A, Vargas O. 2006. Effects of small rodent and large mammal exclusion on seedling recruitment in Costa Rica. Biotropica 38, 196–202. ( 10.1111/j.1744-7429.2006.00117.x) [DOI] [Google Scholar]
- 29.Young HS, McCauley DJ, Helgen KM, Goheen JR, Otárola-Castillo E, Palmer TM, Pringle RM, Young TP, Dirzo R. 2013. Effects of mammalian herbivore declines on plant communities: observations and experiments in an African savanna. J. Ecol. 101, 1030–1041. ( 10.1111/1365-2745.12096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck H, Snodgrass JW, Thebpanya P. 2013. Long-term exclosure of large terrestrial vertebrates: implications of defaunation for seedling demographics in the Amazon rainforest. Biol. Conserv. 163, 115–121. ( 10.1016/j.biocon.2013.03.012) [DOI] [Google Scholar]
- 31.Medellin RA. 1994. Mammal diversity and conservation in the Selva Lacandona, Chiapas, Mexico. Conserv. Biol. 8, 780–799. ( 10.1046/j.1523-1739.1994.08030780.x) [DOI] [Google Scholar]
- 32.Azuara D. 2005. Estimación de abundancia de mamíferos terrestres en un área de la Selva Lacandona, Chiapas. Master's thesis, Universidad Nacional Autónoma de México, Mexico. [Google Scholar]
- 33.Falconi F. 2011. Densidad y abundancia relativa de aves y mamíferos en el sector sur de la Reserva de la Biosfera Montes Azules y comunidades adyacentes de la Selva Lacandona, Chiapas, México. Thesis presented to obtain Bachelor's degree in Biology, Universidad de Ciencias y Artes de Chiapas, Mexico. [Google Scholar]
- 34.Towns VS. 2013. Monitoreo poblacional de algunas especies de mamíferos de talla mayor en la selva alta perennifolia del estado de Chiapas, México. Master's thesis, Universidad Nacional Autónoma de México, Mexico. [Google Scholar]
- 35.Paine CET, Beck H. 2007. Seed predation by neotropical rain forest mammals increases diversity in seedling recruitment. Ecology 88, 3076–3087. ( 10.1890/06-1835.1) [DOI] [PubMed] [Google Scholar]
- 36.DeMattia EA, Curran LM, Rathcke BJ. 2004. Effects of small rodents and large mammals on neotropical seeds. Ecology 85, 2161–2170. ( 10.1890/03-0254) [DOI] [Google Scholar]
- 37.Velho N, Datta A, Isvaran K. 2009. Effect of rodents on seed fate of five hornbill-dispersed tree species in a tropical forest in north-east India. J. Trop. Ecol. 25, 507–514. ( 10.1017/S0266467409990083) [DOI] [Google Scholar]
- 38.Fleming TH. 1974. The population ecology of two species of Costa Rica heteromyid rodents. Ecology 55, 493–510. ( 10.2307/1935142) [DOI] [Google Scholar]
- 39.Chapman CA, Onderdonk DA. 1998. Forests without primates: primate/plant codependency. Am. J. Primatol. 45, 127–141. () [DOI] [PubMed] [Google Scholar]
- 40.Holbrook KM, Loiselle BA. 2009. Dispersal in a Neotropical tree, Virola flexuosa (Myristicaceae): does hunting of large vertebrates limit seed removal? Ecology 90, 1449–1455. ( 10.1890/08-1332.1) [DOI] [PubMed] [Google Scholar]
- 41.Gálvez D, Kranstauber B, Kays RW, Jansen PA. 2009. Scatter hoarding by the Central American agouti: a test of optimal cache spacing theory. Anim. Behav. 78, 1327–1333. ( 10.1016/j.anbehav.2009.08.015) [DOI] [Google Scholar]
- 42.Adler GH, Kestell DW. 1998. Fates of neotropical tree seeds influenced by spiny rats (Proechimys semispinosus). Biotropica 30, 677–681. ( 10.1111/j.1744-7429.1998.tb00109.x) [DOI] [Google Scholar]
- 43.Harrison RD, Tan S, Plotkin JB, Slik F, Detto M, Brenes T, Itoh A, Davies SJ. 2013. Consequences of defaunation for a tropical tree community. Ecol. Lett. 16, 687–694. ( 10.1111/ele.12102) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.28v87.


