Abstract
Background
In this paper, we describe 360° suture trabeculotomy (360°LOT) ab interno and the short-term course in patients who underwent this procedure.
Methods
We prospectively studied 12 patients (12 eyes) with open-angle glaucoma who underwent 360°LOT ab interno at the Sato Eye Clinic between February and July 2014. The surgical procedure involved making a 1.7 mm temporal corneal incision, exposing an approximately 15° opening in the inner wall of Schlemm’s canal (nasal side) using a Trabectome with a gonioscope, and inserting a 5-0 nylon suture rounded at the tip into Schlemm’s canal opened via the anterior chamber. The suture was then threaded around Schlemm’s canal, and the tip of the suture that emerged on the other side was then advanced through the opening to make a circumferential incision. Intraocular pressure (IOP), number of anti-glaucoma medications used, complications, and the surgery completion rate were prospectively studied.
Results
Mean IOP, which was 19.4 mmHg at baseline, showed a significant decrease at each of the monthly observation points, reaching 13.8 mmHg at 6 months after surgery (P=0.0004, paired t-test). The mean number of anti-glaucoma medications decreased from 3.2 at baseline to 1.1 at 6 months after surgery. IOP spikes ≥30 mmHg were seen in 25% of patients, but there were no other serious complications and the surgery completion rate was 92%.
Conclusion
The 360°LOT ab interno procedure preserves the conjunctiva and sclera, and has a high surgery completion rate when using the anterior chamber approach, and could therefore be an effective short-term treatment of open-angle glaucoma.
Keywords: non-penetrating surgery, intraocular pressure, Schlemm’s canal, corneal incision, Trabectome
Video abstract
Introduction
Trabeculotomy is a procedure that decreases intraocular pressure (IOP) by reducing aqueous outflow resistance via an incision in the trabecular meshwork and the inner wall of Schlemm’s canal, and has been shown to be effective in the treatment of not only exfoliation and developmental glaucomas, but also primary open-angle glaucoma.1–3
The 360° suture trabeculotomy (360°LOT) ab externo procedure, which makes a wider incision in Schlemm’s canal, reportedly yields better treatment outcomes for open-angle glaucoma,4–6 and reduces IOP to approximately 13 mmHg without the need for a bleb. However, as we have already reported, due to the complicated technical nature of 360°LOT ab externo, the surgery completion rates range from 74% to 90%.4,6 Furthermore, since this procedure also requires the same conjunctival and scleral incisions as a standard trabeculotomy, it can have a disadvantageous outcome with regard to any future filtering surgery.
In an attempt to overcome these disadvantages and improve the technique, we modified the 360°LOT ab externo method in order to create the 360°LOT ab interno procedure. The new procedure preserves the conjunctiva and sclera and enables a simpler, more reliable 360° incision in Schlemm’s canal via the anterior chamber.
Here, we describe the 360°LOT ab interno procedure and the findings of our study that examined post-surgical short-term IOP, number of anti-glaucoma medications, complications, and surgery completion rate.
Subjects and methods
Subjects
We conducted a prospective, noncomparative, nonrandomized study in accordance with the principles of the Declaration of Helsinki and with the approval of the Sato Eye Clinic’s ethical review board. The subjects were patients who were consecutively treated for open-angle glaucoma at the Sato Eye Clinic between February and July 2014, and who met the defined criteria for surgery. Criteria for surgery included the presence of primary open-angle glaucoma or exfoliation glaucoma with an IOP >21 mmHg, or progression of glaucomatous visual field loss, both of which occurred despite administration of maximally tolerated anti-glaucoma medications. All patients were diagnosed with glaucomatous optic disc changes and associated visual field loss. An optic disc was considered glaucomatous if it had signs of diffuse thinning, focal narrowing, or notching of the optic disc rim with/without diffuse or localized abnormalities of the peripapillary retinal nerve fiber layer, especially at the inferior or superior poles. Visual field loss was defined as a defect corresponding to the retinal nerve fiber layer damage (eg, nasal step, arcuate field defect, or paracentral depression in clusters of the test sites). Progression of visual field loss was determined with trend analyses based on regression of a visual field parameter over time as well as with event analyses which perform a point-by-point comparison between baseline and follow-up examinations.7,8 The study targeted one eye per patient, and in cases of bilateral surgery, only the first eye operated on was included in our analyses.
Exclusion criteria included a previous history of ocular trauma or glaucoma surgery, presence of other pre-existing ocular diseases, a corneal abnormality that precluded reliable applanation tonometry, having only a single functional eye, or undergoing simultaneous cataract surgery.
All patients underwent the same procedure by the same surgeon at our clinic. Prior to surgery, informed consent was obtained from all patients after explaining the anticipated benefits and risks of the procedure and the study aims.
Baseline assessments in all patients included visual acuity testing, slit-lamp microscope examination, gonioscopy, specular microscope (SP-3000P, Topcon Corporation, Tokyo, Japan), IOP measured by Goldmann tonometry, Humphrey Visual Field Analyzer (Carl-Zeiss Meditec, Dublin, CA, USA), dilated fundus examination, and evaluation of past medical history.
360°LOT ab interno
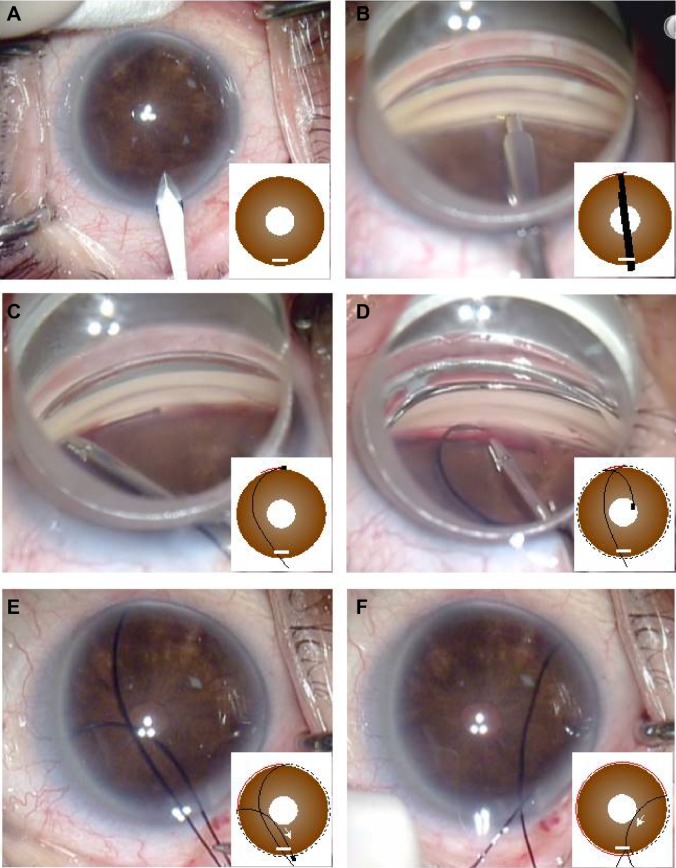
Starting at 1 hour before surgery, three drops of pilocarpine (Sanpilo® 2%, Santen Pharmaceutical Co Ltd, Osaka, Japan), 4% lidocaine (Xylocaine®, Densply Corporation, Tokyo, Japan) and moxifloxacin hydrochloride (Vigamox® Ophthalmic Solution 0.5%, Alcon Japan, Tokyo, Japan) were administered every 5 minutes. Tenon’s capsule anesthesia was administered in the inferior nasal quadrant using 2.0 mL of 2% lidocaine. After making a 1.7 mm incision in the temporal cornea (Figure 1A), the anterior chamber was anesthetized using 1% lidocaine. The patient’s cranial position was tilted to the nasal aspect, and an approximate 15° incision was made on the nasal side of Schlemm’s canal using a Trabectome (Neomedix Corporation, Tustin, CA, USA) while observing the nasal chamber angle with a Swan Jacob gonioprism lens (Figure 1B). The anterior chamber was maintained by filling it with an ocular viscoelastic device (DisCoVisc®, Alcon Japan). The tip of a 5-0 nylon suture (Mani Nylon®, Mani, Tochigi, Japan) was rounded by Paquelin’s cautery (Accu-Temp®, Beaver-Visitec International Japan, Tokyo, Japan) and then inserted into the exposed Schlemm’s canal using a 23 G disposable glass forceps (DSP forceps®, Alcon Japan, Figure 1C). Next, the suture was passed along the outer wall of the canal until emerging from the opening on the opposite side. After clasping the suture tip (Figure 1D), it was pulled through the same opening (Figure 1E) before removing both the inserted and pulled sutures in order to complete the circumferential Schlemm’s canal incision (Figure 1F). If the suture stopped advancing into the Schlemm’s canal, it was removed from the corneal incision to perform the Schlemm’s canal incision for the inserted range. Subsequently, a suture was inserted into the opposite side of the exposed Schlemm’s canal and an incision was made to open the remainder of the canal, thus completing the circumferential incision. Finally, the anterior chamber was washed, with any noted leakage treated by suturing the corneal incision using a 10-0 nylon suture (Mani Nylon), prior to administering a subconjunctival 0.4% betamethasone (Rinderon®, Shionogi, Osaka, Japan) injection to complete the procedure.
Figure 1.
The 360° suture trabeculotomy ab interno procedure.
Notes: (A) 1.7 mm temporal corneal incision, (B) nasal-side Schlemm’s canal incision using a Trabectome, (C) insertion of a 5-0 nylon suture into the exposed Schlemm’s canal, (D) clasp suture emerging on opposite side, and (E, F) removal of suture from same corneal opening, completing the 360° Schlemm’s canal incision.
Postoperative treatment and examination period
Postoperative drug therapy consisted of a 2-week regimen of moxifloxacin hydrochloride, 0.1% betamethasone (Rinderon), and pilocarpine. Patients were examined at 1, 2, and 3 days after surgery, every week thereafter for 1 month, and then every month until 6 months.
Patients with an IOP ≥30 mmHg within 1 month of surgery received additional drug therapy in the form of a carbonic anhydrase inhibitor (Diamox®, Sanwa Kagaku Kenkyusho, Nagoya, Japan) as needed. Anti-glaucoma medications were also administered as needed based on a target IOP ≤20 mmHg at 2 weeks after surgery and a target IOP ≤15 mmHg at 1 month after surgery. Anterior eye examinations and IOP measurements were done at each consultation, while visual acuity testing, gonioscopy, measurement of corneal endothelial cell density, fundus examination, and visual field testing were conducted at suitable times.
Assessment variables and methods
This study examined IOP and percent of IOP reduction from baseline, change in number of anti-glaucoma medications used, surgical complications, and the surgery completion rate. IOP was measured in all patients using a Goldmann tonometer. Baseline IOP was obtained by determining the mean of the three most recent measurements taken on separate days within 1 month of surgery. Postoperative IOP was determined based on measurements taken at 1 day, at 1 and 2 weeks, and at 1, 2, 3, 4, 5, and 6 months after surgery.
The number of anti-glaucoma medications was counted as one for a standard anti-glaucoma drug, and two for a combination drug or carbonic anhydrase inhibitor. Pilocarpine was used for 2 weeks to prevent postoperative peripheral anterior synechia (PAS) and was therefore not counted as an anti-glaucoma medication.
In terms of postoperative complications, an IOP of ≥30 mmHg within 1 month of surgery and a day-to-day increase in the IOP of ≥5 mmHg were defined as IOP spikes. With gonioscopy, focal iris synechia to the scleral spur or posterior meshwork was defined as goniosynechia, while iris adhesions obscuring the meshwork were defined as PAS. The surgery completion rate was determined by the ability to complete the 360° Schlemm’s canal incision as intended.
Statistical analyses were performed using GraphPad Prism version 6.01 statistical software (GraphPad Software, San Diego, CA, USA). Continuous data were expressed as the mean ± standard deviation (SD), with the paired t-test and Wilcoxon matched-pairs signed-rank test used where appropriate. Categorized data were expressed in percentages (%) and compared using Fisher’s test. The significance level was set at P<0.05.
Results
Study patients
The subjects were 12 patients (12 eyes, three men and nine women) with a mean age of 70.8±8.1 years. Glaucoma subtypes included primary open-angle glaucoma in eight eyes and exfoliation glaucoma in four eyes. Baseline IOP was 19.4±2.5 mmHg, number of medications used was 3.2±0.8, mean deviation for the Humphrey visual field score was −8.5±4.9 dB, cup/disc ratio was 0.78±0.15, baseline visual acuity was 0.06±0.16, a past history of cataract surgery was recorded in three eyes, and the observation period was 5.5±0.9 months (Table 1).
Table 1.
Preoperative characteristics of patients
| Sex (male to female) | 3:9 |
| Age (years) | 70.8±8.1 (60–89) |
| Subtype of glaucoma (POAG to XFG) | 8:4 |
| Mean preoperative IOP (mmHg) | 19.4±2.5 (15.3–24.0) |
| Mean number of anti-glaucoma medications | 3.2±0.8 (2–4) |
| Mean deviation (dB) in Humphrey | −8.5±4.9 (−1.05, −15.4) |
| Cup/disc ratio | 0.78±0.15 |
| Mean preoperative best-corrected visual acuity (logMAR) | 0.06±0.16 (−0.08, −0.52) |
| History of cataract surgery (%) | 3 (25.0%) |
| Follow-up period (months) | 5.5±0.9 (3–6) |
Note: Continuous data are expressed as the mean ± standard deviation (range).
Abbreviations: POAG, primary open-angle glaucoma; XFG, exfoliation glaucoma; IOP, intraocular pressure; logMAR, logarithm of the minimum angle of resolution.
Course of IOP
IOP was 19.4±2.5 mmHg (mean ± SD) at baseline, 14.4±6.3 mmHg on day 1, 21.5±13.3 mmHg at week 1, 17.5±8.2 mmHg at week 2, 13.3±3.3 mmHg at month 1, 13.3±2.5 mmHg at month 2, 14.7±3.6 mmHg at month 3, 14.1±2.7 mmHg at month 4, 14.0±2.8 mmHg at month 5, and 13.8±2.7 mmHg at month 6. These results indicate that the IOP decreased significantly from baseline at each time point from 1 month onward (P<0.0001, P<0.0001, P=0.0030, P<0.0001, P=0.0002 and P=0.0004, respectively, paired t-test, Table 2).
Table 2.
Course of IOP and reduction of IOP from baseline
| Time after surgery | IOP (mmHg) reduction (from baseline, %) | P-value* | Eyes (n) |
|---|---|---|---|
| Preoperatively | 19.4±2.5 | 12 | |
| 1 D | 14.4±6.3 (−27.0±24.6) | 0.0041 | 12 |
| 1 W | 21.5±13.3 (12.2±70.5) | 0.5902 | 12 |
| 2 W | 17.5±8.2 (−9.9±42.9) | 0.4315 | 12 |
| 1 M | 13.3±3.3 (−30.9±16.6) | <0.0001 | 12 |
| 2 M | 13.3±2.5 (−30.9±11.5) | <0.0001 | 12 |
| 3 M | 14.7±3.6 (−23.0±22.9) | 0.0030 | 12 |
| 4 M | 14.1±2.7 (−26.7±12.8) | <0.0001 | 11 |
| 5 M | 14.0±2.8 (−26.9±15.4) | 0.0002 | 11 |
| 6 M | 13.8±2.7 (−30.5±12.3) | 0.0004 | 8 |
Notes: Continuous data were expressed as mean ± standard deviation.
P-value compared with preoperative IOP using the paired t-test.
Abbreviations: IOP, intraocular pressure; D, day; W, week; M, month.
Percent reduction of IOP from baseline was −27.0%±24.6% on day 1, 12.2%±70.5% at week 1, −9.9%±42.9% at week 2, −30.9%±16.6% at month 1, 30.9%±11.5% at month 2, −23.0%±22.9% at month 3, −26.7%±12.8% at month 4, −26.9%±15.4% at month 5, and −30.5%±12.3% at month 6 (Table 3).
Table 3.
Number of anti-glaucoma medications
| Time after surgery | Anti-glaucoma medications (n) | P-value* | Eyes (n) |
|---|---|---|---|
| Preoperatively | 3.2±0.8 | 12 | |
| 1 M | 0.7±1.0 | <0.0001 | 12 |
| 2 M | 0.6±0.9 | <0.0001 | 12 |
| 3 M | 0.6±0.9 | <0.0001 | 12 |
| 4 M | 0.7±1.0 | <0.0001 | 11 |
| 5 M | 0.9±1.0 | <0.0001 | 11 |
| 6 M | 1.1±1.4 | 0.0002 | 8 |
Notes: Continuous data are expressed as the mean ± standard deviation.
P-value compared with preoperative number of anti-glaucoma medications using Wilcoxon signed-rank test.
Abbreviation: M, month/months.
Number of anti-glaucoma medications
The number of anti-glaucoma medications used was 3.2±0.8 at baseline, 0.7±1.0 at month 1, 0.6±0.9 at month 2, 0.6±0.9 at month 3, 0.7±1.0 at month 4, 0.9±1.0 at month 5, and 1.1±1.4 at month 6. These results demonstrate that medication use decreased significantly from baseline from 1 to 6 months after surgery (P<0.0001, P<0.0001, P<0.0001, P<0.0001, P<0.0001 and P=0.0020, respectively, Wilcoxon signed-rank test, Table 3).
Complications and surgery completion rate
Complications experienced during and after surgery are shown in Table 4. The only complication seen during surgery was anterior chamber hemorrhage, which occurred in 12 eyes. Although hyphema occurred in eight eyes (67%) after surgery, it was spontaneously absorbed in an average of 6.4 days, and none of the patients required anterior chamber irrigation. IOP spikes were seen in three eyes (25%) but administration of oral acetazolamide reduced IOP to <30 mmHg in 7.7±6.7 days. Gonioscopy revealed focal PAS with a mean range of 13.3% in three eyes (25.0%) and goniosynechia with a mean range of 22.2% in six eyes (50.0%) at 2 months after surgery.
Table 4.
Intraoperative and postoperative complications with the 360° suture trabeculotomy ab interno procedure
| Patients, n (%) | |
|---|---|
| Intraoperative | |
| Anterior chamber hemorrhage | 12 (100) |
| Anterior chamber flattening | 0 (0) |
| Descemet’s membrane detachment | 0 (0) |
| Cyclodialysis | 0 (0) |
| Iris injuries | 0 (0) |
| Postoperative | |
| Hyphema | 8 (67) |
| IOP spike (≥30 mmHg) | 3 (25) |
| Hypotony (<5 mmHg) | 0 (0) |
| Peripheral anterior synechia | 3 (25) |
| Endothelium cell loss ≥10% | 0 (0) |
| Anterior chamber lavage | 0 (0) |
| Infection | 0 (0) |
| Wound leaks | 0 (0) |
| BCVA decrease by 2 or more lines due to cataract | 2 (17) |
| Procedure failure | 1 (8) |
Abbreviations: BCVA, best-corrected visual acuity; IOP, intraocular pressure.
Corrected maximum visual acuity (log of the minimum angle of resolution [logMAR]) was 0.06±0.16 at baseline and 0.13±0.14 at month 2, with a decrease by two or more lines seen in two eyes, one of which underwent cataract surgery at 2 months after surgery. Corneal endothelial cell density was 2,517±312.0/mm2 at baseline and 2,487±304.5/mm2 at month 2, with an average decrease of −1.2%±1.5%. None of the patients showed a decrease of ≥10%. No infections or wound leaks were seen. The procedure was not successful in one eye after the suture failed to advance and the upward 90° could not be performed. Thus, in this case, the Schlemm’s canal incision was performed on the 270° area.
Discussion
We have previously reported the completion rate for 360°LOT ab externo is be around 74%–90%,4,6 as the 360° incision in Schlemm’s canal cannot always be performed as planned. The causes of the previous failures that have occurred when inserting the suture into Schlemm’s canal include early perforation of the anterior chamber, incorrect insertion into the suprachoroidal space, and inability to advance the suture deeper into the canal. Passing a suture around the entire circumference of Schlemm’s canal with a 90% or greater level of certainty has therefore been regarded as technically difficult.
Using the 360°LOT ab interno procedure proved to be much easier than when using the ab externo approach, as we achieved a ≥90% completion rate in making a 360° incision in Schlemm’s canal from the anterior chamber, and it also helped in preserving the conjunctiva and sclera. The reason that the ab interno procedure had a higher completion rate than the ab externo procedure is that suture insertion into Schlemm’s canal in the ab externo approach is performed blind, which leads to a risk of erroneous insertion into the anterior chamber or subchoroidal space. Since suture insertion into Schlemm’s canal in the ab interno approach can be reliably performed under direct gonioscopic observation, there is no risk of incorrect insertion.
With the ab interno technique, the use of a Trabectome to make the incision enables safe and reliable exposure of Schlemm’s canal without causing damage to the outer canal wall.9 While insertion is also possible using a 27 G needle or the like,10 it is rather difficult to incise only the inner wall without damaging the outer wall. Even if the inner wall is opened, it is difficult to insert a 5-0 nylon suture rounded at the tip into the inner canal wall, so this opening needs to be widened by using forceps and a viscoelastic substance.
The IOP after 360°LOT ab interno starting from 1 month onwards was similar to cases using the ab externo approach,4,5 and was significantly lower than at baseline. Furthermore, the number of anti-glaucoma medications used also significantly decreased from 3.2 at baseline to around one after surgery. At 6 months after surgery, the IOP had declined to around 13.8 mmHg, which was approximately 30% lower than at baseline. This suggests that the procedure could be effective in treating open-angle glaucoma.
Similar to the previously reported 360°LOT ab externo studies,4–6 the incidence of complications for the 360°LOT ab interno approach included anterior chamber hemorrhage, postoperative hyphema, and IOP spikes. In all cases, these complications could be controlled by use of conservative treatments. There was a 25% incidence of postoperative PAS with a mean range of 13.3%, suggesting that IOP was unaffected. Decreases in visual acuity due to early postoperative cataract progression were observed in two eyes, one of which required cataract surgery. Since these events were attributed to the surgical instrument coming into contact with the crystalline lens despite use of a miotic agent, care is required to prevent the forceps from touching the crystalline lens by injecting a viscoelastic substance to maintain the depths of the anterior chamber. Although it is possible to treat phakic eyes provided extreme care is taken when working near the crystalline lens, aphakic/pseudophakic eyes are better suited to this procedure.
Limitations of the present study include the use of preliminary data on the 360°LOT ab interno procedure, the small patient population, and the short observation period. Although future studies will require a larger patient population and a longer observation period, we believe that use of the 360°LOT ab interno procedure would be beneficial for patients because it can preserve the conjunctiva and sclera, and can be performed more easily and provide a similar level of short-term efficacy against open-angle glaucoma when compared with the 360°LOT ab externo procedure.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Akimoto M, Tanihara H, Negi A, Nagata M. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol. 1994;112(12):1540–1544. doi: 10.1001/archopht.1994.01090240046024. [DOI] [PubMed] [Google Scholar]
- 2.Tanihara H, Negi A, Akimoto M, Nagata M. Long-term surgical results of combined trabeculotomy ab externo and cataract extraction. Ophthalmic Surg. 1995;26(4):316–324. [PubMed] [Google Scholar]
- 3.Wada Y, Nakatsu A, Kondo T. Long-term results of trabeculotomy ab externo. Ophthalmic Surg. 1994;25(5):317–320. [PubMed] [Google Scholar]
- 4.Chin S, Nitta T, Shinmei Y, et al. Reduction of intraocular pressure using a modified 360-degree suture trabeculotomy technique in primary and secondary open-angle glaucoma: a pilot study. J Glaucoma. 2012;21(6):401–407. doi: 10.1097/IJG.0b013e318218240c. [DOI] [PubMed] [Google Scholar]
- 5.Sato T, Hirata A, Mizoguchi T. Outcomes of 360 degrees suture trabeculotomy with deep sclerectomy combined with cataract surgery for primary open angle glaucoma and coexisting cataract. Clin Ophthalmol. 2014;8:1301–1310. doi: 10.2147/OPTH.S64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato T, Hirata A. Surgical outcome of modified 360° suture trabeculotomy with deep sclerectomy in eyes with primary open-angle glaucoma. Atarashii Ganka. 2014;31(2):271–276. Japanese. [Google Scholar]
- 7.Artes PH, Hutchison DM, Nicolela MT, LeBlanc RP, Chauhan BC. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005;46(7):2451–2457. doi: 10.1167/iovs.05-0135. [DOI] [PubMed] [Google Scholar]
- 8.Vesti E, Johnson CA, Chauhan BC. Comparison of different methods for detecting glaucomatous visual field progression. Invest Ophthalmol Vis Sci. 2003;44(9):3873–3879. doi: 10.1167/iovs.02-1171. [DOI] [PubMed] [Google Scholar]
- 9.Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524–529. doi: 10.1016/j.ajo.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855–861. doi: 10.1016/j.ophtha.2013.11.001. [DOI] [PubMed] [Google Scholar]