Abstract
The type 2 iodothyronine deiodinase (D2) is essential for feedback regulation of TSH by T4. We genetically inactivated in vivo D2 in thyrotrophs using a mouse model of Cga-driven cre recombinase. Pituitary D2 activity was reduced 90% in the Cga-cre D2 knockout (KO) mice compared with control Dio2fl/fl mice. There was no growth or reproductive phenotype. Basal TSH levels were increased 1.5- to 1.8-fold, but serum T4 and T3 were not different from the controls in adult mice. In hypothyroid adult mice, suppression of TSH by T4, but not T3, was impaired. Despite mild basal TSH elevation, the TSH increase in response to hypothyroidism was 4-fold reduced in the Cga-cre D2KO compared with control mice despite an identical level of pituitary TSH α- and β-subunit mRNAs. In neonatal Cga-cre D2KO mice, TSH was also 2-fold higher than in the controls, but serum T4 was elevated. Despite a constant TSH, serum T4 increased 2–3-fold between postnatal day (P) 5 and P15 in both genotypes. The pituitary, but not cerebrocortical, D2 activity was markedly elevated in P5 mice decreasing towards adult levels by P17. In conclusion, a congenital severe reduction of thyrotroph D2 causes a major impairment of the TSH response to hypothyroidism. This would be deleterious to the compensatory adaptation of the thyroid gland to iodine deficiency.
An important role for serum T4 in the feedback regulation of TSH secretion was first suspected from the presence of an elevated serum TSH in combination with a normal serum T3 and a reduced serum T4 in moderately hypothyroid patients (1). This was initially confusing, because in many bioassay experiments, T3 appeared to be the active hormone, and it was already recognized that T4 to T3 conversion occurred (2). Subsequent studies demonstrated that in the hypothyroid rat, the acute suppression of TSH secretion by T4 did not require the formation of circulating T3 but did depend on T3 produced from T4 5′-deiodination within the pituitary by an unidentified, propylthiouracil (PTU)-resistant, 5′-deiodinase (3, 4). Both the intrapituitary T3 production and the suppression of TSH in hypothyroid rats were blocked in parallel by the iodophenol, iopanoic acid, a competitive inhibitor of deiodination (4, 5). Further studies showed that this PTU-resistant deiodinase, subsequently termed type 2 iodothyronine deiodinase (D2), was present in pituitary thyrotrophs, mammotrophs, and somatotrophs as well in the central nervous system (CNS) (largely in astrocytes) and in brown adipose tissue (6).
Interestingly, the activity of D2 in pituitary cells was decreased by thyroid hormone, whereas that of the hepatic D1 was induced at a transcriptional level by the same stimulus (7). Subsequent molecular cloning of both D1 and D2 confirmed that although these proteins were both selenoenzymes that were highly homologous especially in their active centers, they are markedly different in tissue distribution and function and are encoded by different genes (8, 9). The identification of pituitary D2 provided a mechanism through which the thyrotroph could recognize and respond to a decrease in the circulating prohormone T4. Although a global D2 knockout mouse has been developed and shows a clear defect in T4 responsiveness in the pituitary-thyroid axis, the absence of D2 expression in nonpituitary tissues, such as the CNS, complicates the interpretation of the role of thyrotroph D2 deficiency, per se (10, 11).
To define the effects of a selective thyrotroph D2 deficiency, we crossed a mouse with a floxed Dio2 gene with one in which cre-expression is under control of the glycoprotein hormone α-subunit gene (Cga), thus creating a model in which active D2 was depleted in the Cga-expressing thyrotrophs and gonadotrophs, the Cga-cre D2 knockout (KO) mouse (12). This allowed us to examine the functional role of thyrotroph D2 in the euthyroid and hypothyroid state. We found that Cga-cre D2KO mice have an approximately 90% loss of D2 in the pituitary, a normal serum T3 and T4, and a slightly higher but still normal TSH, whereas TSH bioactivity is unchanged. Strikingly, we also found that Cga-cre D2KO mice have an impaired TSH increase during induction of the hypothyroid state. While this work was in progress, Fonseca et al (13) reported studies of adult male mice with inactivated pituitary D2 using the same strategy. Unlike our results, they reported an increase in serum T4 and a goiter despite a normal circulating TSH. A detailed comparison of other differences in the results of the 2 studies is provided in the Discussion. In terms of selective environmental pressures, dietary iodine deficiency is the most serious challenge that the hypothalamic-pituitary-thyroid axis faces in maintaining T3 homeostasis (14). Our results indicate that thyrotroph D2 plays a critical role in meeting that challenge.
Materials and Methods
Animals
All animal studies were approved by the Harvard Medical School Standing Committee on Animals. Mice were fed standard rodent chow and water ad libitum and maintained under a 12-hour light, 12-hour dark cycle. For experiments with neonates, whole litters were euthanized at the indicated time point by isoflurane anesthesia followed by decapitation, and serum and tissues were collected. Mice were then genotyped, and sufficient litters killed to allow for at least 3 mice (range 3–7) for each genotype and gender at each time point. The same animals were used for measurement of serum hormone levels (see Figure 5 below) and tissue deiodinase measurements (see Figure 6 below).
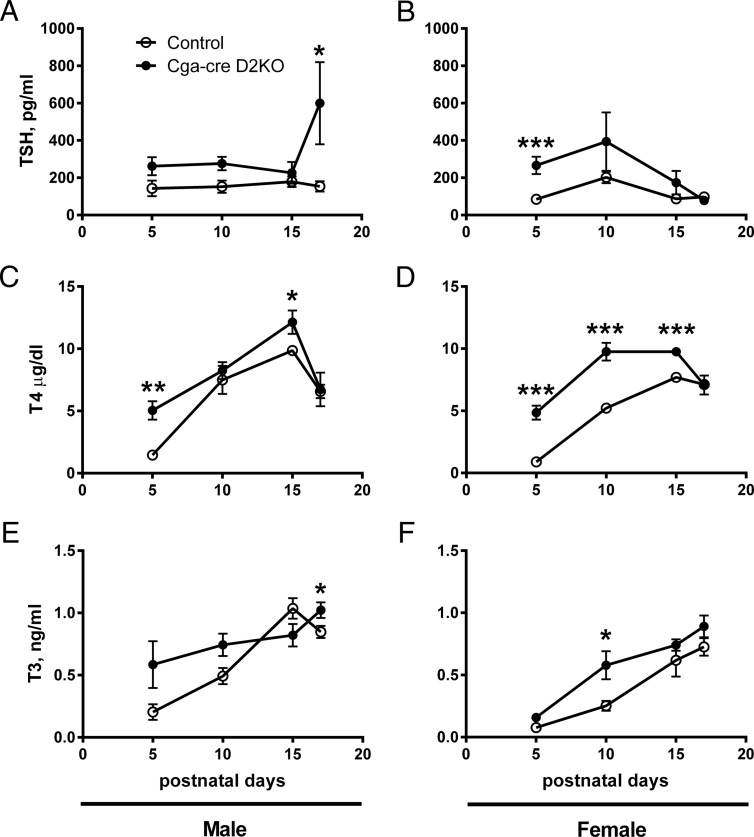
Figure 5.
Serum thyroid hormone profiles of neonatal male and female control and Cga-cre D2KO mice. Serum TSH (A and B), T4 (C and D), and T3 (E and F) levels in male and female mice. Control mice are indicated by open circles, whereas Cga-cre D2KO mice are represented by closed circles. We found significant difference by statistical analysis using two-way ANOVA. *, P < .05; **, P < .01; ***, P < .001 followed by unpaired Student's t test. n = 3–7 per group.
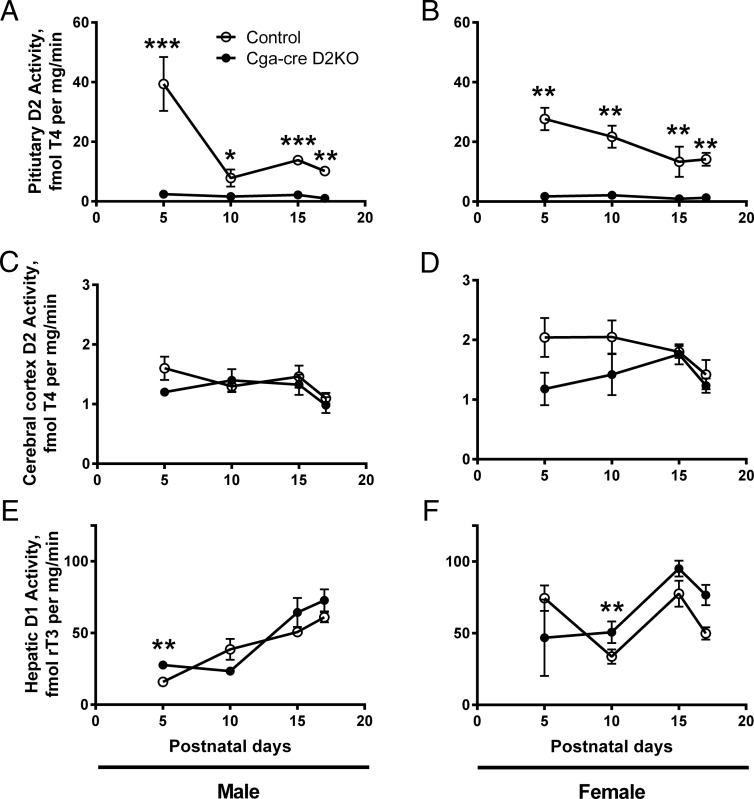
Figure 6.
Deiodinase activity in neonatal male and female control and Cga-cre D2KO mice. Pituitary D2 activity of male (A and B), cerebral cortical D2 activity (C and D), and hepatic D1 activity of male (E and F) male and female mice are shown. These are the same cohort of mice as used in Figure 5, and statistical analyses are as for Figure 5.
Generation of D2fl/fl mice
Initially, a 9132-bp fragment containing exon 2 of the mouse Dio2 gene cloned from BAC: bMQ163j10 (Source BioScience) was inserted by recombination into the PL253 plasmid (15). A LoxP site and a neomycin expression cassette were inserted into the single intron, whereas another LoxP site was inserted into the 3′ untranslated region in exon 2 (Supplemental Figure 1). This construct was confirmed by restriction digests and sequencing and electroporated into mouse ES cells (E14Tg2A). Geneticin-resistant clones were analyzed by PCR and Southern blotting and used for blastocyst injection by the BWH transgenic core. Mice bearing the floxed allele of the Dio2 gene were bred to homozygosity and are referred to as D2fl/fl mice. D2fl/fl mice were crossed with transgenic mice obtained from The Jackson Laboratory (B6;SJL-Tg[Cga-Cre]3Sac/J) developed by Dr Sally Camper (12), in which Cre expression was under control of the mouse Cga. This specifically deleted the floxed fragment of the Dio2 gene in the Cga-expressing thyrotrophs and gonadotrophs of the anterior pituitary. Earlier studies showed that there was low and scattered expression of this promoter in the brain and that Cga-cre expression did not affect TSH expression in thyrotrophs (12, 16).
Serum T3, T4, and TSH measurements
Blood was either collected from the cheek vein, or by terminal cardiac puncture under isoflurane anesthesia, and serum T3 and T4 were measured using COAT-A-COUNT total T4 and T3 kit (DPC), following the manufacturer's instructions. The mouse standard curves were prepared in charcoal-stripped (T4 and T3 deficient) mouse serum as described previously (17). 125I-T3 charcoal uptake was assessed as described previously (18). Serum TSH levels were measured by Milliplex MAP using the rat thyroid hormone TSH panel (EMD Millipore) (19).
Induction of hypothyroidism and T4 sensitivity testing
To induce hypothyroidism, Cga-cre D2KO and D2fl/fl control mice were administered 0.1% methimazole (MMI) (Sigma) and 1% NaClO4 (Fisher Scientific Co) in drinking water for 3–6 weeks as indicated. The sensitivity of the elevated TSH to acute suppression by T4 or T3 was performed as described previously (11). In brief, mice were made hypothyroid by administration of 1% NaClO4 and 0.1% MMI in their drinking water for 3 weeks. On the day of the experiment, blood was obtained from the cheek vein for determination of basal TSH levels. Mice were then injected ip with PTU (2 mg/mouse) to block D1 activity, and 1 hour later given either T4 (3 μg/100 g body weight) or T3 (1.2 μg/100 g body weight) or vehicle alone (PBS). Five hours after hormone administration, mice were euthanized by cardiac puncture under isoflurane anesthesia, and serum and tissues were collected.
Deiodination assays
D1, D2, and D3 activities were assayed as described previously (18).
Gene expression analysis
The hypothalamic tissue was collected using the coronal brain matrix (Braintree Scientific). The hypothalamic region was dissected by coronal cuts located caudal to the optic chiasm and rostral to the mammillary bodies followed by 2 lateral cuts 1 mm to either side of the midline and 2.2 mm from the dura (20). We separated this tissue into 2 regions performing an additional dorso-ventral cut 1 mm from the dura: 1) the upper hypothalamus containing the dorsal hypothalamic area (DHA) and the paraventricular nucleus (PVN) and lateral hypothalamic tissue, and 2) the lower hypothalamus containing the mediobasal hypothalamus (MBH), including tanycytes, the arcuate nucleus, and the median eminence. Total RNA was extracted from tissues using TRIzol (Invitrogen), according to the manufacturer's directions. One microgram of total RNA was reverse transcribed into cDNA using SuperScript VILO (Invitrogen). The cDNAs were amplified by PCR in an i-Cycler (Bio-Rad) using SYBR Green (Bio-Rad). Sequences of primers used are listed in Supplemental Table 1. Quantitative real-time PCR was performed as described previously using the delta delta cycle threshold method, with cyclophilin A used as the housekeeping gene for normalization (21).
TSH biological activity
TSH bioactivity was measured as described previously, with JP26–26 and JP02 cells generously provided by Dr G. Vassart and Dr S. Refetoff (17, 22, 23). cAMP was measured using either the cAMP [125I] RIA kit, catalog number NEK033 (PerkinElmer) for sera from euthyroid mice, or cAMP XP kit for sera from hypothyroid mice (Cell Signaling Technology). The biological activity of TSH in each serum sample was determined by dividing the net pg cAMP induced/pg TSH.
Results
Phenotype and pituitary, cerebrocortical, and hypothalamic deiodinase activities in Cga-cre D2KO mice
We generated mice with 2 LoxP sites flanking the Dio2 gene (Supplemental Figure 1) that were mated with mice harboring a cre recombinase expression cassette under the control of the mouse Cga to remove a portion of the Dio2 coding region from the Cga-expressing thyrotrophs in the pituitary (hereafter Cga-cre D2KOs) (12). The Cga-cre D2KO mice had no significant differences in body weight, growth, litter size, or reproductive capacity in either gender when compared with D2fl/fl littermates (data not shown). These findings are consistent with similar growth hormone mRNA levels in euthyroid 8-week-old Cga-cre, D2KO mice (n = 6) when compared with age-matched controls (n = 4, P = .6723) (data not shown).
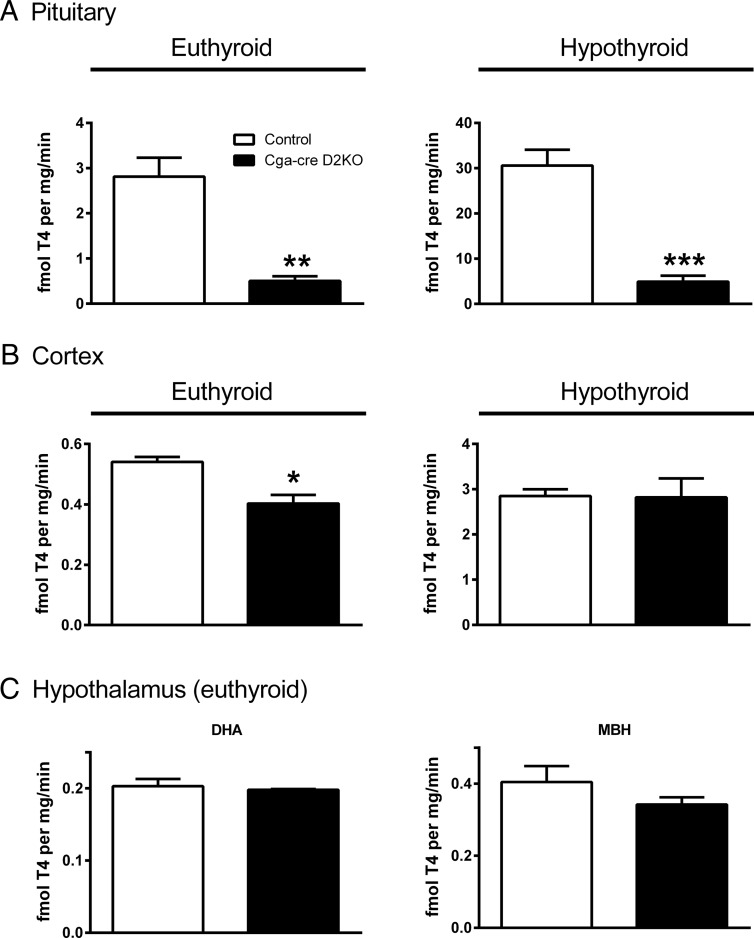
D2 activity was quantified in several tissues to confirm successful and specific recombination. Pituitary D2 was reduced approximately 90% in the euthyroid Cga-cre D2KO mice relative to that in controls indicating significant recombination of the Dio2 gene (P < .01) (Figure 1A). Although the D2 activities in both genotypes were about 10-fold higher when mice were made hypothyroid for 4–6 weeks, D2 activity was still 90% lower in the Cga-cre D2KO mice compared with the controls (P < .001) (Figure 1A). The residual activity in the pituitary of Cga-cre D2KO mice can be explained by either by incomplete recombination and/or the previously demonstrated D2 activity in somatotrophs and mammotrophs (24, 25). D2 activity was reduced 25% in the cerebral cortex of euthyroid Cga-cre D2KOs compared with controls (P < .05), but this difference in D2 activity was lost in hypothyroid mice (Figure 1B). In addition, D2 activity in the DHA containing the PVNs and in the MBH, which included the D2 expressing-tanycytes, was similar between the 2 genotypes (Figure 1C). Likewise, D3 activities were unchanged in pituitary, cerebral cortex, or DHA and MBH of Cga-cre D2KO and control mice (Supplemental Figure 2). We also found no thyroid enlargement despite the slightly higher TSH in the male Cga-cre, D2KO mice (data not shown).
Figure 1.
D2 Activity in control and Cga-cre D2KO mice. D2 activity in pituitary (A) and cerebral cortex (B) of euthyroid (left) and hypothyroid (right) animals. C, D2 activity in hypothalamic areas under euthyroid conditions: DHA (left) and MBH (right). Control animals are shown in the white bars and the Cga-cre D2KO animals with black bars. Data are shown as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001. n = 3–5 mice per group.
The serum TSH concentrations in the adult male and female Cga-cre D2KO mice were 1.5- to 1.8-fold higher, with this difference not reaching statistical significance in the females (Table 1). Despite the slightly higher TSH, the serum T4 and T3 concentrations in control and Cga-cre D2KO mice were not different. The free fraction of T3 in the Cga-cre D2KO mice, as estimated by a T3 charcoal uptake determination, was not different from that of the control mice, indicating that differences in circulating thyroid hormone binding proteins are not a confounding factor and that the total hormone values are representative of the free thyroid hormones (data not shown). We also found no differences in the TSH bioactivity in adult (2 mo old) euthyroid male mice of both genotypes (Supplemental Figure 3). In agreement with the lack of a difference in thyroid hormone levels, hepatic D1 activity, a sensitive indicator of peripheral thyroid status in rodents, was not different between the 2 genotypes (Supplemental Figure 4).
Table 1.
Basal Thyroid Function Test of Control and Cga-cre D2KO Mice
| Male (n = 22–24) | Control | Cga-cre D2KO |
|---|---|---|
| TSH (pg/mL) | 236 ± 30 | 356 ± 43a |
| T4 (μg/dL) | 5.6 ± 0.4 | 5.5 ± 0.8 |
| T3 (ng/mL) | 0.45 ± 0.07 | 0.38 ± 0.06 |
| Female (n = 6–12) | Control | Cga-cre D2KO |
|---|---|---|
| TSH (pg/mL) | 104 ± 15 | 186 ± 38 |
| T4 (μg/dL) | 5.6 ± 0.5 | 5.8 ± 0.8 |
| T3 (ng/mL) | 0.42 ± 0.09 | 0.50 ± 0.04 |
Data as mean ± SEM.
P < .05.
To elucidate the effect of inactivating the pituitary Dio2 gene on the other elements of the hypothalamic-pituitary-thyroid axis, we measured the pre-proTRH mRNA expression in the PVN (Supplemental Figure 5A) and pyroglutamyl peptidase II mRNA, a TRH-inactivating enzyme expressed in tanycytes, in the MBH (Supplemental Figure 5B) by real-time PCR. The expression of these mRNAs was unaffected in the Cga-cre D2KO mice.
Hypothyroid Cga-cre D2KO mice have impaired TSH suppression after acute exposure to T4 but not T3
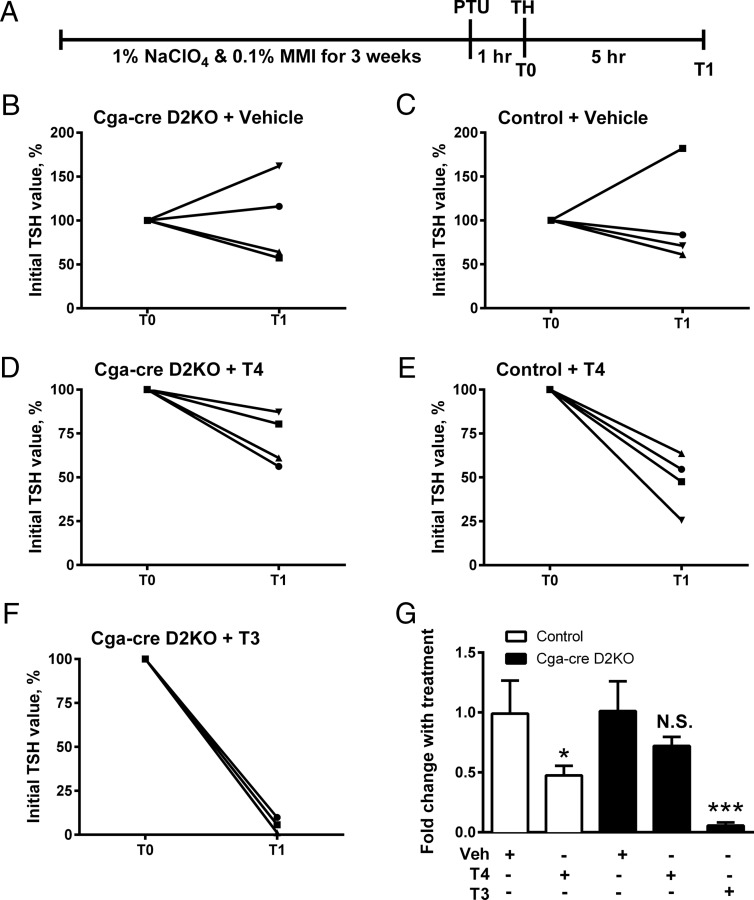
Previous studies in hypothyroid rats showed that iopanoic acid, but not PTU, blocked the acute effect of T4, but not T3, to decrease the elevated TSH in hypothyroid rats. Because blockade of the TSH suppression correlated with the inhibition of pituitary T4 to T3 conversion, this is a D2- and T3-dependent process (4, 5). If this conclusion is accurate, a similar phenomenon should occur in the Cga-cre D2KO mice. To test this, control and Cga-cre D2KO mice were made hypothyroid by the addition of sodium perchlorate and MMI in their drinking water for 3 weeks. Mice were then injected with PTU to block D1 activity and then 1 hour later injected with either T3 or T4 (Figure 2A). Five hours after hormone injection, serum was collected for TSH measurement. TSH was unchanged in both genotypes after vehicle injection (Figure 2, B and C). T4 administration caused a roughly 50% decrease in TSH levels in the controls (P < .05) but not in the Cga-cre D2KO animals (Figure 2, D, E, and G). On the other hand, administration of T3 reduced serum TSH 90% in the Cga-cre D2KO mice (P < .001) (Figure 2, F and G). These results are comparable with those observed in mice with a global loss of D2 (D2KO mice) (10, 11).
Figure 2.
Test of T4 sensitivity in hypothyroid control and Cga-cre D2KO mice. A, Schematic description of experiment. Mice were treated with 1% sodium perchlorate (NaClO4) and 0.1% MMI in the drinking water for 3 weeks to induce hypothyroidism. The serum TSH levels were measured in Cga-cre D2KO and control mice at time 0 (T0), (1 h after PTU pretreatment) and then at T1 5 hours after ip injection of vehicle, or thyroid hormones (THs). Data from individual mice are shown as the ratio T1/T0 (as %) of serum TSH values after administration of PBS (B and C); 3-μg T4/100 g body weight (D and E), or 1.2-μg T3/100 g body weight (F) (11). Average fold change with treatment is shown (mean ± SEM) (G). *, P < .05 when compared with vehicle-treated control by Student's unpaired t test, whereas ***, P < .001 compared with vehicle-treated Cga-cre D2KO by one-way ANOVA. N.S., not significant. n = 4 mice/group.
The TSH response to hypothyroidism is markedly reduced in Cga-cre D2KO mice
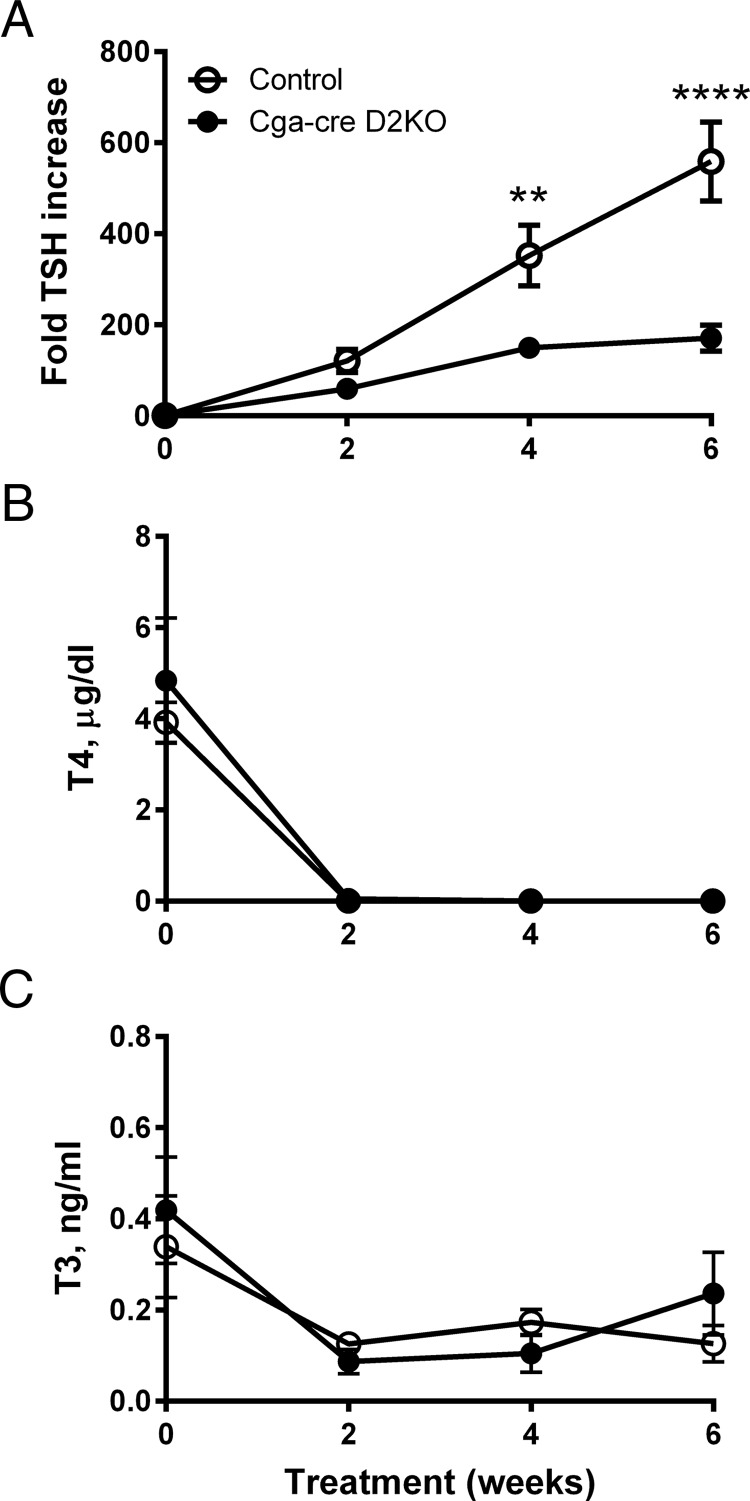
To examine the effect of thyrotroph D2 deficiency on the TSH response to antithyroid drug-induced hypothyroidism, groups of 4 male mice of each genotype were treated with sodium perchlorate and MMI in drinking water for 4–6 weeks. Serum T4 decreased to undetectable levels over the first 2 weeks, whereas the serum T3 concentrations fell to about 1/3 of normal (Figure 3, B and C). Strikingly, the rate of increase in TSH was markedly slower in the Cga-cre D2KO mice than in controls, although both increased substantially (Figure 3A). At 6 weeks, the TSH concentration in the controls was approximately 3-fold greater than in the Cga-cre D2KO mice (P < .0001). In an identical experiment in which mice were treated with antithyroid agents for 4 weeks, serum TSH concentrations in control mice were 5-fold higher (fold TSH increase, 1068 ± 198) than in Cga-cre D2KO mice (fold TSH increase, 199 ± 52; P < .001). These results indicate that thyrotroph D2 is required for a normal TSH response to hypothyroidism.
Figure 3.
Serum TSH, T4 and T3 concentrations in control and Cga-cre D2KO mice during induction of hypothyroidism. Mice received 1% NaClO4 and 0.1% MMI in drinking water. Serial samples of blood were taken for TSH (A), T4 (B), and T3 (C) measurements at the indicated times. TSH is expressed as fold change relative to the value at time 0. Data are shown as mean ± SEM. **, P < .01; ****, P < .0001. n = 3–5 mice/group.
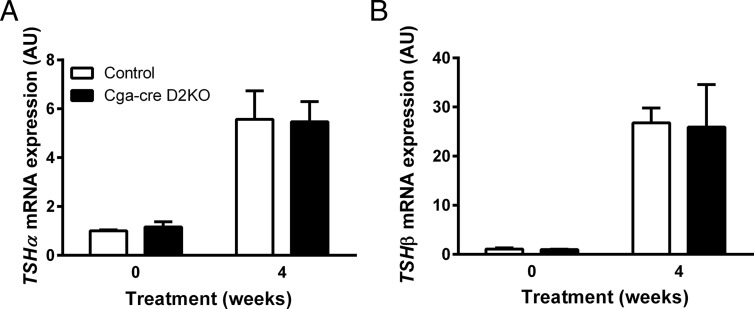
We assessed whether differences in pituitary expression of the TSH α- and β-subunit mRNAs could account for the decreased response of serum TSH in the hypothyroid Cga-cre D2KO mice. The expression of the α and β TSH subunit mRNAs in the euthyroid pituitary was not different in the 2 genotypes, and there were increases of similar magnitude of subunit mRNAs during the induction of hypothyroidism (Figure 4).
Figure 4.
TSHα and TSHβ mRNA expression in the pituitaries of euthyroid and hypothyroid Cga-cre D2KO and control mice. TSHα (A) and TSHβ (B) are shown from euthyroid control (white bars) and Cga-cre D2KO (black bars) male mice at time 0 and at 4 weeks after treatment with 1% sodium perchlorate and 0.1% MMI. Expression levels are normalized to cyclophilin A expression and are expressed relative to euthyroid, control animals. Data are shown as mean ± SEM in arbitrary units (AU) and analyzed using Student's unpaired t test. n = 3–5 animals/group.
Serum hormone concentrations and deiodinase activities in neonatal Cga-cre D2KO pups
Rats exposed to excess thyroid hormone in utero and mice born to mothers with elevations in thyroid hormone during gestation due to a congenital absence of the thyroid hormone-inactivating D3 develop central hypothyroidism after birth (26, 27). Because serum T4 concentrations are elevated in mice with a global D2KO, we wondered whether a mild neonatal thyrotoxicosis could explain the impaired TSH response to hypothyroidism in the Cga-cre D2KO mice. We examined the serum TSH, T4, and T3 concentrations in groups of neonatal control and Cga-cre D2KO mice at several times during postnatal development (Figure 5). As in the adults, the serum TSH levels trended higher in both male and female Cga-cre D2KO mice at postnatal day (P) 5, P10, and P15, although again, these differences were generally not statistically significant (Figure 5, A and B). A higher TSH value as a compensation for a lower biological activity of the TSH in the Cga-cre D2KO mice might also explain this, but we found no differences in this variable between genotypes or gender (Supplemental Figure 3). On the other hand, unlike in the adult, the serum T4 concentrations are higher in the Cga-cre D2KO pups, on days P5 and P15 in males and P5, P10, and P15 in females (Figure 5, C and D). These, and the serum T3 concentrations, increased between P5 and P15 but fell to normal adult levels at P17 (Figure 5, E and F). The higher TSH found in the male, as opposed to the female, Cga-cre D2KO pups at P17 is consistent with higher TSH values in male adult mice (Table 1). The increases in T4 values between P5 and P15 suggest a TSH-driven increase in both neonatal genotypes. We found no differences in the free fraction of T3 in P15 and P17 neonates between genotypes or gender (data not shown). Although the elevated serum T4 results suggested the possibility of a neonatal thyrotoxicosis, TSH suppression did not occur.
We also measured D2 activity in pituitary and cerebral cortex as well as hepatic D1 activity during the neonatal period in these groups of Cga-cre D2KO and control neonatal mice (Figure 6). Surprisingly, the pituitary D2 in the control male and female neonates is quite elevated at P5 (25–50 fmol T4/mg · min) compared with that at P17 (5–10 fmol T4/mg · min), or in the adult animal (3 fmol T4/mg·min) (Figures 1 and 6). In males, the cerebral cortical D2 activity, a protein posttranslationally reduced by T4, was not different between control and Cga-cre D2KO mice despite the higher levels of T4 in the Cga-cre D2KO mice (Figure 5, C and D). On the other hand, although not statistically significant, cerebrocortical D2 activity trended lower in the females at P5 and P10, but not later, perhaps reflecting the consistently relatively greater serum T4 in Cga-cre D2KO females (Figure 5D). Hepatic D1 activity was similar in both genotypes in males and females (Figure 6, E and F). Also, the expression of the T3-sensitive gene hairless was not increased in the cerebral cortex of males or females (Supplemental Figure 6). These results and the similar hepatic D1 activities in both genotypes indicate that the Cga-cre D2KO neonates are not thyrotoxic despite the higher serum T4 than in control pups.
Discussion
A striking result from this study is that 90% of the pituitary D2 is found in Cga-expressing cells, namely, thyrotrophs and gonadotrophs (Figure 1). Given the absence of any significant disruption in reproductive function in the male or female Cga-cre D2KO mice, it is likely that the bulk of this activity is in the thyrotrophs, although more rigorous studies of gonadotroph function are required to confirm this. The fact that nearly all pituitary D2 activity is lost in the Cga-cre D2KO mouse is even more remarkable given the small fraction (∼10%) of the mouse pituitary cells thought to be thyrotrophs. This result is in agreement with the high D2 activity in the transformed thyrotroph cell line, TαT1, and in situ studies of euthyroid and hypothyroid rat pituitaries (28). We found no differences in D3 activity between Cga-cre D2KO and control mice in pituitary, cerebral cortex, and hypothalamus. These results indicate no compensatory changes occurred in the expression of the T3-sensitive Dio3 gene as a consequence of D2 deficiency in the pituitary (Supplemental Figure 2). There is a modestly lower (25%) D2 activity in the cerebral cortex of the euthyroid Cga-cre D2KO mice, but this difference is lost in the hypothyroid animals with higher D2 expression. Interestingly, a similar decrease in baseline cortical D2 was found by Fonseca et al (29), although the difference was not significant due to a high variation in results. This may be due to low levels of Cga-cre expression in brain as reported by Cushman et al (12).
In the adult Cga-cre D2KO mice, the serum T4 and T3 are normal, whereas the TSH is 1.5-fold higher in male mice (Table 1). TSH was also increased to the same degree in females, although this was not statistically significant (P = .057). We interpret the absence of a serum T4 elevation in the adult Cga-cre D2KO mice in the present study as a reflection of the relatively modest increase in the serum TSH. Previous studies have shown that the normal range for TSH can vary from 2- to 4-fold within a given mouse strain (30). The absence of an increase in thyroid weights in the KO mice supports this interpretation. The normal serum T4 concentrations in the present study differ from the elevated values found in the global D2KO mouse and the recently reported mouse model where Cga-driven cre was also used to inactivate thyrotroph and gonadotroph D2 (11, 29, 31, 32). The global D2KO mouse model has a modest, but significant, TSH elevation (10, 17), but surprisingly, the Fonseca et al (13) pituitary-specific D2KO has net normal TSH concentrations (due to the 3-fold increase in TSH levels being mitigated by a 40% decrease in TSH biological activity), even though T4 is elevated and thyroid weights are increased by 60%. The reason for these differences is not clear. As mentioned, we found the serum T4 is elevated in the Cga-cre D2KO mice during the neonatal period as is discussed below, with a transition to a normal T4 by P17 (Figure 5).
The inactivation of D2 in the Cga-expressing thyrotrophs results in a resistance to the acute suppression of TSH release by T4, but not by T3, in the global D2KO hypothyroid mouse and in the recently reported euthyroid thyrotroph-specific D2KO mouse (Figure 2) (10, 13). The loss of thyrotroph D2 can explain this resistance to T4 and it is in agreement with earlier studies in the rat with an iopanoic acid blockade of the D2 enzyme (5). A novel and important result of this study is that in addition to blocking the down-regulation of TSH by T4, thyrotroph D2 deficiency also reduces the TSH response to thyroid hormone deficiency by 3- to 4-fold. What is the basis for this impaired response? One might have predicted that because the basal TSH is higher in the Cga-cre D2KO mice, it would have remained so during the response to reduced thyroid function. This would certainly be the case if the increase was primarily due to increased TRH, because mild hypothyroidism is well known to sensitize the thyrotroph to the effects of TRH (33). However, we found no decrease in D2 activity in the hypothalamus to explain an increase in TRH synthesis or release (Figure 1C), no increase in basal TRH mRNA expression in the PVN (Supplemental Figure 5A), nor was there a decrease in TSH bioactivity in euthyroid or hypothyroid mice that might suggest more subtle reductions in TRH in the Cga-cre D2KO mice (Supplemental Figure 3). Another plausible cause could be a decrease in TSHβ expression in Cga-cre D2KO mice, but this was not found in either the euthyroid or hypothyroid state (Figure 4).
We speculate that one reason for the impaired increase in serum TSH in response to the hypothyroid challenge in the Cga-cre D2KO mice may be the chronic absence of D2-mediated production of thyrotroph T3. Thus, the basal saturation of nuclear thyroid hormone receptors (TRs) is lower in the Cga-cre D2KO thyrotrophs than in the D2-expressing controls, and this is reflected in the somewhat higher TSH. In the euthyroid rat pituitary, the TRs are over 80% occupied by T3 in the euthyroid state and D2-catalyzed T3 in the pituitary accounts for approximately half of this TR-receptor bound T3 (4, 34). The reduction in intranuclear T3 from this source increases the unoccupied TRs as much as 2-fold leading to an approximate doubling of TSH in the Cga-cre D2KO mice (Table 1). As a result, the reduction in the contribution from D2-generated pituitary T3 due to hypothyroidism should cause a 2-fold greater absolute increase in the unoccupied nuclear TRs in the thyrotrophs of control mice than in those with D2 deficiency. The unoccupied TRs bound to the TSHβ gene in the thyrotrophs are thought to be the main driving force to both TSH synthesis and release (35, 36). Because a reduction in serum T4 is an early event during the development of hypothyroidism or iodine deficiency (Figure 3), an increase in TSH could then initiate an enhancement of rate-limiting steps in thyroid hormone synthesis such as iodide trapping and organification as well as increase the T3 to T4 ratio in thyroid secretion (14).
A transiently reduced TSH response to hypothyroidism may result from thyrotoxicosis during development. This has been observed in human newborns of mothers with poorly controlled hyperthyroidism, in rodents after excess T4 administration during early development, and in the global D3-KO mouse, in which both fetal and neonatal serum T3 are elevated (26, 27, 37). A reduced sensitivity to a low-serum T4 in these examples derives from a delayed recovery of the sensitivity of the hypothalamic-pituitary-thyroid axis to low thyroid hormone levels. In the Cga-cre D2KO mouse, however, we propose it may be a chronic deficiency of intracellular thyrotroph T3 derived from T4, which reduces the sensitivity to a further loss of cellular T3 due to a decrease of circulating T4. Importantly, we did not find TRH deficiency or a decrease in bioactivity of TSH (Supplemental Figure 3) in either euthyroid or hypothyroid mice, a secondary consequence of TRH deficiency, unlike what was recently reported by Fonseca et al (Figure 2) (13). We also did not find higher TSH subunit mRNA content in the Cga-cre D2KO mice despite the elevation in the circulating TSH. This suggests that it could be the T3-dependent regulation of TSH release that is less responsive when intracellular pituitary T3 is chronically reduced (38). We have previously shown that this response is more rapid than inhibition of TSH mRNA synthesis in the hypothyroid rat (38).
An intriguing result in newborn Cga-cre D2KO mice was the elevation of serum T4 during the early neonatal period, which did not occur in the adult mice (Figure 5). In global D2KO mice, T4 has also been previously reported to be elevated at P15 (39). The TSH increase in the Cga-cre D2KO neonatal mice was only about 2-fold, not different from that found in the adults. The biggest difference in factors regulating the feedback loop between the neonates and adults is the much higher pituitary D2 levels in the young mice (Figures 1 and 6). The cerebrocortical D2 was identical at P5 and P17, suggesting the elevated D2 activity in the neonatal mouse is specific to the pituitary. The larger difference between the neonatal pituitary D2 in the controls and Cga-cre D2KO mice than in the adults may explain their higher serum T4 throughout the first 2 weeks of life as a reflection of the absence of the T4-feedback through the D2 pathway. Interestingly, the relative T4 elevation in Cga-cre D2KO neonates is greatest at P5, suggesting that it may also be present during the late fetal period. The serum T4 and T3 increase in parallel in both genotypes to several fold above that at P5 as the pituitary D2 falls until P15 when they reach the normal neonatal peak as previously noted. The increase in serum thyroid hormones occurs independently of a change in TSH (Figure 5) in both genders and occurs at a time when hepatic D1 is increasing (Figure 6, E and F). Thus, the increases in thyroid hormones cannot be attributed to a decrease in the deiodinative T4 or T3 clearance. The absence of a further increase in TSH as T4 and T3 increase in both genotypes suggests a gradual increase of the thyroid gland sensitivity to TSH and a reduction in the importance of T4 per se to the feedback regulation of TSH as pituitary D2 falls. These results suggest that the T4 feedback at the thyrotroph level is especially important in the early neonatal period in the mouse and is attenuated by P17. In some sense, this is reminiscent of the marked increase in TSH, and thyroid hormone secretion, at birth in the neonatal human (40). The persistent elevation of TSH, despite the rising serum T4, establishes that neonatal suppression of the thyrotroph does not explain the impaired TSH response of the adult Cga-cre D2KO mouse to hypothyroidism. Taken together, the data argue that the higher serum T4 over the neonatal period in the Cga-cre D2KO mice is a result of an autonomous TSH-mediated thyroidal stimulation due to the reduced effectiveness of T4-mediated suppression in the absence of D2.
Finally, it is well known that there are both transcriptional and posttranslational increases in D2, which can buffer the adverse effects of a deficiency of circulating T4 in the CNS and attenuate the TSH response of the thyrotroph. Nonetheless, previous studies in the rat have demonstrated that the concentration of specifically bound nuclear T3 in the pituitary of the severely hypothyroid rat is substantially reduced, despite the high D2, because of the low-serum T4 (34). Although TRH has a critical role in the regulation of thyroid function, these studies reveal the importance of D2 in modulating the physiological response of the thyrotroph (41). We conclude that the capacity to monitor both the prohormone T4 and the active hormone T3 is essential for the optimal response of the vertebrate to the stress of chronic iodine deficiency or primary hypothyroidism.
Acknowledgments
We thank Dr Stephen Huang and Ms Michelle Maynard for assistance with the D3 assays. JP26–26 and JP02 cells were a generous gift from Dr G. Vassart and S. Refetoff. Blastocyst injection was performed by the BWH Transgenic Core.
Present address for A.M.: Department of Clinical and Experimental Medicine, Section of Endocrinology, University of Pisa, 56126-I Pisa, Italy.
This work was supported by National Institutes of Health Grants R01DK36256, R01DK44128, T32DK007529, and K01DK091403.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Cga
- glycoprotein hormone α-subunit gene
- CNS
- central nervous system
- D2
- type 2 iodothyronine deiodinase
- DHA
- dorsal hypothalamic area
- KO
- knockout
- MBH
- mediobasal hypothalamus
- MMI
- methimazole
- P
- postnatal day
- PTU
- propylthiouracil
- PVN
- paraventricular nucleus
- TR
- thyroid hormone receptor.
References
- 1. Larsen PR. Triiodothyronine: review of recent studies of its physiology and pathophysiology in man. Metabolism. 1972;21:1073–1092. [DOI] [PubMed] [Google Scholar]
- 2. Sterling K, Brenner MA, Newman ES. Conversion of thyroxine to triiodothyronine in normal human subjects. Science. 1970;169:1099–1100. [DOI] [PubMed] [Google Scholar]
- 3. Silva JE, Larsen PR. Pituitary nuclear 3,5,3′-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977;198:617–620. [DOI] [PubMed] [Google Scholar]
- 4. Silva JE, Larsen PR. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978;61:1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larsen PR, Dick TE, Markovitz BP, Kaplan MM, Gard TG. Inhibition of intrapituitary thyroxine to 3.5.3′-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979;64:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. [DOI] [PubMed] [Google Scholar]
- 7. Visser TJ, Kaplan MM, Leonard JL, Larsen PR. Evidence for two pathways of iodothyronine 5′-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983;71:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry MJ, Banu L, Larsen PR. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991;349:438–440. [DOI] [PubMed] [Google Scholar]
- 9. Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest. 1996;98:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148. [DOI] [PubMed] [Google Scholar]
- 11. Marsili A, Sanchez E, Singru P, et al. Thyroxine-induced expression of pyroglutamyl peptidase II and inhibition of TSH release precedes suppression of TRH mRNA and requires type 2 deiodinase. J Endocrinol. 2011;211:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–174. [DOI] [PubMed] [Google Scholar]
- 13. Fonseca TL, Correa-Medina M, Campos MP, et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest. 2013;123:1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riesco G, Taurog A, Larsen R, Krulich L. Acute and chronic responses to iodine deficiency in rats. Endocrinology. 1977;100:303–313. [DOI] [PubMed] [Google Scholar]
- 15. Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao L, Bakke M, Krimkevich Y, et al. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–154. [DOI] [PubMed] [Google Scholar]
- 17. Christoffolete MA, Arrojo e Drigo R, Gazoni F, et al. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology. 2007;148:954–960. [DOI] [PubMed] [Google Scholar]
- 18. Zavacki AM, Ying H, Christoffolete MA, et al. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology. 2005;146:1568–1575. [DOI] [PubMed] [Google Scholar]
- 19. Vella KR, Ramadoss P, Costa-E-Sousa RH, et al. Thyroid hormone signaling in vivo requires a balance between coactivators and corepressors. Mol Cell Biol. 2014;34:1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paxinos A, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed San Diego, CA: Elsevier Science, Technology Books; 2004. [Google Scholar]
- 21. Marsili A, Ramadan W, Harney JW, et al. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology. 2010;151:5952–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perret J, Ludgate M, Libert F, et al. Stable expression of the human TSH receptor in CHO cells and characterization of differentially expressing clones. Biochem Biophys Res Commun. 1990;171:1044–1050. [DOI] [PubMed] [Google Scholar]
- 23. Moeller LC, Kimura S, Kusakabe T, Liao XH, Van Sande J, Refetoff S. Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid-stimulating hormone receptor. Mol Endocrinol. 2003;17:2295–2302. [DOI] [PubMed] [Google Scholar]
- 24. Koenig RJ, Leonard JL, Senator D, Rappaport N, Watson AY, Larsen PR. Regulation of thyroxine 5′-deiodinase activity by 3,5,3′-triiodothyronine in cultured rat anterior pituitary cells. Endocrinology. 1984;115:324–329. [DOI] [PubMed] [Google Scholar]
- 25. Steinsapir J, Harney J, Larsen PR. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J Clin Invest. 1998;102:1895–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azizi F, Vagenakis AG, Bollinger J, Reichlin S, Braverman LE, Ingbar SH. Persistent abnormalities in pituitary function following neonatal thyrotoxicosis in the rat. Endocrinology. 1974;94:1681–1688. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christoffolete MA, Ribeiro R, Singru P, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–1743. [DOI] [PubMed] [Google Scholar]
- 29. Fonseca TL, Werneck-De-Castro JP, Castillo M, et al. Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse. Diabetes. 2014;63:1594–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9:1265–1271. [DOI] [PubMed] [Google Scholar]
- 31. Schneider MJ, Fiering SN, Thai B, et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. [DOI] [PubMed] [Google Scholar]
- 32. de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35:159–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsen PR, Bavli SZ, Castonguay M, Jove R. Direct radioimmunoassay of nuclear 3,5,3′ triiodothyronine in rat anterior pituitary. J Clin Invest. 1980;65:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ortiga-Carvalho TM, Shibusawa N, Nikrodhanond A, et al. Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface. J Clin Invest. 2005;115:2517–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tagami T, Madison LD, Nagaya T, Jameson JL. Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol. 1997;17:2642–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsuura N, Harada S, Ohyama Y, et al. The mechanisms of transient hypothyroxinemia in infants born to mothers with Graves' disease. Pediatr Res. 1997;42:214–218. [DOI] [PubMed] [Google Scholar]
- 38. Silva JE, Larsen PR. Peripheral metabolism of homologous thyrotropin in euthyroid and hypothyroid rats: acute effects of thyrotropin-releasing hormone, triiodothyronine, and thyroxine. Endocrinology. 1978;102:1783–1796. [DOI] [PubMed] [Google Scholar]
- 39. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–1078. [DOI] [PubMed] [Google Scholar]
- 41. Nikrodhanond AA, Ortiga-Carvalho TM, Shibusawa N, et al. Dominant role of thyrotropin-releasing hormone in the hypothalamic-pituitary-thyroid axis. J Biol Chem. 2006;281:5000–5007. [DOI] [PubMed] [Google Scholar]