Abstract
In temperate zones, animals restrict breeding to specific seasons to maximize the survival of their offspring. Birds have evolved highly sophisticated mechanisms of seasonal regulation, and their testicular mass can change 100-fold within a few weeks. Recent studies on Japanese quail revealed that seasonal gonadal development is regulated by central thyroid hormone activation within the hypothalamus, depending on the photoperiodic changes. By contrast, the mechanisms underlying seasonal testicular regression remain unclear. Here we show the effects of short day and low temperature on testicular regression in quail. Low temperature stimulus accelerated short day-induced testicular regression by shutting down the hypothalamus-pituitary-gonadal axis and inducing meiotic arrest and germ cell apoptosis. Induction of T3 coincided with the climax of testicular regression. Temporal gene expression analysis over the course of apoptosis revealed the suppression of LH response genes and activation of T3 response genes involved in amphibian metamorphosis within the testis. Daily ip administration of T3 mimicked the effects of low temperature stimulus on germ cell apoptosis and testicular mass. Although type 2 deiodinase, a thyroid hormone-activating enzyme, in the brown adipose tissue generates circulating T3 under low-temperature conditions in mammals, there is no distinct brown adipose tissue in birds. In birds, type 2 deiodinase is induced by low temperature exclusively in the liver, which appears to be caused by increased food consumption. We conclude that birds use low temperature-induced circulating T3 not only for adaptive thermoregulation but also to trigger apoptosis to accelerate seasonal testicular regression.
Most animals living outside the tropics measure changes in day length to breed at a specific time of year, largely to ensure that offspring are born only when the climate is moderate and abundant food is available. Birds have evolved highly sophisticated photoperiodic mechanisms, probably due to the adaptations necessary for flight. Seasonal changes in testicular mass are typically of the order of 100-fold in birds, as compared with severalfold in mammals (1). Among birds, Japanese quail provides a particularly attractive model for studying these phenomena; recent studies using quail have uncovered the signal transduction pathway that triggers photoperiodic gonadal development.
Light information is received by deep-brain photoreceptors, including Opsin 5-positive cerebrospinal fluid-contacting neurons in the paraventricular organ and then transmitted to the pars tuberalis of the pituitary gland (2–4). The long day (LD)-induced TSH in the pars tuberalis acts on TSH receptors in the ependymal cells within the mediobasal hypothalamus (MBH) to induce DIO2 and to reduce DIO3 expression (5). The regulatory mechanism of pars tuberalis-derived TSH is completely different from that of the classical pars distalis-derived TSH (6). TSH in the pars distalis is regulated by the hypothalamus-pituitary-thyroid axis. However, there is no TRH receptor and thyroid hormone (TH) receptor in the pars tuberalis, and TSH in the pars tuberalis is independent of the hypothalamus-pituitary-thyroid axis (7). DIO2 and DIO3 encode TH-activating (type 2 iodothyronine deiodinase) and -inactivating (type 3 iodothyronine deiodinase) enzymes, respectively. These two genes act as switches to regulate local TH concentration, and LD-induced T3 within the MBH causes testicular development (8, 9). TH is involved in the development and plasticity of the brain (10). Indeed, morphological changes in GnRH nerve terminals and glial processes are observed in the median eminence; these changes are likely to modulate seasonal GnRH secretion (11).
In contrast to the mechanisms that trigger seasonal testicular development, described above, the mechanisms underlying seasonal testicular regression remain unclear. In quail, a low temperature stimulus accelerates short day (SD)-induced testicular regression (12, 13). Therefore, in this study, we examined the effect of SD and short day/low temperature (SL) on quail testis. To this end, we first evaluated germ cell differentiation and apoptosis in the testis. Next, we assessed expression profiles of key genes regulating seasonal reproduction in the brain. We also determined serum hormone levels and detected changes in both LH and T. In addition, low temperature-induced serum T3 was observed at the climax of testicular regression. It is well established that TH is involved in amphibian metamorphosis (14). Therefore, we examined temporal expression profiles of T3 response genes involved in amphibian metamorphosis in the quail testis. We then confirmed that T3 administration mimics the effect of low temperature. In mammals, type 2 deiodinase (DIO2) in the brown adipose tissue (BAT) generates T3, and BAT is considered to be a major source of local and circulating T3 under exposure to a low temperature (15–17). However, birds have no distinct BAT or related thermogenic tissue (18). To identify the tissue responsible for low temperature-induced serum T3, we measured expression of DIO2 in various tissues and detected induction of this gene exclusively in the liver. It has been known that feeding regulates diet-induced thermogenesis, but the involvement of hepatic DIO2 in the avian adaptive thermogenesis remains unclear. Therefore, we also examined the effect of fasting on hepatic DIO2 expression.
Materials and Methods
Animals
Male, 4-week-old Japanese quail (Coturnix japonica) were obtained from a local dealer and kept under SD conditions [6 h light, 18 h dark (6L18D)] for 4 weeks in light-tight boxes in a room held at a temperature of 23 ± 1°C. At 8 weeks of age, quail were transferred to LD conditions (20 h light, 4 h dark). Thirty days after the transfer to LD conditions, quail were transferred to SD or SL conditions (6L18D, 9°C). In the castration experiment, quail were castrated before they were transferred into SL conditions. In the fasting experiment, 3 weeks after the transfer to SD or SL conditions, the measurement of core body temperature by ip loggers (±0.7°C, Thermochron Type-SL; KN Laboratories) and food intake were performed. This study was approved by the Animal Experiment Committee of Nagoya University.
Histology
Paraffin sections (4 μm) of Bouin's fixed testes were used for synaptonemal complex protein 3 (SCP3) immunohistochemistry and analysis of apoptosis. Immunohistochemistry for SCP3 was performed using an anti-SCP3 antibody (NB300-231, 1:1500; Novus Biologicals) as previously described (19, 20). Apoptosis was analyzed by in situ terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) labeling with an ApopTag peroxidase staining kit (Millipore), which was followed by periodic acid-Schiff counterstaining.
Gene expression analysis
Relative mRNA levels were determined by in situ hybridization using 33P-labeled oligonucleotide probes (Supplemental Table 1), as previously described (21). OD was normalized by subtracting the OD at an area of the same section in which no hybridization signal was observed. Densitometric analysis of hybridization signals was performed using Image Gauge (Fujifilm Co Ltd).
Radioimmunoassay
Serum LH concentrations were determined by a RIA using the chicken LH RIA kit (kindly supplied by Dr John A. Proudman, US Department of Agriculture-Agricultural Research Service Beltsville Agricultural Research Center) (5). The labeled antigen was prepared by iodinating purified chicken LH (USDA-cLH-I-3) with Na125I (NEZ033A; PerkinElmer Japan) by the lactoperoxidase method. USDA-cLH-K-3 and USDA-AcLH-5 were used as the standard and the antibody, respectively. For T determination, samples were extracted with diethylether and subjected to a RIA using [1,2,6,7-3H(N)]-T (PerkinElmer Japan) and the rabbit antitestosterone serum (FKA-102; CosmoBio) (22). T3 and T4 concentrations were determined by a RIA using L-3,5,3′-[125I]T3 (NEX110H; PerkinElmer Japan) and anti-T3 (Hycor Biomedicals), and L-[5′-125I]T4 (NEX111H; PerkinElmer Japan) and anti-T4 (Wien Laboratories), respectively (8). Parallelism of inhibition curves was proven between the standard and serial 2-fold dilution of quail samples for each hormone (Supplemental Figure 1). Intra- and interassay coefficients of variation were 7.6% (n = 3) and 11.1% (n = 4) for LH, 5.1% (n = 6) and 7.8% (n = 3) for T, 6.9% (n = 6) and 10.7% (n = 4) for T3, and 7.5% (n = 6) and 11.0% (n = 4) for T4, respectively.
T3 treatment
Quail kept under LD conditions for 30 days (30LD) were transferred to SD conditions or kept continuously under LD conditions. These birds were ip injected with T3 (0, 1, 10, or 100 μg/100 g body weight, once a day) (T2877; Sigma) dissolved in vehicle (0.02 mol/L NaOH) for 4 weeks from 30LD onward. Sera and testes were collected 24 hours after the final injection.
Measurement of DIO2 activity
DIO2 activities were measured in homogenates of the tissues, as previously described (23). Reactions contained 1 nM T4 as the substrate, 100 nM T3 + 0.1 mM propylthiouracil to block the possible interference by other deiodinases, and 25 mM dithiothreitol as the cofactor. The protein concentrations used in the different tests varied from 25 to 2000 μg/mL and the incubation time from 30–240 minutes, depending on the tissue. All activities were calculated as the amount of substrate deiodinated per milligram of protein per minute.
Data analysis
Normally distributed data from time-course samples were analyzed by a parametric test (one way ANOVA with Dunnett's multiple comparison post hoc test), and nonnormally distributed data were analyzed by a nonparametric test (Steel's multiple comparison test) to determine the significance. A Student's t test was performed to compare the results of SD and SL conditions at each time point. All values reported are means ± SEM.
Results
Low temperature stimulus accelerates meiotic arrest
LD (20L4D) stimulus induced testicular development (Figure 1A). Thereafter, the SD (6L18D) stimulus induced partial testicular regression, whereas the SL (6L18D, 9°C) stimulus induced a full and rapid testicular regression (Figure 1A). Between initial SD conditions (LD d 0) to LD conditions (LD d 30), testicular mass increased by 126-fold. To evaluate the occurrence of germ cell differentiation during testicular development and regression, we used immunohistochemistry to examine the expression of a meiotic marker, SCP3 (also known as Sycp3) (19). Although no SCP3-immunopositive cells were observed at the time of the initiation of the LD condition, the number of SCP3-positive cells increased over the course of testicular development (Figure 1, B and C). SD exposure gradually reduced the number of SCP3-positive cells, but these cells did not disappear entirely under the SD condition. In contrast, when a low temperature stimulus was administered in combination with an SD stimulus, only a few SCP3-positive cells were observed at the climax of testicular regression (ie, 20 or 25 d after transfer to SL), suggesting that SL stimuli significantly arrest meiosis (Figure 1, B and C).
Figure 1.
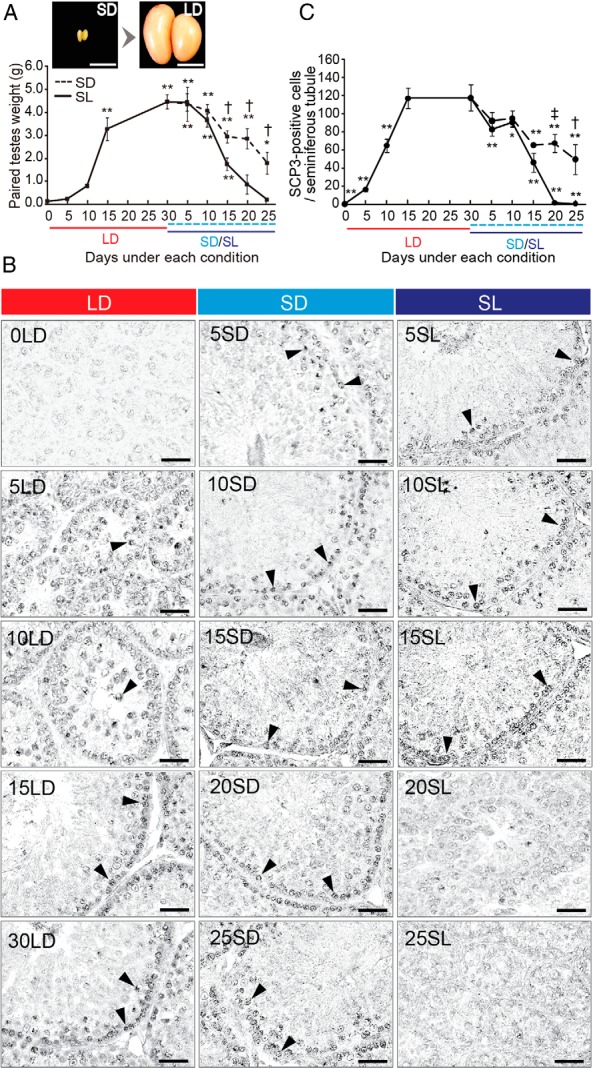
Effect of changing day length and temperature on quail testicular weight and meiosis. A, top panels, Testes of quail kept under SD and LD conditions. Scale bars, 1 cm. Bottom panels, Changes in testicular mass in quail transferred from SD to LD and then to short day/low temperature (SL; solid line) or SD (dashed line). *, P < .05; **, P < .01 vs 0 day of LD condition (0LD) (ANOVA); †, P < .05 SL vs SD (t test, n = 8–10). B, Representative photomicrographs for immunohistochemistry of SCP3, a marker of meiosis (arrowhead). Scale bars, 25 μm. C, Changes in number of SCP3-positive cells. *, P < .05; **, P < .01 vs 30LD (corresponding to 0SD/SL) (ANOVA); †, P < .05; ‡, P < .01 SL vs. SD (t test, n = 4–5).
Low temperature stimulus-induced germ cell apoptosis causes rapid testicular regression
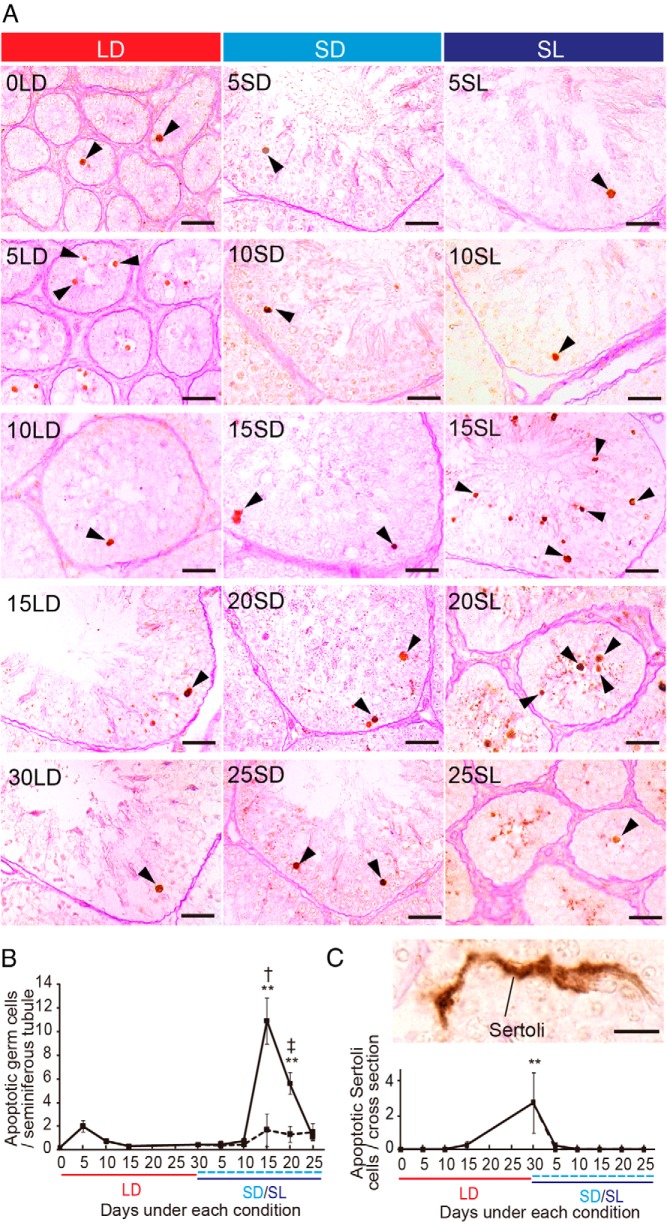
We next assessed apoptosis by in situ TUNEL labeling. A relatively low level of apoptotic cells was observed under the LD conditions (Figure 2, A and B). A substantial number of germ cells underwent apoptosis 15 and 20 days after the transfer to SL conditions (Figure 2, A and B). Although some apoptotic germ cells were observed under SD conditions, their number did not increase significantly (Figure 2, A and B). We observed a few apoptotic Sertoli cells in the fully matured testes but considerably less than germ cells (Figure 2C).
Figure 2.
Changes in number of apoptotic germ cells and Sertoli cells. A, Representative photomicrographs of TUNEL-labeled germ cells (arrowhead). B, The number of apoptotic germ cells per seminiferous tubule. **, P < .01 vs 0LD (ANOVA); †, P < .05 SL vs SD (t test, n = 4–5). C, Representative photomicrograph of a TUNEL-positive Sertoli cell and the number of such cells per cross-section (but not per seminiferous tubule). Scale bars, 25 μm (A) and 10 μm (C).
Photoperiodic response of key genes that regulate seasonal reproduction
When we examined the expression profiles of key genes involved in the regulation of seasonal reproduction, we observed an LD induction of TSHB in the pars tuberalis of the pituitary gland (Figure 3A). In addition, LD exposure induced DIO2 and suppressed DIO3 in the ependymal cells within the MBH (Figure 3, B and C). Although the responses of TSHB and DIO3 tended to occur slightly earlier under the SL conditions than under the SD conditions, there was no significant difference between the two conditions.
Figure 3.
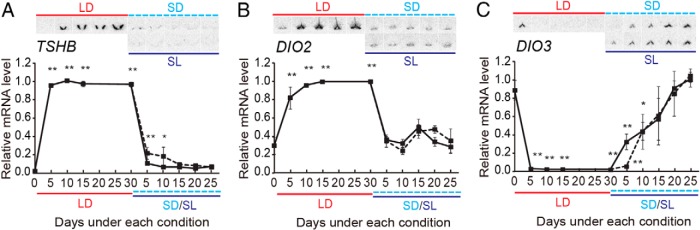
Temporal expression profiles of key genes involved in regulating seasonal reproduction in the quail brain. A–C, Representative autoradiograms and densitometric quantitation are shown: TSHB in the pars tuberalis (A), DIO2 (B), and DIO3 (C) in the ependymal cells within the MBH. *, P < .05; **, P < .01 vs 0LD (n = 4–5, ANOVA). Solid line, SL; dashed line, SD. Note that in Figures 1–5, all samples were collected at 16 hours after dawn to examine the expressions of TSHB and DIO2 in the brain in the same animals. These genes are rhythmically expressed and show high expression levels at around this time of the day.
Changes in serum hormone concentrations
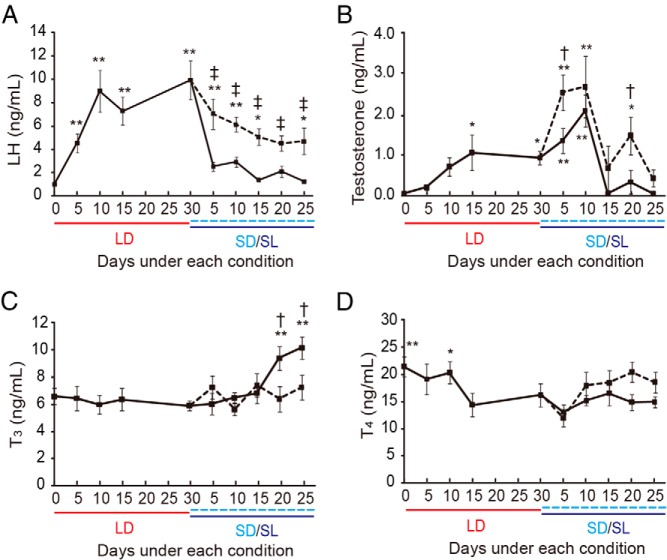
We next assessed serum hormone concentrations by RIA. LH levels changed rapidly, increasing significantly by 5 days after the transfer to LD conditions and decreasing significantly 5 days after the transfer to SL conditions (Figure 4A). In contrast, serum T increased more gradually. A significant increase was first observed 15 days after the transfer to LD conditions, and a further increase was observed after the transfer to SL conditions. However, T rapidly decreased between 10 and 15 days after the transfer to SL conditions (Figure 4B). Although a decrease in serum LH and T was also observed under SD conditions, these reductions were partial and more gradual than under SL conditions (Figure 4, A and B). Because circulating TH is increased by a low temperature stimulus for the purpose of adaptive thermogenesis, we also determined TH concentrations and observed a significant increase in T3 during the climax of testicular regression under SL conditions (ie, 20 and 25 d after transfer to SL conditions) but no such increase under SD conditions (Figure 4C). This result was consistent with the notion that an increase in the capacity for classical adaptive nonshivering thermogenesis takes weeks to develop (24). T4 was slightly higher during the transition from initial SD to LD conditions (Figure 4D).
Figure 4.
Changes in serum hormone concentrations. A, LH. B, T. C, T3. D, T4. *, P < .05; **, P < .01 vs trough value (ANOVA); †, P < .05; ‡, P < .01 SL vs SD (t test, n = 5–10). Solid line, SL; dashed line, SD.
Induction of T3 response genes and reduction of LH response genes during the climax of testicular regression
We next performed an analysis of temporal gene expression in the quail testis. Genes encoding the LH receptor (LHR) and steroidogenic acute regulatory protein (StAR), a rate-limiting factor of steroidogenesis, are both expressed in Leydig cells in an LH-dependent manner (25, 26). Expression of these genes was rapidly induced by an LD stimulus and sustained until 10 days after the transfer to SL conditions, followed by a rapid decrease (Figure 5, A and B). When we examined the expression of T3 response genes involved in amphibian metamorphosis, we observed a high expression of TH receptors (THRA, THRB) and its dimeric partner (RXRA) during the transition from the initial SD to LD conditions as well as during the climax of testicular regression under SL conditions (Figure 5, C–E). Low temperature-induced expression of other T3 response genes, including deiodinases (DIO2, DIO3) and apoptosis-related genes (CASP6, MMP13, TGFB2, TNFAIP2) (14, 27–29), were also observed (Figure 5, F–K). The magnitude of the reduction in LH response genes and the induction in T3 response genes were smaller under the SD than under the SL conditions (Figure 5).
Figure 5.
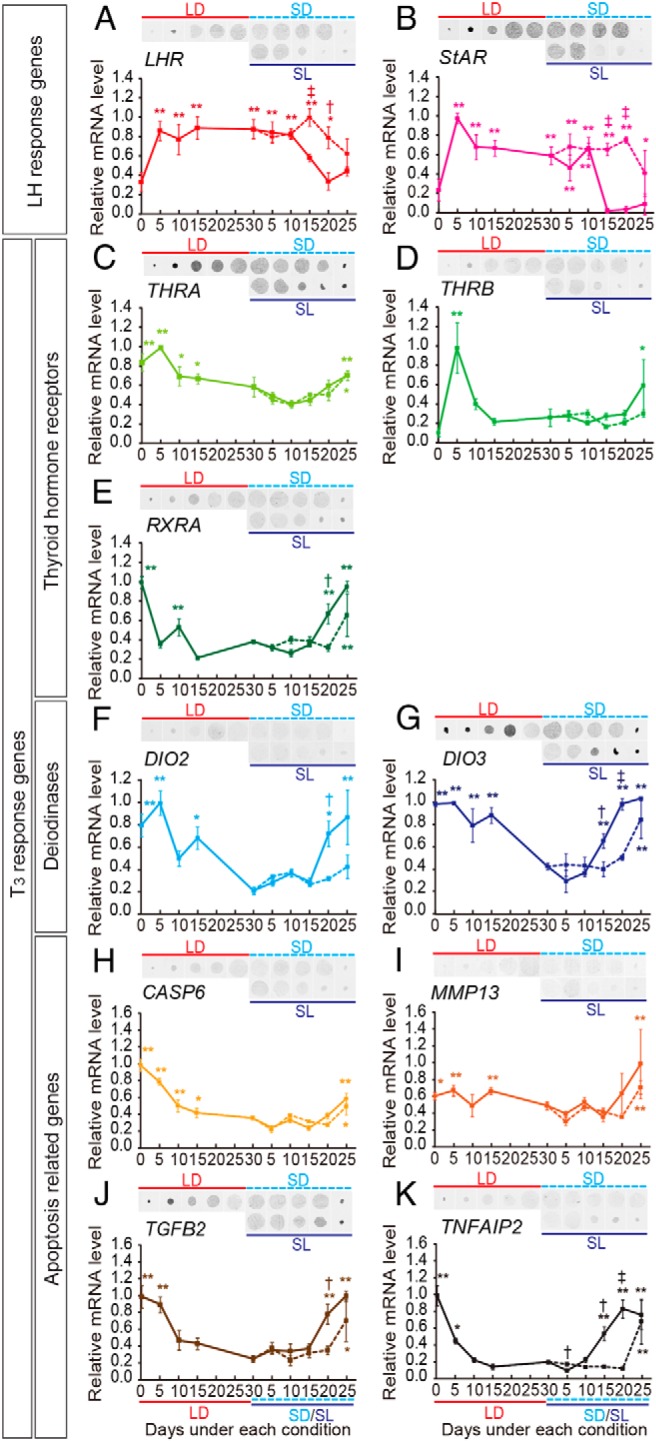
Temporal change in gene expression in the quail testis. A and B, LH-driven genes (LHR, StAR). C–E, TH receptors (THRA, THRB) and their dimeric partner (RXRA). F and G, Deiodinase genes (DIO2, DIO3). H–K, Apoptosis-related genes (CASP6, MMP13, TGFB2, TNFAIP2). *, P < .05; **, P < .01 vs trough value; †, P < .05; ‡, P < .01 SL vs SD (t test, n = 4–5). Solid line, SL; dashed line, SD.
Daily ip T3 administration mimics the effect of low temperature
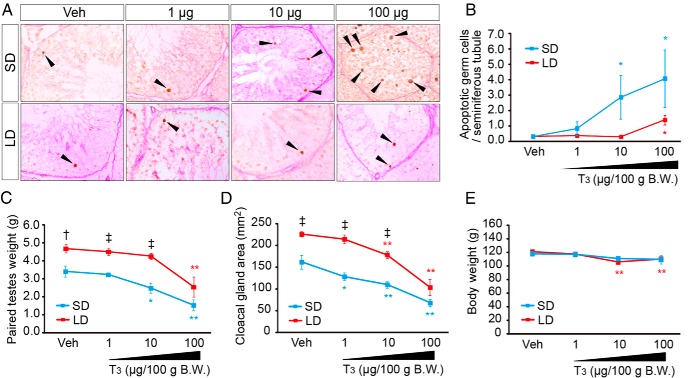
To determine whether T3 mimics the effects of cold stimulus, we examined its effects of T3 administration on germ cell apoptosis, testicular weight, and the size of the cloacal gland area, a T-dependent structure located at the caudal end of the cloaca (30). In a dose-dependent manner, T3 administration increased germ cell apoptosis (Figure 6, A and B) and reduced testicular weight and the size of the cloacal gland area (Figure 6, C and D) under both SD and LD conditions. Effects of T3 administration were more prominent under SD than under LD conditions (Figure 6, A–D). However, T3 treatment did not completely mimic the effect of low temperature under SD (also see Figure 1A). This is probably due to the short half-life of T3 (∼4 h) during circulation (31). Although changes in body weight were also observed, these differences were less than 10% of body weight (Figure 6E), a relatively small change compared with the annual molt, when birds exhibit anorexia and lose 20%–40% of their body weight (32). Accordingly, we believe that the daily T3 injections mimicked physiological conditions.
Figure 6.
Effect of daily T3 administration on apoptosis, testicular mass, cloacal gland area, and body weight. A, Representative photomicrographs of TUNEL-labeled germ cells (arrowheads). B, The number of apoptotic cells. C–E, Effect of daily ip T3 administration on testis mass (C), cloacal gland area (D), and body weight (B.W.; E). *, P < .05; **, P < .01 vs vehicle (Veh); †, P < .05; ‡, P < .01 SL vs SD (t test, n = 5–12). Red line, LD; blue line, SD.
Identification of the tissue responsible for low temperature-induced circulating T3 in birds
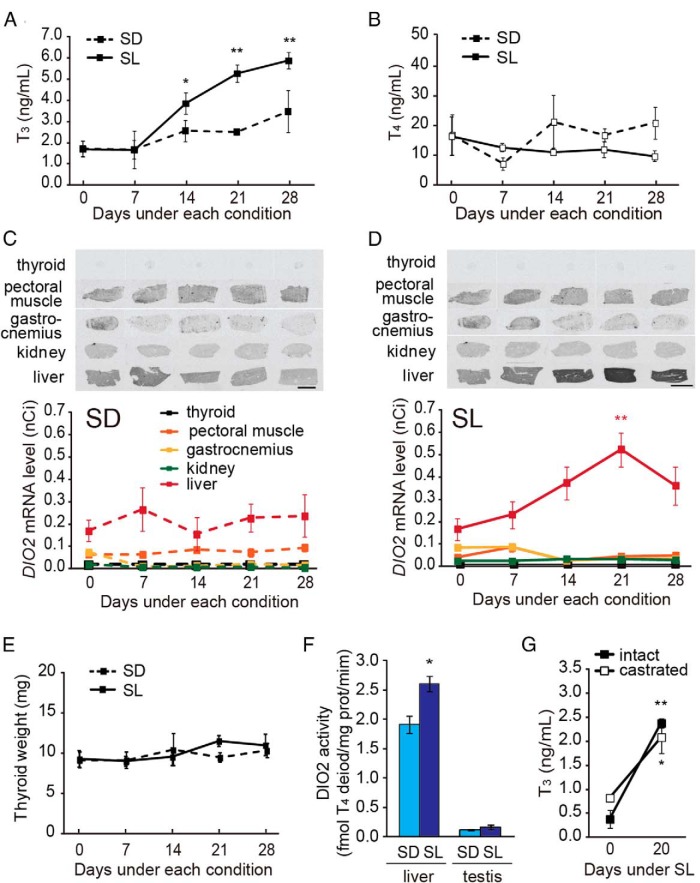
Although DIO2 in the BAT is responsible for low temperature-induced circulating T3 in mammals, there is no distinct BAT or related thermogenic tissue in birds. To identify the tissue responsible for low temperature-induced serum T3, we collected serum and various tissue samples every 7 days after the transfer to SD/SL conditions. Consistent with Figure 4C, a significant increase in serum T3 level was observed under SL conditions but not under SD conditions (Figure 7, A and B). When we examined the expression of DIO2 in various tissues (eg, thyroid gland, pectoral muscle, gastrocnemius, kidney, and liver), we observed low-temperature induction of DIO2 only in the liver (Figure 7D), and this induction did not occur under SD conditions (Figure 7C). Although an increased thyroid gland weight in response to low temperature stimulus was reported several decades ago (33, 34), we did not observe changes in thyroid gland weight (Figure 7E). We also observed low temperature-induced expression of DIO2 in the testis (Figure 5F), but the magnitude of this induction was much smaller than in the liver. Furthermore, DIO2 enzyme activity was significantly increased by low temperature in the liver but not in the testis (Figure 7F), and castration did not attenuate the serum level of T3 induced by low temperature (Figure 7G). These results suggest that the liver, but not the testis, appears to be the source of low temperature-induced circulating T3. Although we also examined the effect of SL on DIO3 expression in the liver, we observed no changes in the expressions of these genes (Supplemental Figure 2). Therefore, we focused on the regulation of liver DIO2 expression.
Figure 7.
Identification of the tissue responsible for low temperature-induced serum T3. A and B, Changes in serum T3 (A) and T4 (B) levels under SD (dashed line) and SL (solid line) conditions. *, P < .05; **, P < .01 vs day 0. C and D, Changes in DIO2 mRNA in various tissues under SD (C) and SL (D) conditions. Representative autoradiograms (top panel) and densitometric quantification (bottom panel) are shown. **, P < .01 vs day 0. Scale bars, 1 cm. E, Size of thyroid gland did not change under SD and SL conditions. F, DIO2 activity in liver and testis under SD and SL conditions. Samples were collected 28 days after transferred to each condition. *, P < .05 (t test, n = 3–6). G, Castration did not affect low-temperature-induced serum T3 level. *, P < .05; **, P < .01 day 0 vs day 20 (t test, n = 6–8). All samples were collected at 3 hours after dawn under SD/SL condition (ie, midday).
Low temperature-induced hepatic DIO2 requires increased food intake
In mammals and birds, increased food intake has been reported under low-temperature conditions (35–37). To test whether food intake is related to cold-induced hepatic DIO2 expression, we first examined the effect of a low-temperature stimulus on food intake. As expected, increased food intake was observed under SL conditions (Figure 8A). When we measured body temperature rhythms, the internal body temperature exhibited clear day-night variation: high during the day and low during the night (Figure 8B, left panel). However, when animals were fasted, their body temperature decreased profoundly during the night phase, regardless of ambient temperature (Figure 8, B right panel, and C). This observation of hypothermia during the inactive phase is consistent with a previous report in quail (38). When we examined hepatic DIO2 expression in these animals, the suppression of the low-temperature DIO2 induction was observed under fasting conditions (Figure 8D). Although a similar tendency was observed under SD conditions, no statistically significant difference was observed. It is interesting to note that the serum T3 levels were suppressed by fasting under both the SL and SD conditions (Figure 8E). These results suggest that increased food intake is required for the low-temperature induction of DIO2 in the liver.
Figure 8.
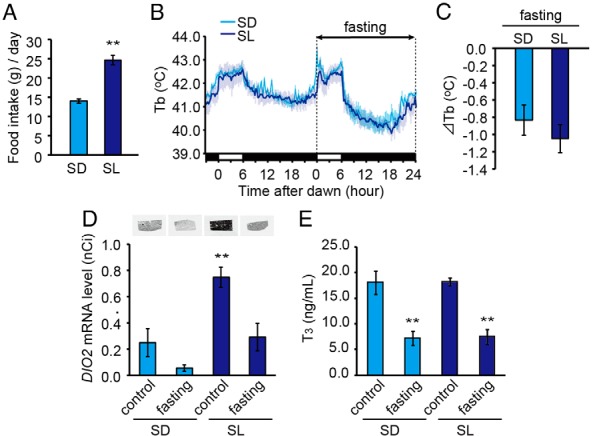
Increased food intake is required for low temperature-induced DIO2 expression in the liver. A, Food intake per day measured at 21 days after transfer to SL and SD conditions. **, P < .01 (t test). B and C, Effect of fasting on body temperature (Tb) at 21 days after transfer to SD (light blue) and SL (dark blue) conditions. Light blue and gray shading indicate the SEM. D, Effect of fasting on hepatic DIO2 expression. Samples were collected 12 hours after dawn when the lowest body temperature was observed. Representative autoradiograms (top panel) and densitometric quantification (bottom panel) are shown. E, Effect of fasting on serum T3 levels (mean ± SEM, n = 4–6). **, P < .01 vs SD control (ANOVA, Dunnett's post hoc test).
Discussion
In this study, we demonstrated dynamic seasonal changes in the quail testis. Although quail raised under LD conditions reach puberty when they are 8 weeks old (30), the testes of 8-week-old quail raised under SD conditions remained small and immature (Figure 1A). However, once quail were transferred to LD conditions, meiosis and rapid testicular development occurred. In quail, a single LD stimulus triggers cascades of gene induction, which results in LH secretion approximately 22 hours after dawn of the first long day; testicular development is accomplished within a few weeks (5, 39–41). However, meiosis continued for a time after the transfer to SD/SL conditions (Figure 1, B and C). The slow rate of initiation of the SD/SL-induced testicular regression observed in this study was in contrast to the rapid rate of photoinduction. It appears that repeated exposure to SD/SL stimuli is required to initiate testicular regression. This mechanism may enable birds to avoid misinterpreting several consecutive rainy, cloudy, or cold days as the start of the autumn stimulus.
Testicular mass gradually decreased under SD conditions (Figure 1A). Because the number of SCP3-positive cells decreased under SD conditions and no significant increase in the number of apoptotic germ cells was observed, the arrest of meiosis appeared to be the primary cause of partial testicular regression under SD conditions (Figures 1 and 2). In marked contrast to SD conditions, a substantial number of apoptotic germ cells was observed 15 and 20 days after transfer into SL conditions. In SL quail, the number of apoptotic cells per seminiferous tubule observed was approximately 10-fold larger than in seasonally breeding mammals (42) but was equivalent to that of European starlings, which exhibit photorefractoriness (42). Photorefractoriness is the insensitivity of gonadal development to the stimulatory effects of LD in birds. The extensive germ cell death observed in these birds seemed to account for the rapid and drastic testicular regression observed in avian species. In addition to germ cells, apoptotic Sertoli cells have been reported in starlings (43). Although we found a few apoptotic Sertoli cells in fully matured quail testes, we did not detect them during the regression process (Figure 2). We therefore conclude that seasonal testicular regression caused by an SL stimulus in quail is mediated by the arrest of germ cell differentiation and apoptosis.
Based on these results, we can propose mechanisms for seasonal testicular development and regression (Supplemental Figure 3). Expression profiles of key genes regulating seasonal reproduction, TSHB, DIO2, and DIO3, simply reflect environmental photoperiodic information (Figure 3). Therefore, when quail raised under SD conditions are transferred to LD conditions, the photoperiodic signaling pathway is immediately switched on and the hypothalamus-pituitary-gonadal (H-P-G) axis is activated. LD-induced LH up-regulates the expression of the LH response genes LHR and StAR in the testis (Figures 4 and 5 and Supplemental Figure 3). Thus, the activation of the LH-dependent steroidogenesis pathway results in increased T production. T is a survival factor for germ cells: withdrawal of T increases germ cell apoptosis, whereas reintroduction decreases it (44). Consequently, activation of the LH-dependent steroidogenesis pathway activates germ cell differentiation and inhibits germ cell apoptosis under LD conditions (Supplemental Figure 3).
When quail were transferred from LD to SD conditions, the photoperiodic signaling pathway (ie, TSHB and DIO2) was switched off, and serum LH gradually decreased (Figures 3 and 4 and Supplemental Figure 3). However, because serum LH and T did not return to basal levels under SD conditions, the arrest of meiosis was partial and the number of apoptotic cells did not increase significantly (Figures 1 and 2). On the other hand, when quail were transferred to SL conditions, we observed a rapid decrease in serum LH (Figure 4). Expression profiles of key genes regulating seasonal reproduction (TSHB, DIO2, and DIO3) did not significantly differ between SD and SL conditions (Figure 3). The photoperiodic response of quail, unlike that of other avian species, has been proposed to be regulated primarily at the level of GnRH secretion rather than GnRH synthesis (1, 11, 45, 46). Therefore, low-temperature stimulus appeared to attenuate GnRH secretion to enhance the effect of SD stimulus, thereby completely shutting down the H-P-G axis (Supplemental Figure 3). LHR-null mice have smaller seminiferous tubules due to apoptosis in spermatocytes (47). Therefore, once serum LH and T decrease to the basal levels under SL conditions, germ cells arrest meiosis (Figure 1) and apoptosis takes place (Figure 2). However, changes in seminiferous tubule diameter are much more moderate in LHR-null mice (diameter in LHR null mice is ∼60% of that in wild type mice: ie, ∼40% reduction) (47) than in quail (diameter in SD quail is ∼20% of that in LD quail: ie, ∼80% reduction). Hence, the SL-induced shutdown of the LH-dependent steroidogenesis pathway (ie, the H-P-G axis) appears to be a component of the mechanisms regulating seasonal testicular regression (Supplemental Figure 3).
TH has multiple functions in development and physiology. Among them, the most visually striking function is amphibian metamorphosis. In frogs, resorption of the tail and formation of the fore- and hindlimbs in tadpoles are both induced by TH (14). Two central questions in metamorphosis research are as follows: 1) how does TH induce opposite morphological responses in different tissues, ranging from outgrowth of limbs to the resorption of the tail? and 2) how is its precise timing achieved (14)? In regard to the former question, it has been well established that low levels of circulating TH induce limb outgrowth, whereas the tail resorbs only when TH is the highest. During the climax of metamorphosis, a surge of TH activates expression of its receptors (autoinduction), deiodinases and apoptosis-related genes (14, 27–29). In this study, serum T4 was slightly elevated during the transition from initial SD to LD conditions (Figure 4). Expression of DIO2 in the testis was also high during this period (Figure 5).
In the mammalian testis, expression of TH receptors and TH response genes, including DIO2, is the highest during fetal and perinatal life; TH plays a pivotal role in the growth and maturation of the testis (48–50). Therefore, we speculate that highly expressed DIO2 in the SD testis locally converts serum-derived T4 into T3 to promote growth and maturation of the testis during the transition from initial SD to LD conditions as in the case of mammalian fetal and perinatal period. On the other hand, we observed a significant increase in serum T3 level (Figure 4) and induction of TH receptors, deiodinases, and apoptosis-related genes during the climax of testicular regression (Figure 5). Furthermore, daily T3 administration mimicked the effects of low temperature on germ-cell apoptosis and testicular regression (Figure 6). Therefore, we conclude that low temperature-induced circulating T3 acts on the testis to activate gene cascades similar to the ones involved in amphibian metamorphosis and causes germ cell apoptosis in the quail testis (Supplemental Figure 3). Metamorphosis is an irreversible biological process that involves robust changes in animal body structure, so it is somewhat surprising that genes involved in the metamorphosis of amphibians are also activated during an annual cyclic event of quail, ie, the regulation of seasonal reproductive activity.
Homeothermic animals maintain body temperature by increasing heat production in response to a low-temperature stimulus. In mammals, DIO2 in the BAT generates T3, and BAT is a major source of circulating T3 during exposure to low temperature (15). Because birds have no distinct BAT or related thermogenic tissue (18), we searched for the tissue responsible for low temperature-induced circulating T3. Although increases in thyroid activity and thyroid weight during cold acclimation were reported several decades ago (33, 34), we did not observe changes in DIO2 expression or thyroid gland weight in this study (Figure 7). In addition, skeletal muscle has been suggested to be a source of heat generation in birds (51–53). However, we did not observe low-temperature induction of DIO2 in muscles (Figure 7), suggesting that skeletal muscle is not the source of circulating T3. In contrast, we observed induction of DIO2 in the liver and the testis. The magnitude of DIO2 induction by low temperature was much smaller in the testis than in the liver. Indeed, low temperature increased the enzyme activity of DIO2 in the liver but not in the testis (Figure 7), and castration did not affect the low temperature-induced rise in serum T3 (Figure 7). Because DIO2 is reportedly absent in human and rat liver (54, 55), its physiological significance has not been recognized. However, it should be noted that the presence of DIO2 in the liver has been reported in mice, chicken, and some species of fish (56–59).
In this study, food deprivation caused nocturnal hypothermia under both SL and SD conditions (Figure 8). This fasting-induced hypothermia during the inactive phase was consistent with a previous report in birds (38) and suggests that food intake is important for body temperature maintenance during the inactive phase, regardless of the ambient temperature. We also observed that fasting suppressed the low-temperature induction of hepatic DIO2 and serum T3 levels (Figure 8). Although serum T3 levels did not completely reflect the hepatic DIO2 expression profiles, these results clearly suggested that increased food intake is important for the low-temperature induction of hepatic DIO2 expression. The mechanism by which food intake induces hepatic DIO2 expression remains to be clarified in future studies. However, these results collectively suggest that the liver is the potential source of low-temperature-induced circulating T3 in birds. TH is the key controller of heat production in birds as well as in mammals (60–62). Therefore, it seems quite reasonable that birds would use T3 not only for adaptive thermoregulation but also for seasonal testicular regression in autumn.
In conclusion, we have demonstrated that a low-temperature stimulus accelerates seasonal testicular regression by arresting meiosis and inducing drastic germ cell apoptosis. Prolonged exposure to SL stimuli is required for the initiation of testicular regression. However, once birds interpret consecutive SL stimuli as the onset of autumn, these mechanisms enable them to achieve full gonadal regression within a short period of time. The reported effects of thyroidectomy or TH treatment on seasonal breeding have often appeared to be contradictory (1). However, our study reveals that TH plays dual roles in the regulation of seasonal reproduction: central action induces seasonal testicular development, whereas peripheral action mediates seasonal testicular regression.
Acknowledgments
We thank the Nagoya University Radioisotope Center for the use of their facilities. We also thank Dr J. A. Proudman for providing the chicken LH RIA kit.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas, “Regulatory Mechanism of Gamete Stem Cells” (Grant 21116504) and Funding Program for Next Generation World Leading Researchers (NEXT Program) initiated by the Council for Science and Technology Policy (Grant LS055), and Japan Society for the Promotion of Science KAKENHI Grant 26000013.
Current address for K.I.: Department of Anatomy and Neurobiology, Kinki University Faculty of Medicine, 377-2 Ohno-Higashi, Osaka-Sayama, Osaka 589-8511, Japan.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- DIO2
- type 2 deiodinase
- H-P-G
- hypothalamus-pituitary-gonadal
- LD
- long day
- 6L18D
- 6 h light, 18 h dark
- 30LD
- LD conditions for 30 days
- MBH
- mediobasal hypothalamus
- SCP3
- synaptonemal complex protein 3
- SD
- short day
- SL
- SD/low temperature
- TH
- thyroid hormone
- TUNEL
- terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- 1. Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythm. 2001;16:365–380. [DOI] [PubMed] [Google Scholar]
- 2. Nakane Y, Ikegami K, Ono H, et al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci USA. 2010;107:15264–15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakane Y, Shimmura T, Abe H, Yoshimura T. Intrinsic photosensitivity of a deep brain photoreceptor. Curr Biol. 2014;24:R596–R597. [DOI] [PubMed] [Google Scholar]
- 4. Nakane Y, Yoshimura T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Front Neurosci. 2014;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakao N, Ono H, Yamamura T, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. [DOI] [PubMed] [Google Scholar]
- 6. Ikegami K, Liao XH, Hoshino H, et al. Tissue-specific post-translational modification allows functional targetinof thyrotropin. Cell Rep. 2014;9:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bockmann J, Böckers T, Winter C. Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3,5,3′-triiodothyronine, thyrotropin-releasing hormone, and Pit-1 independent. Endocrinology. 1997;138:1019–1028. [DOI] [PubMed] [Google Scholar]
- 8. Yoshimura T, Shinobu Y, Watanabe M, et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. [DOI] [PubMed] [Google Scholar]
- 9. Yasuo S, Watanabe M, Nakao N, et al. The reciprocal switching of two thyroid hormone-activating and -inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. [DOI] [PubMed] [Google Scholar]
- 10. Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002;25:268–288. [DOI] [PubMed] [Google Scholar]
- 11. Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology. 2004;145:4264–4267. [DOI] [PubMed] [Google Scholar]
- 12. Oishi T, Konishi T. Effects of photoperiod and temperature on testicular and thyroid activity of the Japanese quail. Gen Comp Endocrinol. 1978;36:250–254. [DOI] [PubMed] [Google Scholar]
- 13. Wada M. Low temperature and short days together induce thyroid activation and suppression of LH release in Japanese quail. Gen Comp Endocrinol. 1993;90:355–363. [DOI] [PubMed] [Google Scholar]
- 14. Furlow JD, Neff ES. A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrionol Metab. 2006;17:40–47. [DOI] [PubMed] [Google Scholar]
- 15. Silva JE, Larsen PR. Potential of brown adipose tissue type II thyroxine 5′-deiodinase as a local and systemic source of triiodothyronine in rats. J Clin Invest. 1985;76:2296–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. [DOI] [PubMed] [Google Scholar]
- 18. Saarela S, Keith JS, Hohtola E, Trayhurn P. Is the “mammalian” brown fat-specific mitochondrial uncoupling protein present in adipose tissues of birds? Comp Biochem Physiol B. 1991;100:45–49. [DOI] [PubMed] [Google Scholar]
- 19. Smith CA, Roeszler KN, Bowles J, Koopman P, Sinclair AH. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev Biol. 2008;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikegami K, Katou Y, Higashi K, Yoshimura T. Localization of circadian clock protein BMAL1 in the photoperiodic signal transduction machinery in Japanese quail. J Comp Neurol. 2009;517:397–404. [DOI] [PubMed] [Google Scholar]
- 21. Yoshimura T, Suzuki Y, Makino E, et al. Molecular analysis of avian circadian clock genes. Brain Res Mol Brain Res. 2000;78:207–215. [DOI] [PubMed] [Google Scholar]
- 22. Shimizu A, Aida K, Hanyu I. Endocrine profiles during the short reproductive cycle of an autumn-spawning bitterling, Acheilognathus rhombea. Gen Comp Endocrinol. 1985;60:361–371. [DOI] [PubMed] [Google Scholar]
- 23. Darras VM, Kotanen SP, Geris KL, Berghman LR, Kühn ER. Plasma thyroid hormone levels and iodothyronine deiodinase activity following an acute glucocorticoid challenge in embryonic compared with posthatch chickens. Gen Comp Endocrinol. 1996;104:203–212. [DOI] [PubMed] [Google Scholar]
- 24. Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. [DOI] [PubMed] [Google Scholar]
- 25. Kero J, Poutanen M, Zhang FP, et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J Clin Invest. 2000;105:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manna P, El-Hefnawy T, Kero J, Huhtaniemi IT. Biphasic action of prolactin in the regulation of murine leydig tumor cell functions. Endocrinology. 2001;142:308–318. [DOI] [PubMed] [Google Scholar]
- 27. Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–1924. [DOI] [PubMed] [Google Scholar]
- 28. St Germain DL, Schwartzman RA, Croteau W, et al. A thyroid hormone-regulated gene in Xenopus laevis encodes a type III iodothyronine 5-deiodinase. Proc Natl Acad Sci USA. 1994;91:7767–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berry DL, Schwartzman RA, Brown DD. The expression pattern of thyroid hormone response genes in the tadpole tail identifies multiple resorption programs. Dev Biol. 1998;203:12–23. [DOI] [PubMed] [Google Scholar]
- 30. Marin RH, Satterlee DG. Cloacal gland and testes development in male Japanese quail selected for divergent adrenocortical responsiveness. Poult Sci. 2004;83:1028–1034. [DOI] [PubMed] [Google Scholar]
- 31. Singh A, Reineke EP, Ringer RK. Thyroxine and triiodothyronine turnover in the chicken and the bobwhite and Japanese quail. Gen. Comp. Endocrinol. 1967;9:353–361. [DOI] [PubMed] [Google Scholar]
- 32. Mrosovsky N, Sherry DF. Animal anorexias. Science. 1980;207:837–842. [DOI] [PubMed] [Google Scholar]
- 33. Straw JA, Fregly MJ. Evaluation of thyroid and adrenal-pituitary function during cold acclimation. J Appl Physiol. 1967;23:825–830. [DOI] [PubMed] [Google Scholar]
- 34. Cadot M, Julien MF, Chevillard L. Estimation of thyroid function in rats exposed or adapted to environments at 5 or 30 °C. Fed Proc. 1969;28:1228–1233. [PubMed] [Google Scholar]
- 35. Abelenda M, Ledesma A, Rial E, Puerta M. Leptin administration to cold-acclimated rats reduces both food intake and brown adipose tissue thermogenesis. J Therm Biol. 2003;28:525–530. [Google Scholar]
- 36. Bénistant C, Duchamp C, Cohen-Adad F, Rouanet JL, Barré H. Increased in vitro fatty acid supply and cellular transport capacities in cold-acclimated ducklings (Cairina moschata). Am J Physiol. 1998;275:R683–R690. [DOI] [PubMed] [Google Scholar]
- 37. Chaînier F, Roussel D, Georges B, et al. Cold acclimation or grapeseed oil feeding affects phospholipid composition and mitochondrial function in duckling skeletal muscle. Lipids. 2000;35:1099–1106. [DOI] [PubMed] [Google Scholar]
- 38. Underwood H, Steele CT, Zivkovic B. Effects of fasting on the circadian body temperature rhythm of Japanese quail. Physiol Behav. 1999;66:137–143. [DOI] [PubMed] [Google Scholar]
- 39. Follett BK, Maung SL. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural daylengths. J Endocrinol. 1978;78:267–280. [DOI] [PubMed] [Google Scholar]
- 40. Follett BK, King VM, Meddle SL. Rhythms and photoperiodism in birds. In: Lumsden PJ, Miller AJ, eds. Biological Rhythms and Photoperiodism in Plants. Oxford, United Kingdom: BIOS Scientific Publishers Ltd; 1998:231–242. [Google Scholar]
- 41. Lin M, Jones RC, Blackshaw AW. The cycle of the seminiferous epithelium in the Japanese quail (Coturnix coturnix japonica) and estimation of its duration. J Reprod Fertil. 1990;88:481–490. [DOI] [PubMed] [Google Scholar]
- 42. Young KA, Zirkin BR, Nelson RJ. Short photoperiods evoke testicular apoptosis in white-footed mice (Peromyscus leucopus). Endocrinology. 1999;140:3133–3139. [DOI] [PubMed] [Google Scholar]
- 43. Young KA, Ball GF, Nelson RJ. Photoperiod-induced testicular apoptosis in European starlings (Sturnus vulgaris). Biol Reprod. 2001;64:706–713. [DOI] [PubMed] [Google Scholar]
- 44. Nandi S, Banerjee PP, Zirkin BR. Germ cell apoptosis in the testes of Sprague Dawley rats following testosterone withdrawal by ethane 1,2-dimethanesulfonate administration: relationship to Fas? Biol Reprod. 1999;61:70–75. [DOI] [PubMed] [Google Scholar]
- 45. Meddle S, Follett BK. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J Neurosci. 1997;17:8909–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Foster RG, Panzica GC, Parry DM, Viglietti-Panzica C. Immunocytochemical studies on the LHRH system of the Japanese quail: influence by photoperiod and aspects of sexual differentiation. Cell Tissue Res. 1988;253:327–335. [DOI] [PubMed] [Google Scholar]
- 47. Lei ZM, Mishra S, Ponnuru P, Li X, Yang ZW, Rao CV. Testicular phenotype in luteinizing hormone receptor knockout animals and the effect of testosterone replacement therapy. Biol Reprod. 2004;71:1605–1613. [DOI] [PubMed] [Google Scholar]
- 48. Jannini EA, Olivieri M, Francavilla S, Gulino A, Ziparo E, D'Armiento M. Ontogenesis of the nuclear 3,5,3′-triiodothyronine receptor in the rat testis. Endocrinology. 1990;126:5–10. [DOI] [PubMed] [Google Scholar]
- 49. Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140:844–851. [DOI] [PubMed] [Google Scholar]
- 50. Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bicudo JE, Vianna CR, Chaui-Berlinck JG. Thermogenesis in birds. Biosci Rep. 2001;21:181–188. [DOI] [PubMed] [Google Scholar]
- 52. Toyomizu M, Ueda M, Sato S, Seki Y, Sato K, Akiba Y. Cold-induced mitochondrial uncoupling and expression of chicken UCP and ANT mRNA in chicken skeletal muscle. FEBS Lett. 2002;529:313–318. [DOI] [PubMed] [Google Scholar]
- 53. Collin A, Buyse J, van As P, et al. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen Comp Endocrinol. 2003;130:70–77. [DOI] [PubMed] [Google Scholar]
- 54. Yoda T, Crawshaw LI, Yoshida K, et al. Effects of food deprivation on daily changes in body temperature and behavioral thermoregulation in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R134–R139. [DOI] [PubMed] [Google Scholar]
- 55. Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest. 1996;98:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salvatore D, Bartha T, Harney J, Larsen P. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology. 1996;137:3308–3315. [DOI] [PubMed] [Google Scholar]
- 57. Gereben B, Bartha T, Tu HM, Harney JW, Rudas P, Larsen PR. Cloning and expression of the chicken type 2 iodothyronine 5′-deiodinase. J Biol Chem. 1999;274:13768–13776. [DOI] [PubMed] [Google Scholar]
- 58. Darras VM, Mol KA, van der Geyten, Kühn ER. Control of peripheral thyroid hormone levels by activating and inactivating deiodinases. Ann NY Acad Sci. 1998;839:80–86. [DOI] [PubMed] [Google Scholar]
- 59. Valverde C, Croteau W, Lafleur GJ, Orozco A, Germain DL. Cloning and expression of a 5′-iodothyronine deiodinase from the liver of Fundulus heteroclitus. Endocrinology. 1997;138:642–648. [DOI] [PubMed] [Google Scholar]
- 60. Danforth E, Jr, Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab. 1984;13:581–595. [DOI] [PubMed] [Google Scholar]
- 61. Assenmacher I. The peripheral endocrine glands. In: Farner DS, King JR, eds. Avian Biology. Vol. 3 New York: Academic Press; 1973:183–386. [Google Scholar]
- 62. Hoch FL. Metabolic effects of thyroid hormones. In: Greer MA, Solomon DH, eds. Handbook of Physiology. Vol 3, section 7 Thyroid. Washington, DC: American Physiological Society; 1974:391–411. [Google Scholar]