Abstract
Although the processing of facial identity is known to be sensitive to the orientation of the face, it is less clear whether orientation sensitivity extends to the processing of facial expressions. To address this issue, we used functional MRI (fMRI) to measure the neural response to the Thatcher illusion. This illusion involves a local inversion of the eyes and mouth in a smiling face—when the face is upright, the inverted features make it appear grotesque, but when the face is inverted, the inversion is no longer apparent. Using an fMRI-adaptation paradigm, we found a release from adaptation in the superior temporal sulcus—a region directly linked to the processing of facial expressions—when the images were upright and they changed from a normal to a Thatcherized configuration. However, this release from adaptation was not evident when the faces were inverted. These results show that regions involved in processing facial expressions display a pronounced orientation sensitivity.
Keywords: face perception, facial expressions, neuroimaging, facial features, cognitive neuroscience
It is well established that facial identity is harder to process when faces are inverted than when they are upright (Diamond & Carey, 1986; Valentine, 1988; Yin, 1969). However, studies often show a much smaller effect of inversion on the perception of facial expressions (Birgit, Seidel, Kainz, & Carbon, 2009; Calvo & Nummenmaa, 2008; Fallshore & Bartholow, 2003; McKelvie, 1995; Prkachin, 2003), with the recognition of some expressions, such as happiness, not being affected at all by inversion (Calvo & Nummenmaa, 2008; McKelvie, 1995).
Findings showing relatively small costs of inversion on the perception of facial expressions form a noticeable contrast to findings on the Thatcher illusion, which involves turning the eyes and the mouth upside down relative to the rest of the face (a transformation called Thatcherization). Following Thatcherization, the facial expression appears grotesque when the face is upright (Bartlett & Searcy, 1993; Parks, Coss, & Coss, 1985; Thompson, 1980). Strikingly, however, when the image is inverted, the grotesque appearance is no longer visible. Although the Thatcher illusion is often thought to result from a disruption of configural or holistic processing that in an upright face allows the perception of the grotesque expression (Bartlett & Searcy, 1993; Boutsen & Humphreys, 2003; Leder, Candrian, Huber, & Bruce, 2001; Rhodes, Brake, & Atkinson, 1993), this explanation does not account for all aspects of the illusion (see Thompson, Anstis, Rhodes, Jeffery, & Valentine, 2009).
The Thatcher illusion demonstrates a degree of independence between the processing of facial identity and facial expression. The identity of a Thatcherized face can be recognized when the face is upside down, albeit with some difficulty, whereas the ability to perceive the grotesque facial expression is completely lost. Inversion appears to have a differential effect on the processing of facial expression and facial identity. This dissociation is consistent with a variety of evidence that facial identity and expression are processed along parallel streams (Haxby, Hoffman, & Gobbini, 2000; Young & Bruce, 2011).
Despite the importance of the Thatcher illusion for showing the selectivity of face processing, the precise neural processes underlying this phenomenon remain unclear. When comparing Thatcherized faces with normal faces, studies using event-related potentials (ERPs) and functional MRI have shown both increased responses (Carbon, Schweinberger, Kaufmann, & Leder, 2005; Milivojevic, Clapp, Johnson, & Corballis, 2003; Rotshtein, Malach, Hadar, Graif, & Hendler, 2001) and decreased responses (Boutsen, Humphreys, Praamstra, & Warbrick, 2006) to Thatcherized images. However, these studies have not directly measured sensitivity to changes from normal to Thatcherized images that arise for upright faces only—the perceptual hallmark of the Thatcher illusion. It is this strong dissociation between the perception of expression in upright and inverted stimuli that makes the Thatcher illusion such a striking perceptual phenomenon, and understanding how the dissociation arises is essential to understanding the illusion.
In the research reported here, we used the powerful functional-magnetic-resonance (fMR)-adaptation technique (Grill-Spector, Henson, & Martin, 2006) with a robust block design to probe the neural correlates of this key perceptual property of the Thatcher illusion—the loss of sensitivity to the change in expression between inverted normal faces and inverted Thatcherized faces. A functional localizer scan was used to identify core face-selective regions in visual cortex (Haxby et al., 2000). We then tested the sensitivity of each region to the Thatcherization of upright and inverted facial expressions.
The principle behind fMR adaptation is that repetition of a stimulus causes a reduction or habituation in the neural response, which leads to a lower fMR signal. The sensitivity of the neural representation can then be determined for different changes to the stimulus. If the underlying neural representation is insensitive to a particular type of change in the stimulus, the reduction in fMR signal for this type of change will be similar to the overall reduction produced by repetitions of identical stimuli. However, if the underlying neural representation is sensitive to this change, the fMR signal will remain at its original (nonadapted) level. In the present study, we compared responses to blocks of stimuli in which normal and Thatcherized images alternated with responses to blocks of stimuli in which the images were all normal or all Thatcherized. Our reasoning was that any region that contributes to the perception of the Thatcher illusion should show a greater response to a series of images that keeps changing from normal to Thatcherized expressions across a block of trials than to a series of images that are all normal or all Thatcherized across a block. Moreover, this difference in response should be evident for upright but not inverted faces.
Method
Participants and stimuli
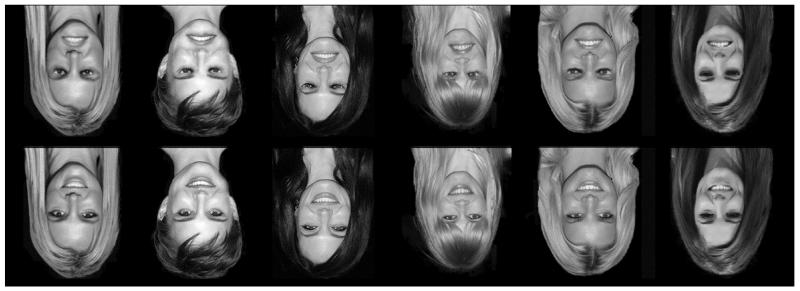
Ten participants (6 female, 4 male; mean age = 23 years, SD = 2.1) took part in a behavioral experiment, and 27 participants (18 female, 9 male; mean age = 22.5 years, SD = 3.0) took part in an imaging experiment. Written consent was obtained for all participants, and the study was approved by the York Neuroimaging Centre Ethics Committee. Photographs of six upright familiar female faces (Britney Spears, Natalie Portman, Angelina Jolie, Claudia Schiffer, Jessica Simpson, and Cheryl Cole) were used. Each face was Thatcherized by inverting the mouth and eye areas by 180°, which created a second set of six images (Fig. 1). Finally, the full non-Thatcherized (i.e., “normal”) and Thatcherized images were themselves rotated by 180° to produce two additional sets of inverted images. Visual stimuli (6° × 8°) were presented approximately 57 cm from the participants’ eyes. In the scanner, images were back-projected onto the screen located inside the magnetic bore.
Fig. 1.
The six identities used in the experiment. Identities were shown in both Thatcherized (top row) and normal (bottom row) configurations. Thatcherization consists of turning the eyes and the mouth upside down relative to the rest of the face. On each trial, stimuli were presented either in an inverted orientation (shown here) or in an upright orientation.
Behavioral experiment
A behavioral experiment was used to validate the stimuli and demonstrate the difficulty of perceiving physical changes between Thatcherized and non-Thatcherized images when they are inverted. Participants viewed two upright or two inverted images presented consecutively on each trial and had to indicate by pressing a button whether the two images were physically the same or different in any way. Each image was presented for 800 ms, and images were separated by an interval of 200 ms.
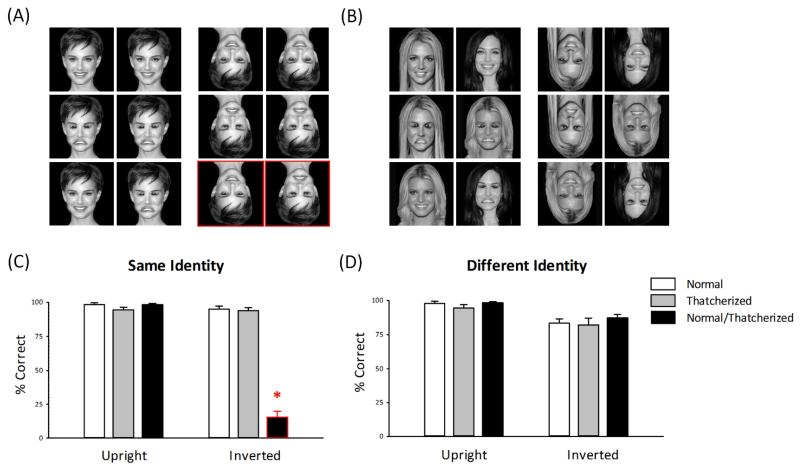
There were six different conditions (Figs. 2a and 2b):
-
(a)
The normal-normal, same-identity condition consisted of two identical images of a normal face.
-
(b)
The Thatcherized-Thatcherized, same-identity condition consisted of two identical images of a Thatcherized face.
-
(c)
The normal-Thatcherized, same-identity condition consisted of the normal face and the Thatcherized face of the same person.
-
(d)
The normal-normal, different-identity condition consisted of normal face images of two different people.
-
(e)
The Thatcherized-Thatcherized, different-identity condition consisted of Thatcherized face images of two different people.
-
(f)
The normal-Thatcherized, different-identity condition consisted of a normal face and a Thatcherized face of two different people.
Fig. 2.
Stimulus conditions (a, b) and results from the behavioral experiment (c, d). On each trial, a pair of faces with either (a) the same identity or (b) different identities were presented in the upright or inverted orientation. Faces could both be normal (i.e., non-Thatcherized; top row), both be Thatcherized (middle row), or one could be normal and one Thatcherized (bottom row). Participants were asked to report whether the images were completely identical or different in any way. The graphs show the mean percentage of correct responses as a function of face orientation and stimulus condition, separately for (c) same-identity trials and (d) different-identity trials. The border indicates stimuli for which performance was below chance (< 50% correct). Error bars represent standard errors of the mean. The asterisk indicates a significant difference between conditions (**p < .001).
In each run, there were 18 trials for each condition, giving a total of 108 trials. The experiment involved two separate runs, in each of which the images were all inverted or all upright.
fMRI experiment
To determine the neural correlates of the Thatcher illusion, we measured responses to normal and Thatcherized faces using an fMRI-adaptation paradigm with the same stimulus conditions as in the behavioral experiment. Our prediction was that, if a face-selective region is sensitive to the perceptual change created by Thatcherization of face images, it should show a significantly greater response to alternations between the normal and Thatcherized images (normal-Thatcherized condition) than to alterations between images that were all normal (normal-normal condition) or all Thatcherized (Thatcherized-Thatcherized condition). In contrast, if a region is not sensitive to Thatcherized images, it should show a similar response to all conditions. We measured this sensitivity to Thatcherization for upright and for inverted images.
In contrast to the behavioral experiment, images in the fMRI experiment were presented in a blocked design. There were six images in each block. In a same-identity block, the same face identity was repeated six times. In a different-identity block, six different facial identities were presented. In the normal-normal and Thatcherized-Thatcherized blocks, the six face images were either all normal or all Thatcherized, respectively. In the normal-Thatcherized blocks, alternate images were normal or Thatcherized. Images in each block were shown for 800 ms, followed by a 200-ms interval (these timings were identical to those used in the behavioral experiment). The use of six images per block gave an overall duration of 6 s per block. Blocks were separated by a 9-s gray screen with a fixation cross. Each of the six conditions was repeated six times in a pseudorandomized, counterbalanced design, giving a total of 36 blocks. There were two experimental runs. In the first run, images were shown in an inverted orientation. In the second run, images were shown in an upright orientation.
To maintain a consistent attentional load across stimulus blocks, we superimposed a red dot on 16% of the images. Participants were told to respond with a button press as soon as they saw the image with the red dot. Other than this red-dot task, the experiment involved passive viewing of the face images. Participants correctly reported the occurrence of the red dot on more than 95% of trials (upright faces: 98.6%, SD = 0.75; inverted faces: 98.3%, SD = 0.67%). Across the different conditions, there was no significant difference in the rate of detection— inverted faces: F(2, 52) = 0.19, p = .82; upright faces: F(2, 52) = 0.53, p = .59—or in reaction time—inverted faces: F(2, 52) = 0.62, p = .54; upright faces: F(2, 52) = 1.25, p = .29.
To identify face-selective regions of interest, we performed a separate localizer scan for each participant. There were four conditions: faces, objects, places, and scrambled faces. Images from each condition were presented in a blocked design with five images in each block. Each image was presented for 1 s, followed by a 200-ms fixation cross. Blocks were separated by a 9-s gray screen. Each condition was repeated five times in a counterbalanced design.
All experimental scans were acquired using a General Electric Signa HD Excite 3T high-definition MRI scanner at the York Neuroimaging Centre at the University of York. An eight-channel, phased-array, head-dedicated gradient insert coil tuned to 127.4 MHz was used to acquire MRI data. A gradient-echo echo-planar imaging sequence was used to collect data from 38 contiguous axial slices (repetition time = 3 s, echo time = 32.7-45 ms, flip-angle = 90°, field of view = 288 mm × 288 mm, in-plane resolution = 2.25 mm × 2.25 mm, slice thickness = 3 mm).
Statistical analysis of the fMRI data was carried out using the FEAT tool in the FMRIB Software Library (fsl.fmrib.ox.ac.uk/fsl/fslwiki/FEAT). The initial 9 s of data from each scan were removed to minimize the effects of magnetic saturation. Motion correction was followed by spatial smoothing (Gaussian, full-width at half-maximum, 6 mm) and temporal high-pass filtering (cut off = 0.01 Hz). Face-selective regions were determined from the localizer scan using a standard localizer approach (Andrews, Davies-Thompson, Kingstone, & Young, 2010). The averaged statistical map for the following contrasts was set at a threshold of p < .001 (uncorrected): faces greater than places, faces greater than objects, and faces greater than scrambled faces. Regions were defined independently for each individual. The time series of each voxel within a region was converted from units of image intensity to percentage signal change. All voxels in a given region were then averaged to give a single time series in each region for each participant. The peak response was then calculated 9 s after the onset of the block.
Results
Behavioral experiment
Participants in the behavioral experiment were simply asked to indicate by pressing a button whether the two images on each trial were completely identical or different in any way. Accuracy judgments (Figs. 2c and 2d) showed that participants were able to perform this task at well above chance level (50%) in all conditions, except when an inverted normal image was paired with an inverted Thatcherized image with the same identity. The high error rate in this condition reflects a failure to notice differences between normal and Thatcherized versions of the same person’s face when these images are inverted.
Separate 3 × 2 analyses of variance (ANOVAs) on same-identity and different-identity faces determined the effect of condition (normal-normal, Thatcherized-Thatcherized, normal-Thatcherized) and orientation (upright, inverted). For the same-identity images, there was a significant effect of condition, F(2, 18) = 168.7, p < .001, and orientation, F(1, 9) = 209.7, p < .001. There was also a significant interaction between condition and orientation, F(2, 18) = 172.6, p < .001. This reflects the fact that participants were unable to judge the difference between a normal and a Thatcherized image when they were both inverted. Accuracy on normal-Thatcherized trials (15.6%, SD = 4.4 %) was significantly lower than on normal-normal trials (95.0%, SD = 2.3 %), t(9) = −13.42, p < .001, or Thatcherized-Thatcherized trials (93.9%, SD = 2.1 %), t(9) = 15.66, p < .001, when the faces were inverted. However, there was no significant difference between the normal-Thatcherized and the normal-normal conditions, t(9) = 0.01, p = .99, or between the normal-Thatcherized and the Thatcherized-Thatcherized conditions, t(9) = 2.69, p = .08, when the faces were upright. For the different-identity images, there was a significant effect of orientation, F(1, 9) = 18.33, p < .01, but no significant effect of condition, F(2, 18) = 1.82, p = .19, or any interaction between condition and orientation, F(2, 18) = 0.46, p = .64. The effect of orientation was due to a lower accuracy for inverted compared with upright images.
fMRI experiment
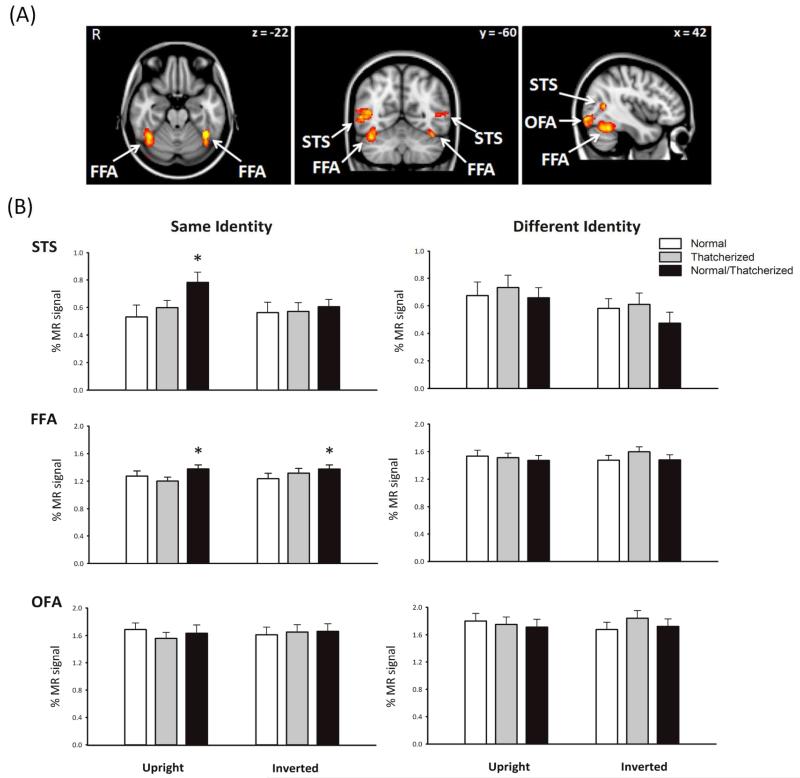
Figure 3a shows the location of the three face-selective regions in the occipital and temporal lobes identified by the functional localizer scan (Haxby et al., 2000): the fusiform face area (FFA), occipital face area (OFA), and superior temporal sulcus (STS). The coordinates of each region are shown in Table 1. Each region was defined separately for each individual, and all further analyses were performed on the mean time courses of voxels in the regions of interest. There was no significant difference between the patterns of response between the right and left hemispheres (F < 0.7, p > .08) nor any significant interactions between condition and hemisphere (F < 2.0, p > .16). Therefore, in all further analyses, right and left hemisphere voxels were combined in each region of interest.
Fig. 3.
Functional MRI results. The brain images (a) show the locations of the three face-selective regions defined by an independent localizer scan: the fusiform face area, occipital face area, and superior temporal sulcus. The graphs (b) show the mean percentage signal change as a function of face orientation and stimulus condition, separately for same-identity and different-identity trials. Results are presented for each of the three regions identified in the localizer scan. Error bars represent standard errors of the mean. The asterisks indicate significant differences between conditions (*p < .05). R = right.
Table 1.
Mean Montreal Neurological Institute Coordinates of the Identified Face-Selective Regions of Interest
| Region and hemisphere | Number of participants showing activationsa |
Coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Fusiform face area | ||||
| Left | 26 | −40 | −54 | −22 |
| Right | 26 | 42 | −56 | −22 |
| Occipital face area | ||||
| Left | 22 | −38 | −86 | −12 |
| Right | 25 | 42 | −82 | −12 |
| Superior temporal sulcus | ||||
| Left | 12 | −46 | −60 | 6 |
| Right | 22 | 48 | −60 | 6 |
N = 27.
Figure 3b shows the response to upright and inverted faces across all image conditions of the same or a different identity. The peak responses of face-selective regions were analyzed using a two-way ANOVA (Condition × Orientation) on same-identity or different-identity faces. In the STS, there was a main effect of condition, F(2, 46) = 3.76, p < .05, and orientation, F(1, 23) = 4.14, p < .05, for same-identity faces. There was also an interaction between condition and orientation, F(2, 46) = 3.03, p < .05. This interaction was due to a greater response to faces in the normal-Thatcherized condition compared with faces in both the normal-normal, t(21) = 2.80, p < .05, and Thatcherized-Thatcherized, t(21) = 2.26, p < .05, conditions in the upright orientation. Consistent with the behavioral and perceptual properties of the Thatcher illusion, there was no difference between the normal-Thatcherized condition and either the normal-normal, t(21) = 0.72, p = .48, or Thatcherized-Thatcherized, t(21) = 0.56, p = .58, conditions when the images were inverted. For different-identity faces, there was no main effect of condition, F(2, 46) = 2.05, p = .14, or orientation, F(1, 23) = 3.41, p = .078, and no significant interaction between condition and orientation, F(2, 46) = 0.71, p = .50.
This orientation-sensitive response to Thatcherized faces was not evident in other face-selective regions. In the FFA, there was a main effect of condition, F(2, 52) = 4.83, p < .05, but no effect of orientation, F(1, 26) = 0.70, p = .41, and no significant interaction between condition and orientation, F(2, 52) = 1.81, p = .17, for same-identity faces. The main effect of condition was due to a smaller response to faces in the normal-normal or Thatcherized-Thatcherized conditions compared with faces in the normal-Thatcherized condition for both the upright orientation, t(26) = 2.99, p < .01, and inverted orientation, t(26) = 2.41, p < .05. For different-identity images, there were no main effect of condition, F(2, 52) = 2.46, p = .095, or orientation, F(1, 26) = 0.065, p = .80, nor was there any significant interaction between condition and orientation, F(2, 52) = 1.58, p = .22, which suggests that patterns of response did not differ across conditions.
In the OFA, we found no main effect of condition, F(2, 50) = 0.444, p = .644, or orientation, F(1, 25) = 0.11, p = .74, and no significant interaction between condition and orientation, F(2, 50) = 1.77, p = .18, for same-identity faces. Similarly, for the different-identity conditions, there was no significant effect of condition, F(2, 50) = 1.99, p = .15, or orientation, F(1, 25) = 0.023, p = .88, and there was no significant interaction between condition and orientation, F(2, 50) = 2.19, p = .12. This suggests that the OFA shows a similar pattern of responses across all conditions and orientations.
Discussion
In the present experiments, we used the Thatcher illusion to examine whether the neural processes involved in judgments of facial expression are sensitive to the orientation of the image. We found that the ability to discriminate behaviorally between a normal and a Thatcherized image of the same person’s face was substantially impaired when the images were inverted. In contrast, participants could easily make this discrimination when the faces were upright. A neural correlate of this behavioral effect was evident in the STS—a face-selective region that is thought to be involved in the processing of facial expressions (Allison, Puce, & McCarthy, 2000; Baseler, Harris, Young, & Andrews, 2012; Engell & Haxby, 2007; Harris, Young, & Andrews, 2012). We found an increased response in the STS when there was a change from a normal to a Thatcherized face during a block of trials. Consistent with the behavioral findings, the fMRI results showed that this sensitivity to a change from a normal to a Thatcherized face was no longer apparent when the faces were inverted.
The selectivity of the response in the STS can be seen by contrasting it with the responses of other face-selective regions. The FFA—a region involved in processing facial identity (Grill-Spector, Knouf, & Kanwisher, 2004; Rotshtein, Henson, Treves, Driver, & Dolan, 2005)—was sensitive to a change between normal and Thatcherized faces, but this response was evident for both upright and inverted faces. In contrast, activity in the OFA was not sensitive to the Thatcherization of face images and revealed no difference in response between upright and inverted images.
Previous studies have failed to find a consensus on the critical neural processes that underpin the orientation sensitivity to Thatcherized expressions that is the hallmark of the illusion. ERP studies in humans have shown that Thatcherization increases the ERP response to faces and that this increase is attenuated when the face is inverted (Carbon et al., 2005; Milivojevic et al., 2003). However, other studies have reported that Thatcherization reduces the evoked response to faces but that this effect is reduced by inversion (Boutsen et al., 2006).
Although these ERP studies revealed the timing of neural responses to Thatcherized images, they were not able to relate this to specific face-processing pathways. To address this issue, Rotshtein et al. (2001) used fMRI to compare responses to upright and inverted images in different regions of the visual cortex. They found that upright Thatcherized images elicited a significantly greater response compared with upright normal faces in the fusiform gyrus, lateral occipital lobe, and amygdala. However, contrary to findings for the Thatcher illusion, a similar pattern of response was also evident with inverted faces. Donnelly et al. (2011) compared neural activity between simultaneously presented normal and Thatcherized faces. They reported a distributed pattern of response in which face-selective regions such as the FFA were more responsive when discriminating inverted images than upright images, whereas an increased response to upright faces was evident in regions associated with social and emotional cognition.
The inability of previous research to show an orientation-sensitive neural response that can explain the Thatcher illusion may reflect a key difference between the present design and that of previous studies. Rather than determining the neural sensitivity to a change from a normal to a Thatcherized image, these earlier studies simply compared the overall response to normal face images with the overall response to Thatcherized images. In the present research, we instead used fMR adaptation to directly measure sensitivity to a change from a normal to a Thatcherized image. We found that the face-selective region in the STS was more responsive to a change between normal and Thatcherized images compared with when the images were either all normal or all Thatcherized. Critically, we showed that the sensitivity of the STS to Thatcherization was not evident when the faces were inverted. Our finding that the STS is sensitive to the orientation of Thatcherized images confirms the critical role of this region for the processing of facial expressions (Allison et al., 2000; Baseler et al., 2012; Engell & Haxby, 2007; Harris et al., 2012).
To be socially meaningful, changes in expression and gaze direction must often be tracked across an individual whose invariant features (identity) remain constant. The increased response in the STS to sequences of faces that change from a normal to a Thatcherized configuration but do not change in identity is therefore consistent with the role of this region in social communication (Allison et al., 2000; Engell & Haxby, 2007; Harris et al., 2012). Indeed, this result integrates well with recent studies that have shown that the STS is most sensitive to changes in expressions of faces with the same identity (Andrews & Ewbank, 2004; Baseler et al., 2012). Presumably, this reflects the critical social importance of monitoring changes in a particular individual’s expression.
The majority of studies on face-inversion effects have focused on the perception of facial identity. A variety of evidence has shown that judgments of facial identity are impaired when faces are turned upside down (Valentine, 1988; Yin, 1969). These findings have been complemented by neuroimaging studies that have focused on the effect of face inversion in face-selective regions of the fusiform gyrus (Aguirre, Singh, & D’Esposito, 1999; Haxby et al., 1999; Kanwisher, Tong, & Nakayama, 1998; Mazard, Schiltz, & Rossion, 2006; Schiltz & Rossion, 2006; Yovel & Kanwisher, 2004, 2005). Although they differ in the magnitude of the inversion effect they revealed, the majority of studies report a decreased response in the fusiform gyrus to inverted faces. These studies also found reduced fMR adaptation to facial identity in the FFA in response to inverted compared with upright faces (Mazard et al., 2006, Schiltz & Rossion, 2006; Yovel & Kanwisher, 2004). Rather than explore a release from adaptation to changes in identity, we measured the sensitivity to changes in expression. We found a release from adaptation in the FFA when there was a change in expression from a normal to a Thatcherized image. However, in contrast to the response in the STS and the perception of the Thatcher illusion, this increased FFA response was still evident when the faces were presented upside down. It is interesting that the release from adaptation to a Thatcherized expression was only apparent when the identity of the faces was unchanged within a block. When the identity of the images was varied, there was no additional increase in response to Thatcherized images. This is likely to reflect the sensitivity of the FFA to image changes that are associated with changes in facial identity (Davies-Thompson, Newling, & Andrews, 2013).
In conclusion, our results demonstrate clear evidence for orientation-dependent sensitivity to changes in facial expression in a key component of the neural network underlying face perception. We found that activity in the STS was sensitive to changes between normal and Thatcherized images when the faces were upright but that there was no difference in response when the faces were inverted. In contrast, the FFA was sensitive to Thatcherized face images in both an upright and inverted configuration. This functional dissociation provides a neural explanation for the Thatcher illusion and confirms that the STS plays a key role in the perception of facial expressions. The implication of these results is that the neural processing of facial expressions is sensitive to the orientation of the image.
Acknowledgments
Funding
This work was supported by a grant from the Wellcome Trust (WT087720MA).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Aguirre GK, Singh R, D’Esposito M. Stimulus inversion and the responses of face and object-sensitive cortical areas. NeuroReport. 1999;10:189–194. doi: 10.1097/00001756-199901180-00036. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Davies-Thompson J, Kingstone A, Young AW. Internal and external features of the face are represented holistically in face-selective regions of visual cortex. Journal of Neuroscience. 2010;30:3544–3552. doi: 10.1523/JNEUROSCI.4863-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in human visual cortex. NeuroImage. 2004;23:905–913. doi: 10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Searcy J. Inversion and configuration of faces. Cognitive Psychology. 1993;25:281–316. doi: 10.1006/cogp.1993.1007. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Harris RJ, Young AW, Andrews TJ. Neural responses to expression and gaze in the posterior superior temporal sulcus interact with facial identity. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs360. Advance online publication. doi:10.1093/cercor/bhs360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgit D, Seidel EM, Kainz E, Carbon CC. Recognition of emotional expression is affected by inversion and presentation time. Perception. 2009;38:1849–1862. doi: 10.1068/p6448. [DOI] [PubMed] [Google Scholar]
- Boutsen L, Humphreys GW. The effect of inversion on the encoding of normal and “Thatcherized” faces. Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 2003;56:955–975. doi: 10.1080/02724980244000774. [DOI] [PubMed] [Google Scholar]
- Boutsen L, Humphreys W, Praamstra P, Warbrick T. Comparing neural correlates of configural processing in faces and objects: An ERP study of the Thatcher illusion. NeuroImage. 2006;32:352–367. doi: 10.1016/j.neuroimage.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Nummenmaa L. Detection of emotional faces: Salient physical features guide effective visual search. Journal of Experimental Psychology: General. 2008;137:471–494. doi: 10.1037/a0012771. [DOI] [PubMed] [Google Scholar]
- Carbon C, Schweinberger R, Kaufmann M, Leder H. The Thatcher illusion seen by the brain: An event-related brain potentials study. Cognitive Brain Research. 2005;24:544–555. doi: 10.1016/j.cogbrainres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Davies-Thompson J, Newling K, Andrews TJ. Image-invariant responses in face-selective regions do not explain the perceptual advantage for familiar face recognition. Cerebral Cortex. 2013;23:370–377. doi: 10.1093/cercor/bhs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology: General. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Donnelly N, Zürcher NR, Cornes K, Snyder J, Naik P, Hadwin J, Hadjikhani N. Discriminating grotesque from typical faces: Evidence from the Thatcher illusion. PLoS ONE. 2011;6(8):e23340. doi: 10.1371/journal.pone.0023340. Retrieved from http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0023340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, Haxby JV. Facial expression and gaze-direction in human superior temporal sulcus. Neuropsychologia. 2007;45:3234–3241. doi: 10.1016/j.neuropsychologia.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Fallshore M, Bartholow J. Recognition of emotion from inverted schematic drawings of faces. Perceptual & Motor Skills. 2003;96:236–244. doi: 10.2466/pms.2003.96.1.236. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Young AW, Andrews TJ. Morphing between expressions dissociates continuous from categorical representations of facial expression in the human brain. Proceedings of the National Academy of Sciences, USA. 2012;109:21164–21169. doi: 10.1073/pnas.1212207110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A. The effect of face inversion on activity in human neural systems for face and object perception. Neuron. 1999;22:189–199. doi: 10.1016/s0896-6273(00)80690-x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Tong F, Nakayama K. The effect of face inversion on the human fusiform face area. Cognition. 1998;68:B1–B11. doi: 10.1016/s0010-0277(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Leder H, Candrian G, Huber O, Bruce V. Configural features in the context of upright and inverted faces. Perception. 2001;30:73–83. doi: 10.1068/p2911. [DOI] [PubMed] [Google Scholar]
- Mazard A, Schiltz C, Rossion B. Recovery from adaptation to facial identity is larger for upright than inverted faces in the human occipito-temporal cortex. Neuropsychologia. 2006;44:912–922. doi: 10.1016/j.neuropsychologia.2005.08.015. [DOI] [PubMed] [Google Scholar]
- McKelvie SJ. Emotional expression in upside-down faces: Evidence for configurational and componential processing. British Journal of Social Psychology. 1995;34:325–334. doi: 10.1111/j.2044-8309.1995.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Milivojevic B, Clapp C, Johnson W, Corballis C. Turn that frown upside down: ERP effects of Thatcherization of misoriented faces. Psychophysiology. 2003;40:967–978. doi: 10.1111/1469-8986.00115. [DOI] [PubMed] [Google Scholar]
- Parks TE, Coss RG, Coss CS. Thatcher and the Cheshire cat: Context and the processing of facial features. Perception. 1985;14:747–754. doi: 10.1068/p140747. [DOI] [PubMed] [Google Scholar]
- Prkachin G. The effects of orientation on detection and identification of facial expressions of emotions. British Journal of Psychology. 2003;94:45–62. doi: 10.1348/000712603762842093. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Brake S, Atkinson P. What’s lost in inverted faces? Cognition. 1993;47:25–57. doi: 10.1016/0010-0277(93)90061-y. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nature Neuroscience. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Malach R, Hadar U, Graif M, Hendler T. Feeling or features: Different sensitivity to emotion in high-order visual cortex and amygdala. Neuron. 2001;32:747–757. doi: 10.1016/s0896-6273(01)00513-x. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Rossion B. Faces are represented holistically in the human occipito-temporal cortex. NeuroImage. 2006;32:1385–1394. doi: 10.1016/j.neuroimage.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Thompson P. Margaret Thatcher: A new illusion. Perception. 1980;9:483–484. doi: 10.1068/p090483. [DOI] [PubMed] [Google Scholar]
- Thompson P, Anstis S, Rhodes G, Jeffery L, Valentine T. Thompson’s 1980 paper. Perception. 2009;38:921–932. [Google Scholar]
- Valentine T. Upside-down faces: A review of the effect of inversion upon face recognition. British Journal of Psychology. 1988;79:471–491. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Yin RK. Looking at upside-down faces. Journal of Experimental Psychology. 1969;81:141–145. [Google Scholar]
- Young A, Bruce V. Understanding person perception. British Journal of Psychology. 2011;102:959–974. doi: 10.1111/j.2044-8295.2011.02045.x. [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. Face perception: Domain specific, not process specific. Neuron. 2004;44:889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. The neural basis of the behavioral face-inversion effect. Current Biology. 2005;15:2256–2262. doi: 10.1016/j.cub.2005.10.072. [DOI] [PubMed] [Google Scholar]