Abstract
Polychlorinated biphenyls are among the most well-studied endocrine-disrupting chemicals (EDCs) for their neurobehavioral effects, especially neurodevelopment and cognitive performance. In addition, past research has demonstrated effects of PCBs on circulating hormones and associated changes in reproductive behaviors. This article will focus on recent advances that have been made in characterizing developmental PCB effects on reproductive function, broader social and affective behaviors, and the neuroendocrine mechanisms behind such changes. In general, PCBs seem to inhibit reproductive function by suppressing multiple aspects of the associated hypothalamic circuitry. Additionally, PCBs may also reduce motivation for social behaviors and induce depressive-like symptoms via overall reductions in dopaminergic and glutamatergic functions in the limbic system. However, more work with human-relevant exposure paradigms is needed to fully support these conclusions.
Keywords: development, endocrine disrupting chemical, dopamine, glutamate, estrogen, testosterone, polychlorinated biphenyl
Introduction
Gonadal Hormones and Development
In all vertebrates, reproductive development, behavior, and physiological function are directed via the hypothalamic-pituitary-gonadal (HPG) axis [1]. From the hypothalamus, gonadotropin-releasing hormone (GnRH) is secreted from about 1000 neurons to stimulate the release of the pituitary gonadotropins. These travel through the blood stream to the gonads to promote steroidogenesis of estrogens (E), progesterone (P), and testosterone (T) from the ovaries and testes, and to drive gametogenesis. The gonadal steroids exert their actions via steroid hormone receptors that are located throughout the body and brain, including estrogen receptors (ERs), androgen receptors (ARs), and progesterone receptors (PRs). Activation of steroid hormone receptors stimulates expression of target genes to control biological processes such as the growth of tissues, cellular proliferation or death, and hormone-dependent behaviors. In terms of neuroendocrine function, receptors in the hypothalamus and pituitary can also act to regulate activity of the HPG axis via negative feedback in response to circulating hormones, and (in females) positive feedback that leads to ovulation. Given the ubiquity of gonadal hormonal action throughout the body, any disruption of the careful balance of steroid actions in the nervous system can have major health consequences.
Early development, especially in the fetus and infant, are periods of life when the brain and body are particularly sensitive to effects of gonadal hormones on tissue structure and function in sex-specific ways. In contrast to the relatively short term ‘activating’ effects present when hormones are in circulation, hormones can also have long-term ‘organizing’ effects on tissue structure and function during these sensitive periods [2]. When the tissue in question is neural, functional changes are often associated with changes in sex-typical behaviors. These effects are best characterized in rodents, where testosterone from the male fetus is converted to estradiol to determine size, cell number, receptor expression, and dendritic spine formation in brain regions important in regulating sexual behavior [3]. Importantly, these effects can persist into adulthood, independent of circulating gonadal hormones. As such, any disruption of gonadal hormones during early development can have far reaching consequences for brain and behavior.
Polychlorinated biphenyls (PCBs) are Endocrine Disrupting Chemicals (EDCs)
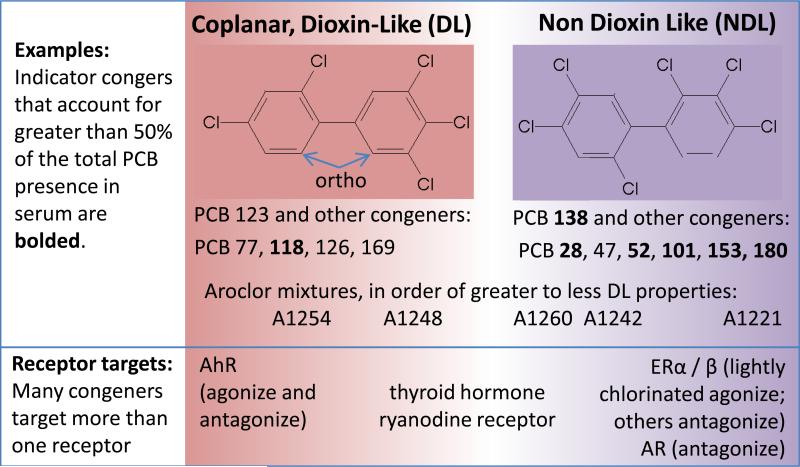
An EDC is an exogenous chemical, or mixture of chemicals, that can interfere with any aspect of hormone action [4]. Much of our knowledge about EDC effects on the brain come from studies on PCBs, a family of chemicals within the industrial organohalogens and persistent organic pollutants. There are 209 different PCB congeners of the same basic structure: two connected phenolic rings with 1-10 chlorine molecules at different positions around the rings (Figure 1). Multiple attempts have been made to group congeners into functional categories [5-7]. One of the broadest categorizations is based on physical structure and likeness to dioxin, as dictated by having zero or one ortho substitutions and substitutions at both para and meta positions. Dioxin-like (DL) compounds can bind the aryl hydrocarbon receptor (AhR) and induce the cytochrome P450 monooxygenase enzyme (CYP) 1A subfamily, and are classically thought to be the most toxic and anti-estrogenic. Toxic Equivalency Factors (TEFs) have been used to relate the ability of DL congeners to induce AhR activity to that of dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) itself [8]. However, TEFs cannot characterize other biologically active capacities of the more varied group of Non Dioxin-like (NDL) congeners. These can induce the CYP2B enzyme, induce both CYP1A and 2B enzymes, or have estrogenic or anti-androgenic properties through actions on steroid hormone receptors in lighter and more heavily chlorinated congeners, respectively [9,10]. Additional mechanisms of action at thyroid hormone receptors and ryanodine receptors are beyond the scope of this review, but are described well elsewhere [11,12]. Because exposures to PCBs typically occur in mixtures, and even single PCB congeners can act at more than one pathway, PCB actions as EDCs are quite diverse [13].
Figure 1.
There are 209 different congeners of PCBs, and numbers and locations of chlorine atoms dictate structure and mechanism of action, as described above. Many studies use industrial mixtures of ~50 PCBs, called Aroclors. The degree of chlorination is indicated by the last two digits in each Aroclor name; for example; A1254 is 54% chlorine, with an average of 5 chlorines per molecules. Given that many of the receptor targets interact and that congeners (and their metabolites) exist in the environment in mixes, the physiological and behavioral outcomes of PCB exposure are always composites of multiple mechanisms interacting.
Used in industrial processes from 1930s until being banned in 1977 in the US, PCBs are persistent, lipophilic molecules. With half lives over decades (an average of 10 to 18 years for light and heavy congeners, respectively), some congeners work their way up the food chain and accumulate in fatty fish and breast milk [14]. Ingested sources account for the bulk of human exposure; however lighter congeners can also be volatile and inhaled, especially in house dust [15]. Occupational exposure from years ago also remains a contributor to human body burden in exposed individuals. In capacitor workers exposed to PCBs 28 years earlier, total PCB concentrations in serum was ~6ng/g in 2011 [14]; this is ~10 times greater than normal US population samples from 2003-2004 at 0.820 ng/g [16,17]. An estimate of adult human PCB exposure is 2-6 ng/kg/day via food [18,19], but estimates vary greatly by age and population. For example, infants are often exposed to higher levels via breast milk (an average of 3 ug/kg) [20], especially in heavily contaminated environments (up to 10ug/kg/day) [21]. These infant exposure levels are above the minimum risk level (MRL) derived by the ATSDR of 0.03ug/kg/day for daily oral exposure less than one year, based off of a LOAEL of 0.0075mg/kg/day for neurobehavioral effects in infant monkeys [13]. Additionally, humans are exposed to PCBs throughout their lives; therefore, normal levels of PCB exposure still pose risks for subtle neurobiological effects.
Toxic effects of PCBs in humans were first indicated by acute high exposure in adulthood, either from high occupational exposure or contaminated food, resulting in skin rashes, weakness, and liver disease [22-24]. Further studies have also demonstrated the importance of developmental exposure, as the offspring of the acutely exposed adults show signs of reduced fetal and childhood growth and neurodevelopmental issues [25-27]. Chronic exposure during development via maternal consumption of contaminated lake fish or at “background” levels has long been tied to reduced fetal growth and neurodevelopmental problems in infants and young children [28-33]. Similar effects have been found in more recent studies that measured specific congeners in maternal blood or placental tissue in humans, including shorter gestational length with mono-chlorinated NDL congener [34], reduced IQ with heavily chlorinated NDL congener [35], reduced motor development with DL and NDL congeners [36]. A thorough analysis of these neurodevelopmental effects can be found elsewhere [37] and is beyond the scope of the current review.
Given the estrogenic and anti-androgenic effects of PCBs in cellular assays, effects of PCBs on hormone-sensitive developmental processes are of great interest. Effects of PCBs on reproductive function and behavior, and the neuroendocrine systems that regulate them, have been well-described through 2011 [38,39]. Thus, this review will attempt to bring the reader up to date on recent findings (since 2011). Additionally, reproductive behaviors occur in a broader context of social motivation, on which the effects of PCBs have not been well defined. Ones’ behaviors are dictated by the motivation to avoid negative stimuli and approach positive stimuli. In social species, interactions with conspecifics include stimuli that can be attractive (a new friend or potential mate) or aversive (a new location with the potential for predation). Perceptions of the balance between these rewarding and aversive stimuli eventually determine our actions. Importantly, these internal calculations are hormone- and, thus potentially, PCB-sensitive. As such, this review will also attempt to describe the effects of developmental PCB exposure on broader social behavior and neural correlates of negative and positive motivation.
Effects of Developmental PCB Exposure on the HPG Axis.
Exposure to PCBs has long been associated with reproductive dysfunction in humans, including decreased sperm motility [40], decrease in fecundity [41], earlier menarche [42], altered sex ratio [43], and altered gonadal hormones in newborns [44]. However, many of these earlier studies were limited by technical analysis of PCB congeners, and failed to provide possible mechanisms of action. More recent findings in humans, and data from rodents indicating neuroendocrine dysfunctions, are described below.
Human data
Recent epidemiological studies focused on the effects of developmental exposure on adult physiological function. As in newborns, greater concentrations of NDL serum PCBs was associated with reduced T in adolescent (PCB 138 153, 180) and adult (PCB74, 99, 153, and 206) males [45,46]. Exposure to specific congeners, as measured in the blood of mothers immediately following parturition, also affected time to pregnancy in daughters; specifically, higher levels of PCBs 187, 156 and 99 were associated with longer time to pregnancy, while higher levels of PCBs 105, 138 and 183 was associated with shorter time to pregnancy [47]. Interestingly, the effect of the congener was somewhat independent of the classical functional groupings, highlighting the need for continued congener-specific analysis. One recent study provides a possible mechanism of action: exposure to mixed inducers of CYP1A and CYPIIB subfamilies was associated with upregulation of aromatase enzyme and estrogen receptor beta gene expression in blood samples from adult daughters of Great Lakes fish-eaters [6]. However, this effect alone cannot explain both an increase and decrease in time to pregnancy; therefore, I turn to more mechanistic rodent studies.
Rodent data
PCB effects on rodent reproduction and the HPG axis are well-characterized and described in Table 1 and Figure 2. As in humans, A1221 increased the number of mating trials required for female offspring to reach a successful pregnancy [48]. This could be the result of PCB-associated changes in female paced mating [48], as it is known that the ability of a female to control of the pace of mating is important for reproductive success [49]. Alternatively, or in addition to the possibility that PCBs could be reducing fecundity by altering behavior, HPG axis dysfunction could be responsible. As in humans, reductions in circulating E, T, and P were observed in neonate and adult male and female rats in response to A1221 and a mix of NDL PCBs [50,51]. Changes in circulating hormones may result from PCB-induced alterations in synthesis enzymes [52], but these findings cannot explain all hormonal changes. Because regulation of the HPG axis is orchestrated by a network of hormones, neuropeptides and neurotransmitters in the preoptic area (POA) and anteroventral periventricular (AVPV) regions of the hypothalamus, several studies have investigated how developmental PCB exposure affects these neural substrates. Broadly speaking, A1221 tended to masculinize the complexity of gene expression regulatory networks in the female AVPV across development [53]. A1221 caused a reduction in ERalpha and AR expression in adult female POAs [50], and masculinized the pattern of expression of G-protein coupled estrogen receptor in the AVPV and AR in AVPV and arcuate nucleus in rats during development [53]. Hypothalamic PR was also decreased by embryonic A1254 in male and female rats [54].
Table 1.
PCB exposure details of recent rodent studies on PCB effects on social and affective behavior and neural correlates cited herein.
| PCB Congeners | Dose (PCB/BW) per day | Exposure Period | Exposure Route | Test Period | Sex Tested | Species | Effect |
|---|---|---|---|---|---|---|---|
| A1221 | 0.1-10mg/kg | E16, E18 | ip to dam | Adult | F | Rat | 1mg/kg Dec in Fecundity; Inc return latency in paced mating |
| A1221 | 1mg/kg | E16, E18 | ip to dam | Adult | M&F | Rat | Dec P in M. Dec ERalpha, AR in POA in F; Dec in GnRH Fos colabel in F; Dec Kiss IR in F POA |
| Mix of PCBs 138, 153, 180 (all NDL) | 1mg/kg | E16, E18 | ip to dam | Adult | M&F | Rat | Dec NR2b expression in F; Dec Kiss IR in F POA; Dec T and P in M |
| A1221 | 1mg/kg | E16, E18 | ip to dam | P1 | M&F | Rat | Dec in P in F; Inc NR2b expression in POA in F not M |
| Mix of PCBs 138, 153, 180 (all NDL) | 1mg/kg | E16, E18 | ip to dam | P1 | M | Rat | Dec T in M; Dec Kiss IR in POA in M not F |
| Mix of PCBs 126 (DL), 138, 153, 180 (NDL) | 10mg/kg | E15-E19 | subQ to dam | Juv. & Adult | M&F | Rat | Inc aromatase in M at P21; Dec 5alpha-R1 in M&F at P60; Inc 5alpha-R2 in F at P60; Reduction in male sex beh.; no effect on depressive or anxiety behavior |
| A1221 | 1mg/kg | E16, E18 | ip to dam | Juv. & Adult | F | Rat | Masculinize hyp gene expression network, GPER in AVPV and AR |
| A1254 | 10mg/kg | E10 to E18 | subQ to dam | Adult | M&F | Rat | Dec in basal PR in F hypothalamus; Dec in PR after E challenge in M&F hypothalamus |
| A1221 | 0.01 - 100uM | in vitro | Rat GT1-7 cells | Inc GnRH mRNA and peptide, neurite outgrowth | |||
| A1254 | Inc and Dec in GnRH mRNA with Inc dose. No effect on peptide | ||||||
| A1253, PCB77, 118, 153 | 0.1-100uM | in vitro | GT1-7 cell line | Dose dependent Inc / Dec GnRH release; ER dependent. Inc cell death (apoptosis and necrosis). | |||
Compare doses with the estimated range of 2 ng/kg - 10ug/kg total daily human oral exposure, taking into account the number of exposures in study design. Abbreviations: Embryonic (E) and Postnatal (P) days of age; Male (M) and Female (F). Increase (Inc) and Decrease (Dec).
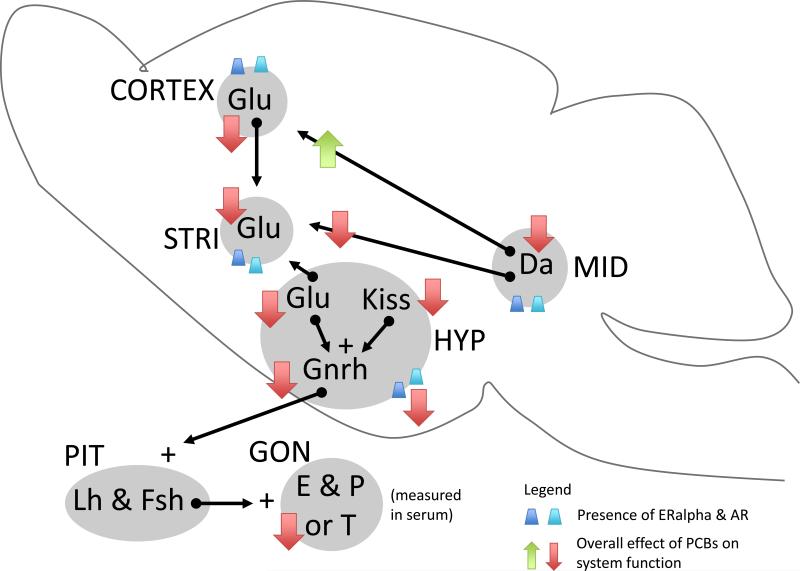
Figure 2.
PCBs effects on neuroendocrine circuitry regulating reproductive physiology and sociosexual function. Brain regions and endocrine glands involved are shown as grey areas, labeled in all capital letters (STRI, striatum; HYP, hypothalamus; MID, midbrain; PIT, pituitary; GON, gonad) and containing appropriate neurotransmitters (Da, dopamine; Glu, glutamate; Kiss, kisspeptin), and hormones (Gnrh, Gonadotropin releasing hormone; Lh, leutinizing hormone; Fsh, follicle stimulating hormone; E, estradiol; P, progesterone; T, testosterone), within. Simplified functional connections between areas are shown with arrows; when possible a stimulatory (+) or inhibitory (-) effect on the downstream region is shown. All areas are responsive to gonadal hormones, as indicated by presence of ER and AR in dark and light blue cones, respectively. Overall effects of PCBs on neural systems (cell viability, synthesis, release, receptors, etc) are indicated by nearby red or green arrow, indicating an increase or decrease in function, respectively.
There is some evidence that PCBs affect GnRH cells, the master driver of the HPG system. A1254 and specific DL and NDL congeners caused an increase and then decrease of GnRH mRNA expression and peptide in hypothalamic GT1-7 cells as dose and duration of treatment increased, suggestive of a non-monotonic dose-response curve in vitro [55,56]. The PCB-suppression of GnRH peptide release was prevented by co-treatment with an ER antagonist, indicating PCB action at the ER on these cells [56]. Finally, the same NDL and DL congeners also increased apoptosis in the GnRH cells in an inverse U curve, with 0.1uM and 1uM having greater effects than higher doses [56]. The micromolar doses used in these two studies were relevant to human exposures, as PCBs have been found at levels of 50 ppb in neonatal human brains [57]. In vivo data support these findings, as a reduction in GnRH activity (as indicated by the immediate early gene product Fos co-expression in the cells) was also demonstrated in adult females treated with A1221 [50].
More is known about PCB effects on the hypothalamic neurotransmitters and neuropeptides that regulate GnRH neurosecretion and play roles in other neuroendocrine and behavioral functions. For example, the neurotransmitter glutamate is the primary excitatory neurotransmitter in the nervous system and is important in neuroendocrine development and function in adulthood [58]. Glutamate signals through NMDA receptors (in addition to others), and expression of NMDA receptor subunits in the hypothalamus is generally reduced by PCBs. A1221- and a NDL PCB mix reduced the NMDA-NR2b subunit mRNA expression in adult female POAs [50]. Interestingly, and consistent with other EDC literature on the brain, the observed effects of A1221 are dependent upon the age at which gene expression is measured. In contrast with adult measures, A1221 increased NR2b receptor mRNA when measured in neonatal female POAs [51]. Similarly, age and sex specific effects of A1221 on NR1 and NR2d was found in the AVPV [53].
The hypothalamic neuropeptide kisspeptin is also critical for reproductive function and sensitive to EDCs [59,60]. A NDL PCB mix reduced kisspeptin receptor expression in the POA and A1221 reduced Kiss1 gene expression in the AVPV of young and adult males [50,53]. In contrast, A1221 increased Kiss1 gene expression in the AVPV of females [53]. However, these changes in mRNA are not paralleled by protein expression in all regions, as A1221 and PCB mix reduced kisspeptin immunoreactivity in adult female POAs [51]. A1221 also reduced the expression of hypothalamic growth factors that regulate GnRH cells in neonatal male POA, and female adult POAs [50,51].
As a whole, these data suggest that neurotransmitters, neurotrophic factors, and steroid hormone receptors involved in the control of GnRH activity are suppressed by PCB exposure (Figure 2). The outcome of these changes is a reduction in GnRH output and diminished reproductive function. While the evidence for PBCs is somewhat limited, they are consistent with other EDCs like BPA that are also known to target the GnRH regulatory circuitry [61]. Moreover, all of these hypothalamic molecules play much broader roles in neuroendocrine control systems involved in stress, lactation, metabolism, and growth. Thus, while these endpoints were not investigated in the literature summarized above, these are important targets for future mechanistic research on PCB effects.
Effects of Developmental PCB Exposure on Social Behavior and Neuroendocrine Mechanisms.
Effects of PCBs on hormones and hypothalamic neural circuits involved in neuroendocrine control extend to links among hormones and behavior. Not only are gonadal hormones important for reproductive behaviors; they also affect cognitive function and affective state in humans and rodent models, including reward-seeking, depressive, and anxiety-like behaviors. However few studies have investigated the effects of PCBs on affective behaviors, especially depressive traits in humans. Mixed results have been reported: higher levels of heavily chlorinated PCBs that induce CYP2B in serum increased the incidence of depressive like symptoms in older adults [62], but not diagnoses of depression in young adults [63]. Different effects are even found among capacitor workers occupationally exposed to PCBs earlier in their life: in a German study, higher measures of DL and NDL congeners were associated with major increases in symptoms of depression but not anxiety [64], whereas in a US study, no associations were found [65]. Findings of increased depressive symptoms are also true in studies of exposure to other EDCs, especially pesticides [66]. Rodent studies have attempted to characterize effects of developmental PCBs on social and affective behaviors, and also indicate potential mechanisms of PCB action on the function of dopaminergic and glutamatergic systems in the limbic system (Table 1).
Social and Affective Behavior
Sociosexual interactions are the result of opposing approach and avoidance motivating factors, and are highly sensitive to hormones. In rodents, the neural correlates of these behaviors are well-studied, providing an excellent model with which to test the hypothesis that PCBs alter the neural correlates of affective behavior. In general, the literature shows that PCBs reduce social behavior in female rats. Gestational exposure to A1221, A1254, DL and NDL PCBs at a range of doses reduced lordosis behavior and increased the amount of time a female rat gave herself between male sex acts in paced mating paradigms [48,67-70]. The ability to pace interactions imparts many of the rewarding aspects of sexual interaction [71]; therefore, these findings are consistent with reduced motivation for sexual interactions. Similarly, PCB 77 reduced the preference for a male over a female stimulus animal in exposed females [72]. No effects of PCB 47 or 77 were found on masculine reproductive behaviors in rats [69,73], however, a more heavily chlorinated mix of PCBs caused an increase in latency to perform sex behavior in males [52]. One study of general social behavior found that exposure to a DL and NDL mix of two PCBs also reduced approach and memory of a social stimulus after isolation in juvenile and adult male rats [74]. The effect on memory may or may not be specific to social interactions, as studies on spatial memory have provided mixed results [52,75-79].
Changes in social behavior may be explained by one or more mechanisms. Sensory deficits would certainly affect behavior, and PCBs were shown to affect auditory and visual but not olfactory functions [80-82]. Increased anxiety and aversion to novel situations could also limit social interactions, however earlier work found contradictory effects of PCBs on anxiety [52,83]. More recently, developmental exposure to a NDL PCB mix caused an increase in anxiety-like behaviors in the elevated plus maze and light dark box in mice [75]. In contrast, a reduction in anxiety in an open field test was found in response to A1254 in juvenile female but not male mice [84]. Notably, these studies point to potentially age-dependent effects of PCBs on depressive-like behavior, reminiscent of findings on human depression. PCBs may also alter perceptions of pleasurable or rewarding social interactions, thereby affecting social behaviors. PCBs do not affect performance in classic tests of depressive-like behavior as measured in tail suspension and forced swim paradigms [52,75,84]. However, an alternative test to assess depressive-like behavior indicates an effect of PCBs on anhedonia (a consistent marker of depression): gestational exposure to relatively high doses of specific congeners increased or decreased intake of sweetened solutions in rats in congener and sex-specific ways [85].
Neural Mechanisms
The behaviors described above are dependent on several complex and not completely characterized neural circuits including the frontal cortex, striatum, hippocampus, and midbrain, simply described in Figure 2. Within these neural pathways, glutamate and dopamine are essential, and may be the proximate mechanisms for effects of PCBs on behavior. Glutamatergic signaling throughout the limbic system drives neural activation relevant to social behavior, and is affected by PCBs in similar ways as discussed in the hypothalamus. Exposure to a heavily chlorinated PCB mix reduced NMDA receptor binding in the cortex but not hippocampus of adult male rats [86]. Exposure to A1254 decreased NMDA receptor binding in striatum, frontal cortex and hippocampus in female adolescent mice [84]. Adult exposure to A1254 also inhibited expression of glial glutamate transporter in forebrain of chronically exposed adult male and female rats [87]. Exposure to NDL PCBs reduced NMDA-receptor induction of extracellular cGMP and NMDA-NR1 subunit in cerebellum [88]. The same exposure paradigm reduced extracelluar glutamate and the effect of metabotropic glutamate receptor activation on glutamate and dopamine release in the nucleus accumbens [67].
Dopamine (DA) has long been a focus of PCB studies since early work revealed that NDL PCBs decreased DA tissue content and increased extracellular DA in dopaminergic cell lines or striatal slices, as reviewed in [73] and [89]. The striatum and prefrontal cortex receive large dopaminergic projections from the midbrain, and are involved in coordinating movement and rewarding behavior; therefore effects seen in striatum and prefrontal cortex likely interact to affect locomotor and reward seeking sexual behavior. These effects could be the result of PCB-induced disruptions in synthesis [90], reuptake of DA from the synapse via dopamine transporter (DAT) competitive inhibition [91,92], or packaging of DA into vesicles by vesicular monoamine transporter [93]. More recent studies support the striatal findings, as PCB 180 increased basal extracellular DA in ventral striatum in male and female adult rats [67], and NDL PCBs inhibited DAT binding in male rat synaptosomes in vitro [87, 94]. Adult exposure to A1254 also reduced TH and DAT levels in the striatum in male mice [95]. There may be some congener or regional specificity in the effects on DA regulation, as specific PCBs increased DA in frontal cortex tissues of adolescent and adult male and female rats [96]. In addition to affecting DA and transporters, effects on DA receptors have also been demonstrated. PCBs do not seem to affect D1 receptor family in females, but PCB 153 decreased D1 receptor density in male cortex and striatum [97]. PCB 153 also decreased D2 receptor affinity, while at the same time, increased cortical but not striatal expression of D2 receptor density in male juveniles and male and female adult rats [97]. Similarly, a mixture of Aroclors caused increased striatal dopamine autoreceptor (D2) sensitivity in male but not female adult rats [98]. Importantly, effects of PCBs on striatal DAT in human populations have also been found [99].
While dopaminergic and glutamatergic neural circuitries may be a proximate mechanism for effects of PCBs on behavior, the cells themselves may or may not respond directly to PCBs (Figure 2). It is possible that PCBs act elsewhere in the body to regulate circulating gonadal hormones, which then modify activity of the cells in question. Alternatively, cells throughout the striatum, cortex, and hippocampus express estrogen and androgen receptors, and therefore could be sites of direct action of NDL PCBs. Midbrain dopaminergic cells also express AhR, making them a direct target of DL congeners [100]. These sensitivities could serve to alter cellular function or even induce neurotoxic effects [101,102]. The mechanisms underlying neurotoxicity also include glutamate-mediated effects. A1254-induced cell death in vitro was inhibited by a NMDA receptor antagonist, indicating the importance of NMDA associated calcium handling in PCB-induced neurotoxicity [103]. Dopamine dysregulation is also capable of generating reactive oxygen species, and 4 weeks of adult exposure to a high dose of A1254 caused DA (and non-DA) cell death in the midbrain and increased oxidative stress markers [95]. Similarly, 30 days of adult exposure to a lower dose of A1254 also increased markers of oxidative stress in the hippocampus [104], lending some support for this possible mechanism of action.
Complications and Conclusions
Taken together, PCBs disrupt reproductive function and sociosexual behaviors via subtle changes in both gonadal hormones and (directly or indirectly) neural function. The specific mechanisms of action of PCBs mixes are still difficult to determine. The effects of specific congeners may be the result of actions at hormone receptors, thereby changing circulating gonadal hormones or regulating gene expression in target cells anywhere along the HPG axis. Alternatively, some congeners act at AhR, inducing cytotoxic effects. The negative and positive feedback loops in the HPG axis and interactions between dopaminergic and glutamatergic systems add another layer of complexity when trying to determine the primary neural target of PCBs. Therefore, to suggest one specific mechanism of PCB action would be overly simplistic, and potentially underestimate risks of exposure to typical mixtures of congeners. A thorough determination of NOAELs for more subtle behavioral alterations in social and affected behaviors is needed, but may not even be completely illustrative: hormones often act in non-monotonic dose-response curves, and the same is likely true of estrogenic or anti-androgenic PCB congeners, as in the GnRH studies cited above [105]. Regardless, future animal work should focus on lower daily exposures, in the ug/kg range instead of the mg/kg range to allow better comparisons to human studies.
Highlights.
Polychlorinated biphenyls affect a range of social and reproductive behaviors.
Effects may be mediated via hormonal, dopaminergic, and/or glutamateric mechanisms.
Most effects depend on specific congeners, dose, exposure time, and sex of animal.
Additional research using human-relevant exposure levels and mixtures is needed.
Acknowledgements
The author wishes to thank Dr. Andrea Gore for her helpful feedback on the manuscript. The word was funded by NIEHS: R01-ES020662 (to Andrea Gore), T32-ES7247-20 (to MRB), F32-ES023291 (to MRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
Conflict of Interest: Nothing declared.
Literature Cited
- 1.Gore AC. GnRH: The Master Molecule of Reproduction. Kluwer Academic Publishers; 2002. [Google Scholar]
- 2.Blaustein JD, McCarthy MM. Phoenix, Goy, Gerall, and Young, Endocrinology, 1959: 50 years young and going strong. Endocrinology. 2009;150:2501. doi: 10.1210/en.2009-0414. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Saal Vom FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ. Health Perspect. 1997;105:13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner J, Osuch JR, Karmaus W, Landgraf JR, Taffe B, O'Keefe M, Mikucki D, Haan P. Common classification schemes for PCB congeners and the gene expression of CYP17, CYP19, ESR1 and ESR2. Sci. Total Environ. 2012;414:81–89. doi: 10.1016/j.scitotenv.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 7.McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ. Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- 10*.Hamers T, Kamstra JH, Cenijn PH, Pencikova K, Palkova L, Simeckova P, Vondracek J, Andersson PL, Stenberg M, Machala M. In Vitro Toxicity Profiling of Ultrapure Non-Dioxin-like Polychlorinated Biphenyl Congeners and Their Relative Toxic Contribution to PCB Mixtures in Humans. Toxicol. Sci. 2011;121:88–100. doi: 10.1093/toxsci/kfr043. [While in vitro assays are not always ideal to determine overall physiological consequences of a toxin, this study indicates previously under-studied effects of a range of congeners on the androgen receptor.] [DOI] [PubMed] [Google Scholar]
- 11.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agency for Toxic Substances and Disease Registry Toxicological Profile for Polychlorinated Biphenyls. 2000. [PubMed]
- 14.Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Factor SA, et al. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. Journal of Exposure Science and Environmental Epidemiology. 2011;21:234–246. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- 15.Rudel RA, Seryak LM, Brody JG. PCB-containing wood floor finish is a likely source of elevated PCBs in residents' blood, household air and dust: a case study of exposure. Environ Health. 2008;7:2. doi: 10.1186/1476-069X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson DG, Wong L-Y, Turner WE, Caudill SP, Dipietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, et al. Levels in the U.S. population of those persistent organic pollutants (2003-2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ. Sci. Technol. 200943:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control Fourth National Report on Human Exposure to Environmental Chemicals. 2009.
- 18.Gunderson EL. FDA Total Diet Study, July 1986-April 1991, dietary intakes of pesticides, selected elements, and other chemicals. J AOAC Int. 199578:1353–1363. [PubMed] [Google Scholar]
- 19.Newsome WH, Davies DJ, Sun WF. Residues of polychlorinated biphenyls (PCB) in fatty foods of the Canadian diet. Food Addit Contam. 1998;15:19–29. doi: 10.1080/02652039809374596. [DOI] [PubMed] [Google Scholar]
- 20.Dekoning EP, Karmaus W. PCB exposure in utero and via breast milk. A review. J Expo Anal Environ Epidemiol. 2000;10:285–293. doi: 10.1038/sj.jea.7500090. [DOI] [PubMed] [Google Scholar]
- 21.Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Sampson EJ, Jorgensen PJ, Vahter M. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ. Res. 1995;71:29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- 22.Hsu ST, Ma CI, Hsu SK, Wu SS, Hsu NH, Yeh CC, Wu SB. Discovery and epidemiology of PCB poisoning in Taiwan: a four-year followup. Environ. Health Perspect. 1985;59:5–10. doi: 10.1289/ehp.59-1568088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuratsune M, Yoshimura T, Matsuzaka J, Yamaguchi A. Epidemiologic study on Yusho, a Poisoning Caused by Ingestion of Rice Oil Contaminated with a Commercial Brand of Polychlorinated Biphenyls. Environ. Health Perspect. 1972;1:119–128. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischbein A, Wolff MS, Lilis R, Thornton J, Selikoff IJ. Clinical findings among PCB-exposed capacitor manufacturing workers. Ann. N. Y. Acad. Sci. 1979;320:703–715. doi: 10.1111/j.1749-6632.1979.tb56645.x. [DOI] [PubMed] [Google Scholar]
- 25.Guo YL, Lambert GH, Hsu CC. Growth abnormalities in the population exposed in utero and early postnatally to polychlorinated biphenyls and dibenzofurans. Environ. Health Perspect. 1995;103(Suppl 6):117–122. doi: 10.1289/ehp.95103s6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita F, Hayashi M. Fetal PCB syndrome: clinical features, intrauterine growth retardation and possible alteration in calcium metabolism. Environ. Health Perspect. 1985;59:41–45. doi: 10.1289/ehp.59-1568075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am J Public Health. 1994;84:415–421. doi: 10.2105/ajph.84.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. J. Pediatr. 1984;105:315–320. doi: 10.1016/s0022-3476(84)80139-0. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson JL, Jacobson SW, Humphrey HE. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J. Pediatr. 1990;116:38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 1985;56:853–860. [PubMed] [Google Scholar]
- 31.Darvill T, Lonky E, Reihman J, Stewart P, Pagano J. Prenatal exposure to PCBs and infant performance on the fagan test of infant intelligence. Neurotoxicology. 2000;21:1029–1038. [PubMed] [Google Scholar]
- 32.Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol. 2003;25:11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 33.Rogan WJ, Gladen BC. PCBs, DDE, and child development at 18 and 24 months. Ann Epidemiol. 1991;1:407–413. doi: 10.1016/1047-2797(91)90010-a. [DOI] [PubMed] [Google Scholar]
- 34.Kezios KL, Liu X, Cirillio PM, Kalantzi OI, Wang Y, Petreas MX, Park J-S, Bradwin G, Cohn BA, Factor-Litvak P. Prenatal polychlorinated biphenyl exposure is associated with decreased gestational length but not birth weight: archived samples from the Child Health and Development Studies pregnancy cohort. Environ Health. 2012;11 doi: 10.1186/1476-069X-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berghuis SA, Soechitram SD, Hitzert MM, Sauer PJJ, Bos AF. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants. Neurotoxicology. 2013;38:124–130. doi: 10.1016/j.neuro.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Engel SM, Wolff MS. Causal inference considerations for endocrine disruptor research in children's health. Annu Rev Public Health. 2013;34:139–158. doi: 10.1146/annurev-publhealth-031811-124556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 39.Gore AC, Dickerson SM. Endocrine Disruptors and The Developing Brain. Morgan and Claypool Life Sciences. 2012 [Google Scholar]
- 40.Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med. 2010;56:122–131. doi: 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- 41.Faroon OM, Keith S, Jones D, de Rosa C. Effects of polychlorinated biphenyls on development and reproduction. Toxicol Ind Health. 2001;17:63–93. doi: 10.1191/0748233701th097oa. [DOI] [PubMed] [Google Scholar]
- 42.Schell LM, Gallo MV. Relationships of putative endocrine disruptors to human sexual maturation and thyroid activity in youth. Physiol. Behav. 2010;99:246–253. doi: 10.1016/j.physbeh.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karmaus W, Huang S, Cameron L. Parental concentration of dichlorodiphenyl dichloroethene and polychlorinated biphenyls in Michigan fish eaters and sex ratio in offspring. J. Occup. Environ. Med. 2002;44:8–13. doi: 10.1097/00043764-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Cao Y, Winneke G, Wilhelm M, Wittsiepe J, Lemm F, Fuerst P, Ranft U, Imoehl M, Kraftg M, Oesch-Bartlomowicz B, et al. Environmental exposure to dioxins and polychlorinated biphenyls reduce levels of gonadal hormones in newborns: Results from the Duisburg cohort study. Int J Hyg Environ Health. 2008;211:30–39. doi: 10.1016/j.ijheh.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Goncharov A, Rej R, Negoita S, Schymura M, Santiago-Rivera A, Morse G, Carpenter DO, Environm ATF. Lower Serum Testosterone Associated with Elevated Polychlorinated Biphenyl Concentrations in Native American Men. Environ. Health Perspect. 2009;117:1454–1460. doi: 10.1289/ehp.0800134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Grandjean P, Gronlund C, Kjaer IM, Jensen TK, Sorensen N, Andersson A-M, Juul A, Skakkebaek NE, Budtz-Jorgensen E, Weihe P. Reproductive hormone profile and pubertal development in 14-year-old boys prenatally exposed to polychlorinated biphenyls. Reprod. Toxicol. 2012;34:498–503. doi: 10.1016/j.reprotox.2012.07.005. [A recent study showing a negative correlation between PCBs in neonatal cord blood and juvenile serum testosterone in the Faroe Islands. The longitudinal design makes this especially insightful.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohn BA, Cirillo PM, Sholtz RI, Ferrara A, Park J-S, Schwingl PJ. Polychlorinated biphenyl (PCB) exposure in mothers and time to pregnancy in daughters. Reprod. Toxicol. 2011;31:290–296. doi: 10.1016/j.reprotox.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav. Brain Res. 2004;153:295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol. Appl. Pharmacol. 2011;252:36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol. Appl. Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 53**.Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol. Endocrinol. 2014;28:99–115. doi: 10.1210/me.2013-1270. [An exhaustive characterization of masculinizing effects of estrogenic PCBs on hypothalamic gene expression throughout development. The study shows age and sex dependent effects of PCBs that dramatically restructure the organization of hypothalamic gene networks.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faass O, Ceccatelli R, Schlumpf M, Lichtensteiger W. Developmental effects of perinatal exposure to PBDE and PCB on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen. Comp. Endocrinol. 2013;188:232–241. doi: 10.1016/j.ygcen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Gore AC, Wu TJ, Oung T, Lee JB, Woller MJ. A novel mechanism for endocrine-disrupting effects of polychlorinated biphenyls: Direct effects on gonadotropin-releasing hormone neurones. J. Neuroendocrinol. 2002;14:814–823. doi: 10.1046/j.1365-2826.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 56.Dickerson SM, Guevara E, Woller MJ, Gore AC. Cell death mechanisms in GT1-7 GnRH cells exposed to polychlorinated biphenyls PCB74, PCB118, and PCB153. Toxicol. Appl. Pharmacol. 2009;237:237–245. doi: 10.1016/j.taap.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanting CI, Huisman M, Muskiet F, Van Der Paauw CG, Essed CE, Boersma ER. Polychlorinated biphenyls in adipose tissue, liver, and brain from nine stillborns of varying gestational ages. Pediatr. Res. 1998;44:222–225. doi: 10.1203/00006450-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol. Endocrinol. 2011;25:2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 60.Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schütz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. doi: 10.1038/ncomms3492. [DOI] [PubMed] [Google Scholar]
- 61.McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. 2013;36:55–62. doi: 10.1016/j.neuro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, Jansing RL, Hwang S-A, Hicks HE. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ. Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strøm M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, Halldorsson TI. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ Int. 2014;68:41–48. doi: 10.1016/j.envint.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 64**.Gaum PM, Esser A, Schettgen T, Gube M, Kraus T, Lang J. Prevalence and incidence rates of mental syndromes after occupational exposure to polychlorinated biphenyls. Int J Hyg Environ Health. 2014;217:765–774. doi: 10.1016/j.ijheh.2014.04.001. [A recent study demonstrating effects of previous occupational PCB exposure on current day symptoms of depression; effects are large and true of dioxin-like and non-dioxin-like congeners.] [DOI] [PubMed] [Google Scholar]
- 65.Seegal RF, Fitzgerald EF, McCaffrey RJ, Shrestha S, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons PJ, Molho ES, et al. Tibial bone lead, but not serum polychlorinated biphenyl, concentrations are associated with neurocognitive deficits in former capacitor workers. J. Occup. Environ. Med. 2013;55:552–562. doi: 10.1097/JOM.0b013e318285f3fd. [DOI] [PubMed] [Google Scholar]
- 66.Kajta M, Wójtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65:1632–1639. doi: 10.1016/s1734-1140(13)71524-x. [DOI] [PubMed] [Google Scholar]
- 67.Boix J, Cauli O, Leslie H, Felipo V. Differential long-term effects of developmental exposure to polychlorinated biphenyls 52, 138 or 180 on motor activity and neurotransmission. Gender dependence and mechanisms involved. Neurochem. Int. 2011;58:69–77. doi: 10.1016/j.neuint.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol. 1999;62:664–670. doi: 10.1007/s001289900925. [DOI] [PubMed] [Google Scholar]
- 69.Wang XQ, Fang J, Nunez AA, Clemens LG. Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol. Behav. 2002;75:689–696. doi: 10.1016/s0031-9384(02)00673-x. [DOI] [PubMed] [Google Scholar]
- 70.Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol. Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- 71.Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav. Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 72.Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiol. Behav. 2008;95:471–475. doi: 10.1016/j.physbeh.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 73.Seegal RF, Brosch KO, Bush B. Polychlorinated biphenyls produce regional alterations of dopamine metabolism in rat brain. Toxicol. Lett. 1986;30:197–202. doi: 10.1016/0378-4274(86)90103-7. [DOI] [PubMed] [Google Scholar]
- 74.Jolous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol. Lett. 2010;199:136–143. doi: 10.1016/j.toxlet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Elnar AA, Diesel B, Desor F, Feidt C, Bouayed J, Kiemer AK, Soulimani R. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (Sigma 6 NDL-PCBs) in mice. Toxicology. 2012;299:44–54. doi: 10.1016/j.tox.2012.05.004. [The authors use low levels of human-relevant PCBs and demonstrate congener specific effects on a range of behaviors throughout the lifespan.] [DOI] [PubMed] [Google Scholar]
- 76.Gilbert ME, Mundy WR, Crofton KM. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol. Sci. 2000;57:102–111. doi: 10.1093/toxsci/57.1.102. [DOI] [PubMed] [Google Scholar]
- 77.Sugawara N, Ohba T, Nakai K, Kakita A, Nakamura T, Suzuki K, Kameo S, Shimada M, Kurokawa N, Satoh C, et al. Effects of perinatal coexposure to methylmercury and polychlorinated biphenyls on neurobehavioral development in mice. Arch. Toxicol. 2008;82:387–397. doi: 10.1007/s00204-007-0254-x. [DOI] [PubMed] [Google Scholar]
- 78.Curran CP, Nebert DW, Genter MB, Patel KV, Schaefer TL, Skelton MR, Williams MT, Vorhees CV. In Utero and Lactational Exposure to PCBs in Mice: Adult Offspring Show Altered Learning and Memory Depending on Cyp1a2 and Ahr Genotypes. Environ. Health Perspect. 2011;119:1286–1293. doi: 10.1289/ehp.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curran CP, Altenhofen E, Ashworth A, Brown A, Kamau-Cheggeh C, Curran M, Evans A, Floyd R, Fowler J, Garber H, et al. Ahr(d)Cyp1a2(−/−) mice show increased susceptibility to PCB-induced developmental neurotoxicity. Neurotoxicology. 2012;33:1436–1442. doi: 10.1016/j.neuro.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental Exposure to Polychlorinated-Biphenyls (Aroclor-1254) Reduces Circulating Thyroid-Hormone Concentrations and Causes Hearing Deficits in Rats. Toxicol. Appl. Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- 81.Saint-Amour D, Roy M-S, Bastien C, Ayotte P, Dewailly E, Despres C, Gingras S, Muckle G. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology. 2006;27:567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 82.Cromwell HC, Johnson A, McKnight L, Horinek M, Asbrock C, Burt S, Jolous-Jamshidi B, Meserve LA. Effects of polychlorinated biphenyls on maternal odor conditioning in rat pups. Physiol. Behav. 2007;91:658–666. doi: 10.1016/j.physbeh.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orito K, Gotanda N, Murakami M, Ikeda T, Egashira N, Mishima K, Fujiwara M. Prenatal exposure to 3,3“,4,4,”5-pentachlorobiphenyl (PCB126) promotes anxiogenic behavior in rats. Tohoku J. Exp. Med. 2007;212:151–157. doi: 10.1620/tjem.212.151. [DOI] [PubMed] [Google Scholar]
- 84.Tian Y-H, Hwan Kim S, Lee S-Y, Jang C-G. Lactational and postnatal exposure to polychlorinated biphenyls induces sex-specific anxiolytic behavior and cognitive deficit in mice offspring. Synapse. 2011;65:1032–1041. doi: 10.1002/syn.20934. [DOI] [PubMed] [Google Scholar]
- 85.Lilienthal H, Heikkinen P, Andersson PL, van der Ven LTM, Viluksela M. Dopamine-dependent Behavior in Adult Rats after Perinatal Exposure to Purity-controlled Polychlorinated Biphenyl Congeners (PCB52 and PCB180). Toxicol. Lett. 2013 doi:10.1016/j.toxlet.2013.10.016. [PubMed] [Google Scholar]
- 86.Altmann L, Mundy WR, Ward TR, Fastabend A, Lilienthal H. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: Effects on long-term potentiation and [H-3]MK-801 binding in occipital cortex and hippocampus. Toxicol. Sci. 2001;61:321–330. doi: 10.1093/toxsci/61.2.321. [DOI] [PubMed] [Google Scholar]
- 87.Strużyoska L, Sulkowski G, Dąbrowska-Bouta B. Aroclor 1254 selectively inhibits expression of glial GLT-1 glutamate transporter in the forebrain of chronically exposed adult rat. Toxicology. 2012;300:12–18. doi: 10.1016/j.tox.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Boix J, Cauli O, Felipo V. Developmental Exposure to Polychlorinated Biphenyls 52, 138 or 180 Affects Differentially Learning or Motor Coordination in Adult Rats. Mechanisms Involved. Neuroscience. 2010;167:994–1003. doi: 10.1016/j.neuroscience.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 89.Tilson HA, Kodavanti P. Neurochemical effects of polychlorinated biphenyls: An overview and identification of research needs. Neurotoxicology. 1997;18:727–743. [PubMed] [Google Scholar]
- 90.Choksi NY, Kodavanti P, Tilson HA, Booth RG. Effects of polychlorinated biphenyls (PCBs) on brain tyrosine hydroxylase activity and dopamine synthesis in rats. Fundam Appl Toxicol. 1997;39:76–80. doi: 10.1006/faat.1997.2351. [DOI] [PubMed] [Google Scholar]
- 91.Rosin DL, Martin BR. Neurochemical and Behavioral-Effects of Polychlorinated-Biphenyls in Mice. Neurotoxicology. 1981;2:749–764. [PubMed] [Google Scholar]
- 92.Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- 93.Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol. Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- 94.Wigestrand MB, Stenberg M, Walaas SI, Fonnum F, Andersson PL. Non-dioxin-like PCBs inhibit [H-3]WIN-35,428 binding to the dopamine transporter: A structure-activity relationship study. Neurotoxicology. 2013;39:18–24. doi: 10.1016/j.neuro.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Lee DW, Notter SA, Thiruchelvam M, Dever DP, Fitzpatrick R, Kostyniak PJ, Cory-Slechta DA, Opanashuk LA. Subchronic Polychlorinated Biphenyl (Aroclor 1254) Exposure Produces Oxidative Damage and Neuronal Death of Ventral Midbrain Dopaminergic Systems. Toxicol. Sci. 2012;125:496–508. doi: 10.1093/toxsci/kfr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seegal RF, Brosch KO, Okoniewski RJ. Coplanar PCB congeners increase uterine weight and frontal cortical dopamine in the developing rat: implications for developmental neurotoxicity. Toxicological Sciences. 2005;86:125–131. doi: 10.1093/toxsci/kfi174. [DOI] [PubMed] [Google Scholar]
- 97.Coccini T, Roda E, Castoldi AF, Poli D, Goldoni M, Vettori MV, Mutti A, Manzo L. Developmental exposure to methylmercury and 2,2 ',4,4 ‘,5,5 ’-hexachlorobiphenyl (PCB153) affects cerebral dopamine D1-like and D2-like receptors of weanling and pubertal rats. Arch. Toxicol. 2011;85:1281–1294. doi: 10.1007/s00204-011-0660-y. [DOI] [PubMed] [Google Scholar]
- 98*.Fielding JR, Rogers TD, Meyer AE, Miller MM, Nelms JL, Mittleman G, Blaha CD, Sable HJK. Stimulation-Evoked Dopamine Release in the Nucleus Accumbens Following Cocaine Administration in Rats Perinatally Exposed to Polychlorinated Biphenyls. Toxicol. Sci. 2013;136:144–153. doi: 10.1093/toxsci/kft171. [Electrophysiological study of PCB effects on dopamine release, with implications for reward seeking behavior. It is rare that electrophysiological studies are paired with developmental exposures, so this is very helpful to assess functional repercussions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seegal RF, Marek KL, Seibyl JP, Jennings DL. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: A β-CIT imaging study. Neurobiology of Disease. 2010;38:219–225. doi: 10.1016/j.nbd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanida T, Tasaka K, Akahoshi E, Ishihara-Sugano M, Saito M, Kawata S, Danjo M, Tokumoto J, Mantani Y, Nagahara D, et al. Fetal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin transactivates aryl hydrocarbon receptor-responsive element III in the tyrosine hydroxylase immunoreactive neurons of the mouse midbrain. J Appl Toxicol. 2014;34:117–126. doi: 10.1002/jat.2839. [DOI] [PubMed] [Google Scholar]
- 101.Fischer LJ, Seegal RF, Ganey PE, Pessah IN, Kodavanti PRS. Symposium Overview: Toxicity of Non-Coplanar PCBs. Toxicol. Sci. 1998;41:49–61. doi: 10.1006/toxs.1997.2386. [DOI] [PubMed] [Google Scholar]
- 102.Giesy JP, Kannan K. Dioxin-Like and Non-Dioxin-Like Toxic Effects of Polychlorinated Biphenyls (PCBs): Implications For Risk Assessment. Crit. Rev. Toxicol. 1998;28:511–569. doi: 10.1080/10408449891344263. [DOI] [PubMed] [Google Scholar]
- 103.Ndountse LT, Chan HM. Role of N-methyl-D-aspartate receptors in polychlorinated biphenyl mediated neurotoxicity. Toxicol. Lett. 2009;184:50–55. doi: 10.1016/j.toxlet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 104.Selvakumar K, Bavithra S, Ganesh L. Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: Anxiolytic-like effects of quercetin. Toxicol. Lett. 2013;222:45–54. doi: 10.1016/j.toxlet.2013.06.237. [DOI] [PubMed] [Google Scholar]
- 105*.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DRJ, Lee D-H, Shioda T, Soto AM, Saal vom FS, Welshons WV, et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [An excellent review of an endocrinologist perspective to the toxicology of endocrine disrupting chemicals.] [DOI] [PMC free article] [PubMed] [Google Scholar]