Abstract
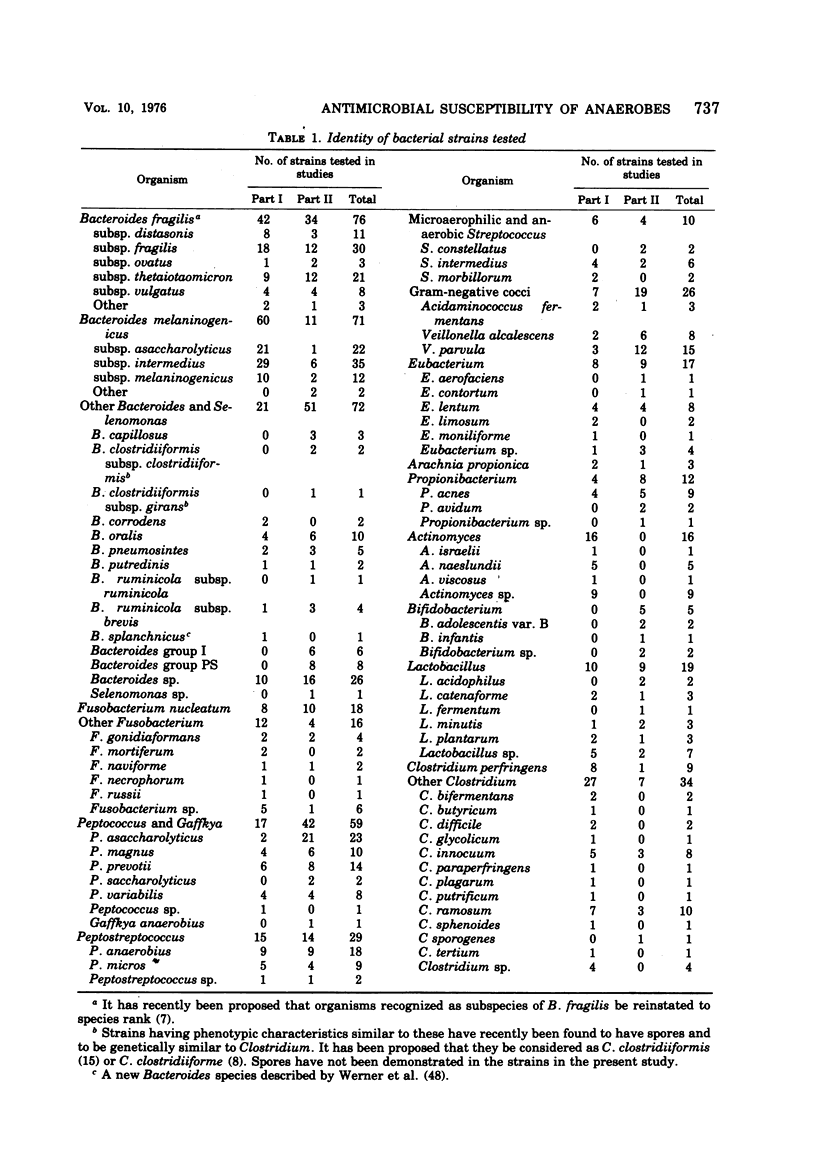
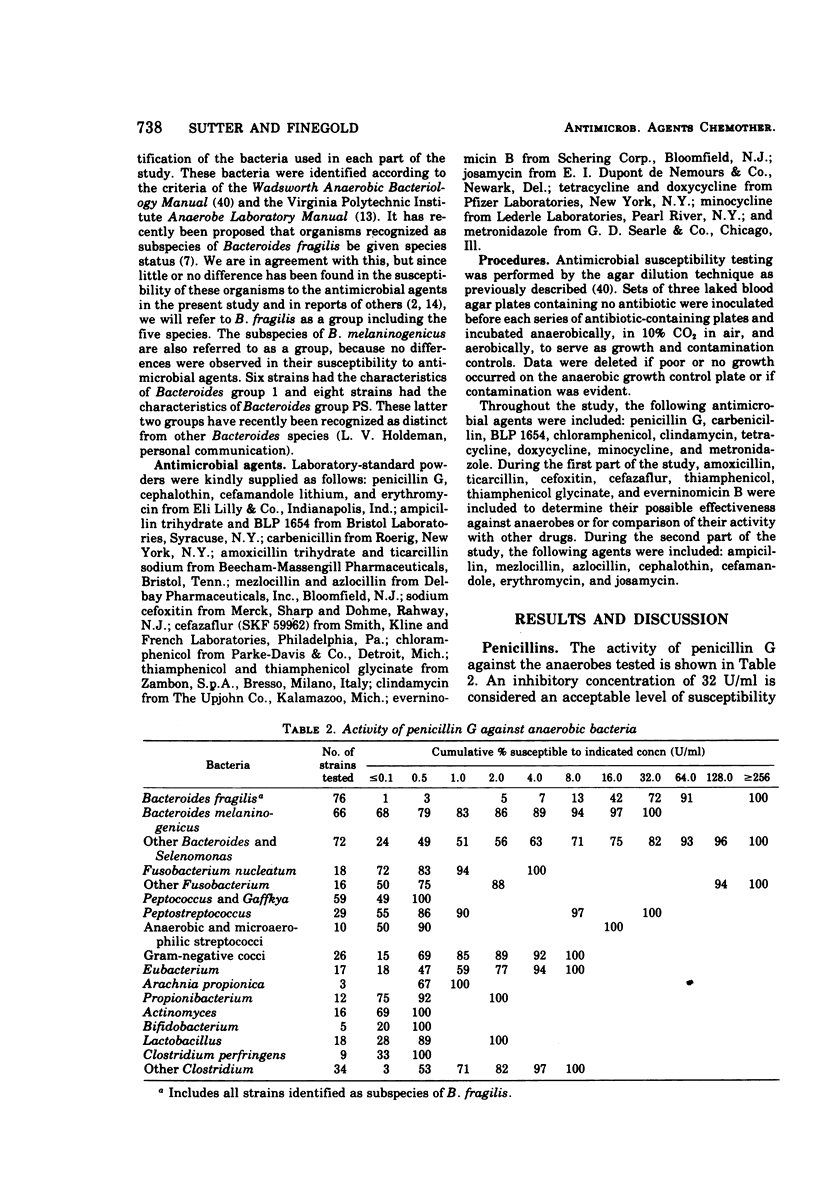
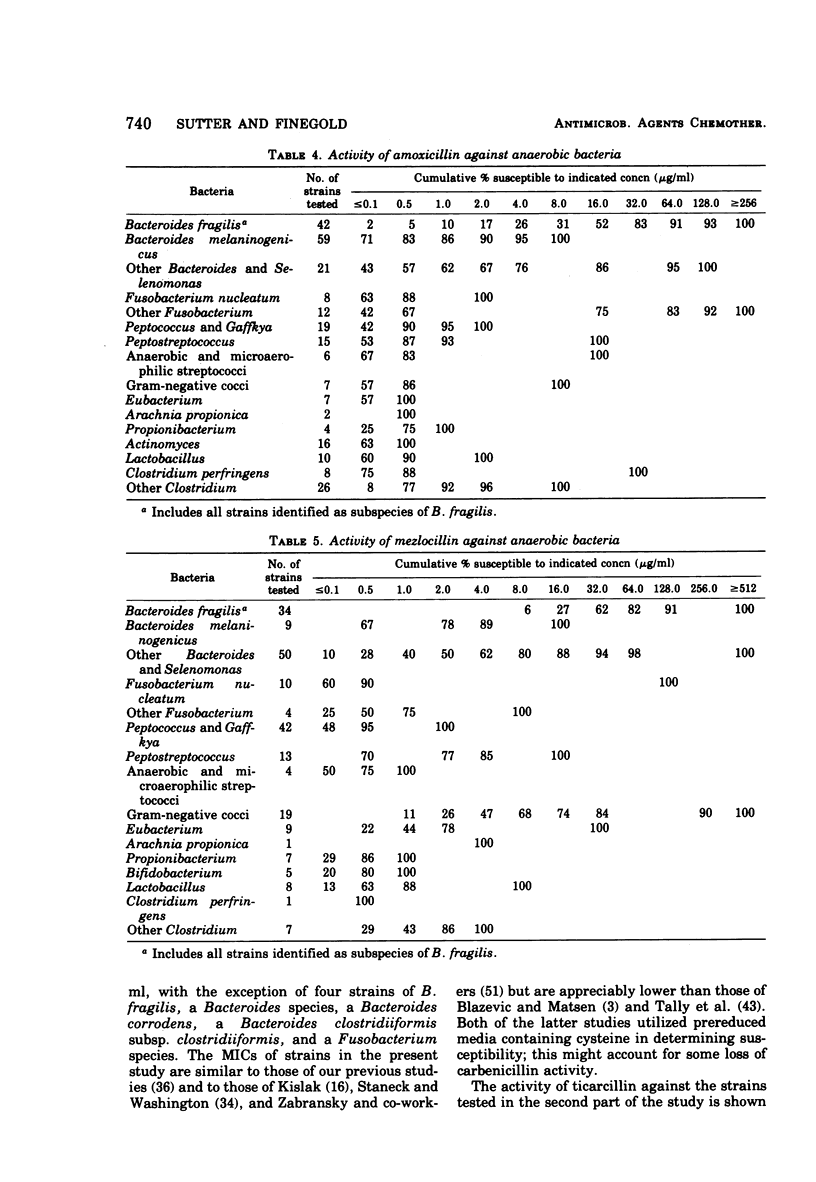
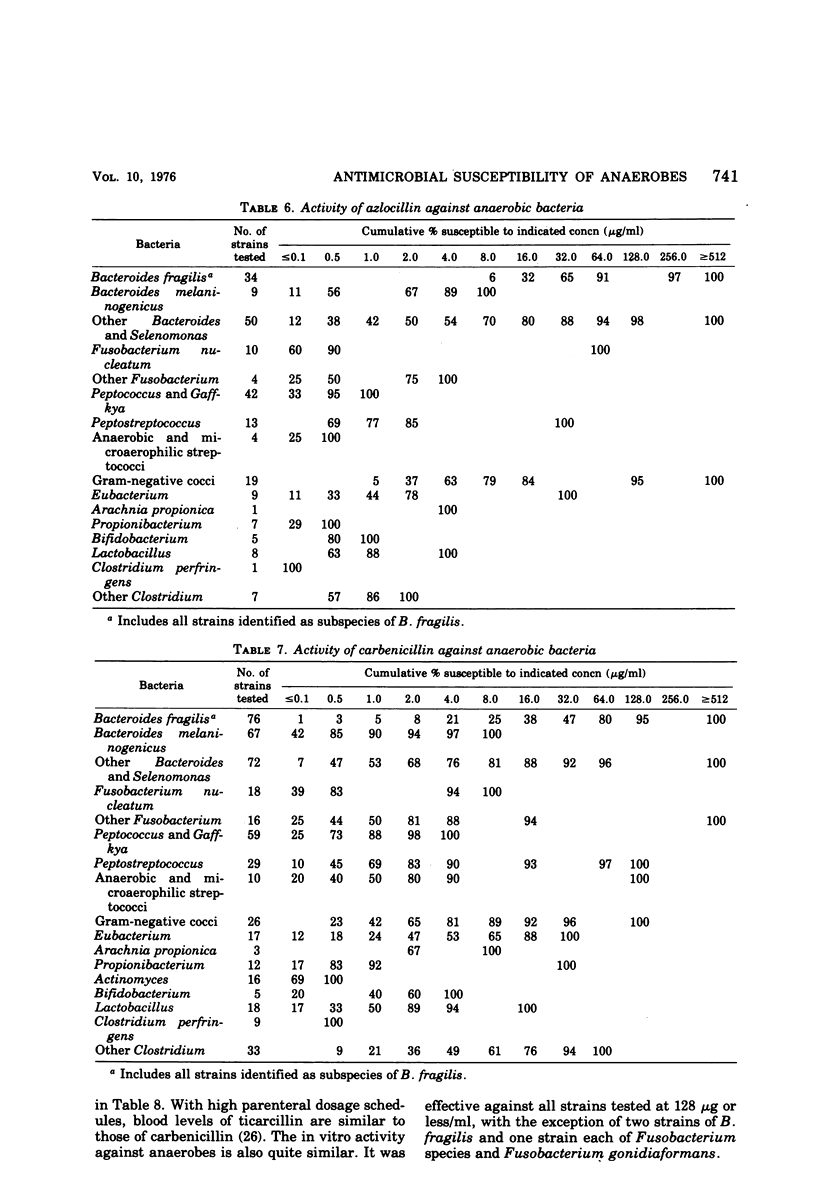
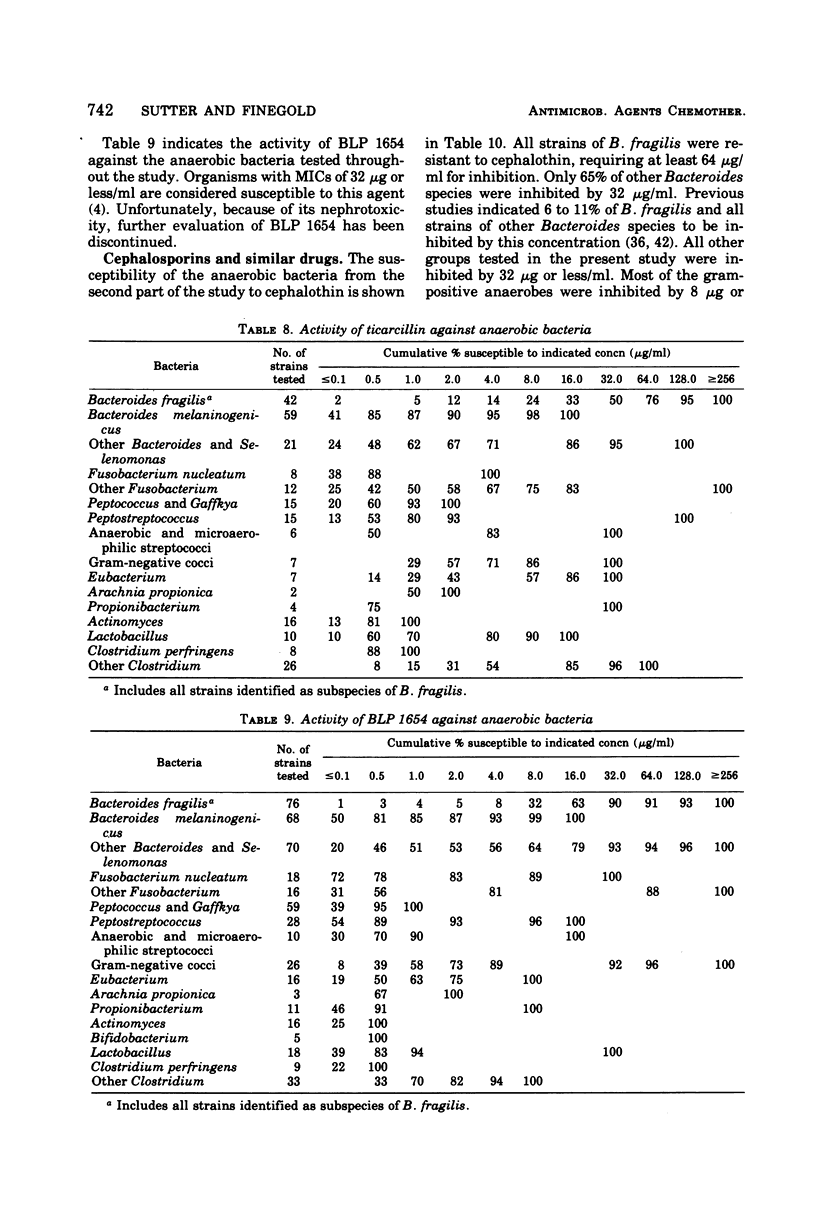
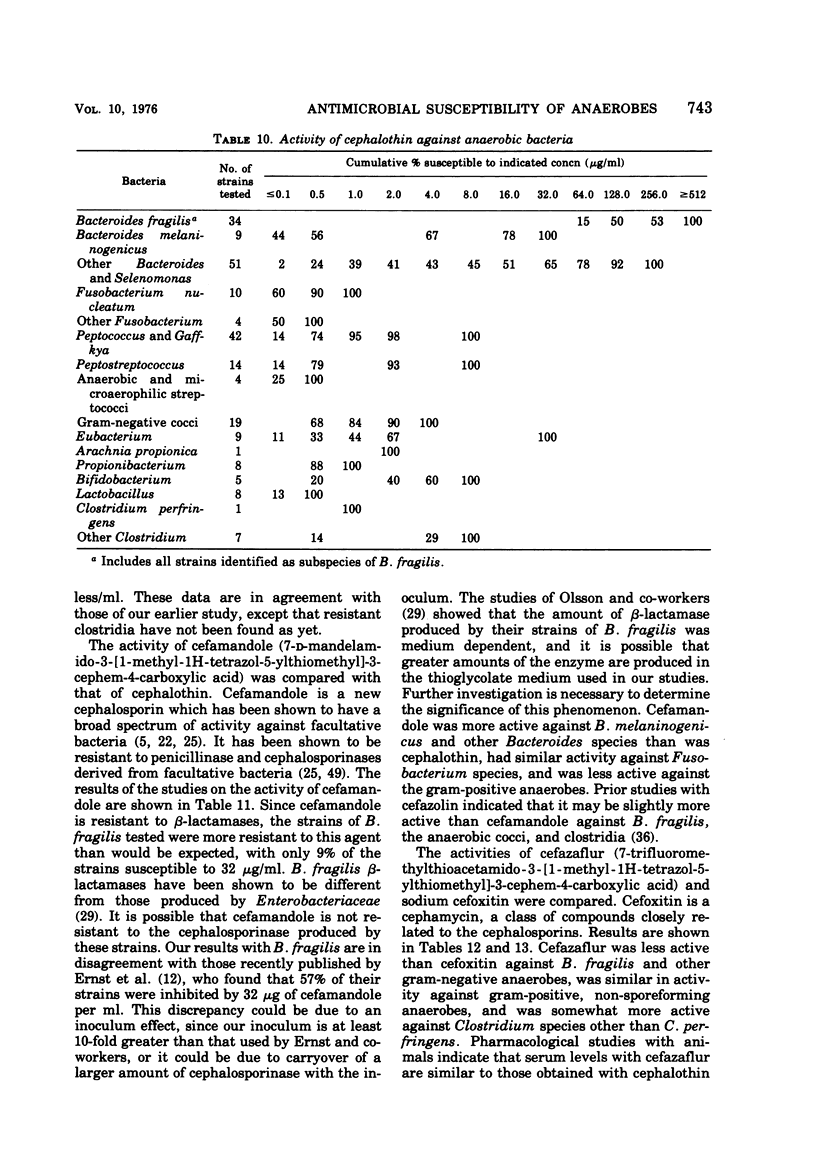
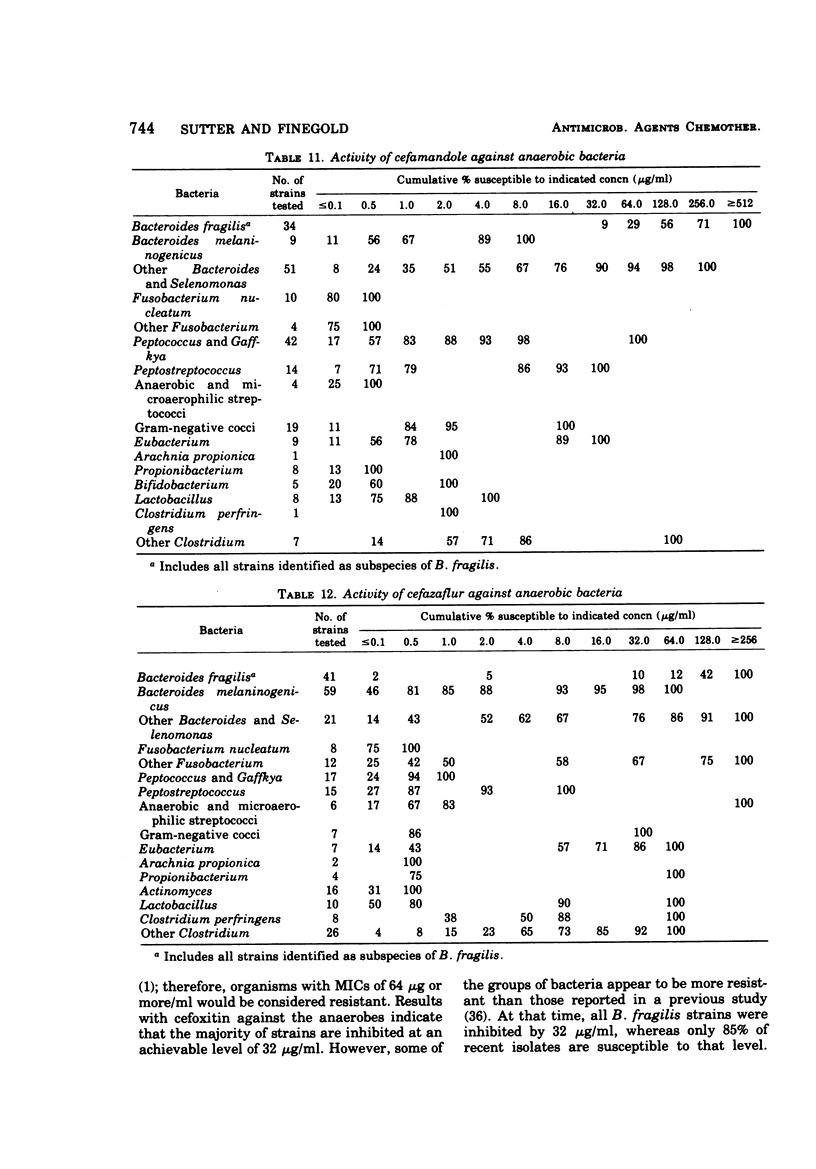
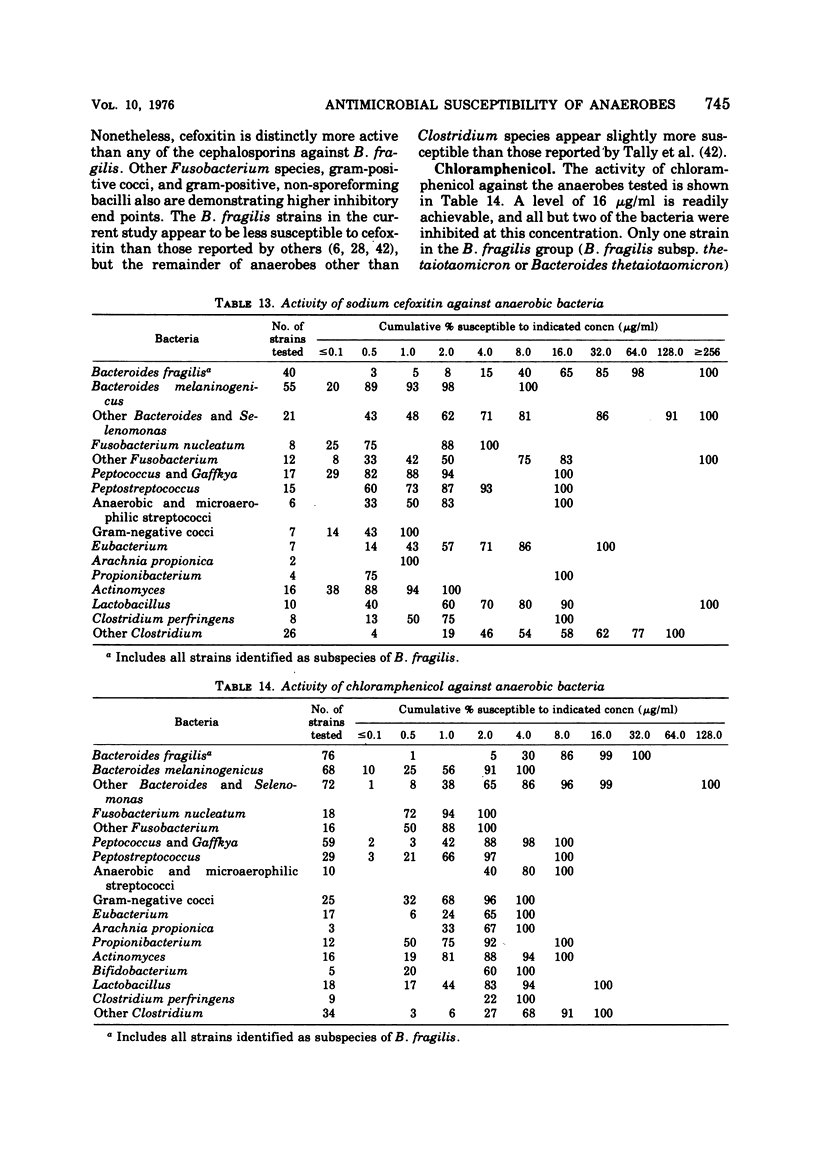
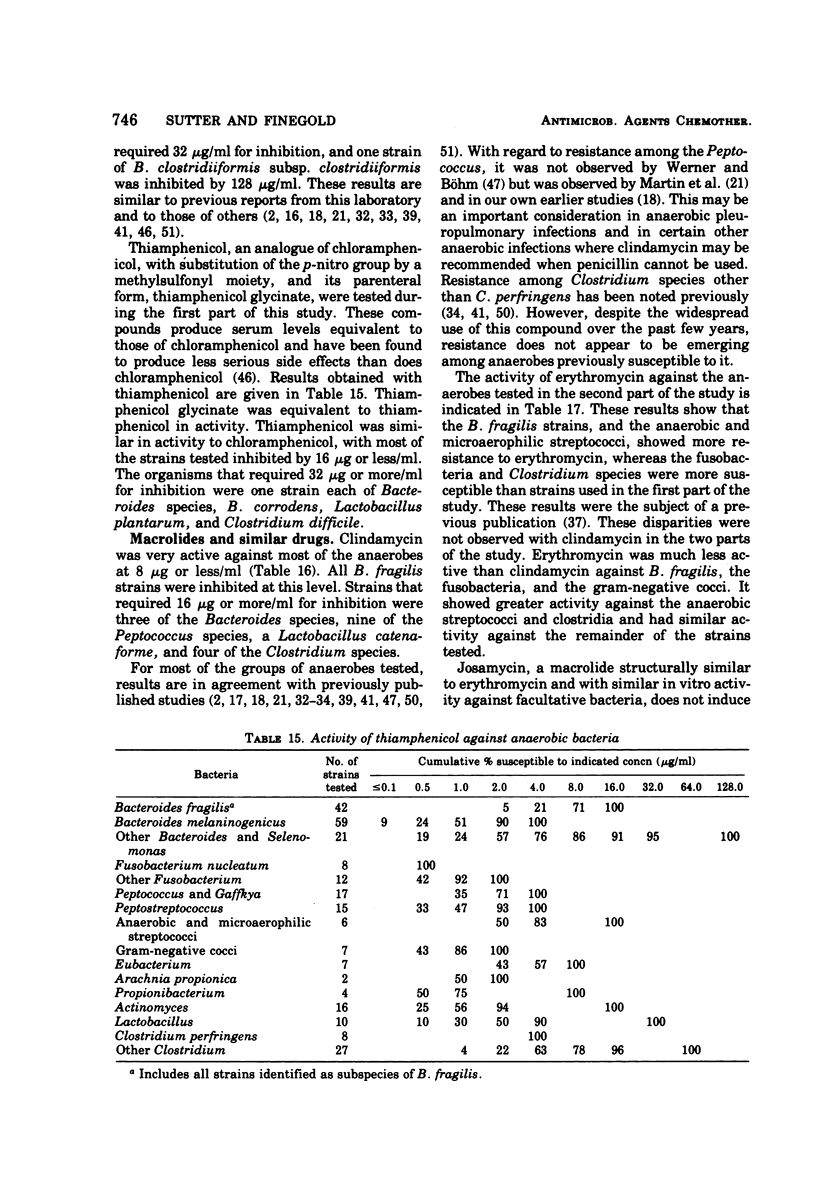
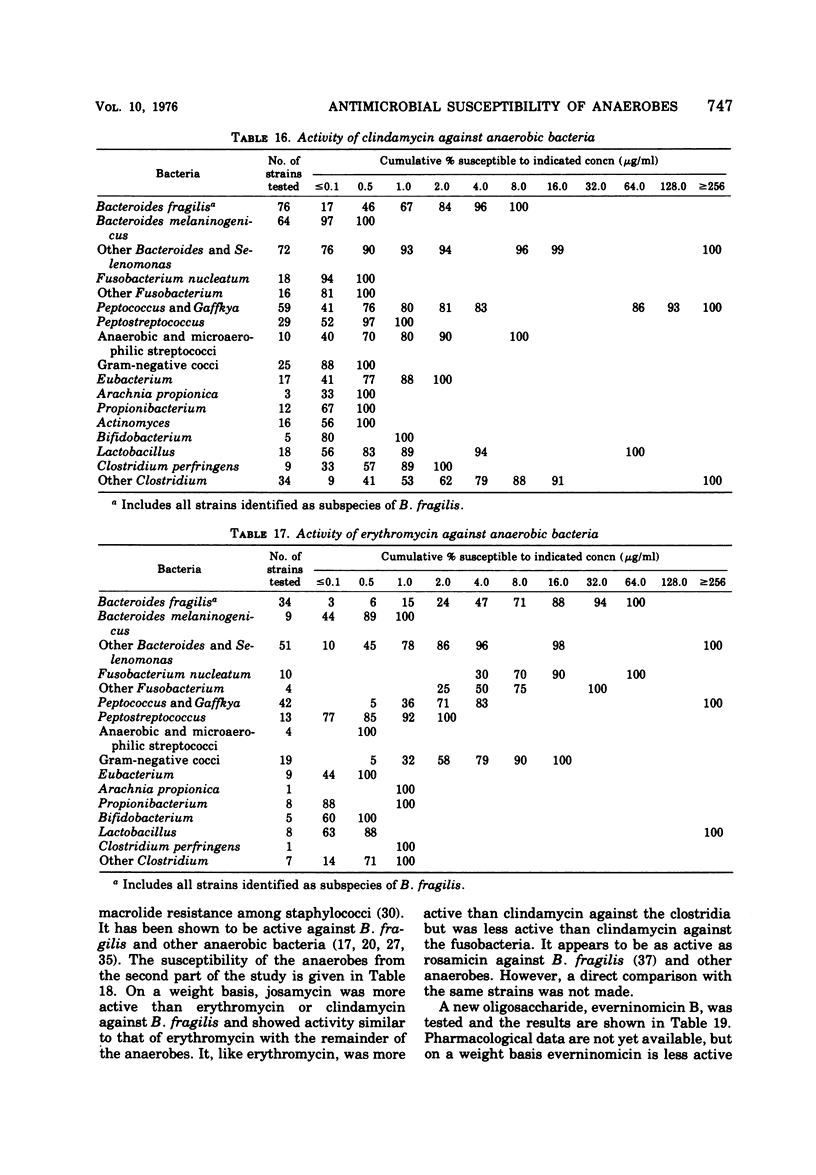
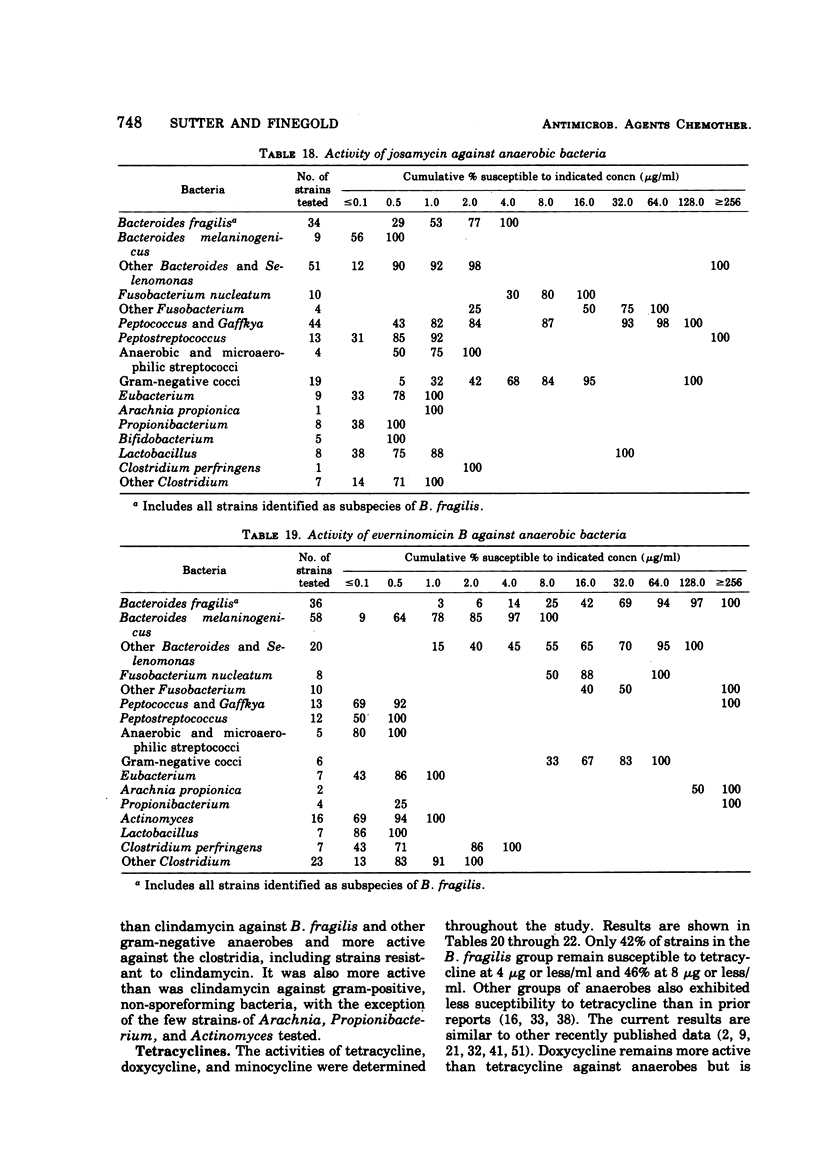
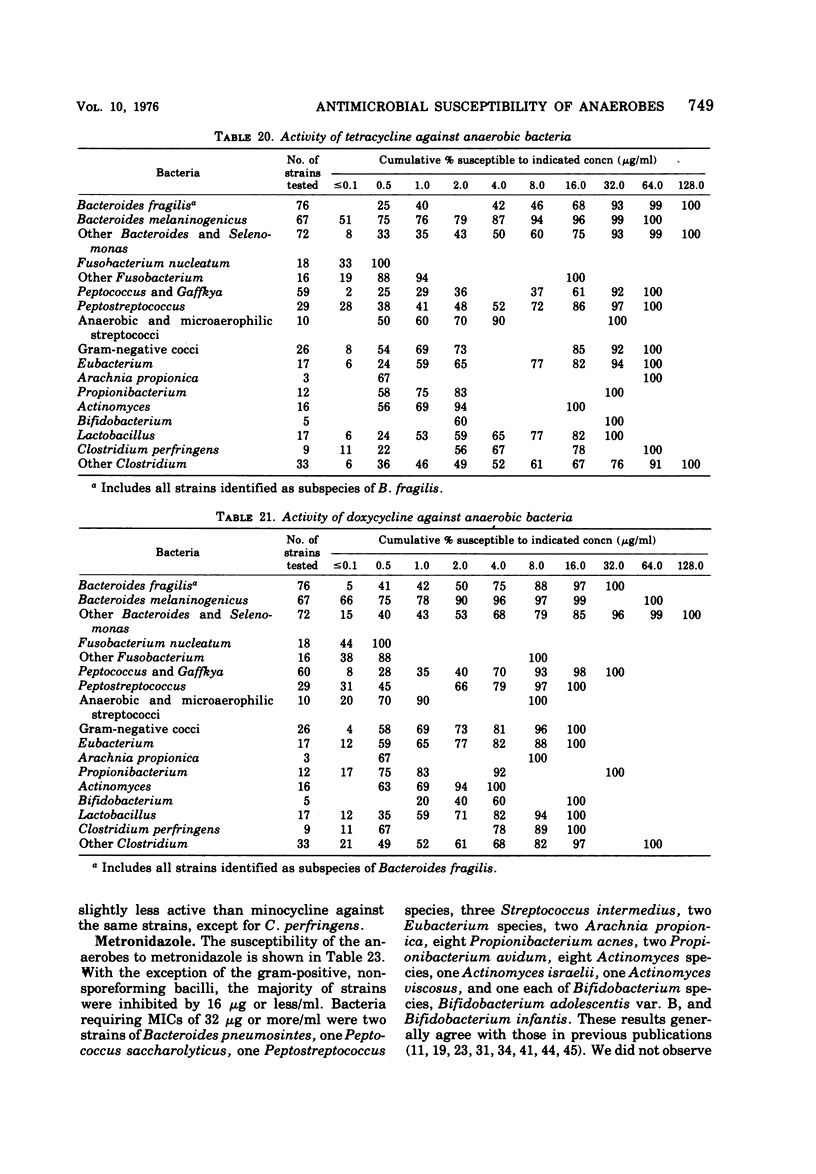
The antimicrobial susceptibility of 492 anaerobic bacteria, the majority of which were recent clinical isolates, was determined by the agar dilution technique. Penicillin G was active against most of the strains tested at 32 U or less/ml, but only 72% of Bacteroides fragilis strains were susceptible at this level and 9% required 256 U or more/ml. Ampicillin was effective against most of the strains except B. fragilis at 16 μg or less/ml. Amoxicillin was active against only 31% of B. fragilis, 76% of other Bacteroides species, and 67% of Fusobacterium species at 8 μg/ml. Two new penicillins, mezlocillin and azlocillin, were similar to ampicillin in their activity. Carbenicillin and ticarcillin inhibited all but a few strains at 128 μg or less/ml. BLP 1654 was somewhat more active than penicillin G against B. fragilis but had similar activity against other anaerobes. Cephalothin was inactive against B. fragilis, and only 65% of other Bacteroides species were inhibited by 32 μg or less/ml. It was effective against all other anaerobes at that level. Cefamandole showed somewhat greater activity than cephalothin against B. fragilis but generally less activity against gram-positive organisms. Cefazaflur (SKF 59962) was comparable to cephalothin against B. fragilis. Cefoxitin was distinctly more active than cephalothin against B. fragilis. These latter two agents were less active than cephalothin against the gram-positive anaerobes. Chloramphenicol remains active against anaerobic bacteria at 16 μg or less/ml, with rare exceptions. Thiamphenicol was similar to chloramphenicol in its activity. Clindamycin was very active against most of the anaerobes at 8 μg or less/ml. Erythromycin and josamycin were also tested, with josamycin showing greater activity against B. fragilis than either erythromycin or clindamycin. A new oligosaccharide, everninomicin B, was less active than clindamycin against B. fragilis but more active against clostridia and some of the other strains tested. Most of the groups of bacteria tested demonstrated a trend toward resistance to tetracycline. Doxycycline and minocycline were somewhat more active than was tetracycline. Metronidazole was active against the majority of the anaerobes tested; resistance ws demonstrated by some of the gram-positive cocci and gram-positive, non-sporeforming bacilli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actor P., Uri J. V., Guarini J. R., Zajac I., Phillips L., Sachs C. S., DeMarinis R. M., Hoover J. R., Weisbach J. A. A new parenteral cephalosporin. SK&F 59962: in vitro and in vivo antibacterial activity and serum levels in experimental animals. J Antibiot (Tokyo) 1975 Jun;28(6):471–476. doi: 10.7164/antibiotics.28.471. [DOI] [PubMed] [Google Scholar]
- Beers D. V., Schoutens E., Vanderlinden M. P., Yourassowsky E. Comparative in vitro acitvity of chloramphenicol and thiamphenicol on common aerobic and anaerobic gram-negative bacilli(Salmonella and Shigella excluded). Chemotherapy. 1975;21(2):73–81. doi: 10.1159/000221849. [DOI] [PubMed] [Google Scholar]
- Blazevic D. J. Antibiotic susceptibility of the subspecies of Bacteroides fragilis. Antimicrob Agents Chemother. 1976 Mar;9(3):481–484. doi: 10.1128/aac.9.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazevic D. J., Matsen J. M. Susceptibility of anaerobic bacteria to carbenicillin. Antimicrob Agents Chemother. 1974 May;5(5):462–465. doi: 10.1128/aac.5.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Rodriguez V., Horikoshi N., Valdivieso M. Diagnosis and management of infections associated with immunosuppression. New antibiotics of potential importance for immunosuppressed patients. Transplant Proc. 1973 Sep;5(3):1227–1231. [PubMed] [Google Scholar]
- Bodey G. P., Weaver S. In vitro studies of cefamandole. Antimicrob Agents Chemother. 1976 Mar;9(3):452–457. doi: 10.1128/aac.9.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Kosmidis J., Hamilton-Miller J. M., Gilchrist J. N. Cefoxitin and cephalothin: antimicrobial activity, human pharmacokinetics, and toxicology. Antimicrob Agents Chemother. 1974 Sep;6(3):290–299. doi: 10.1128/aac.6.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Patten V., Guze L. B. Comparative susceptibility of anaerobic bacteria to minocycline, doxycycline, and tetracycline. Antimicrob Agents Chemother. 1975 Jan;7(1):46–49. doi: 10.1128/aac.7.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Patten V., Guze L. B. Susceptibility of anaerobic bacteria to metronidazole: relative resistance of non-spore-forming gram-positive baccilli. J Infect Dis. 1975 Feb;131(2):182–185. doi: 10.1093/infdis/131.2.182. [DOI] [PubMed] [Google Scholar]
- Dornbusch K., Nord C. E. In vitro effect of metronidazole and tinidazole on anaerobic bacteria. Med Microbiol Immunol. 1974;160(4):265–267. doi: 10.1007/BF02121441. [DOI] [PubMed] [Google Scholar]
- Ernst E. C., Berger S., Barza M., Jacobus N. V., Tally F. P. Activity of cefamandole and other cephalosporins against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1976 May;9(5):852–855. doi: 10.1128/aac.9.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C. Identification and antimicrobial susceptibility of 250 Bacteriodes fragilis subspecies tested by broth microdilution methods. Antimicrob Agents Chemother. 1976 Apr;9(4):719–721. doi: 10.1128/aac.9.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislak J. W. The susceptibility of Bacteroides fragilis to 24 antibiotics. J Infect Dis. 1972 Mar;125(3):295–299. doi: 10.1093/infdis/125.3.295. [DOI] [PubMed] [Google Scholar]
- Kwok Y. Y., Tally F. P., Sutter V. L., Finegold S. M. Disk susceptibility testing of slow-growing anaerobic bacteria. Antimicrob Agents Chemother. 1975 Jan;7(1):1–7. doi: 10.1128/aac.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner P. I. Susceptibility of pathogenic actinomycetes to antimicrobial compounds. Antimicrob Agents Chemother. 1974 Mar;5(3):302–309. doi: 10.1128/aac.5.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. S., Mueller S., Swenson R. M. In vitro susceptibilities of anaerobic bacteria to josamycin. Antimicrob Agents Chemother. 1976 May;9(5):859–860. doi: 10.1128/aac.9.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers B. R., Leng B., Hirschman S. Z. Cefamandole: antimicrobial activity in vitro of a new cephalosporin. Antimicrob Agents Chemother. 1975 Dec;8(6):737–741. doi: 10.1128/aac.8.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastro L. J., Finegold S. M. Bactericidal activity of five antimicrobial agents against Bacteroides fragilis. J Infect Dis. 1972 Jul;126(1):104–107. doi: 10.1093/infdis/126.1.104. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Antimicrobial activity and human pharmacology of amoxicillin. J Infect Dis. 1974 Jun;129(0):suppl–suppl:S131. doi: 10.1093/infdis/129.supplement_2.s123. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Cefamandole, a cephalosporin antibiotic with an unusually wide spectrum of activity. Antimicrob Agents Chemother. 1974 Aug;6(2):177–182. doi: 10.1128/aac.6.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Garvey G. J. Comparative in vitro activity and clinical pharmacology of ticarcillin and carbenicillin. Antimicrob Agents Chemother. 1975 Oct;8(4):457–462. doi: 10.1128/aac.8.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby R., Brorsson J. E., Seeberg S. Comparative study of the in vitro antibacterial activity of cefoxitin, cefuroxine, and cephaloridine. Antimicrob Agents Chemother. 1976 Mar;9(3):506–510. doi: 10.1128/aac.9.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. V., Hamilton-Miller J. M., Brumfitt W. A comparison of the in vitro activity of metronidazole, tinidazole, and nimorazole against Gram-negative anaerobic bacilli. J Clin Pathol. 1975 Oct;28(10):775–778. doi: 10.1136/jcp.28.10.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotilie C. A., Fass R. J., Prior R. B., Perkins R. L. Microdilution technique for antimicrobial susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1975 Mar;7(3):311–315. doi: 10.1128/aac.7.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Washington J. A., 2nd Antimicrobial susceptibilities of anaerobic bacteria: recent clinical isolates. Antimicrob Agents Chemother. 1974 Sep;6(3):311–315. doi: 10.1128/aac.6.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh L. J., Dilworth J. A., Gwaltney J. M., Jr, Sande M. A. In vitro susceptibility studies with josamycin and erythromycin. Antimicrob Agents Chemother. 1976 Mar;9(3):546–548. doi: 10.1128/aac.9.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Rosamicin: in vitro activity against anaerobes and comparison with erythromycin. Antimicrob Agents Chemother. 1976 Feb;9(2):350–351. doi: 10.1128/aac.9.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of Anaerobic bacteria to carbenicillin, cefoxitin, and related drugs. J Infect Dis. 1975 Apr;131(4):417–422. doi: 10.1093/infdis/131.4.417. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y., Finegold S. M. Susceptibility of Bacteroides fragilis to six antibiotics determined by standardized antimicrobial disc susceptibility testing. Antimicrob Agents Chemother. 1973 Feb;3(2):188–193. doi: 10.1128/aac.3.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Armfield A. Y., Dowell V. R., Jr, Kwok Y. Y., Sutter V. L., Finegold S. M. Susceptibility of Clostridium ramosum to antimicrobial agents. Antimicrob Agents Chemother. 1974 Jun;5(6):589–593. doi: 10.1128/aac.5.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Bartlett J. G., Gorbach S. L. In vitro activity of penicillins against anaerobes. Antimicrob Agents Chemother. 1975 Apr;7(4):413–414. doi: 10.1128/aac.7.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Bartlett J. G., Gorbach S. L. Susceptibility of anaerobes to cefoxitin and other cephalosporins. Antimicrob Agents Chemother. 1975 Feb;7(2):128–132. doi: 10.1128/aac.7.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Sutter V. L., Finegold S. M. Metronidazole versus anaerobes. In vitro data and initial clinical observations. Calif Med. 1972 Dec;117(6):22–26. [PMC free article] [PubMed] [Google Scholar]
- Werner H., Böhm G. Empfindlichkeit sporenloser Anaerobier der Gattungen Bacteroides, Fusobacterium, Sphaerophorus, Peptococcus und Peptostreptococcus gegen Clindamycin. Zentralbl Bakteriol Orig A. 1974;229(3):401–408. [PubMed] [Google Scholar]
- Werner H., Rintelen G., Kunstek-Santos H. Eine neue buttersäurebildende Bacteroides-Art: B. splanchnicus n. sp. Zentralbl Bakteriol Orig A. 1975;231(1-3):133–144. [PubMed] [Google Scholar]
- Wick W. E., Preston D. A. Biological properties of three 3-heterocyclic-thiomethyl cephalosporin antibiotics. Antimicrob Agents Chemother. 1972 Mar;1(3):221–234. doi: 10.1128/aac.1.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Resistance of some species of Clostridium to clindamycin. Antimicrob Agents Chemother. 1973 Jan;3(1):136–137. doi: 10.1128/aac.3.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J., Johnston J. A., Hauser K. J. Bacteriostatic and bactericidal activities of various antibiotics against Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Feb;3(2):152–156. doi: 10.1128/aac.3.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]



