Abstract
The heterogeneous nature of rheumatoid arthritis (RA) complicates early recognition and treatment. In recent years, a growing body of evidence has demonstrated that intervention during the window of opportunity can improve the response to treatment and slow—or even stop—irreversible structural changes. Advances in therapy, such as biologic agents, and changing approaches to the disease, such as the treat to target and tight control strategies, have led to better outcomes resulting from personalized treatment to patients with different prognostic markers. The various biomarkers identified either facilitate early diagnosis or make it possible to adjust management to disease activity or poor outcomes. However, no single biomarker can bridge the gap between disease onset and prescription of the first DMARD, and traditional biomarkers do not identify all patients requiring early aggressive treatment. Furthermore, the outcomes of early arthritis cohorts are largely biased by the treatment prescribed to patients; therefore, new challenges arise in the search for prognostic biomarkers. Herein, we discuss the value of traditional and new biomarkers and suggest the need for intensive treatment as a new surrogate marker of poor prognosis that can guide therapeutic decisions in the early stages of RA.
Keywords: Early arthritis, biomarker, IL-15, VIP.
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by chronic inflammation of diarthrodial joints leading to joint destruction and, therefore, severe disability. In addition, the prevalence of comorbid conditions is high, and systemic inflammatory manifestations are common in patients with poorly controlled long-term disease [1, 2]. Consequently, RA causes impaired quality of life, loss of working-hours due to disability, high direct and indirect care costs, and decreased life expectancy [1, 3-11]. However, during the last 20 years, rheumatologists have observed a change in the clinical course of patients with RA attended at their clinics [12]. Improved diagnostic tests and management paradigms have led to better disease control. One of the most striking and dramatic improvements in the management of RA is the availability of biologic agents. Furthermore, changes in the mentality of rheumatologists, who no longer consider the disease benign, have led to more favorable outcomes in patients with RA [13]. Some of these new models are discussed below.
WINDOW OF OPPORTUNITY
The “window of opportunity” concept is a key component of current management of RA. During the late 1980s, rheumatologists realized that available treatment strategies were unable to control the disease adequately in many patients. In 1989, Healey and Wilske proposed discontinuing the pyramid strategy and treating patients more efficiently from the earliest stages [14, 15]. Although some authors disagree with a more proactive approach [16], ie, prescription of disease-modifying antirheumatic drugs (DMARD), the paper by McCarty entitled “Suppress rheumatoid inflammation early and leave the pyramid to the Egyptians” encouraged many rheumatologists to adopt the new approach [17]. During the 1990s, a growing body of evidence indicated that treating RA earlier would lead to better outcomes [18]. This notion was further supported in 2000 by Anderson et al. [19], whose meta-analysis of clinical trials investigating various DMARDs showed that disease duration was a critical factor for worse rate of response, irrespective of the drug prescribed. The authors anticipated that the window of opportunity could be open during the first 2 years of the disease. However, during the first decade of this century, several clinical trials and cohort studies suggested that the window of opportunity may close progressively around the onset of symptoms; consequently, rheumatologists moved the decision to start DMARDs from <1-2 years of symptoms (early RA) to <12 weeks of symptoms (very early RA) [20, 21]. At the same time, it became clear that new diagnostic criteria were necessary to classify patients when disease duration is <12 weeks and/or no typical radiological findings are present [22]. These criteria for classification of early RA were published in 2010 [23]. Furthermore, many rheumatologists consider that some patients not fulfilling the 2010 criteria could require DMARDs to avoid progression to RA [24-26]. The term “undifferentiated arthritis” had been used during the 1980s to refer to a subpopulation of patients that falls within the spectrum of spondyloarthritis. However, during the first decade of this century, undifferentiated arthritis was more commonly used to refer to a disease that could be considered a pre-RA disorder, at least in a subset of patients [27-30].
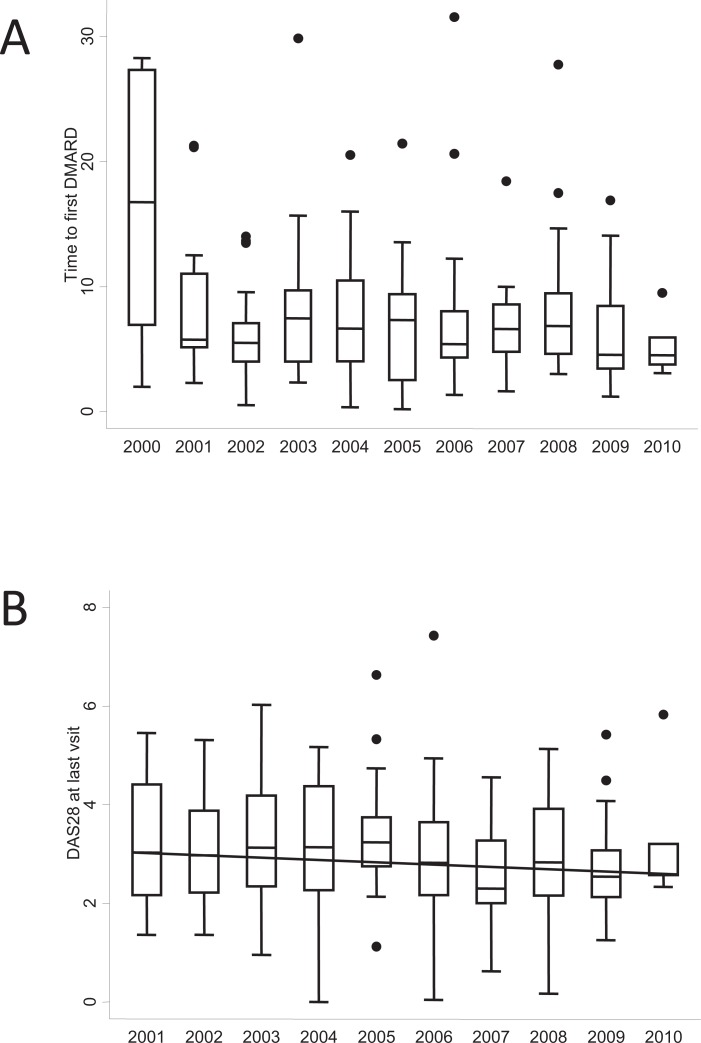
These findings led rheumatologists to treat RA patients with DMARDs as soon as possible in an attempt to achieve remission or at least the best possible response. However, the lag time between onset of symptoms and prescription of the first DMARD does not depend only on the rheumatologist. In 1999 in Spain, the emAR Study revealed a median lag time to first DMARD of 19 months [31]. The reasons for such a long lag time were related to patients (years of schooling and other sociodemographic variables), primary care physicians, and the organization of rheumatology services [31, 32]. During the first decade of this century, the Spanish Society of Rheumatology (SER) promoted a series of media campaigns to improve awareness of RA among the general population. Furthermore, in 2004, the SER supported nationwide implementation of early arthritis clinics (EAC) through the SERAP program. This initiative was designed to facilitate closer cooperation between primary care physicians and rheumatology departments so that patients with potential early arthritis had fast access to treatment [33]. The results of this huge effort were excellent. The Rheumatology Service of Hospital Universitario de La Princesa (HUP) also participated in the study, although our EAC had already been running for 4 years. During the last 13 years, the median lag time to prescription of the first DMARD in our unit has decreased progressively from 26 to 3 months Fig. (1a). In addition, we observed a trend towards better control of disease activity during the first 2 years of follow-up compared with before the implementation of the EAC Fig. (1b). This finding in a local population was confirmed throughout Spain in the SERAP program [34].
Fig. (1).
Benefits of establishing the Early Arthritis Clinic at Hospital Universitario La Princesa. A) Lag time to prescription of the first disease-modifying antirheumatic drug (DMARD). B) Disease activity after 2 years of follow-up. Data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the lag time from the date of onset of symptoms to the date of prescription of the first DMARD (A) or the DAS28 at the end of the second year of follow-up (B). Dots represent the outliers. The solid black line in figure B represents the trend of medians over the years.
TREAT TO TARGET
The other key element in achieving optimal outcome for RA patients is the “treat to target” strategy. Various studies, such as FIN-RAco, TICORA, CAMERA, and BeSt, have shown that, irrespective of the therapeutic approach used, patient outcomes can be improved when treatment is adjusted to achieve a pre-established goal [35-38]. Essentially, treat to target involves stepping up or adjusting treatment with glucocorticoids or DMARDs (whether synthetic or biologic) until the therapeutic objective is achieved. Intensification is based on a predefined schedule analyzed in clinical trials that have confirmed this approach [35, 38, 39]. Nevertheless, in daily practice, therapeutic strategies based on management protocols are not the rule, and prescription of DMARDs varies widely depending on the patient’s and the physician’s preferences [40-42]. In order ensure acceptance by rheumatologists of the treat to target strategy, an international task force formulated 10 recommendations for better application of the approach in daily practice, leading to more accurate control of disease activity [43]. Despite these efforts, implementation is challenging, and the rheumatologists most likely to use the strategy are those involved in the management of EACs and in research.
RA IS A HETEROGENEOUS DISEASE
Despite a widespread trend toward applying earlier and more aggressive treatment in patients with RA, rheumatologists are aware that the heterogeneous nature of the disease means that a substantial proportion of patients could achieve spontaneous remission. This is especially true of patients with early arthritis, who achieve remission rates ranging from 10% to 50% [44]. The heterogeneous genetic background underlying the broad clinical spectrum of RA is addressed elsewhere in the review issue by Rodríguez et al., who believe that the wide range of responses to treatment is probably due to genetic variability and environmental factors. Therefore, given that patients outside the clinical trial setting show considerable variability in age at onset, number of comorbid conditions, socioeconomic status, and lifestyle, application of a pre-established therapeutic schedule to every patient attended at an EAC does not seem appropriate. It would be advantageous to develop composite tools/algorithms combining sociodemographic items with biological markers in order to customize the treat to target strategy, thus avoiding unnecessary therapy and undesirable toxicity in those patients with a low risk of developing severe disease, but allowing rapid control in those patients with a poor prognosis. However, testing the prognostic value of biomarkers in untreated cohorts merely to study their impact on the natural history of the disease is subject to ethical limitations. Thus, at present, the outcomes of early arthritis cohorts are largely biased by the treatment prescribed.
NEED FOR TREATMENT AS A SURROGATE OUTCOME FOR DISEASE SEVERITY IN RA
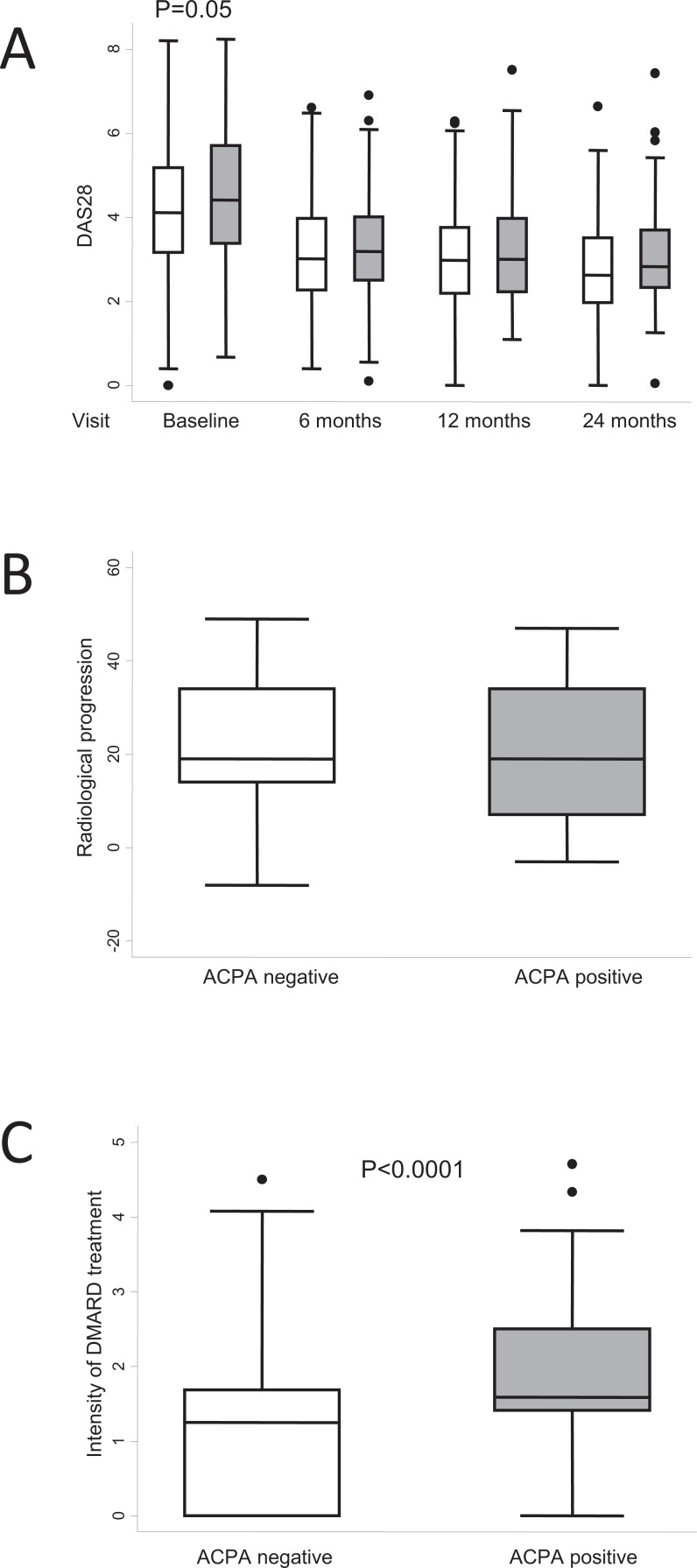
The changes in the management of RA described above are difficult to assess when observational studies searching for biomarkers of poor prognosis are based on populations attended at an EAC. This concern had already been raised in 2003 by Boers, who suggested that confounding by indication may decrease the effect of prognostic indicators on outcome (either disease activity or radiological progression) if physicians are aware of the importance of the biomarker [45]. The effect of confounding by indication is clearly seen in our early arthritis population, where physicians stressed the relevance of anticitrullinated protein antibody (ACPA) positivity. Our EAC has no pre-established therapeutic protocol, and when it was established in September 2000, the prognostic value of ACPA for poor outcome had already been proposed [46, 47]. Therefore, as shown in Fig. (2a), although ACPA-positive patients showed slightly but significantly higher baseline scores in the Disease Activity Score measured in 28 joints (DAS28), these differences disappeared during the 2-year follow-up. In addition, no significant differences were observed in radiological progression during these 2 years Fig. (2b).
Fig. (2).
Differences in clinical course between patients with positive and negative anticitrullinated peptide antibody (ACPA) in the Early Arthritis Clinic at Hospital Universitario La Princesa. A) Disease activity assessed using DAS28. White boxes represent ACPA-negative patients (n = 168); grey boxes represent ACPA-positive patients (n = 136). B) Radiological progression was estimated as the variation in the Sharp score with the modification of van der Heijde between baseline and the 24-month visit (n = 97). C) Intensity of treatment with disease-modifying antirheumatic drugs (DMARD) was estimated as described in the section “Need for treatment as a surrogate outcome for disease severity in RA” (n = 304). Data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the respective variables. Dots represent outliers. Statistical significance was determined using the Mann-Whitney test.
To overcome this bias, several groups analyzed variables associated with treatment. Verstappen et al. reported that failure of the first DMARD is a marker of the need for biologic agents in patients with early inflammatory polyarthritis [48]. These authors used therapeutic variables as prognostic factors of outcomes. Other possible therapeutic outcomes could be of the need for combined therapy with DMARDs or the classic cumulative dose of glucocorticoids.
In our EAC registry, we defined a variable termed “intensity of DMARD treatment” (IDT), which represents the cumulative prescription of all drugs (whether synthetic or biologics) during the first 2 years of follow-up weighted by their effectiveness, as follows:
IDT = [(1 x number of days with antimalarials) + (1.5 x number of days with methotrexate, leflunomide, sulphasalazine, parenteral gold salts) + (2 x number of days with biologic therapy)]/number of days of follow-up [49].
The factor used to customize the weight of the different drugs was based on the meta-analysis by Felson, who suggested that methotrexate, sulfasalazine, and gold salts are more efficacious than antimalarials [50] and similar to leflunomide [51]. In addition, it is commonly accepted that biologics represent a step beyond synthetic DMARDs.
Using this tool, we observed that ACPA-positive patients received more intense treatment with DMARDs than ACPA-negative patients Fig. (2c). Therefore, measuring the intensity of treatment could be a useful surrogate marker of disease severity in populations that routinely received early treat to target strategies in an EAC. In fact, the 2010 criteria for classification of RA were based on a similar concept, where initiating methotrexate within the first 12 months of follow-up was the gold standard for classification of RA [23].
Obviously, prescription of DMARDs could be biased by factors such as age, educational or socioeconomic status, physician preferences, and features of the health system. However, the analysis of this variable can be easily adjusted for these confounders, and physicians at hospitals with an EAC are usually less heterogeneous in their prescription habits.
BIOMARKERS OF THE NEED FOR INTENSIVE TREATMENT
Sociodemographic Data
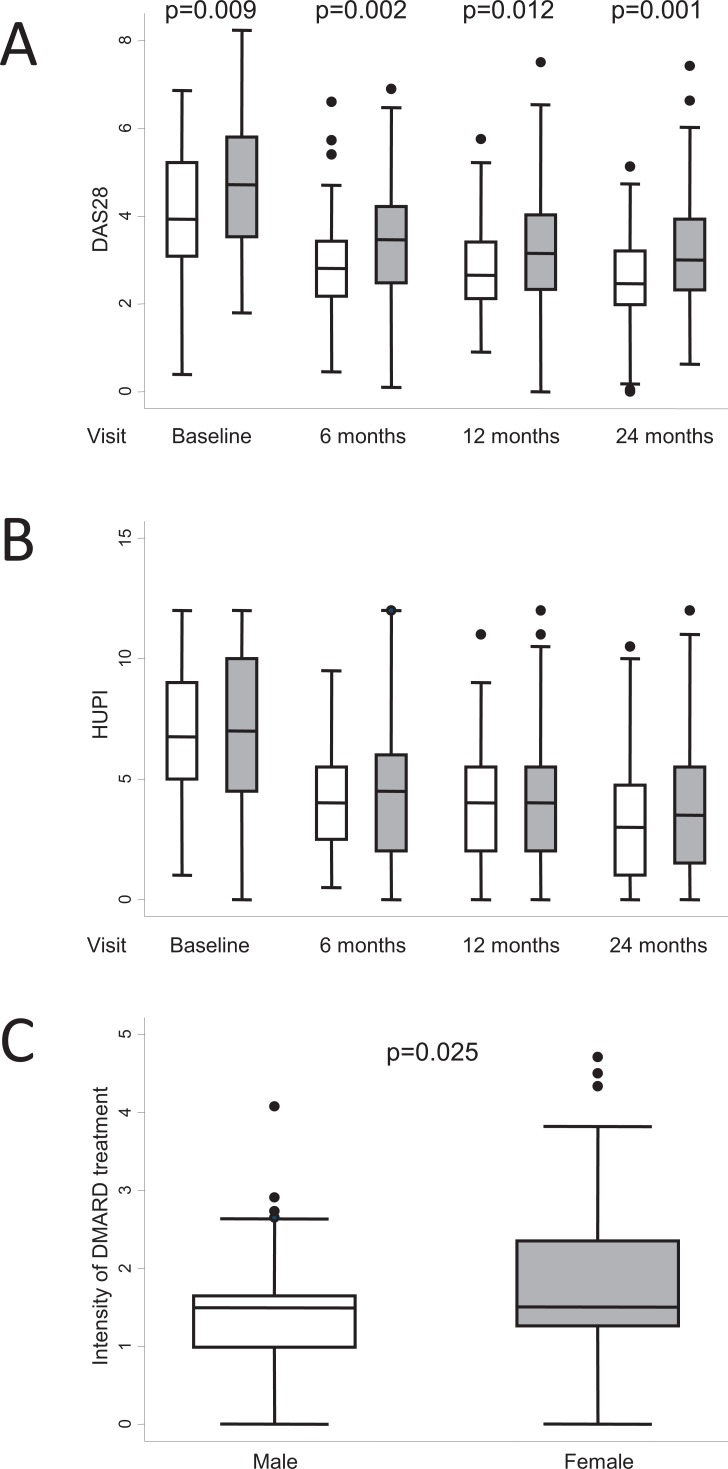
Female gender has traditionally been considered an indicator of poor prognosis in RA, since women display higher disease activity scores Fig. (3a) [1, 52]. However, some authors consider that this finding could be due to a bias in the tools used for the assessment of disease activity [53, 54]. In this regard, we recently described a new composite index, the HUPI (Hospital Universitario La Princesa Index), which prevents gender bias associated with tender joints and erythrocyte sedimentation rate [55]. Nevertheless, slightly higher disease activity scores are seen in women, even with this index Fig. (3b). We propose that female gender should continue to be considered an indicator of poor prognosis, since in our early arthritis population, women display similar or higher disease activity (depending on the indexes) despite receiving more intensive DMARD therapy Fig. (3c). This finding was associated with a trend towards more frequent prescription of combined therapy to women than to men (47.7% vs 38%, p=0.26) and a significantly higher percentage of tumor necrosis factor (TNF) blockers in women (11.8% vs 2.9%; p=0.046).
Fig. (3).
Differences in clinical course by gender (female, grey boxes; male, white boxes) in the population of the Early Arthritis Clinic at Hospital Universitario La Princesa. A) Disease activity assessed by DAS28 (n = 285, 230, 238, and 248 patients at baseline, 6, 12, and 24 months, respectively). B) Disease activity assessed using the Hospital Universitario La Princesa Index (HUPI; n = 304, 236, 251, and 256 patients at baseline, 6, 12, and 24 months, respectively). C) The intensity of treatment with disease-modifying antirheumatic drugs (DMARD) was estimated as described in the section “Need for treatment as a surrogate outcome for disease severity in RA” (n = 304). Data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the respective variables. Dots represent outliers. Statistical significance was determined using the Mann-Whitney test.
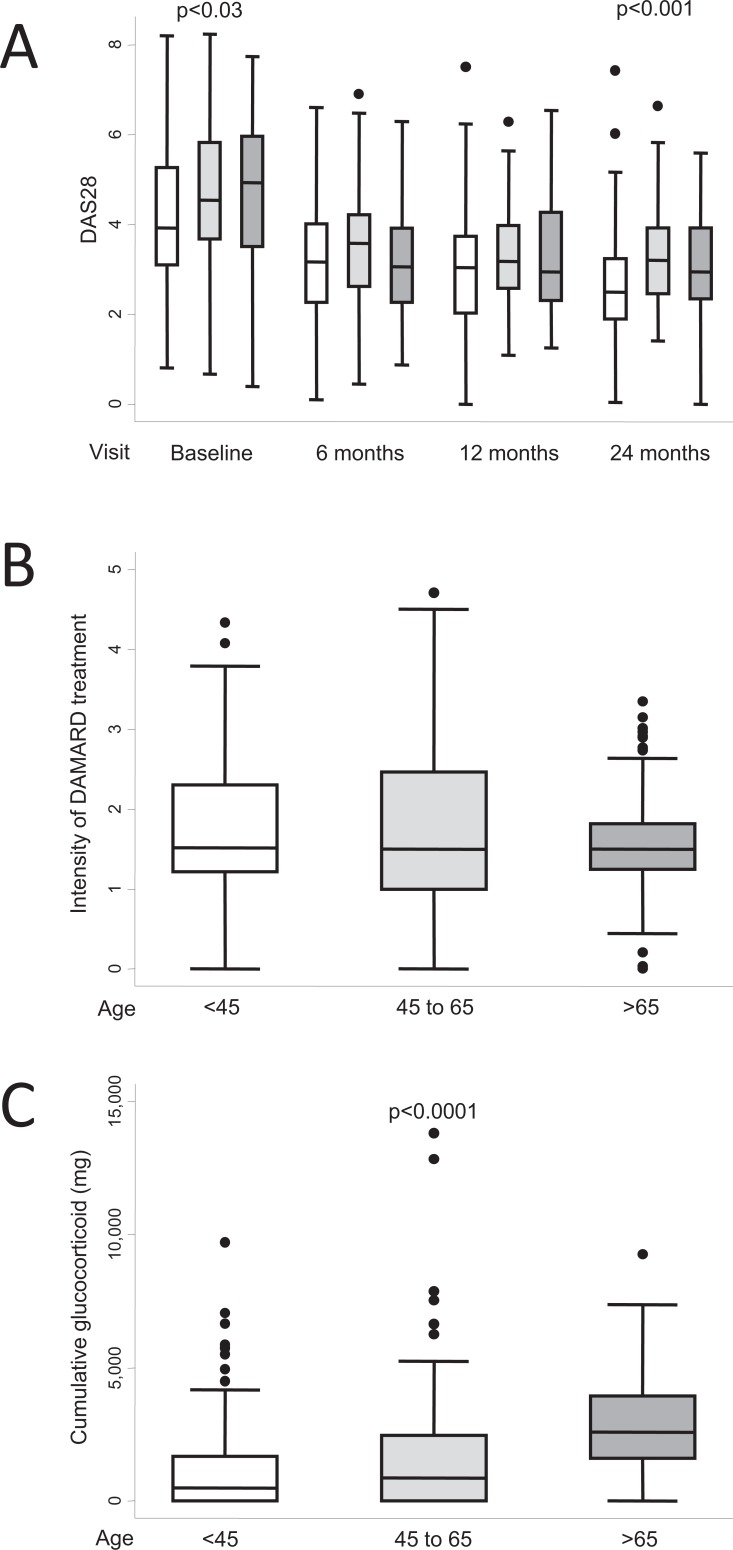
Age at disease onset is also a controversial prognostic factor. Traditionally, late onset had been associated with less severe disease [56]. However, in 1999, Pease et al. reported that patients with late onset RA in an EAC had a clinical course comparable to that of patients with early onset RA in terms of disability and presence of erosions [56]. In addition, more frequent prescription of glucocorticoids and less frequent prescription of DMARDs were also noted in the late onset population. The latter finding was confirmed in a recent German study in which DMARDs (synthetic or biologic) were prescribed less frequently in patients aged >65 years [57]. This finding could be explained by the higher prevalence of comorbid conditions in elderly patients, which leaves rheumatologists more reluctant to intensify treatment. In our EAC registry, patients 65 years displayed higher disease activity scores at baseline and after a 2-year follow-up Fig. (4a). Regarding DMARDs, the median IDT values were similar in patients >65 years compared to either patients aged<45 years or to the subset aged 45-65 years Fig. (4b). However, few elderly patients received very intensive DMARD treatment (IDT>2 Units); Fig. (4b), probably reflecting less frequent prescription of combined therapy in this population (33%) than in patients aged 45-65 years (48%) and patients aged<45 years (53%). Accordingly, in the Norfolk Arthritis Register, Verstappen et al. recently reported younger age to be a predictor for prescription of biologics (48) and older age to be associated with reduced use of biologic agents in the US (58). Taken together, these findings suggest that aggressive therapeutic approaches such as combined therapy or biologics are avoided in elderly patients in an attempt to prevent adverse events. In contrast with this safety-guided management, patients aged >65 years received more glucocorticoids, both in our practice Fig. (4c) and in reports from other European cohorts [56]. However, glucocorticoids have also been associated with an increased risk of infection in elderly patients [59-61]. Therefore, this treatment schedule should be used with caution in elderly people, and further studies should be performed to determine which treatments carry an increased risk for patients aged >65 years.
Fig. (4).
Differences in clinical course by age (white boxes, age ≤45 years; light grey 45-65 years; dark grey, >65 years) in the population of the Early Arthritis Clinic at Hospital Universitario La Princesa. A) Disease activity assessed by DAS28 (n = 285, 230, 238, and 248 patients at baseline, 6, 12, and 24 months respectively). B) The intensity of treatment with disease-modifying antirheumatic drugs (DMARD) was estimated as described in the section “Need for treatment as a surrogate outcome for disease severity in RA” (n = 304). C) Cumulative dose of glucocorticoids during the 2 years of follow-up (n=304). Data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the respective variables. Dots represent outliers. Statistical significance was determined using the Kruskal-Wallis test.
Finally, other sociodemographic factors associated with poor prognosis or worse response to treatment are educational level, smoking, and marital status [62]. We did not find clearly worse outcomes of RA associated with these variables, except for a lower cumulative dose of glucocorticoids in patients with a high educational level.
Variables Associated with RA
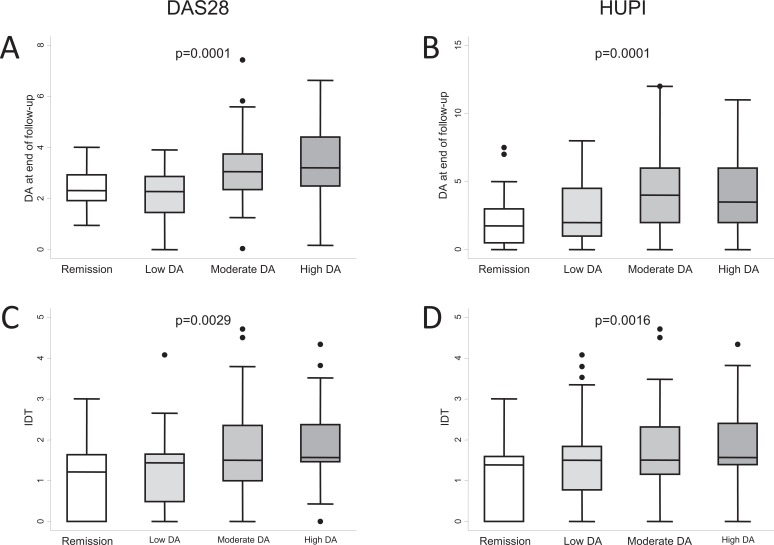
The characteristics of RA at onset, such as baseline DAS28 or Health Assessment Questionnaire (HAQ) scores, have been proposed as predictors of subsequent disease progress. However, baseline DAS28 remains a controversial prognostic marker of disease severity in the long term. In fact, as reported by Emery and Dorner, a low DAS28 at the beginning of treatment with TNF blockers could be a marker for achieving remission according to the EULAR criteria, whereas a high DAS28 could be a marker for achieving a response according to the ACR criteria [63]. In addition to this discrepancy, our registry data show that higher disease activity at baseline correlates with higher disease activity after 2 years of follow-up, irrespective of the index used Fig. (5A,B). Furthermore, we obtained these results even though patients with moderate or high disease activity at baseline were prescribed more intensive DMARD therapy during these 2 years Fig. (5C,D).
Fig. (5).
Differences in clinical course of the population of the Early Arthritis Clinic at Hospital Universitario La Princesa compared with the baseline level of disease activity assessed with either DAS28 (A and C) or HUPI (B and D). A) Disease activity (DA) at the end of follow-up measured by DAS28 (n = 23, 23, 98, and 83 at remission, low, moderate, or high disease activity, respectively). B) Disease activity at the end of follow-up measured by HUPI (n = 28, 64, 69, and 91 at remission, low, moderate, or high disease activity, respectively). C and D) Intensity of treatment with disease-modifying antirheumatic drugs (DMARD) was estimated as described in the section “Need for treatment as a surrogate outcome for disease severity in RA”. Data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the respective variables. Dots represent outliers. Statistical significance was determined using the Kruskal-Wallis test.
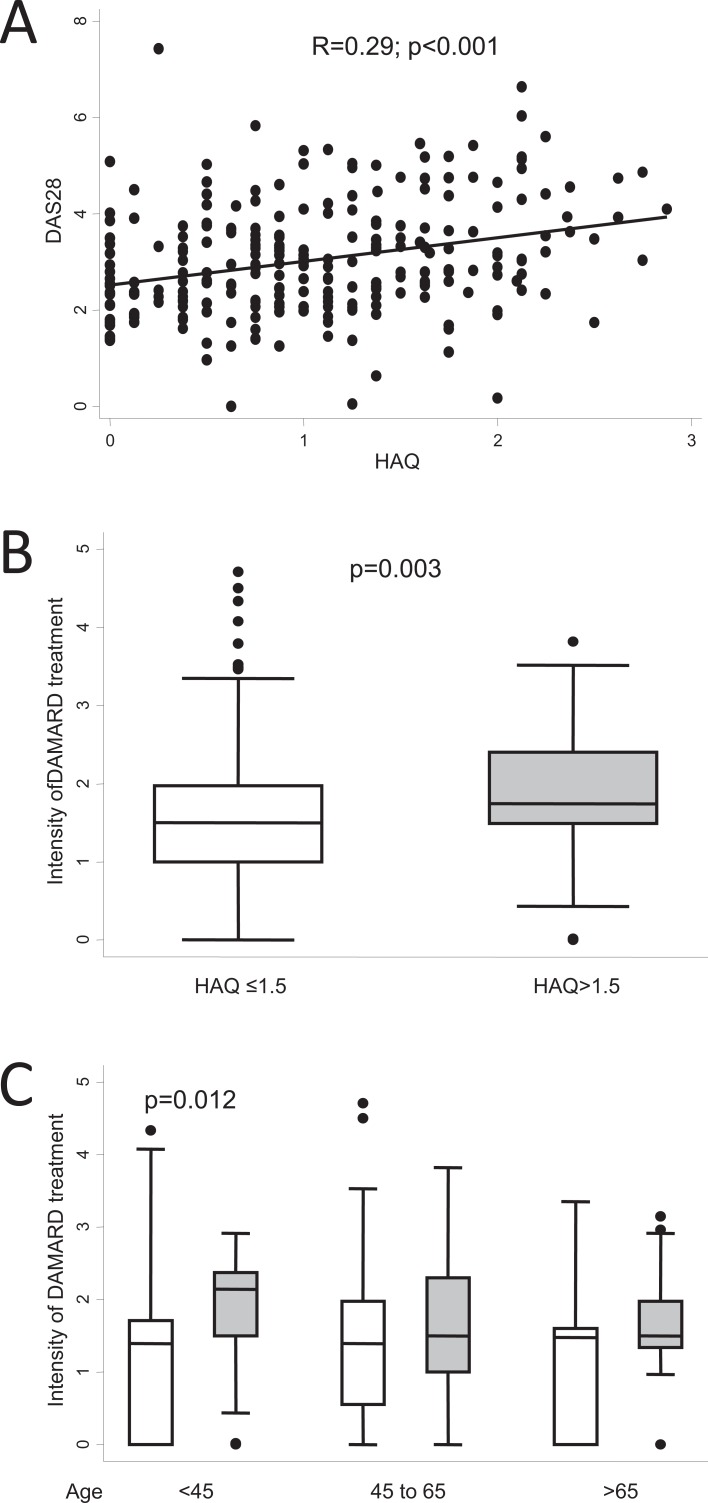
The usefulness of the baseline HAQ score as a predictor of poor prognosis is more complex to analyze, since age and comorbid conditions have a considerable impact on the progress of the score [64-66]. This might be the reason why a high HAQ score is a good predictor of mortality in RA [13]. Nevertheless, as the baseline HAQ score showed an acceptable correlation with DAS28 after 2 years Fig. (6a), those patients with a baseline HAQ score >1.5 required significantly more intensive DMARD therapy than those with a baseline HAQ ≤1.5 Fig. (6b). However, when the age-stratified analysis was performed, a baseline HAQ >1.5 only predicted higher IDT values in patients younger than 45 years Fig. (6c). This finding highlights the need for complex analysis involving adjustment for confounding or modifying variables when searching for biomarkers to predict poor prognosis.
Fig. (6).
Differences in the clinical course of the population of the Early Arthritis Clinic at Hospital Universitario La Princesa considering the baseline level of disability measured by the Health Assessment Questionnaire (HAQ). A) Correlation between disease activity at the end of follow-up measured by DAS28 and baseline HAQ score (n = 248). Data are shown as a dot plot, and the linear prediction was obtained with the lfit command of Stata 12. Statistical significance was determined using the Pearson test. B) Intensity of treatment with disease-modifying antirheumatic drugs (DMARD) was estimated as described in the section “Need for treatment as a surrogate outcome for disease severity in RA” (n = 195 and 75 if HAQ ≤1.5 or >1.5 respectively). C) Intensity of DMARD treatment clustered by HAQ level and age. In B and C, data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the respective variables. Dots represent outliers. Statistical significance was determined using the Mann-Whitney test. In panel C, considering that multiple comparisons were performed, statistical significance was set at <0.017.
Serum Biomarkers
Practical biomarkers must be both accurate and feasible [67]. In this regard, detection in serum enables a biomarker to be tested in large populations and become widely used. Such is the case of rheumatoid factor (RF), which has been largely considered a marker of poor prognosis. In recent decades, ACPA testing has been shown to improve the diagnostic and prognostic yield of RA, mostly in patients who test negative for RF [2]. The predictive value of both biomarkers increases when high levels are detected; this concept has been included in the 2010 RA classification criteria [23]. As previously mentioned, ACPA positivity has become a reliable marker of poor outcome that prompts clinicians to intensify therapeutic efforts Fig. (2c), including prescription of biologics [48], which can result in improved outcomes Fig. (2A,B). Those findings stand in contrast to the poor outcome of ACPA-positive patients in historic cohorts of RA and support evidence that the search for risk factors in current early arthritis cohorts remains a challenge, since a single variable rarely provides a reliable estimation of prognosis. More accurate statistical strategies based on a multivariate approach are needed to highlight new prognostic biomarkers.
In addition to autoantibodies, cytokines are potential biomarkers that can be measured in serum. For the last 10 years, our Unit has been involved in the study of the predictive value of serum cytokine levels when assessing the need for treatment or the response to drugs.
Our first publication in this field analyzed the receptor activator of nuclear factor kappaB ligand (RANKL) and osteoprotegerin (OPG) [68]. The balance between these 2 molecules regulates osteoclast activation, which is an essential feature in RA bone erosions. Higher serum and tissue levels of both proteins have been described in RA patients than in healthy controls [69, 70]. In addition, the baseline RANKL/OPG ratio had been reported to predict radiological progression in early arthritis [71]. In view of the major effect of TNF blockers in arresting radiological damage in RA patients, we decided to study whether the levels of these cytokines could predict the response to pharmacological TNF blockade. TNF blockers did not decrease total serum RANKL levels, although a significant decrease in OPG levels was observed [68]. Interestingly, lower baseline serum levels of both total RANKL and the RANKL/OPG ratio were associated with achieving remission after 3 and 7 months of treatment [68]. One of the concerns with this biomarker is the fact that several variables, such as gender, age, and disease activity, can affect both RANKL levels and the assessment of response. Nevertheless, multivariate logistic regression analysis showed that after adjustment for gender, age, and baseline DAS28, lower levels of RANKL in serum were predictive of remission after prescription of TNF blockers [68]. Therefore, we studied the potential correlation between RANKL or the RANKL/OPG ratio and greater need for treatment in patients with early arthritis. A slight positive correlation was observed between baseline serum RANKL/OPG ratio and the variable IDT (r=0.16; p=0.14), as well as a trend toward higher baseline values for this variable in patients requiring DMARDs than in those managed without these drugs, although no significant differences were recorded.
A second molecule, interleukin (IL) 15 has been researched in-depth by our group. IL15 is involved in several steps of the pathogenesis of RA, ranging from activation of Th17 cells to induce IL17 production [72-75] to modulation of intercellular contacts driving TNF production [76-78]. It may also be involved in the survival and activation of neutrophils, B cells, and NK cells [79-81]. In addition, IL15 has been associated with osteoclastogenesis and, therefore, with bone erosion in RA [82-84]. Knevel et al. reported that genetic variants in the IL15 gene are associated with differences in the radiological progression of patients with RA [85]. The possible mechanism underlying this association is the link between IL15, IL17, and RANKL, a relationship that we proposed in response to the article by Knevel et al.. Furthermore, blockade of IL15 has proven to prevent collagen-induced arthritis in mice [86]. Less impressive results were observed in human trials, where only a subpopulation of RA patients achieved considerable improvement [87]. A possible explanation for this disappointing result comes from the observation that dysregulation of IL15 production is observed in<30% of patients with RA [88]. Interestingly, patients with increased serum levels of IL15 showed a greater need for treatment, which was assessed using IDT (see above) [49]. As proposed in the Introduction, these findings reinforce the challenge of discovering new prognostic biomarkers in current early arthritis cohorts because of the treat to target approach and the bias of indication. Multivariate longitudinal analyses adjusting for conventional poor prognostic markers and for drug prescription were needed to unravel the association between increased serum IL15 levels and increased disease activity [49]. Moreover, patients with increased levels of IL15 or ACPA positivity were more frequently treated with combined therapy or TNF blockers, although physicians were only aware of the result of ACPA and not serum IL15 levels [49].
During the last 20 years, vasoactive intestinal peptide (VIP), a neuropeptide synthesized by immune cells, has emerged as a therapeutic agent owing to its notable immunoregulatory and anti-inflammatory effects [89-92].
Treatment with exogenous VIP decreased the frequency and severity of symptoms in a murine model of collagen-induced arthritis [93]. The therapeutic effect of VIP has been attributed to its capacity to downregulate inflammatory factors, to regulate various lymphocyte subsets (Th1, Th2, Treg, and Th17), and to protect against bone erosion by altering the RANK/RANKL/OPG system [94, 95]. Ex vivo assays with fibroblast-like synoviocytes (FLS) and peripheral blood lymphocytes from RA patients have shown that VIP modulates different proinflammatory pathways suggesting beneficial effects of this molecule on the functional capacity of human lymphocytes [96-100].
Interestingly, FLS from RA patients show lower expression of VIP than FLS from osteoarthritis (OA) patients [101]. Recently, Jiang et al. reported decreased VIP expression in the synovial fluid and joint cartilage of patients with severe OA [102], as well as it occurs in patients with juvenile idiopathic arthritis compared to healthy controls [103].
Considering these findings, we have recently tested the utility of measuring serum VIP as a clinical biomarker in our population of early arthritis patients [104]. Several important conclusions arose from this study. First, baseline serum levels of VIP were lower in patients who show a worse clinical course and increased requirements for treatment in the first two years of disease monitoring [104]. Interestingly, measurement of serum VIP levels at baseline extended the predictive value of poor outcome of ACPA since ACPA-negative patients with low VIP serum levels had a higher degree of disease activity than ACPA-negative patients with normal serum VIP levels and an even higher degree of disease activity than ACPA-positive patients [104]. Moreover, VIP levels showed no variation during follow-up [104] suggesting that assessment of VIP concentrations could provide prognostic information for EA patients independently of the treatment prescribed and the phase of the disease, an improvement of potential clinical relevance.
Finally, in addition to the problem of confounding by indication caused by conventional prognostic factors, serum cytokine levels raise additional concerns, such as their low accuracy as biomarkers. The heterogeneous nature of cytokine expression profiles in the general population further complicates statistical analysis, and technical reliability is limited by wide inter-assay variability. Furthermore, depending on the target, treatment with synthetic or biologic DMARDs can modify cytokine levels. Therefore, in order to obtain more accurate and robust markers, researchers are directing their efforts towards genetic biomarkers capable of overcoming the noise caused by fluctuations in cytokine expression.
Genetic Biomarkers
Many genetic studies have assessed the risk of developing RA, and many studies analyze the predictive value of genetic variants in the prognosis of RA, focusing mainly on radiological progression. In addition, genetic studies have tried to predict the response to the most commonly used drugs, methotrexate and TNF blockers. These topics are widely described elsewhere in this review issue. However, to our knowledge, no studies have focused on the usefulness of genetic markers to predict the need for more intensive treatment as a surrogate marker of disease severity.
We recently showed that patients who are homozygous for the T allele of the single-nucleotide polymorphism rs7574865 in STAT4 experience more severe disease [105]. In this work, we also studied the effect of carrying the shared epitope (SE) in HLADRB1 or the minor allele of rs2476601 in PTPN22 on the disease activity scores of patients with early RA. The statistical model was adjusted for several confounders such as age, gender, and the presence of ACPA. Under these conditions, carrying the SE was not associated with worse disease progress, probably because only smokers with the SE develop ACPA [105]. In addition, we observed a trend toward less active disease in patients carrying the T allele of rs2476601 in PTPN22 [105], although the prevalence of this genetic variant is clearly lower in Spain than in northern Europe [106].
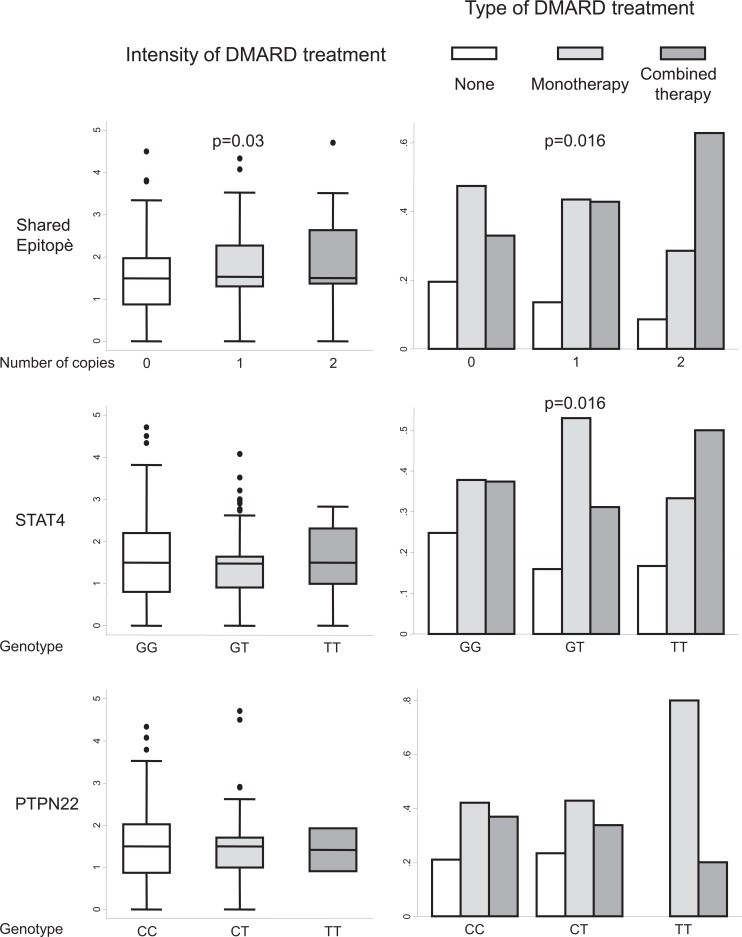
Considering the IDT variable, the presence of the SE was associated with an increased need for treatment Fig. (7, upper left panel). However, neither the genetic variant of STAT4 nor that of PTPN22 was associated with higher IDT values Fig. (7, left middle and lower panels, respectively). By contrast, the presence of both SE and the T allele of rs7574865 in STAT4 was associated with wider prescription of combined therapy and with a lower frequency of non-DMARD treatment. The controversial findings with STAT4 can be explained by the fact that the rheumatologists were blind to the result of testing for these genetic variants and patients with a slowly worsening course could neglected owing to a delay in prescription of combined therapy. Consequently, IDT could be lower than expected in those patients. By contrast, most patients carrying the SE also displayed ACPA positivity; therefore, physicians were more likely to prescribe intense treatment earlier, leading to higher IDT values [105]. As a consequence, we did not observe a worse clinical course in patients carrying SE, as we did in those who were homozygous for the T allele of rs7574865 in STAT4 [105].
CONCLUSION
In this article, we discuss the need for treatment, under conditions of routine clinical practice, as a pertinent surrogate marker of disease severity in early arthritis cohorts. Our experience shows that intensity of DMARD treatment can be useful to explore prognostic biomarkers in patients with early arthritis. We also emphasize that currently recognized markers of poor prognosis could act as an environmental factor that modifies the behavior of rheumatologists and somehow invalidates the efficacy of classic outcomes (radiological progression, level of disease activity) in detecting new biomarkers. Therefore, simple statistical analysis (bivariate) is no longer valid, since many factors now act as confounders. Adjustment for these confounders or modifiers of disease progress is needed. Gender, age at onset, and presence of ACPA should be systematically considered when assessing the value of biomarkers in predicting outcomes in EAC populations.
Studies of this type are affected by 2 drawbacks. First, in addition to confounders, interactions between biomarkers might mask the effect of a new one. To overcome this shortcoming, we are committed to more complex analysis to explore these interactions; therefore, larger sample populations are required. It is essential to increase sample sizes by means of collaborative efforts between different groups. Second, many of these biomarkers could be hidden, since they have no effect on disease incidence, although they can modulate its intensity.
In summary, changing paradigms such as the early treat to target strategy are improving the outcome of patients with newly diagnosed RA and making the search for new biomarkers of prognosis more exciting and challenging. Surrogate markers of disease severity such as need for biologic agents or estimation of the intensity of treatment could provide useful results.
Fig. (7).
Need for treatment in patients with early arthritis clustered by the number of copies of alleles encoding for the shared epitope in HLADRB1, the T allele in rs7574865 of STAT4, or the T allele of rs2476601 in PTPN22. In the left panels, data are presented as the interquartile range (p75 upper edge, p25 lower edge, p50 midline), as well as the p95 (line above the box) and p5 (line below the box), of the intensity of treatment with disease-modifying antirheumatic drugs (DMARD) (see the section “Need for treatment as a surrogate outcome for disease severity in RA” for definition). Dots represent outliers. Statistical significance was established using the Kruskal-Wallis test. In the right panels, data are shown as the percentage of patients with each type of DMARD treatment (none, monotherapy, or combined therapy) in each subpopulation. Statistical significance was established using the Fisher exact test.
ACKNOWLEDGEMENTS
This work was supported by the Fondo de Investigacion Sanitaria, Instituto de Salud Carlos III (PI08/0025, PI11/00195, PI11/00505, PI11/551, PI12/0758, RETICS RD08/0075, RD12/0009).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ABBREVIATIONS
- ACPA
= Anticitrullinated Protein Antibody
- ACR
= American College of Rheumatology
- B cells
= B lymphocytes
- DAS28
= Disease Activity Score measured in 28 joints
- DMARD/DMARDs
= Disease-Modifying Antirheumatic Drugs
- EA
= Early Arthritis
- EAC/EACs
= Early Arthritis Clinics
- EULAR
= The European League Against Rheumatism
- Fig
= Figure
- FLS
= Fibroblast-Like Synoviocytes
- HAQ
= Health Assessment Questionnaire
- HLADRB1
= HLA class II Histocompatibility Antigen, DRB1-9 beta chain
- HUP
= Hospital Universitario de La Princesa
- HUPI
= Hospital Universitario La Princesa Index
- IDT
= Intensity of DMARD Treatment
- IL-15
= Interleukin 15
- IL17
= Interleukin 17
- NK cells
= Natural Killer cells
- OA
= Osteoarthritis
- OPG
= Osteoprotegerin
- p25
= percentile 25
- p5
= percentile 5
- p50
= percentile 50
- p75
= percentile 75
- p95
= percentile 95
- PTPN22
= Protein Tyrosine Phosphatase, Non-receptor type 22
- RA
= Rheumatoid Arthritis
- RANK
= Receptor Activator of Nuclear Factor kappaB
- RANKL
= Receptor Activator of Nuclear Factor kappaB Ligand
- RF
= Rheumatoid Factor
- SE
= Shared Epitope
- SER
= Spanish Society of Rheumatology
- STAT4
= Signal Transducer and Activator of Transcription 4
- Th1
= T helper 1 lymphocytes
- Th17
= T helper 17 lymphocytes
- Th2
= T helper 2 lymphocytes
- TNF
= Tumor Necrosis Factor
- Treg
= Regulatory T lymphocytes
- US
= United States
- VIP
= Vasoactive Intestinal Peptide
The following abbreviations correspond to specific names of patient cohorts of early arthritis or to studies and projects carried out in these cohorts
emAR
SERAP
FIN-RAco
TICORA
CAMERA
BeSt
REFERENCES
- 1.Carmona L, Cross M, Williams B, Lassere M, March L. Rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2010;24:733–45. doi: 10.1016/j.berh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 3.Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–17. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Bansback N, Marra CA, Finckh A, Anis A. The economics of treatment in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2009;23:83–92. doi: 10.1016/j.berh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Lajas C, Abasolo L, Bellajdel B , et al. Costs and predictors of costs in rheumatoid arthritis a prevalence-based study. Arthritis Rheum. 2003;49:64–70. doi: 10.1002/art.10905. [DOI] [PubMed] [Google Scholar]
- 6.Yelin E, Wanke LA. An assessment of the annual and long-term direct costs of rheumatoid arthritis the impact of poor function and functional decline. Arthritis Rheum. 1999;42:1209–18. doi: 10.1002/1529-0131(199906)42:6<1209::AID-ANR18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Lundkvist J, Kastang F, Kobelt G. The burden of rheumatoid arthritis and access to treatment health burden and costs. Eur J Health Econ. 2008;8(Suppl 2: ): S49–60. doi: 10.1007/s10198-007-0088-8. [DOI] [PubMed] [Google Scholar]
- 8.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130–6. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Navarro-Cano G, Del Rincon I, Pogosian S, Roldan JF, Escalante A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis Rheum. 2003;48:2425–33. doi: 10.1002/art.11127. [DOI] [PubMed] [Google Scholar]
- 10.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–72. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 11.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis 2008 update. Clin Exp Rheumatol. 2008;26(5 ) Suppl 51 :S35–61. [PubMed] [Google Scholar]
- 12.Bergstrom U, Book C, Lindroth Y, Marsal L, Saxne T, Jacobsson L. Lower disease activity and disability in Swedish patients with rheumatoid arthritis in 1995 compared with 1978. Scand J Rheumatol. 1999;28:160–5. doi: 10.1080/03009749950154239. [DOI] [PubMed] [Google Scholar]
- 13.Pincus T, Brooks RH, Callahan LF. Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med. 1994;120:26–34. doi: 10.7326/0003-4819-120-1-199401010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Healey LA, Wilske KR. Reforming the pyramid.A plan for treating rheumatoid arthritis in the. Rheum Dis Clin North Am. 1990;1989, 15:615–9. [PubMed] [Google Scholar]
- 15.Wilske KR, Healey LA. Remodeling the pyramid--a concept whose time has come. J Rheumatol. 1989;16:565–7. [PubMed] [Google Scholar]
- 16.Hess EV, Luggen ME. Remodeling the pyramid--a concept whose time has not yet come. J Rheumatol. 1989;16:1175–6. [PubMed] [Google Scholar]
- 17.McCarty DJ. Suppress rheumatoid inflammation early and leave the pyramid to the Egyptians. J Rheumatol. 1990;17:1115–8. [PubMed] [Google Scholar]
- 18.O'Dell Jr. Treating rheumatoid arthritis early a window of opportunityκ. Arthritis Rheum. 2002;46:283–5. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis the importance of disease duration. Arthritis Rheum. 2000;43:22–9. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2004;43:906–14. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 21.Gremese E, Salaffi F, Bosello SL , et al. Very early rheumatoid arthritis as a predictor of remission a multicentre real life prospective study. Ann Rheum Dis. 2013;72:858–62. doi: 10.1136/annrheumdis-2012-201456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aletaha D, Breedveld FC, Smolen JS. The need for new classification criteria for rheumatoid arthritis. Arthritis Rheum. 2005;52:3333–6. doi: 10.1002/art.21410. [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ , et al. 2010 Rheumatoid arthritis classification criteria an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 24.Verstappen SM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DP. Beneficial effects of a 3-week course of intramuscular glucocorticoid injections in patients with very early inflammatory polyarthritis results of the STIVEA trial. Ann Rheum Dis. 2010;69:503–9. doi: 10.1136/ard.2009.119149. [DOI] [PubMed] [Google Scholar]
- 25.Emery P, Durez P, Dougados M , et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis a clinical and imaging study of abatacept (the ADJUST trial). Ann Rheum Dis. 2010;69:510–6. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleem B, Mackie S, Quinn M , et al. Does the use of tumour necrosis factor antagonist therapy in poor prognosis, undifferentiated arthritis prevent progression to rheumatoid arthritisκ. Ann Rheum Dis. 2008;67:1178–80. doi: 10.1136/ard.2007.084269. [DOI] [PubMed] [Google Scholar]
- 27.Quinn MA, Green MJ, Marzo-Ortega H , et al. Prognostic factors in a large cohort of patients with early undifferentiated inflammatory arthritis after application of a structured management protocol. Arthritis Rheum. 2003;48:3039–45. doi: 10.1002/art.11269. [DOI] [PubMed] [Google Scholar]
- 28.Raza K, Filer A. Predicting the development of RA in patients with early undifferentiated arthritis. Best Pract Res Clin Rheumatol. 2009;23:25–36. doi: 10.1016/j.berh.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 29.van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis how to guide individual treatment decisions. Arthritis Rheum. 2007;56:433–40. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 30.Verpoort KN, van Dongen H, Allaart CF, Toes RE, Breedveld FC, Huizinga TW. Undifferentiated arthritis--disease course assessed in several inception cohorts. Clin Exp Rheumatol. 2004;22(5 ) Suppl 35 :S12–7. [PubMed] [Google Scholar]
- 31.Hernandez-Garcia C, Vargas E, Abasolo L , et al. Lag time between onset of symptoms and access to rheumatology care and DMARD therapy in a cohort of patients with rheumatoid arthritis. J Rheumatol. 2000;27:2323–8. [PubMed] [Google Scholar]
- 32.Gonzalez-Alvaro I, Hernandez-Garcia C. El estudio emAR.Variabilidad en el uso de recursos terap uticos emAR Estudio sobre el manejo de la Artritis Reumatoide. Barcelona TINGS Servicios de Comunicaci n. 2001:92–128. [Google Scholar]
- 33.Villaverde V, Descalzo MA, Carmona L, Bascones M, Carbonell J. Characteristics of early arthritis units that may be associated with better referral efficiency survey of SERAP units. Reumatol Clin. 2011;7:236–40. doi: 10.1016/j.reuma.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Descalzo MA, Carbonell J, Gonzalez-Alvaro I , et al. Effectiveness of a clinical practice intervention in early rheumatoid arthritis. Arthritis Care Res. 2012;64:321–30. doi: 10.1002/acr.20682. [DOI] [PubMed] [Google Scholar]
- 35.Grigor C, Capell H, Stirling A , et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 36.Allaart CF, Breedveld FC, Dijkmans BA. Treatment of recent-onset rheumatoid arthritis lessons from the BeSt study. J Rheumatol . 2007;80(Suppl. ):25–33. [PubMed] [Google Scholar]
- 37.Mottonen T, Hannonen P, Leirisalo-Repo M , et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis a randomised trial.FIN-RACo trial group. Lancet. 1999;353:1568–73. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 38.Verstappen SM, Jacobs JW, van der Veen MJ , et al. Intensive treatment with methotrexate in early rheumatoid arthritis aiming for remission.Computer Assisted Management in Early Rheumatoid Arthritis (CMERA an open-label strategy trial). Ann Rheum Dis. . 2007;66:1443–9. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF , et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Alvaro I, Hernandez-Garcia C, Villaverde Garcia V, Vargas E, Ortiz AM. Variations in the drug treatment of rheumatoid arthritis in Spain. Med Clin (Barc). 2002;118:771–6. doi: 10.1016/s0025-7753(02)72526-8. [DOI] [PubMed] [Google Scholar]
- 41.Zink A, Listing J, Ziemer S, Zeidler H. Practice variation in the treatment of rheumatoid arthritis among German rheumatologists. J Rheumatol. 2001;28:2201–8. [PubMed] [Google Scholar]
- 42.Sokka T, Envalds M, Pincus T. Treatment of rheumatoid arthritis a global perspective on the use of antirheumatic drugs. Mod Rheumatol. 2008;18:228–39. doi: 10.1007/s10165-008-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smolen JS, Aletaha D, Bijlsma JW , et al. Treating rheumatoid arthritis to target recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huizinga TW, van der Helm-van Mil A. A quantitative approach to early rheumatoid arthritis. Bull Hosp Jt Dis. 2011;69:116–21. [PubMed] [Google Scholar]
- 45.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1771–4. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 46.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schellekens GA, Visser H, de Jong BA , et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Verstappen SM, Lunt M, Bunn DK, Scott DG, Symmons DP. In patients with early inflammatory polyarthritis, ACPA positivity, younger age and inefficacy of the first non-biological DMARD are predictors for receiving biological therapy results from the Norfolk Arthritis Register. Ann Rheum Dis. 2011;70:1428–32. doi: 10.1136/ard.2010.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Alvaro I, Ortiz AM, Alvaro-Gracia JM , et al. Interleukin 15 levels in serum may predict a severe disease course in patients with early arthritis. PLoS One. 2011;6:e29492. doi: 10.1371/journal.pone.0029492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Felson DT, Anderson JJ, Meenan RF. Use of short-term efficacy/toxicity tradeoffs to select second-line drugs in rheumatoid arthritis.A metaanalysis of published clinical trials. Arthritis Rheum. 1992;35:1117–25. doi: 10.1002/art.1780351003. [DOI] [PubMed] [Google Scholar]
- 51.Laan RF, van Riel PL, van de Putte LB. Leflunomide and methotrexate. Curr Opin Rheumatol. 2001;13:159–63. doi: 10.1097/00002281-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Alarcon GS. Epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:589–604. [PubMed] [Google Scholar]
- 53.Leeb BF, Haindl PM, Maktari A, Nothnagl T, Rintelen B. Disease activity score-28 values differ considerably depending on patient's pain perception and sex. J Rheumatol. 2007;34:2382–7. [PubMed] [Google Scholar]
- 54.Ahlmen M, Svensson B, Albertsson K, Forslind K, Hafstrom I. Influence of gender on assessments of disease activity and function in early rheumatoid arthritis in relation to radiographic joint damage. Ann Rheum Dis. 2010;69:230–3. doi: 10.1136/ard.2008.102244. [DOI] [PubMed] [Google Scholar]
- 55.Castrejon I, Carmona L, Ortiz AM, Belmonte MA, Martinez-Lopez JA, Gonzalez-Alvaro I. Development and validation of a new disease activity index as a numerical sum of four variables in patients with early arthritis. Arthritis Care Res. 2013;65:518–25. doi: 10.1002/acr.21854. [DOI] [PubMed] [Google Scholar]
- 56.Pease CT, Bhakta BB, Devlin J, Emery P. Does the age of onset of rheumatoid arthritis influence phenotypeκ: a prospective study of outcome and prognostic factors. Rheumatology (Oxford). 1999;38:228–34. doi: 10.1093/rheumatology/38.3.228. [DOI] [PubMed] [Google Scholar]
- 57.Huscher D, Sengler C, Gromnica-Ihle E , et al. Clinical presentation, burden of disease and treatment in young-onset and late-onset rheumatoid arthritis a matched-pairs analysis taking age and disease duration into account. Clin Exp Rheumatol. 2013;31:256–62. [PubMed] [Google Scholar]
- 58.DeWitt EM, Lin L, Glick HA, Anstrom KJ, Schulman KA, Reed SD. Pattern and predictors of the initiation of biologic agents for the treatment of rheumatoid arthritis in the United States an analysis using a large observational data bank. Clin Ther. 2009;31:1871–80. doi: 10.1016/j.clinthera.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon WG, Abrahamowicz M, Beauchamp ME , et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis a nested case-control analysis. Ann Rheum Dis. 2012;71:1128–33. doi: 10.1136/annrheumdis-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford). 2013;52:53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 61.Winthrop KL. Infections and biologic therapy in rheumatoid arthritis our changing understanding of risk and prevention. Rheum Dis Clin North Am. 2012;38:727–45. doi: 10.1016/j.rdc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–65. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- 63.Emery P, Dorner T. Optimising treatment in rheumatoid arthritis a review of potential biological markers of response. Ann Rheum Dis. 2011;70:2063–70. doi: 10.1136/ard.2010.148015. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Alvaro I, Descalzo MA, Carmona L. Trends towards an improved disease state in rheumatoid arthritis over time influence of new therapies and changes in management approach analysis of the EMECAR cohort. Arthritis Res Ther. 2008;10:R138. doi: 10.1186/ar2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michaud K, Wallenstein G, Wolfe F. Treatment and nontreatment predictors of health assessment questionnaire disability progression in rheumatoid arthritis a longitudinal study of 18,485 patients. Arthritis Care Res. 2011;63:366–72. doi: 10.1002/acr.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Louie GH, Reveille JD, Ward MM. Challenges comparing functional limitations in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009;27(4 ) Suppl 55 :S83–91. [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson WH, Lindstrom TM, Cheung RK, Sokolove J. Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat Rev Rheumatol. 2013;9:267–76. doi: 10.1038/nrrheum.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Alvaro I, Ortiz AM, Tomero EG , et al. Baseline serum RANKL levels may serve to predict remission in rheumatoid arthritis patients treated with TNF antagonists. Ann Rheum Dis. 2007;66:1675–8. doi: 10.1136/ard.2007.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziolkowska M, Kurowska M, Radzikowska A , et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum. 2002;46:1744–53. doi: 10.1002/art.10388. [DOI] [PubMed] [Google Scholar]
- 70.Crotti TN, Smith MD, Weedon H , et al. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61:1047–54. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geusens PP, Landewe RB, Garnero P , et al. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 2006;54:1772–7. doi: 10.1002/art.21896. [DOI] [PubMed] [Google Scholar]
- 72.Cho ML, Ju JH, Kim KW , et al. Cyclosporine A inhibits IL-15-induced IL-17 production in CD4+ T cells via down-regulation of PI3K/Akt and NF-kappaB. Immunol Lett. 2007;108:88–96. doi: 10.1016/j.imlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Alvaro I, Ortiz Garcia AM, Dominguez-Jimenez C , et al. Inhibition of TNF and IL-17 production by leflunomide involves the JAK/STAT pathway. Ann Rheum Dis. 2009;68:1644–50. doi: 10.1136/ard.2008.096743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshihara K, Yamada H, Hori A, Yajima T, Kubo C, Yoshikai Y. IL-15 exacerbates collagen-induced arthritis with an enhanced CD4+ T cell response to produce IL-17. Eur J Immunol. 2007;37:2744–52. doi: 10.1002/eji.200737229. [DOI] [PubMed] [Google Scholar]
- 75.Ziolkowska M, Koc A, Luszczykiewicz G , et al. High levels of IL-17 in rheumatoid arthritis patients IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–8. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez-Alvaro I, Dominguez-Jimenez C, Ortiz AM , et al. Interleukin-15 and interferon-gamma participate in the cross-talk between natural killer and monocytic cells required for tumour necrosis factor production. Arthritis Res Ther. 2006;8:R88. doi: 10.1186/ar1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis [see comments]. Nat Med. 1997;3:189–95. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 78.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis effect of methotrexate. J Immunol. 2004;173:1463–76. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 79.Benito-Miguel M, Garcia-Carmona Y, Balsa A , et al. IL-15 Expression on RA Synovial Fibroblasts Promotes B Cell Survival. PLoS One. 2012;7:e40620. doi: 10.1371/journal.pone.0040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carroll HP, Paunovic V, Gadina M. Signalling, inflammation and arthritis Crossed signals the role of interleukin-15 and -18 in autoimmunity. Rheumatology (Oxford). 2008;47:1269–77. doi: 10.1093/rheumatology/ken257. [DOI] [PubMed] [Google Scholar]
- 81.Liu CC, Perussia B, Young JD. The emerging role of IL-15 in NK-cell development. Immunol Today. 2000;21:113–6. doi: 10.1016/s0167-5699(99)01581-9. [DOI] [PubMed] [Google Scholar]
- 82.Miranda-Carus ME, Benito-Miguel M, Balsa A , et al. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54:1151–64. doi: 10.1002/art.21731. [DOI] [PubMed] [Google Scholar]
- 83.Ogata Y, Kukita A, Kukita T , et al. A novel role of IL-15 in the development of osteoclasts inability to replace its activity with IL-2. J Immunol. 1999;162:2754–60. [PubMed] [Google Scholar]
- 84.Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Mechanisms of Disease the link between RANKL and arthritic bone disease. Nat Clin Prac Rheumatol. 2005;1:47–54. doi: 10.1038/ncprheum0036. [DOI] [PubMed] [Google Scholar]
- 85.Knevel R, Krabben A, Brouwer E , et al. Genetic variants in IL15 associate with progression of joint destruction in rheumatoid arthritis a multicohort study. Ann Rheum Dis. 2012;71:1651–7. doi: 10.1136/annrheumdis-2011-200724. [DOI] [PubMed] [Google Scholar]
- 86.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis a role for IL-15 in development of antigen- induced immunopathology. J Immunol. 1998;160:5654–60. [PubMed] [Google Scholar]
- 87.Baslund B, Tvede N, Danneskiold-Samsoe B , et al. Targeting interleukin-15 in patients with rheumatoid arthritis A proof-of-concept study. Arthritis Rheum. 2005;52:2686–92. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- 88.Lamana A, Ortiz AM, Alvaro-Gracia JM , et al. Characterization of serum interleukin-15 in healthy volunteers and patients with early arthritis to assess its potential use as a biomarker. Eur Cytokine Netw. 2010;21:186–94. doi: 10.1684/ecn.2010.0203. [DOI] [PubMed] [Google Scholar]
- 89.Abad C, Gomariz RP, Waschek JA. Neuropeptide mimetics and antagonists in the treatment of inflammatory disease focus on VIP and PACAP. Curr Top Med Chem. 2006;6:151–63. doi: 10.2174/156802606775270288. [DOI] [PubMed] [Google Scholar]
- 90.Gomariz RP, Juarranz Y, Abad C, Arranz A, Leceta J, Martinez C. VIP-PACAP system in immunity new insights for multitarget therapy. Ann N Y Acad Sci. 2006;1070:51–74. doi: 10.1196/annals.1317.031. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Rey E, Ganea D, Delgado M. Neuropeptides keeping the balance between pathogen immunity and immune tolerance. Curr Opin Pharmacol. 2010;10:473–81. doi: 10.1016/j.coph.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomariz RP, Gutierrez-Canas I, Arranz A , et al. Peptides targeting Toll-like receptor signalling pathways for novel immune therapeutics. Curr Pharm Des. 2010;16:1063–80. doi: 10.2174/138161210790963841. [DOI] [PubMed] [Google Scholar]
- 93.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–8. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 94.Juarranz Y, Abad C, Martinez C , et al. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1034–45. doi: 10.1186/ar1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leceta J, Gomariz RP, Martinez C, Carrion M, Arranz A, Juarranz Y. Vasoactive intestinal peptide regulates Th17 function in autoimmune inflammation. Neuroimmunomodulation. 2007;14:134–8. doi: 10.1159/000110636. [DOI] [PubMed] [Google Scholar]
- 96.Gutierrez-Canas I, Juarranz Y, Santiago B , et al. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology (Oxford). 2006;45:527–32. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- 97.Gutierrez-Canas I, Juarranz Y, Santiago B , et al. Immunoregulatory properties of vasoactive intestinal peptide in human T cell subsets implications for rheumatoid arthritis. Brain Behav Immun. 2008;22:312–7. doi: 10.1016/j.bbi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Carrion M, Juarranz Y, Perez-Garcia S , et al. RNA sensors in human osteoarthritis and rheumatoid arthritis synovial fibroblasts immune regulation by vasoactive intestinal peptide. Arthritis Rheum. 2011;63:1626–36. doi: 10.1002/art.30294. [DOI] [PubMed] [Google Scholar]
- 99.Perez-Garcia S, Juarranz Y, Carrion M , et al. Mapping the CRF-urocortins system in human osteoarthritic and rheumatoid synovial fibroblasts effect of vasoactive intestinal peptide. J Cell Physiol. 2011;226:3261–9. doi: 10.1002/jcp.22687. [DOI] [PubMed] [Google Scholar]
- 100.Carrion M, Juarranz Y, Martinez C , et al. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford). 2013;52:2177–86. doi: 10.1093/rheumatology/ket315. [DOI] [PubMed] [Google Scholar]
- 101.Juarranz Y, Gutierrez-Canas I, Santiago B, Carrion M, Pablos JL, Gomariz RP. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–95. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- 102.Jiang W, Gao SG, Chen XG , et al. Expression of synovial fluid and articular cartilage VIP in human osteoarthritic knee a new indicator of disease severityκ. Clin Biochem. 2012;45:1607–12. doi: 10.1016/j.clinbiochem.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 103.El-Sayed ZA, Mostafa GA, Aly GS, El-Shahed GS, El-Aziz MM, El-Emam SM. Cardiovascular autonomic function assessed by autonomic function tests and serum autonomic neuropeptides in Egyptian children and adolescents with rheumatic diseases. Rheumatology (Oxford). 2009;48:843–8. doi: 10.1093/rheumatology/kep134. [DOI] [PubMed] [Google Scholar]
- 104.Martinez C, Ortiz AM, Juarranz Y , et al. Serum levels of vasoactive intestinal Peptide as a prognostic marker in early arthritis. PLoS One. 2014;9:e85248. doi: 10.1371/journal.pone.0085248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lamana A, Balsa A, Rueda B , et al. The TT Genotype of the STAT4 rs7574865 Polymorphism Is Associated with High Disease Activity and Disability in Patients with Early Arthritis. PLoS One. 2012;7:e43661. doi: 10.1371/journal.pone.0043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune diseaseκ. FEBS Lett. 2011;585:3689–98. doi: 10.1016/j.febslet.2011.04.032. [DOI] [PubMed] [Google Scholar]