Abstract
Background
Fibroids are the most common benign tumors in women. One-third of all women of reproductive age undergo treatment for symptomatic fibroids. In recent years, the spectrum of available treatments has been widened by the introduction of new drugs and interventional procedures.
Methods
Selective literature review on the treatment of uterine fibroids, including consideration of several Cochrane Reviews.
Results
Fibroids can be treated with drugs, interventional procedures (uterine artery embolization [UAE] and focused ultrasound treatment [FUS]), and surgery. The evidence regarding the various available treatments is mixed. All methods improve symptoms, but only a few comparative studies have been performed. A meta-analysis revealed that recovery within 15 days is more common after laparoscopic enucleation than after open surgery (odds ratio [OR], 3.2). A minimally invasive hysterectomy, or one performed by the vaginal route, is associated with a shorter hospital stay and a more rapid recovery than open transabdominal hysterectomy. UAE is an alternative to hysterectomy for selected patients. The re-intervention rates after fibroid enucleation, hysterectomy, and UAE are 8.9–9%, 1.8–10.7%, and 7–34.6%, respectively. The main drugs used to treat fibroids are gonadotropin-releasing hormone analogs and selective progesterone receptor modulators.
Conclusion
Multiple treatment options are available and enable individualized therapy for symptomatic fibroids. The most important considerations in the choice of treatment are the question of family planning and, in some cases, the technical limitations of the treatments themselves.
Uterine fibroids are the most common benign tumors in women; 80% to 90% of women have them. Fibroids become clinically relevant in about 25% to 30% of all women aged between 30 and 50 years (e1). Apart from the ovarian sexual steroids estrogen and progesterone, the development and growth of fibroids are influenced by a variety of other factors: genetic changes in the myometrium, growth factors, cytokines, and the extracellular matrix (1). An increasing number of somatic mutations have been found, especially in association with increasing fibroid size (e2).
Symptoms associated with fibroids
The symptoms and the form and extent of complaints in the individual case depend on the location, number, and size of the fibroids (Figure 1). Complaints are often subjective and are perceived differently by different individuals. Some patients do not report any complaints.
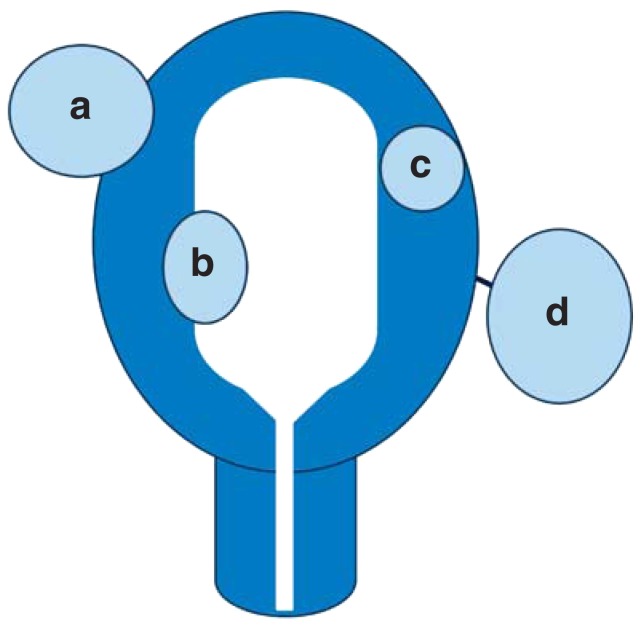
Figure 1.
Fibroid locations (schematic):
a) subserosal fibroid,
b) submucosal fibroid,
c) intramural fibroid,
d) pedunculated fibroid
Submucosal and intracavitary fibroids impair the endometrium or its function, impair the contractility of the uterus, and give rise to mainly menstrual disorders in the form of severe (hypermenorrhea) and prolonged bleeding (menorrhagia) which can even result in anemia (Figures 1, 2). In an international study of 21 500 women, just under 60% of women with fibroids complained of hypermenorrhea, whereas the prevalence of hypermenorrhea in women without fibroids was 37.4% (2). Painful bleeding (dysmenorrhea) can also be associated with fibroids (e3).
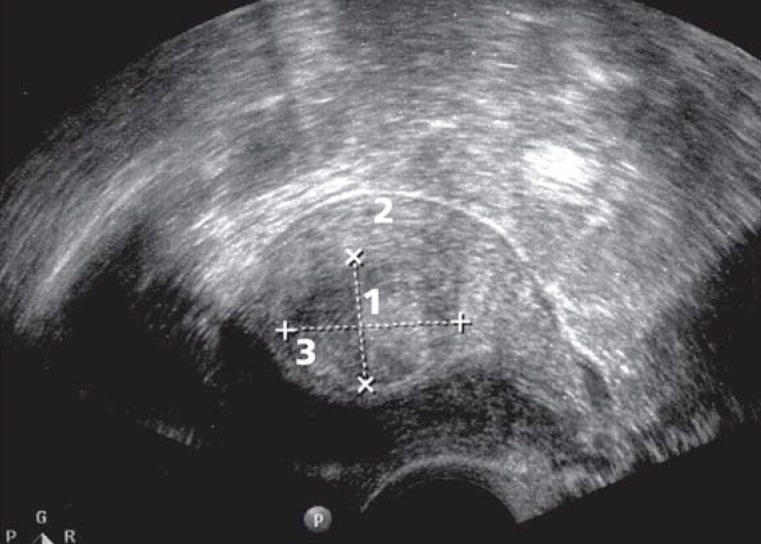
Figure 2.
Ultrasonography:
1 Submucosal posterior wall fibroid distorting the endometrial cavity
2 Myometrium
3 Endometrium (uterine cavity)
Subserosal and pedunculated fibroids may become clinically manifest through pressure symptoms or a disturbing foreign-body sensation, with negative effects on sexual intercourse, micturition, or bowel movements (e.g., dyspareunia, pollakisuria, and constipation). The occurrence of the symptoms described correlates significantly with the size of the fibroids (e3).
Fertility and pregnancy
The general question of whether fibroids can impair fertility is a subject of debate. Among women who undergo fertility treatment, fibroids are the only identifiable cause in 1% to 2.4% (3).
In the absence of randomized studies, it has not so far been possible to confirm that enucleation of small fibroids leads to a general improvement in fertility (4). Women with submucosal fibroids have a clearly increased rate of spontaneous abortion, and this rate can be significantly reduced by operative resection of the fibroids, as was shown in a nonrandomized study (e4). One retrospective study found that intramural fibroids reduced the birth rate and prolonged the time to conception significatly (e5). Women with multiple fibroids or fibroids larger than 5 cm can show the following pathologies during pregnancy (5, 6):
Increased rate of spontaneous abortion
Anomalous fetal presentation
Higher rate of cesarean section and of postpartum hemorrhage.
Diagnosis
Transvaginal ultrasonography (US), combined if necessary with abdominal US, is the gold standard for diagnosing uterine fibroids (e6) (Figure 2). Sonographic screening for fibroids in asymptomatic patients does not seem worthwhile and is not reimbursed by the statutory health insurance companies (GKV, gesetzliche Krankenkasse) in Germany. Where ultrasound conditions are very poor, magnetic resonance imaging (MRI) may be necessary to obtain precise information about the number, size, location, and perfusion of the fibroids.
Leiomyosarcoma (incidence 0.2%) cannot be diagnosed preoperatively by any imaging technique, and cannot be distinguished with certainty from a benign fibroid (e6). In everyday clinical routine, the typical clinical and sonographic picture of a benign leiomyoma (fibroid) is assumed to be just that. Highly differentiated leiomyosarcomas differ histomorphologically from fibroids only in showing a higher number of mitoses, while all other microscopic features are the same; other sarcoma entities, by contrast, show additional morphological changes.
Therapeutic options
In women with symptomatic fibroids, drug therapy, surgical therapy—now as minimally invasive operations—whether organ-preserving or in the form of hysterectomy, and other interventional techniques such as uterine artery embolization (UAE) and fibroid treatment using highly focused ultrasound (FUS) can be offered. As a general rule, women with fibroids should only receive specific treatment when the fibroids are causing complaints (i.e., specific symptoms), or they wish to retain their fertility or are planning to conceive, and have multiple fibroids or a fibroid that is larger than 5 cm in diameter (5– 7).
The evidence base relating to these various therapies is heterogeneous. In particular, there is not enough evidence from randomized controlled trials (RCTs) to form a judgment about whether fertility is improved after enucleation of fibroids (4). By contrast, alleviation of symptoms and improvement in quality of life after medical and surgical treatment and UAE are very well supported by evidence from randomized studies and meta-analyses (8– 10).
Medical therapy
In premenopausal women who have menstrual disorders without having fibroids, oral progesterones and progesterone-releasing intrauterine pessaries (IUP) are successfully used as first-line treatment, but in women with fibroids these therapeutic options are of only limited efficacy (11). Neither progesterone nor mifepristone (a progesterone receptor antagonist) leads to any significant reduction in fibroid volume. However, mifepristone did reduce fibroid-related hypermenorrhea (12). There are no randomized studies for the drug danazol, which ceased to be licensed in Germany in 2005 (13). Despite the existence of large randomized studies, it has not yet been possible to evaluate herbal preparations targeted specifically at fibroid symptom relief (14). Although a direct comparison of letrozole (an aromatase inhibitor) and gonadotropin-releasing hormone analogs (GnRH analogs) showed fibroid volume reduction by 46% after letrozole treatment, no effect on symptoms was seen, and the lack of blinding must be seen as a further limitation in the studies (15). Unwanted effects included dizziness and hot flushes, and also, after long-term ingestion, loss of bone density (e7, 16).
Hence, two drug classes are basically available to treat uterine fibroids: GnRH analogs and selective progesterone receptor modulators (SPRMs). The primary indication for drug therapy is pretreatment before surgery. There are no studies showing improved pregnancy or birth rates.
Although pretreatment with GnRH analogs leads to a reduction in fibroid size and in symptoms, neither improved resectability nor reduced operative time has been demonstrated (17, e8). The main disadvantage of treatment with GnRH analogs is the suppression of ovarian steroid hormone production, and the strong vasomotor symptoms triggered as a consequence (8); and also, with prolonged hypoestrogenemia, the associated loss of bone density. For this reason, the use of GnRH analogs is usually limited to 3 to 6 months.
Since February 2012, the SPRM ulipristal acetate has been licensed for pretreatment before scheduled surgery. The main advantage of ulipristal acetate over GnRH analogs (leuprorelin acetate) is the lower incidence of unwanted effects (8, 18). Particularly worth noting is its rapid effect on disordered menstruation (bleeding ceased within a week in over 90% of cases) compared with leuprorelin acetate (8). The direct action of ulipristal acetate on the endometrium leads to reversible benign histological changes (progesterone receptor modulator–associated endometrial changes) (18).
GnRH analogs are not suitable for long-term treatment of fibroids because the fibroid shrinkage reverses after treatment stops, and within a short time the fibroids have returned to their original size (e9, e10). In the PEARL-III study (open label—all enrolled patients received ulipristal acetate, there was no control group), the mean volume reduction of the three largest fibroids after 3 months was 59.8% (range, 21.0–72.2%) (19). The main unwanted effects were hot flushes, which were reported less frequently the longer the drug continued to be taken.
Minimally invasive surgery—organ-preserving
A recent review and a meta-analysis of 6 RCTs on convalescence, blood loss, postoperative pain, and general complication rate concluded that in terms of these factors, laparoscopic surgery is advantageous; pregnancy rates and recurrence rates were the same (4, 20, e11). The probability of complete recovery after 15 days was markedly higher after a laparoscopic procedure than after open surgery (odds ratio [OR] 3.2 [1.3–8.2]) (20). The largest diameter of fibroid accessible to laparoscopic enucleation is 10–12 cm (e12). The reintervention rate is around 9%.
Possible indications for fibroid resection or enucleation are the wish to retain fertility or the uterus. Depending on the location of the fibroids, they may be removed hysteroscopically (intracavitary and submucosal fibroids, Figure 3) or laparoscopically (intramural and transmural fibroids, Figure 4). Unlike after hysteroscopic resection, after laparoscopic removal of fibroids the myometrium has to be closed again, thus restoring the continuity of the muscular uterus.
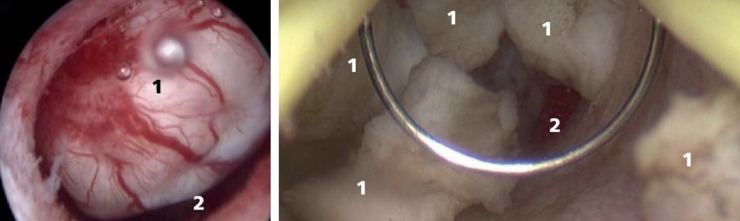
Figure 3.
Intracavitary fibroid before hysteroscopic resection (left), hysteroscopic enucleation of a fibroid (right). (1, Fibroid and fibroid fragments; 2, uterine cavity)
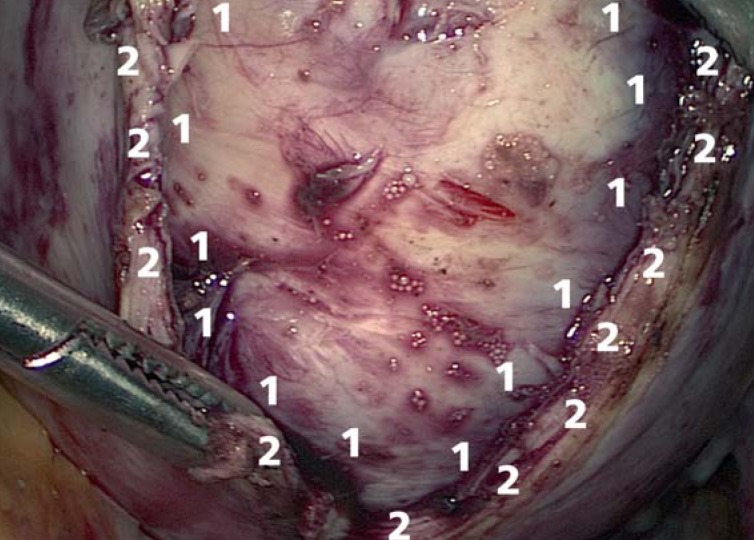
Figure 4.
Incision of the myometrium for laparoscopic enucleation of a fibroid
1 Fibroid
2 Myometrium
After successful resection or enucleation of fibroids, there is no general recommendation for delivery by cesarean section (21). In our experience, the extent of the uterine wound and the reconstruction required should be stated in the operative report, so that the obstetrician has the necessary information to hand when advising the patient about attempting vaginal delivery or planning primary cesarean section. The risk of rupture of a uterus that has not previously been operated on and is free of fibroids is estimated at about 1 in 17 000 pregnancies (e13). The estimated risk of uterine rupture after fibroid enucleation varies widely in the literature, between 1% and 10% (22, 23, e14, e15).
Minimally invasive surgery—hysterectomy
Total laparoscopic hysterectomy (TLH) and laparoscopic supracervical (subtotal) hysterectomy (LASH—which requires a normal cervical carcinoma screening result) are both suitable methods (after full information and counseling of the patient) for treatment of symptomatic uterine fibroids or fibroids in women who do not wish to preserve their fertility. Once the uterus has been mobilized and detached from the vagina, it is extracted through the vagina. In the treatment of very large fibroids, or in LASH procedures, an electric morcellator is also employed. Because of the theoretical risk of tumor seeding if an occult sarcoma or endometrial carcinoma should prove to be present, the patient must be given adequate information about electric morcellation (FDA Warning Letter of 17 April 2014). We believe that, in centers with enough experience in laparoscopic hysterectomy, conversion to open hysterectomy is rare. In a study of over 500 patients, there was only one case in which laparotomy became necessary after TLH had begun (24). The advantages of LASH consist in the 1.7% complication rate (25, 26), although in 3.7% of patients secondary removal of the cervical stump is required because of persistent complaints, bleeding, or histological abnormalities (26). Another minimally invasive technique is the classical vaginal hysterectomy, as this also has advantages such as low complication rate, short hospital stay, and speedy return of the patient to normal activities (27). Basically, all techniques for hysterectomy (reintervention rate 1.8% to 10.7%) in patients with menstrual disorders and/or symptomatic uterine fibroids lead to a marked improvement in quality of life (27). No studies have yet shown that preservation of the cervix confers any postoperative advantages compared to the other hysterectomy techniques (25).
Uterine artery embolization
UAE as a uterine-preserving form of treatment for symptomatic uterine fibroids has been available to women since 1989 (e16). Embolization of the uterine artery is a possible alternative to hysterectomy, especially in women with
multiple fibroids,
very large fibroids,
restricted operability, or
a history of multiple operative procedures in the abdomen (e17).
Fibroid embolization is a treatment procedure supported by evidence from randomized studies (9, 10, e18– e20). In Germany a national consensus has been attained between the various scientific medical societies and is continually updated (28). Embolization of the uterine artery is associated with low blood loss, short procedural times, and a short hospital stay (29). This technique is associated with a higher risk of unwanted effects and a higher reintervention rate (7% to 34.6%) (29). Specific risks include complete amenorrhea in 3.9% of cases (30). Data suggest that a risk of subclinical deterioration of ovarian function exists, particularly in women over 45 years of age (e21). Reintervention or secondary hysterectomy rates of 26.4% to 34.6% during a 5-year follow-up period have been reported (31).
In terms of the number and size of fibroids, there is no restriction for UAE. Fibroid size reduces permanently by about 50% (0.2% to 89.1%) (e22) and patients’ symptoms improve markedly (32). UAE is not a suitable method of choice in the treatment of women who wish to preserve their fertility.
MR-guided focused ultrasound
Because of the apparatus required, this technique is not available everywhere in Germany, and is in fact only offered at a few centers. No randomized long-term studies have yet been performed. This relatively new option for the treatment of uterine fibroids combines two familiar technologies. Magnetic resonance imaging (MRI) is used for treatment planning and synchronous treatment monitoring. Ultrasound is used for punctal heating of the fibroid to 60 to 80 °C, which leads to necrosis of the treated area of tissue and hence to a reduction in fibroid size. Compared to the baseline situation, patients reported an average improvement in symptoms of 40% (after 6 months) using a symptom severity score validated for fibroids (33).
Whether treatment with focused ultrasound is possible in a given case depends on various factors. Its applicability and success rate are restricted by factors such as perfusion of the fibroid, its size and location, and bowel loops in the ultrasound field (34). Large fibroid size is not in itself a contraindication. Absolute contraindications are ongoing pregnancy and all contraindications to MRI. Reported complication rates after focused ultrasound treatment vary greatly, between 1.9% and 39% (33, 34). Possible complications are skin burns, pain, nausea, and allergic reactions (34). Unlike for UAE, increased rates of spontaneous abortion and of placental disorders have not been reported (e23).
Summary
Patients with symptomatic uterine fibroids can be offered a number of different treatment options. The choice of therapeutic technique depends first and foremost on whether the patient still wishes to bear children. For those who do, or who wish to retain their uterus, the methods of choice are hysteroscopic myomectomy for intracavitary and submucosal fibroids and laparoscopic enucleation for intramural or transmural fibroids—although it must be mentioned that the evidence that fibroid enucleation carries any advantage for women who wish to preserve their fertility is thin (e4). The results of a few small studies show at most that hysteroscopic fibroid resection or removal of intermural fibroids seems to have some advantage, but there are no large RCTs on this topic (5, e5). Possible complications of pregnancy can be reduced by operative removal of multiple fibroids or those that are larger than 5 cm before the pregnancy is started (5, 6). Drug therapy with ulipristal acetate can be beneficial before the surgical removal of very large fibroids. At present it is too early to judge whether drug therapy alone is sufficient, but it does at least appear to lead to alleviation of symptoms and shrinking of the fibroids (8, 18).
One possible alternative to surgery for women with intramural fibroids who wish to preserve their fertility is to use focused ultrasound. Data relating to successful full-term pregnancies after focused ultrasound treatment are limited, but those from the first available case reports appear to be similar to data about the surgical options (e23). For women who do not wish to preserve their fertility, the minimally invasive hysterectomy techniques and uterine artery embolization are the treatments of choice. However, hysterectomy rates vary between countries (Table 1) (35). Drug therapy alone without further treatment will rarely be sufficient, and then only in perimenopausal women until they reach menopause. Most data on complication rates and postoperative satisfaction relate to the hysterectomy techniques. The published data on short-term complications after UAE vary widely. Complications are often very differently defined (e.g., pain and postinterventional raised temperature defined as complications). Published data on short- and medium-term complications of the various interventional techniques range from <10% to >70% (33). A more valid parameter is the reintervention rate, which is lowest after fibroid enucleation and hysterectomy and highest after UAE. The reintervention rate after supracervical hysterectomy is 3.7% due to secondary removal of the cervix, and it is lowest (1.8%) after complete hysterectomy (26). The various therapies are compared in Tables 2 and 3.
Table 1. Hysterectomy rates in women with benign disease (per 1000 person-years) (35).
| Country | Rate (%) |
|---|---|
| Germany | 3.6 |
| Sweden | 2.1 |
| USA | 4.9 |
| Australia | 5.4 |
Table 2. Comparison of different forms of interventional treatment.
| Hysterectomy (incl. supracervical) |
Fibroid enucleation |
Uterine artery embolization |
Focused ultrasound |
|
|---|---|---|---|---|
| Evidence base | Randomized controlled trials | Controlled studies | Randomized controlled trials | Controlled studies |
| Hospital stay | 2–5 days | 0–3 days | 1 day | No |
| Histological confirmation | Yes | Yes | No | No |
| Fertility preserved | No | Yes | Potentially not | Yes |
| Depends on number and size of fibroids | No | Yes | No | Yes |
| Reintervention rate (Reference) | 1.8–10.7% (26. 31) | 8.9–9%*1 (39. 40) | 7–34.6% (34. 36. 37) | n. d.*2 (38) |
| Advantages | Patient satisfaction low complication rate |
Fertility preserved | No general anesthesia | Outpatient procedure. no general anesthesia |
*1Lower rate possible if followed by delivery; *2 retrospective study. no long-term data
Table 3. Comparison of different drug therapies.
| Ulipristal acetate (8. 18. 19) |
Leuprolide acetate (8) |
Mifepristone (12) |
Herbal preparations (14) |
Gestagens (11) |
|
|---|---|---|---|---|---|
| Drug group | Selective progesterone receptor modulator | GnRH analogs | Progesterone receptor antagonist | Progesterone receptor agonist | |
| Evidence base | 3 RCTs | 1 RCT | Metaanalysis (3 RCTs) |
Metaanalysis (21 RCTs) |
Metaanalysis (3 RCTs) |
| Number of study participants | 756 | 307 | 112 | 2222 | 104 |
| Remarks on studies | High quality of studies; 1 RCT open-label |
High-quality study |
Small number of participants | Poor study quality |
Small number of participants |
| Fibroid volume reduction | 36–59.8% after 3 months |
53% after 3 months |
No | Yes | Marginal |
| Improvement of symptoms | 90–98% of patients |
89% of patients |
Yes | n.d. | Yes. especially with intrauterine administration |
| Improvement of fertility | n.d. | n.d. | n.d. | n.d. | n.d. |
| Most frequent unwanted drug effects | Vasomotor complaints |
Vasomotor complaints |
Uterine cramps |
n.d. | Headache, abdominal pain |
RCT. randomized controlled trial; GnRH. gonadotropin-releasing hormone; n.d.. no data
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Römer has received consultancy fees, reimbursement of conference fees and travel and accommodation expenses, and fees for preparing scientific educational events from Gedeon Richter.
Dr. Boosz, Prof. Reimer, Dr. Matzko, and Prof. Müller declare that no conflict of interest exists.
References
- 1.Ciavattini A, Di Giuseppe J, Stortoni P, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int. 2013:173–184. doi: 10.1155/2013/173184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12 doi: 10.1186/1472-6874-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002;17:1424–1430. doi: 10.1093/humrep/17.6.1424. [DOI] [PubMed] [Google Scholar]
- 4.Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev. 2012 Nov 14; doi: 10.1002/14651858.CD003857.pub3. CD003857. [DOI] [PubMed] [Google Scholar]
- 5.Ciavattini A, Clemente N, Delli Carpini G, Di Giuseppe J, Giannubilo SR, Tranquilli AL. Number and size of uterine fibroids and obstetric outcomes. J Matern Fetal Neonatal Med. 2014;5:1–5. doi: 10.3109/14767058.2014.921675. [DOI] [PubMed] [Google Scholar]
- 6.Shavell V, Thakur M, Sawant A, et al. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertil Steril. 2012;97:107–110. doi: 10.1016/j.fertnstert.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332–338. doi: 10.1097/GCO.0b013e3283630e10. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Tomaszewski J, Vázquez F, et al. PEARL II Study Group. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 9.Manyonda IT, Bratby M, Horst JS, Banu N, Gorti M, Belli AM. Uterine artery embolization versus myomectomy: impact on quality of life-results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovasc Intervent Radiol. 2012;35:530–536. doi: 10.1007/s00270-011-0228-5. [DOI] [PubMed] [Google Scholar]
- 10.van der Kooij SM, Hehenkamp WJ, Birnie E, et al. The effect of treatment preference and treatment allocation on patients’ health-related quality of life in the randomized EMMY trial. Eur J Obstet Gynecol Reprod Biol. 2013;169:69–74. doi: 10.1016/j.ejogrb.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BW. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Syst Rev. 2013 Feb 28; doi: 10.1002/14651858.CD008994.pub2. CD008994. [DOI] [PubMed] [Google Scholar]
- 12.Tristan M, Orozco LJ, Steed A, Ramírez-Morera A, Stone P. Mifepristone for uterine fibroids. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD007687.pub2. CD007687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ke LQ, Yang K, Li J, Li CM. Danazol for uterine fibroids. Cochrane Database Syst Rev. 2009;3 doi: 10.1002/14651858.CD007692.pub2. CD007692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JP, Yang H, Xia Y, Cardini F. Herbal preparations for uterine fibroids. Cochrane Database Syst Rev. 2013;4 doi: 10.1002/14651858.CD005292.pub3. CD005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song H, Lu D, Navaratnam K, Shi G. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev. 2013;10 doi: 10.1002/14651858.CD009505.pub2. CD009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhan N, Madaan S, Sen J. Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. Eur J Obstet Gynecol Reprod Biol. 2013;171:329–332. doi: 10.1016/j.ejogrb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Mavrelos D, Ben-Nagi J, Davies A, Lee C, Salim R, Jurkovic D. The value of pre-operative treatment with GnRH analogues in women with submucous fibroids: a double-blind, placebo-controlled randomized trial. Hum Reprod. 2010;25:2264–2269. doi: 10.1093/humrep/deq188. [DOI] [PubMed] [Google Scholar]
- 18.Donnez J, Tatarchuk TF, Bouchard P, et al. PEARL I Study Group: Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 19.Donnez J, Vázquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101:1565–1573. doi: 10.1016/j.fertnstert.2014.02.008. e1-18. [DOI] [PubMed] [Google Scholar]
- 20.Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14–21. doi: 10.1016/j.ejogrb.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Sentilhes L, Vayssière C, Beucher G, et al. Delivery for women with a previous cesarean: guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF) Eur J Obstet Gynecol Reprod Biol. 2013;170:25–32. doi: 10.1016/j.ejogrb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Uhm YK, Kim JY, Jee BC, Kim YB. Obstetric outcomes after uterine myomectomy: Laparoscopic versus laparotomic approach. Obstet Gynecol Sci. 2013;56:375–381. doi: 10.5468/ogs.2013.56.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardi TS, Radosa MP, Weisheit A, et al. Laparoscopic myomectomy: a 6-year follow-up single-center cohort analysis of fertility and obstetric outcome measures. Arch Gynecol Obstet. 2014;290:87–91. doi: 10.1007/s00404-014-3155-2. [DOI] [PubMed] [Google Scholar]
- 24.Mueller A, Boosz A, Koch M, et al. The Hohl instrument for optimizing total laparoscopic hysterectomy: results of more than 500 procedures in a university training center. Arch Gynecol Obstet. 2012;285:123–127. doi: 10.1007/s00404-011-1905-y. [DOI] [PubMed] [Google Scholar]
- 25.Lethaby A, Mukhopadhyay A, Naik R. Total versus subtotal hysterectomy for benign gynaecological conditions. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD004993.pub3. CD004993. [DOI] [PubMed] [Google Scholar]
- 26.Boosz A, Lermann J, Mehlhorn G, et al. Comparison of re-operation rates and complication rates after total laparoscopic hysterectomy (TLH) and laparoscopy-assisted supracervical hysterectomy (LASH) Eur J Obstet Gynecol Reprod Biol. 2011;158:269–273. doi: 10.1016/j.ejogrb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD003677.pub4. CD003677. [DOI] [PubMed] [Google Scholar]
- 28.Kröncke TJ, David M. Uterusarterienembolisation (UAE) zur Myombehandlung. RöFo. 2013;185:461–463. doi: 10.1055/s-0033-1335339. [DOI] [PubMed] [Google Scholar]
- 29.Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5 doi: 10.1002/14651858.CD005073.pub3. CD005073. [DOI] [PubMed] [Google Scholar]
- 30.Toor SS, Jaberi A, Macdonald DB. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR 2012. 199:1153–1163. doi: 10.2214/AJR.11.8362. [DOI] [PubMed] [Google Scholar]
- 31.van der Kooij SM, Shandra Bipat MD, et al. Uterine artery embolization versus surgery in the treatment of symptomatic fibroids: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205 doi: 10.1016/j.ajog.2011.03.016. e1-18. [DOI] [PubMed] [Google Scholar]
- 32.Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: Long-term results. Eur J Radiol. 2013;82:2265–2269. doi: 10.1016/j.ejrad.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Kamp JE, David M, Scheurig-Muenkler C, Hengst S, Beck A. Clinical outcome of magnetic-resonance-guided focused ultrasound surgery (MRgFUS) in the treatment of symptomatic uterine fibroids. Rofo. 2013;185:136–143. doi: 10.1055/s-0032-1325512. [DOI] [PubMed] [Google Scholar]
- 34.Trumm CG, Stahl R, Clevert DA, et al. Magnetic resonance imaging-guided focused ultrasound treatment of symptomatic uterine fibroids: Impact of technology advancement on ablation volumes in 115 patients. Invest Radiol. 2013;48:359–365. doi: 10.1097/RLI.0b013e3182806904. [DOI] [PubMed] [Google Scholar]
- 35.Stang A, Merrill RM, Kuss O. Hysterectomy in Germany: a DRG-based nationwide analysis. Dtsch Arztebl Int 2011. 2005-2006;108:508–514. doi: 10.3238/arztebl.2011.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spies JB, Bruno J, Czeyda-Pommersheim F, Magee ST, Ascher SA, Jha RC. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106:933–939. doi: 10.1097/01.AOG.0000182582.64088.84. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin SC, Spies JB, Worthington-Kirsch R, et al. Uterine artery embolization frp treatment of leiomyoma: long-term outcomes from FIBROID registry. Obstet & Gynecol. 2008;111:22–32. doi: 10.1097/01.AOG.0000296526.71749.c9. [DOI] [PubMed] [Google Scholar]
- 38.Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet & Gynecol. 2007;110:279–287. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 39.Saccardi C, Conte L, Fabris A, et al. Hysteroscopic Enucleation in toto of submucous type 2 myomas: Long-term follow-up in women affected by menorrhagia. J Minim Invasive Gynecol. 2014;21:426–430. doi: 10.1016/j.jmig.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105:877–881. doi: 10.1097/01.AOG.0000156298.74317.62. [DOI] [PubMed] [Google Scholar]
- e1.Robboy SJ, Bentley RC, Butnor K, Anderson MC. Pathology and pathophysiology of uterine smooth-muscle tumors. Environ Health Perspect. 2000;108:779–784. doi: 10.1289/ehp.00108s5779. [DOI] [PubMed] [Google Scholar]
- e2.Rein MS, Powell WL, Walters FC, et al. Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod. 1998;4:83–86. doi: 10.1093/molehr/4.1.83. [DOI] [PubMed] [Google Scholar]
- e3.David M, Krätschell R. Uterusmyome: Korrelation von Befundkenntnisstand und Beschwerden. Frauenarzt. 2013;54:119–123. [Google Scholar]
- e4.Shokeir TA. Hysteroscopic management in submucous fibroids to improve fertility. Arch Gynecol Obstet. 2005;273:50–54. doi: 10.1007/s00404-005-0729-z. [DOI] [PubMed] [Google Scholar]
- e5.Bernard G, Darai E, Poncelet C, Benifla JL, Madelenat P. Fertility after hysteroscopic myomectomy: effect of intramural myomas associated. Eur J Obstet Gynecol Reprod Biol. 2000;88:85–90. doi: 10.1016/s0301-2115(99)00123-2. [DOI] [PubMed] [Google Scholar]
- e6.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- e7.Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93:192–198. doi: 10.1016/j.fertnstert.2008.09.064. [DOI] [PubMed] [Google Scholar]
- e8.Campo S, Garcea N. Laparoscopic myomectomie in premenopausal women with and without preoperative treatment using gonadotropin-releasing hormone analogues. Human Repord. 1999;14:44–48. doi: 10.1093/humrep/14.1.44. [DOI] [PubMed] [Google Scholar]
- e9.Felberbaum RE, Germer U, Ludwig M, et al. Treatment of uterine fibroids with a slow-release formulation of the gonadotrophin releasing hormone antagonist Cetrorelix. Hum Reprod. 1998;13:1660–1668. doi: 10.1093/humrep/13.6.1660. [DOI] [PubMed] [Google Scholar]
- e10.Kettel LM, Murphy AA, Morales AJ, Rivier J, Vale W, Yen SS. Rapid regression of uterine leiomyomas in response to daily administration of gonadotropin-releasing hormone antagonist. Fertil Steril. 1993;60:642–646. doi: 10.1016/s0015-0282(16)56214-1. [DOI] [PubMed] [Google Scholar]
- e11.Falcone T, Parker WH. Surgical management of leiomyomas for fertility or uterine preservation. Obstet Gynecol. 2013;121:856–868. doi: 10.1097/AOG.0b013e3182888478. [DOI] [PubMed] [Google Scholar]
- e12.Ribeiro SC, Reich H, Rosenberg J, Guglielminetti E, Vidali A. Laparoscopic myomectomy and pregnancy outcome in infertile patients. Fertil Steril. 1999;71:571–574. doi: 10.1016/s0015-0282(98)00483-x. [DOI] [PubMed] [Google Scholar]
- e13.Miller DA, Goodwin TM, Gherman RB, Paul RH. Intrapartum rupture of the unscarred uterus. Obstet Gynecol. 1997;89:671–673. doi: 10.1016/s0029-7844(97)00073-2. [DOI] [PubMed] [Google Scholar]
- e14.Stotland NE, Lipschitz LS, Caughey AS. Delivery strategies for women with a previous classic cesarean delivery: adecision analysis. Am J Obstet Gynecol. 2002;187:1203–1208. doi: 10.1067/mob.2002.127123. [DOI] [PubMed] [Google Scholar]
- e15.Fukuda M, Tanaka T, Kamada M, et al. Comparison of the perinatal outcomes after laparoscopic myomectomy versus abdominal myomectomy. Gynecol Obstet Invest. 2013;76:203–208. doi: 10.1159/000355098. [DOI] [PubMed] [Google Scholar]
- e16.De Wilde R, Hucke J. Brauchen wir die Uterusarterien-Embolisation? Frauenarzt. 2006;47 [Google Scholar]
- e17.Kröncke TJ, David M, Ricke J, et al. Uterusarterien-Embolisation zur Myombehandlung. Frauenarzt. 2006;47:412–415. [Google Scholar]
- e18.Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73–85. doi: 10.1007/s00270-007-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Pinto I, Chimeno P, Romo A, et al. Uterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment—a prospective, randomized, and controlled clinical trial. Radiology. 2003;226:425–431. doi: 10.1148/radiol.2262011716. [DOI] [PubMed] [Google Scholar]
- e20.Ruuskanen A, Hippeläinen M, Sipola P, Manninen H. Uterine artery embolisation versus hysterectomy for leiomyomas: primary and 2-year follow-up results of a randomised prospective clinical trial. Eur Radiol. 2010;20:2524–2532. doi: 10.1007/s00330-010-1829-0. [DOI] [PubMed] [Google Scholar]
- e21.Kaump GR, Spies JB. The impact of uterine artery embolization on ovarian function. J Vasc Interv Radiol. 2013;24:459–467. doi: 10.1016/j.jvir.2012.12.002. [DOI] [PubMed] [Google Scholar]
- e22.Lee MS, Kim MD, Jung DC, et al. Apparent diffusion coefficient of uterine leiomyoma as a predictor of the potential response to uterine artery embolization. J Vasc Interv Radiol. 2013;24:1361–1365. doi: 10.1016/j.jvir.2013.05.054. [DOI] [PubMed] [Google Scholar]
- e23.Rabinovici J, David M, Fukunishi H, et al. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199–209. doi: 10.1016/j.fertnstert.2008.10.001. [DOI] [PubMed] [Google Scholar]