Abstract
Background
Inflammation is involved in the mechanism of inflammatory bowel disease (IBD). Omentin, a newly discovered adipokine, is thought to play an anti-inflammatory role. This study aimed to determine whether serum levels of omentin-1 are associated with the presence and disease activity of IBD.
Material/Methods
This study consisted of 192 patients with IBD: 100 with Crohn’s disease [CD], 92 with ulcerative colitis [UC], and 104 healthy subjects. Serum levels of omentin-1 were measured using enzyme-linked immunosorbent assay (ELISA).
Results
Serum omentin-1 levels were significantly decreased in CD and UC patients compared with healthy controls. Multivariable logistic regression analysis revealed that serum omentin-1 levels were inversely associated with the presence of CD and UC. Active CD and UC patients both had significantly decreased levels of serum omentin-1 compared with inactive CD and UC patients. In both CD and UC patients, serum omentin-1 levels were significantly associated with decreased levels of body mass index (BMI) and C-reactive protein (CRP).
Conclusions
Decreased serum omentin-1 levels could be considered as an independent predicting marker of the presence and disease activity of IBD.
MeSH Keywords: Adipokines, Inflammation, Inflammatory Bowel Diseases
Background
White adipose tissue (WAT) can express and secrete various bioactive proteins and multifunctional molecules, collectively called adipokines. These adipokines include leptin, resistin, adiponectin, and visfatin. Recent evidence indicates that adipokines play a key role in the inflammatory pathways [1].
Inflammatory bowel disease (IBD), comprised of Crohn’s disease (CD) and ulcerative colitis (UC), is a group of inflammatory disorders of the gastrointestinal tract [2]. IBD is associated with alterations in fat mass and fat distribution. Patients with CD have accumulation of intraabdominal fat and mesenteric adipose tissue hypertrophy [3], whereas submucosal fat deposition (fat halo sign) is found in both CD and UC [4]. These alterations in fat mass and fat distribution were long considered to make no sense; however, it was recently suggested that this may result in alterations in the secretion of adipokines and thereby play an important role in IBD [5].
Omentin, a recently discovered adipokine, is produced and secreted mainly by visceral adipose tissue. Omentin is codified by 2 genes, omentin-1 and omentin-2, and the former is the major circulating form [6]. Decreased serum omentin-1 levels were found to be associated with obesity [7], polycystic ovary syndrome [8], diabetes [9], and coronary artery disease [10]. Recently, omentin was suggested to play an anti-inflammatory role. In vascular smooth muscle cells, omentin was shown to serve as an anti-inflammatory mediator by inhibiting TNF-α-induced superoxide production [11]. Fat mass and fat distribution alterations during IBD may lead to changed production of adipokines, including omentin. Therefore, omentin is hypothesized to be involved in the pathophysiology of IBD.
The aim of this study was to determine the serum levels of omentin-1 in patients with IBD to assess its role in the pathophysiology of IBD.
Material and Methods
Patients
A total of 192 patients with IBD – 100 with Crohn’s disease [CD] and 92 with ulcerative colitis [UC] – were enrolled in this study. The diagnoses of CD and UC were established by endoscopic, histological, radiological, and clinical findings. Clinical disease activity of CD and UC patients was determined according to the Crohn’s disease activity index (CDAI) and clinical activity index (CAI), respectively. Inactive disease was defined as a CDAI score ≤150 [12] or a CAI score ≤3 [13], and active disease as a CDAI score >150 or a CAI score >3. Patients with malignancies, chronic obstructive pulmonary disease, acute infectious diseases, cardiovascular diseases, type 2 diabetes, or strokes were excluded. The control group consisted of 104 healthy check-up examinees matched to the cases by age, sex, and body mass index (BMI). Subjects with any findings of IBD were excluded from the control group.
This study was approved by the ethics committee of our hospital, and informed consent was obtained from all participants.
Measurements
At first examinations, height and weight were measured. Venous blood was collected after a minimum of 10 h of fasting. White blood cell (WBC) count and C-reactive protein (CRP) were tested. Serum omentin-1 levels were measured by an ELISA (BlueGene Biotech Co., Shanghai, China). BMI was calculated as weight in kilograms divided by height squared in meters (kg m−2).
Statistical analysis
Data are presented as means ±SD or median (interquartile range). Data normality was analyzed using the Kolmogorov-Smirnov test. Comparison of the characteristics between patients with IBD and healthy controls were performed by one-way ANOVA, chi-square tests, or Kruskal-Wallis test. Univariate analysis was performed and the variables with a P<0.10 were then entered into a backward stepwise multivariate logistic regression model to calculate the odds ratio (OR) values and 95% confidence intervals (CI) for the presence of CD and UC. Mann-Whitney U test was used to compare the difference of serum omentin-1 levels between inactive patients and active patients. The correlation between serum omentin-1 and other parameters were analyzed using Spearman correlation analysis. Because serum omentin-1 and CRP levels were not normally distributed, logarithmic (log) transformed values were used for multiple linear regression analysis. Statistical analysis was carried out using SPSS version 13.0 software (SPSS Inc, Chicago, IL). Statistical significance was accepted at a level of P less than 0.05.
Results
Baseline clinical characteristics
Table 1 shows the clinical and laboratory characteristics of IBD patients and control subjects. CD and UC patients both had elevated levels of WBC and CRP compared with healthy controls. No significant differences were found in age, sex, and BMI among the 3 groups.
Table 1.
Clinical and biochemical characteristics of IBD patients and healthy controls.
| Control | CD patients | UC patients | P value | |
|---|---|---|---|---|
| N | 104 | 100 | 92 | |
| Age (years) | 40.64±4.78 | 40.84±4.61 | 41.23±5.17 | 0.696 |
| Gender (M/F) | 51/53 | 53/47 | 44/48 | 0.751 |
| BMI (Kg/m2) | 23.32±2.68 | 23.10±2.86 | 23.41±2.48 | 0.704 |
| WBC | 6.48±0.95 | 7.41±1.05* | 7.24±1.10a | <0.001 |
| CRP (mg/L) | 1.70 (0.90–2.18) | 3.25 (2.13–5.48)* | 3.05 (1.93–5.30)* | <0.001 |
| Omentin-1 (ng/mL) | 28.62 (24.71–33.21) | 14.63 (11.42–18.12)* | 14.74 (11.52–18.16)* | <0.001 |
| Treatment | ||||
| Steroids | – | 81 | 83 | |
| 5-Aminosalicylic acid | – | 54 | 48 | |
Significant versus control subjects.
Serum omentin-1 levels in IBD patients
Serum levels of omentin-1 in IBD patients and healthy controls are presented in Table 1. Serum omentin-1 levels were significantly lower in CD and UC patients compared with healthy controls (P<0.001 and P<0.001, respectively). Simple logistic regression analysis indicated that WBC, CRP, and serum omentin-1 levels showed a trend (P<0.10) toward an association with the presence of CD (Table 2). All these parameters were then entered into a multivariate logistic regression model. Serum omentin-1 levels remained adversely associated with the presence of CD (OR 0.210, 95% CI 0.083 to 0.530; P<0.001) (Table 2). Furthermore, simple logistic regression analysis indicated that WBC, CRP, and serum omentin-1 levels showed a trend (P<0.10) toward an association with the presence of UC (Table 3). All these parameters were then entered into a multivariate logistic regression model. Serum omentin-1 levels remained to be adversely associated with the presence of UC (OR 0.266, 95% CI 0.132 to 0.535; P<0.001) (Table 3).
Table 2.
Logistic regression analysis for the presence of CD.
| Simple regression | Multiple regression | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (years) | 1.038 (0.823–1.309) | 0.752 | ||
| Gender (M/F) | 0.154 (0.010–2.380) | 0.180 | ||
| BMI (Kg/m2) | 1.030 (0.678–1.566) | 0.889 | ||
| WBC (109/L) | 2.760 (0.828–9.196) | 0.098 | 2.595 (0.960–7.014) | 0.060 |
| CRP (mg/L) | 3.608 (1.370–9.497) | 0.009 | 3.894 (1.538–9.856) | 0.004 |
| Omentin-1 (ng/mL) | 0.161 (0.044–0.592) | 0.006 | 0.210 (0.083–0.530) | 0.001 |
Table 3.
Logistic regression analysis for the presence of UC.
| Simple regression | Multiple regression | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (years) | 0.969 (0.779–1.206) | 0.779 | ||
| Gender (M/F) | 0.338 (0.034–3.372) | 0.355 | ||
| BMI (Kg/m2) | 0.865 (0.580–1.290) | 0.478 | ||
| WBC (109/L) | 9.563 (1.927–47.444) | 0.006 | 7.439 (1.890–29.276) | 0.004 |
| CRP (mg/L) | 4.481 (1.727–11.621) | 0.002 | 4.034 (1.681–9.681) | 0.002 |
| Omentin-1 (ng/mL) | 0.216 (0.085–0.544) | 0.001 | 0.266 (0.132–0.535) | <0.001 |
Serum omentin-1 levels with the disease activity of IBD
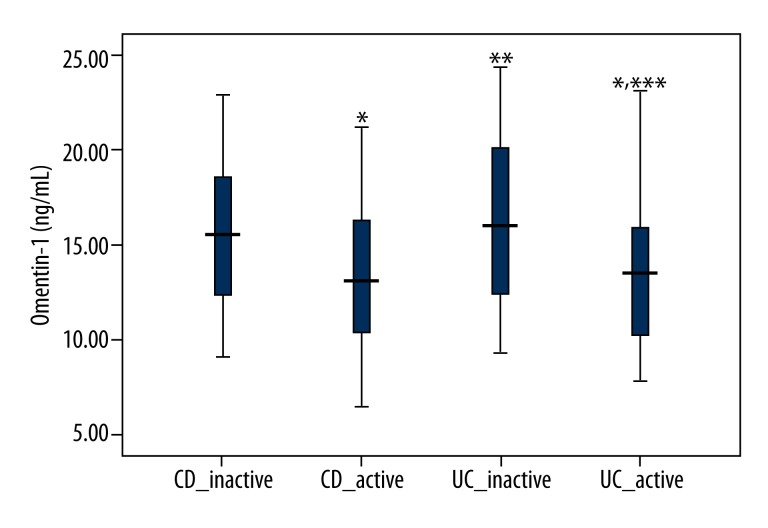
Serum omentin-1 levels in inactive and active patients are displayed in Figure 1. Active CD patients showed significantly decreased levels of serum omentin-1 compared with inactive CD patients (P=0.012). In addition, active UC patients showed significantly decreased levels of serum omentin-1 compared with inactive UC patients (P=0.001). However, there were no significant difference in serum omentin-1 levels between active CD and active UC patients, or between inactive CD and inactive UC patients (P=0.992 and P=0.310, respectively).
Figure 1.
Serum omentin-1 levels in active and inactive IBD patients. Active CD and UC patients showed significantly decreased levels of serum omentin-1 compared with inactive CD and UC patients, respectively (P=0.012 and P=0.001, respectively). * Significant versus serum omentin-1 levels in inactive CD patients; ** Significant versus serum omentin-1 levels in active CD patients; *** Significant versus serum omentin-1 levels in inactive UC patients.
The association of serum omentin-1 level with other clinical characteristics
Spearman correlation analysis showed that serum omentin-1 levels in CD and UC patients were both negatively correlated with BMI and CRP (Table 4).
Table 4.
Correlation of serum omentin-1 levels with other clinical parameters in CD and UC patients.
| Parameters | CD patients | UC patients | ||
|---|---|---|---|---|
| r | P value | r | P value | |
| Age (years) | −0.059 | 0.559 | 0.054 | 0.611 |
| Gender (M/F) | −0.075 | 0.459 | −0.070 | 0.505 |
| BMI (Kg/m2) | −0.240 | 0.016 | −0.283 | 0.006 |
| WBC (109/L) | −0.163 | 0.105 | −0.178 | 0.090 |
| CRP (mg/L) | −0.287 | 0.004 | −0.316 | 0.002 |
Discussion
The current study indicated that serum omentin-1 levels were significantly decreased in CD and UC patients compared with healthy subjects. Active CD and UC patients showed significantly decreased levels of serum omentin-1 compared with inactive CD and UC patients, respectively. In addition, serum levels of omentin-1 were negatively correlated with BMI and CRP in CD and UC patients. This is the first study to demonstrate that decreased levels of serum omentin-1 are associated with the presence and disease activity of IBD.
The differential diagnosis of IBD and differentiating between CD and UC are very difficult. Clinicians often have to combine laboratory, radiologic, endoscopic, and histological findings to confirm the diagnosis. However, endoscopic and histological evaluations are limited due to the difficulty in performing, invasiveness, time, and cost. Recently, biomarkers have been utilized to assess the risk of various diseases. Serum biomarkers have been used to evaluate the risk of IBD and differentiate either IBD from non-IBD, CD from UC, or active IBD from inactive IBD [14]. Our study revealed that serum omentin-1 levels were significantly decreased in IBD patients compared with healthy subjects. This indicates that omentin may be involved in the pathophysiology of IBD. Decreased levels of serum omentin-1 are correlated with the presence of IBD. Recent studies have focused on the important role of adipokines in the mechanism of IBD. Some other adipokines, such as adiponectin, leptin, resistin, and chemerin, were also demonstrated to be associated with the development of IBD [15,16]. These results indicate that adipose tissue and adipokine may play an important role in the pathophysiology of IBD. Our results also revealed that active CD and UC patients had significantly lower serum omentin-1 levels compared with inactive CD and UC patients. This indicated that serum omentin-1 levels were associated with the disease activity of IBD. Hence, serum omentin-1 levels were suggested to be associated with the presence and disease activity of IBD.
Chronic inflammation is a well-known characteristic of IBD, mainly of CD [17]. Elevated levels of various proinflammatory cytokines have been suggested to be associated with IBD [18]. Omentin-1, a newly discovered adipokine, could serve as an anti-inflammatory mediator. Omentin was shown to inhibit TNF-induced vascular inflammation in human endothelial cells [19]. In vascular smooth muscle cells, omentin was also found to inhibit TNF-α-induced vascular cell adhesion molecule-1 (VCAM-1) expression [11]. These results point to the anti-inflammatory role of omentin. The current results indicate that serum omentin-1 levels were negatively correlated with CRP. This is consistent with other studies. Serum omentin concentration was negatively correlated with CRP in both normal glucose tolerance and impaired glucose tolerance subjects [20].
Obesity is associated with IBD, particularly CD. Obese CD patients tend to have increased perianal complications and a higher level of disease activity on an annual basis. Omentin is closely correlated with obesity. Serum levels of omentin-1 were found to be negatively associated with BMI in patients with metabolic syndrome (MetS) [21], type 2 diabetes [22], and coronary artery disease [23]. Similar results were found in our study. The current results indicated that serum levels of omentin-1 were negatively correlated with BMI. This indicates that omentin may be involved in the crosstalk of obesity, particularly fat deposition in gastrointestinal tract, and IBD.
The present study has several limitations. First, the sample size was relatively small. Further study in a larger sample is required to determine the differences of serum omentin-1 levels between IBD patients and healthy controls. Second, this study was cross-sectional; therefore, our findings should be validated in long-term prospective studies.
Conclusions
This study shows that serum omentin-1 levels were decreased in IBD patients compared with healthy controls. In addition, serum omentin-1 levels were correlated with the disease activity of IBD. Decreased serum omentin-1 levels are suggested to be associated with the presence and disease activity of IBD.
Footnotes
Source of support: Departmental sources
References
- 1.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–93. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim A, Jung BH, Cadet P. A novel pathway by which the environmental toxin 4-Nonylphenol may promote an inflammatory response in inflammatory bowel disease. Med Sci Monit Basic Res. 2015;21:47–54. doi: 10.12659/MSMBR.890644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue – emerging role in intestinal and mesenteric diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:103–11. doi: 10.1038/ncpgasthep0090. [DOI] [PubMed] [Google Scholar]
- 4.Ahualli J. The fat halo sign. Radiology. 2007;242:945–46. doi: 10.1148/radiol.2423041600. [DOI] [PubMed] [Google Scholar]
- 5.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–83. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. 2010;20:143–48. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 8.Mahde A, Shaker M, Al-Mashhadani Z. Study of Omentin1 and Other Adipokines and Hormones in PCOS Patients. Oman Med J. 2009;24:108–18. doi: 10.5001/omj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan P, Liu D, Long M, et al. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119:257–63. doi: 10.1055/s-0030-1269912. [DOI] [PubMed] [Google Scholar]
- 10.Zhong X, Zhang HY, Tan H, et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32:873–78. doi: 10.1038/aps.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazama K, Usui T, Okada M, et al. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686:116–23. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–44. [PubMed] [Google Scholar]
- 13.Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–96. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 14.Wolf DC, Abraham BP, Afzali A, et al. Community Perspectives: Combining Serology, Genetics, and Inflammation Markers for the Diagnosis of IBD and Differentiation Between CD and UC. Gastroenterol Hepatol (N Y) 2012;8:1–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Weigert J, Obermeier F, Neumeier M, et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm Bowel Dis. 2010;16:630–37. doi: 10.1002/ibd.21091. [DOI] [PubMed] [Google Scholar]
- 16.Karmiris K, Koutroubakis IE, Xidakis C, et al. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–5. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 17.Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 18.Nanau RM, Neuman MG. Metabolome and inflammasome in inflammatory bowel disease. Transl Res. 2012;160:1–28. doi: 10.1016/j.trsl.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Yamawaki H, Kuramoto J, Kameshima S, et al. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–43. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Navarrete JM, Ortega F, Castro A, et al. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity (Silver Spring) 2011;19:1552–59. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93:21–25. doi: 10.1016/j.diabres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Shibata R, Ouchi N, Kikuchi R, et al. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis. 2011;219:811–14. doi: 10.1016/j.atherosclerosis.2011.08.017. [DOI] [PubMed] [Google Scholar]