dear editor, Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, inflammatory skin disease affecting terminal hair follicles in apocrine-gland-bearing skin.1 Associated comorbidities include depression,2 obesity3 and metabolic syndrome.4 The objective of the current analysis of patients with moderate-to-severe HS was to identify the most common comorbidities, their prevalence according to the level of HS disease burden (high vs. medium) and any association between baseline characteristics and the risk for the comorbidity. These patients, representing one of the largest HS groups to be evaluated to date, were adults from a 52-week, phase 2, randomised, double-blind, placebo-controlled trial of adalimumab treatment,5 who had at least moderate disease [HS Physician's Global Assessment (HS-PGA) grade ≥ 3; 0–5 scale]. Additional inclusion/exclusion criteria were published previously.5
Baseline comorbidities were identified with patient reports and medical histories. The following conditions were defined: hypertension, use of antihypertensive medication and/or self-reported history; uncontrolled hypertension, systolic/diastolic blood pressure (SBP/DBP) ≥ 140/≥ 90 mmHg; depression, Patient Health Questionnaire 9 (PHQ-9) score ≥ 10;6 morbid obesity, body mass index (BMI) ≥ 40 kg m−2; hyperlipidaemia, total cholesterol ≥ 240 mg dL−1; high HS disease burden, HS-PGA > 3 and/or Hurley stage III; and medium HS disease burden, HS-PGA ≤ 3 and Hurley stage II.5
All patients with available baseline values were included in this analysis. All statistical tests were two-sided and significant at 0·05. Associations between the most common comorbidities and baseline characteristics were evaluated by logistic regression. The odds ratio (OR) with 95% Wald confidence interval (CI) was provided. Final models were chosen by stepwise selection with a P-value of 0·15 for both entry and stay. Model selection was conducted per Akaike information criteria and Bayesian information criteria, which confirmed the final models selected by the stepwise selection method.
Of the 154 patients in this analysis, 60 (39·0%) had high HS disease burden and 94 (61·0%) had medium burden. Mean high-sensitivity C-reactive protein (CRP) was almost four times higher in the high vs. medium disease burden groups (32·7 mg L−1 vs. 8·7 mg L−1). Combining self-report and medical examination results, 39·6% of patients had hypertension, 38·3% were morbidly obese and 48·1% had depression. The incidence of modifiable cardiovascular risk factors (Table1) revealed that > 50% of patients were smokers, overweight or had hypertension. Other cardiovascular risk factors included hyperlipidaemia (11·7%) and diabetes mellitus (6·5%). Over one-third of patients (35·7%) had two cardiovascular risk factors (Table1).
Table 1.
Baseline status of enrolled patients with hidradenitis suppurativa (HS)
| All patients, n = 154 | HS severity | ||
|---|---|---|---|
| High disease burden, n = 60 | Medium disease burden, n = 94 | ||
| Demographics | |||
| Age (years), mean ± SD | 36·3 ± 11·76 | 37·2 ± 12·90 | 35·8 ± 11·00 |
| Age < 40 years, n (%) | 98 (63·6) | 37 (62) | 61 (65) |
| Female, n (%) | 110 (71·4) | 35 (58) | 75 (80) |
| Race, n (%) | |||
| White | 110 (71·4) | 42 (70) | 68 (72) |
| Black | 29 (18·8) | 12 (20) | 17 (18) |
| Other | 15 (9·7) | 6 (10) | 9 (10) |
| Characteristics | |||
| Nicotine use, n (%) | |||
| Ever used | 108 (70·1) | 44 (73) | 64 (68) |
| Current user | 85 (55·2) | 40 (67) | 45 (48) |
| Former user | 23 (14·9) | 4 (7) | 19 (20) |
| Nonuser | 46 (29·9) | 16 (27) | 30 (32) |
| Body weight (kg), mean ± SD | 97·2 ± 24·80 | 100·1 ± 27·61 | 95·4 ± 22·79 |
| BMI (kg m−2), mean ± SD | 34·0 ± 8·56 | 34·9 ± 9·72 | 33·5 ± 7·75 |
| BMI 30–40, n (%) | 58 (37·7) | 17 (28) | 41 (44) |
| BMI > 40, n (%) | 43 (27·9) | 22 (37) | 21 (22) |
| Blood pressure (mmHg), systolic/diastolic, mean ± SD | 125 ± 13·9/79 ± 1·0 | 125 ± 14·2/79 ± 9·6 | 125 ± 13·9/80 ± 10·2 |
| HS disease duration (years), mean ± SD | 11·9 ± 9·52 | 12·0 ± 9·11 | 11·8 ± 9·82 |
| Family history of HS, n (%) | 43 (27·9) | 20 (33) | 23 (24) |
| HS-PGA, n (%) | |||
| Moderate or less | 105 (68·2) | 11 (18) | 94 (100) |
| Severe/very severe | 49 (31·8) | 49 (82) | 0 |
| Hurley stage, n (%) | |||
| I/II (mild/moderate) | 109 (70·8) | 15 (25) | 94 (100) |
| III (severe/very severe) | 45 (29·2) | 45 (75) | 0 |
| Prior therapies/medications, n (%) | |||
| Topical | 76 (49·4) | 30 (50) | 46 (49) |
| Systemic | 151 (98·1) | 58 (97) | 93 (99) |
| hsCRPa (mg L−1), mean ± SD | 17·5 ± 26·02 (n = 117) | 32·7 ± 36·79 (n = 43) | 8·7 ± 9·09 (n = 74) |
| VAS skin pain score,b mean ± SD | 54·3 ± 26·46 | 65·9 ± 24·64 | 46·8 ± 24·96 |
| PHQ-9 scorec (0–27), mean ± SD | 9·5 ± 6·69 (n = 153) | 11·0 ± 6·49 | 8·5 ± 6·66 (n = 93) |
| Modifiable cardiovascular risk factors | |||
| History of diabetes mellitus, n (%) | 10 (6·5) | ||
| Current tobacco use, n (%) | 85 (55·2) | ||
| BMI ≥ 30 and/or obesity, n (%) | 103 (66·9) | ||
| TC ≥ 240 mg dL−1 or medical history of hyperlipidaemia, n (%) | 18 (11·7) | ||
| SBP ≥ 140 and/or DBP ≥ 90 mmHg or history of hypertension, n (%) | 39·6 (61·0) | ||
| Number of risk factors, n (%) | |||
| 2 | 55 (35·7) | ||
| 3 | 28 (18·2) | ||
| 4 | 7 (4·5) | ||
| 5 | 2 (1·3) | ||
Percentages are based on patients with nonmissing values. BMI, body mass index; DBP, diastolic blood pressure; hsCRP, high-sensitivity C-reactive protein; HS-PGA, HS Physician's Global Assessment; PHQ, Patient Health Questionnaire; SBP, systolic blood pressure; TC, total cholesterol; VAS, visual analogue scale. aNormal range < 3·1 mg L−1. bVAS ranging from 0 (no pain) to 100 (worst pain). cPHQ-9 scores for depression severity: 0–4 none, 5–9 mild, 10–14 moderate, 15–19 moderately severe, 20–27 severe.
Of the 40·3% of patients who had and/or were diagnosed with hypertension, or were receiving antihypertensive medication (Fig.1a), 53% had been diagnosed with hypertension, and 31% had been both diagnosed and treated. Of the latter, 53% had reached the treatment goal (SBP/DBP < 140/< 90 mmHg). Similarly, a minority with hyperlipidaemia were both diagnosed and receiving medication (Fig.1a).
Figure 1.
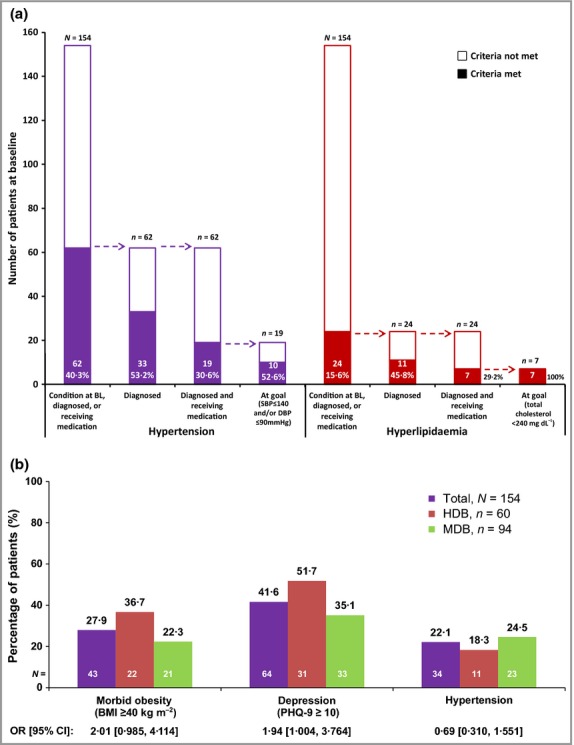
Comorbidities in patients with hidradenitis suppurativa: study population. (a) Number and percentage of patients with hypertension or hyperlipidaemia at baseline (BL). (b) Prevalence of main comorbidities in patients with high disease burden (HDB) vs. medium disease burden (MDB). Hypertension was identified by treatment with antihypertensive medication and/or self-reported history at BL. BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; OR, odds ratio; PHQ, Patient Health Questionnaire; SBP, systolic blood pressure.
The percentage of patients with morbid obesity or depression was 14% and 17% higher, respectively, in patients with high vs. medium disease burden (Fig.1b). The percentage of patients with hypertension was 6% lower in patients with high vs. medium disease burden (Fig.1b).
Multiple logistic regression identified the most influential factors for morbid obesity and depression. An association with increased odds of morbid obesity was seen for high HS disease burden (OR 2·13, 95% CI 1·00–4·53), and a trend towards association was seen for depression (OR 1·74, 95% CI 0·82–3·68). Smoking was associated with reduced odds of morbid obesity (OR 0·47, 95% CI 0·22–0·99). High HS disease burden (OR 2·12, 95% CI 1·04–4·31), female sex (OR 2·57, 95% CI 1·13–5·85) and smoking (OR 2·35, 95% CI 1·15–4·81) were associated with increased odds of depression.
High HS disease burden was significantly associated with increased prevalence of morbid obesity and depression, but not hypertension, partially contradicting a previous report that also demonstrated the high prevalence of obesity and depression in patients with HS, but not significant association between disease severity and BMI or depression.7 Our findings are novel because we demonstrate that the magnitude of HS disease burden appears to be correlated with the risk of depression and morbid obesity, even after controlling for possible confounding variables.
Based on these findings, instructive parallels and differences can be drawn between HS and psoriasis. Positive correlations between psoriasis disease severity and obesity8 and between psoriasis disease severity and CRP elevation9 have been demonstrated. However, patients with psoriasis have lower CRP levels,9 and psoriasis disease severity correla-tes with hypertension prevalence.8 More than just skin diseases, both HS and psoriasis are systemic diseases associated with high systemic inflammation and numerous co-morbidities.
This analysis had several limitations. A cross-sectional study cannot assess causality. This population may not reflect the entire spectrum of patients with HS because it was limited to clinical trial participants, for whom previous treatment with tumour necrosis factor-α inhibitors, cardiac insufficiency (New York Heart Association class III or greater), active skin diseases and tumours were exclusion criteria.9 Meaningful correlations were difficult to establish due to the limited population size. Finally, patient-reported prevalence of comorbidities is subject to recall bias.
Acknowledgments
The authors would like to thank Jody Bennett, employee of AbbVie, for assistance in writing the first draft of this publication.
Funding sources
AbbVie Inc. funded this study and participated in the study design, data collection, data management, data analysis and preparation of the manuscript. All authors had full access to the data and were involved in the analysis of data, development and revision of the manuscript, and decision to submit the manuscript for publication.
Conflicts of interest
J.J.C. has received honoraria and grants from AbbVie and Amgen for participation on ad boards and as a speaker and investigator, and grants from Astra-Zeneca, Celgene, Janssen, Lilly, Pfizer, Merck and Regeneron for participation as an investigator. J.R.M. declares no conflicts of interest; his department was reimbursed by AbbVie for his participation as an investigator in this clinical trial. C.C.Z. has received honoraria from AbbVie and Stiefel/GlaxoSmithKline for participation on advisory boards, and as an investigator and speaker; from Galderma for participation on advisory boards; from LEO Pharma for participation as a consultant; and from Bayer Health Care, Bioderma, Biogen-Idec, General Topics and Glenmark for his participation as a speaker; his department received grants from AbbVie, Biogen-Idec, BMS, Immundiagnostik AG, LVMH, Merz, Pierre Fabre and UCB for his participation as an investigator, and from Intendis for his participation on an advisory board. N.S. has received payments from AbbVie and Celgene for participation as an investigator; honoraria from Medicis, Merz, Stiefel and Valeant for participation on advisory boards; and receives a salary as an employee of Optigenex, Inc. A.K. is a consultant and investigator for Janssen, AbbVie and Amgen, and has received fellowship funding from Janssen. F.K. has received honoraria from AbbVie, Amgen, Astellas, Galderma, Janssen and Medicis for participation as a speaker; and has received grants from AbbVie for participation as an investigator. M.S., Y.G. and M.M.O. receive a salary as AbbVie employees, and may also receive AbbVie stock, stock options and/or stock grants.
Some data from this manuscript were presented at the 71st Annual Meeting of the American Academy of Dermatology (AAD) at Miami Beach, FL, U.S.A., 1–5 March 2013.
References
- Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa) Dermatoendocrinol. 2010;2:9–16. doi: 10.4161/derm.2.1.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdijk AJ, van der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2013;27:473–8. doi: 10.1111/j.1468-3083.2012.04468.x. [DOI] [PubMed] [Google Scholar]
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case–control studies. J Am Acad Dermatol. 2008;59:596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS ONE. 2012;7:e31810. doi: 10.1371/journal.pone.0031810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846–55. doi: 10.7326/0003-4819-157-12-201212180-00004. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County. Minnesota. J Invest Dermatol. 2013;133:97–103. doi: 10.1038/jid.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Oliveira H, Reis F, et al. C-reactive protein and leucocyte activation in psoriasis vulgaris according to severity and therapy. J Eur Acad Dermatol Venereol. 2010;24:789–96. doi: 10.1111/j.1468-3083.2009.03527.x. [DOI] [PubMed] [Google Scholar]


