Abstract
The physiological role of serotonin, or 5-hydroxytryptamine (5-HT), in pancreatic β-cell function was previously elucidated using a pregnant mouse model. During pregnancy, 5-HT increases β-cell proliferation and glucose-stimulated insulin secretion (GSIS) through the Gαq-coupled 5-HT2b receptor (Htr2b) and the 5-HT3 receptor (Htr3), a ligand-gated cation channel, respectively. However, the role of 5-HT in β-cell function in an insulin-resistant state has yet to be elucidated. Here, we characterized the metabolic phenotypes of β-cell-specific Htr2b−/− (Htr2b βKO), Htr3a−/− (Htr3a knock-out [KO]), and β-cell-specific tryptophan hydroxylase 1 (Tph1)−/− (Tph1 βKO) mice on a high-fat diet (HFD). Htr2b βKO, Htr3a KO, and Tph1 βKO mice exhibited normal glucose tolerance on a standard chow diet. After 6 weeks on an HFD, beginning at 4 weeks of age, both Htr3a KO and Tph1 βKO mice developed glucose intolerance, but Htr2b βKO mice remained normoglycemic. Pancreas perfusion assays revealed defective first-phase insulin secretion in Htr3a KO mice. GSIS was impaired in islets isolated from HFD-fed Htr3a KO and Tph1 βKO mice, and 5-HT treatment improved insulin secretion from Tph1 βKO islets but not from Htr3a KO islets. Tph1 and Htr3a gene expression in pancreatic islets was not affected by an HFD, and immunostaining could not detect 5-HT in pancreatic islets from mice fed an HFD. Taken together, these results demonstrate that basal 5-HT levels in β-cells play a role in GSIS through Htr3, which becomes more evident in a diet-induced insulin-resistant state.
Obesity induces insulin resistance, which increases the insulin demand of the body. In response to the increased insulin demand, pancreatic β-cell mass and insulin secretion increase (1–5). Type 2 diabetes develops when β-cells fail to compensate for the insulin resistance. Similar to obesity, pregnancy is associated with insulin resistance attributable to placental hormones and maternal adiposity (6). To adapt to pregnancy, β-cells compensate for insulin resistance by increasing their mass and glucose-stimulated insulin secretion (GSIS). Failure of these adaptive processes results in gestational diabetes (7).
Recently, serotonin, or 5-hydroxytryptamine (5-HT), has been implicated in the adaptation of β-cells to pregnancy. In response to lactogenic signaling, the expression of tryptophan hydroxylase 1 (Tph1), the rate-limiting enzyme of 5-HT synthesis, and 5-HT production increase in pancreatic islets during pregnancy, even before maternal insulin resistance develops (8–10). Islet-derived 5-HT acts in an autocrine/paracrine manner through the Gαq-coupled 5-HT2b receptor (Htr2b) to increase β-cell proliferation and mass at midgestation and through the Gαi-coupled 5-HT1d receptor to reduce β-cell mass at the end of gestation (10). In addition, 5-HT increases GSIS through 5-HT3 receptor (Htr3) during pregnancy (11). Htr3 is a ligand-gated cation channel composed of 5 identical Htr3a subunits (homopentamer) or a combination of Htr3a and one of the other subunits (heteropentamer) (12, 13). Opening of the channel in response to 5-HT activates an inward current and depolarizes the membrane (14, 15). Glucose also depolarizes β-cells: increased ATP from glycolysis induces ATP-sensitive K+ channels to close, and the resulting depolarization causes a Ca2+ influx through voltage-gated Ca2+ channels, thereby triggering insulin granule exocytosis (16). Thus, depolarization via Htr3 activation renders β-cells more sensitive to glucose stimulation (11).
Considering the role of 5-HT in β-cell compensation during pregnancy and the similarity of β-cell compensation mechanisms for insulin resistance induced by pregnancy and obesity, it is intriguing to investigate the functions of 5-HT in an obesity-induced insulin-resistant state. In the present study, we characterized the metabolic phenotypes of β-cell-specific Htr2b−/− (Htr2b βknock-out [KO]), Htr3a−/− (Htr3a KO), and β-cell-specific Tph1−/− (Tph1 βKO) mice on a high-fat diet (HFD) and showed that 5-HT is necessary for normal β-cell compensation for an HFD.
Materials and Methods
Animal experiments
Mice were housed on a 12-hour light, 12-hour dark cycle in climate-controlled, pathogen-free barrier facilities, and chow and water were provided ad libitum. The Institutional Animal Care and Use Committee at Korea Advanced Institute of Science and Technology and Kyorin University approved the animal experiment protocols for this study. Htr3a KO (17), MIP-CreER (18), Htr2b floxed (Htr2b fl/fl), and Tph1 floxed (Tph1 fl/fl) (19) mice have been described previously. To create the Htr2b βKO and Tph1 βKO mice, Htr2b floxed and Tph1 floxed mice were crossed with MIP-CreER mice, and Cre recombination was induced by the ip injection of 3 doses of 2 mg of tamoxifen (Sigma) over a period of 1 week. Tamoxifen was freshly dissolved in corn oil at 20 mg/mL. All the mice were backcrossed with C57BL/6J mice for more than 10 generations, and 10-week-old male mice were used in the experiments. The mice were divided into 2 groups and were fed either a standard chow diet (SCD) or a HFD (60% kcal fat; Research Diets, Inc) from 4 to 10 weeks of age. For the glucose tolerance tests, mice were fasted for 16 hours before an ip injection (2 g/kg body weight) of D-glucose in deionized water. For the insulin tolerance tests, mice were fasted for 4 hours before an ip injection (0.75 U/kg body weight) of Humulin R (Eli Lilly). Glucose levels were measured from tail vein bleeds using a GlucoDr Plus glucometer (Allmedicus). For the in vivo GSIS experiments, mice that fasted for 16 hours received an ip injection of D-glucose (2 g/kg body weight), and the glucose levels were measured from tail vein bleeds after 15 minutes using a glucometer.
Immunostaining and β-cell mass measurements
Immunofluorescent staining of the mouse pancreas and β-cell mass measurements were performed as described previously (10). Pancreata were fixed in 10% neutral buffered formalin (Sigma) for 6 hours at 4°C and then washed with deionized water. After tissue processing using an automatic tissue processor (Leica), the tissues were embedded in molten paraffin wax. Tissue sections were cut to a 5-μm thickness and were mounted on adhesive slides (Marienfeld). The slides were deparaffinized and rehydrated. Antigen retrieval was then performed by placing the slides in sodium citrate buffer (10mM sodium citrate; pH 6.0) and incubating them for 30 minutes at 95°C. After cooling for 30 minutes, the slides were washed in PBS for 5 minutes. The samples were blocked with 1% goat serum in PBS for 1 hour at room temperature and incubated for 3 hours with the next primary antibodies: antiinsulin (guinea pig, 1:1000; Dako), antiglucagon tk;2(mouse, 1:1000; Sigma), or anti-5-HT (rabbit, 1:1000; ImmunoStar). After washing in PBS for 5 minutes, the samples were incubated for 2 hours with the next secondary antibodies: fluorescein isothiocyanate conjugated goat antiguinea pig IgG (1:1000; Jackson ImmunoResearch), rhodamine-conjugated goat antirabbit IgG (1:1000; Jackson ImmunoResearch), or cyanine 3-conjugated goat antimouse IgG (1:1000; Abcam) (see Table 1). The samples were incubated for 5 minutes with 4′,6-diamidino-2-phenylindole (1:2000; Invitrogen), washed with PBS and mounted with fluorescence mounting medium (Dako). Images were acquired using a confocal microscope (LSM 510; Carl Zeiss). For the β-cell mass measurements, 10 insulin-3,3′-diaminobenzidine-stained sections (at least 100 μm apart) per pancreas were imaged using brightfield and stereoscopic microscopes (Leica). The insulin-positive area and the pancreatic area were measured using ImageJ (NIH). The β-cell area was calculated by dividing the insulin-positive area by the total pancreatic tissue area.
Table 1.
Antibodies Used for Immunostaining
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Insulin | Polyclonal guinea pig antiinsulin | Dako, A0564 | Guinea pig; polyclonal | 1:1000 | |
| Glucagon | Monoclonal antiglucagon | Sigma, G2654 | Mouse; monoclonal | 1:1000 | |
| 5-HT | Serotonin rabbit antibody | ImmunoStar, 20080 | Rabbit; polyclonal | 1:1000 |
Insulin secretion from isolated islets and perfused pancreata and the pancreatic insulin content
Mouse pancreatic islets were isolated by collagenase digestion as described previously with slight modifications (20). Islets were incubated overnight in RPMI 1640 medium (Thermo Scientific) containing 10% fetal bovine serum (Thermo Scientific) and 100-U/mL penicillin-streptomycin (Thermo Scientific) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Next, the islets were transferred to Krebs-Ringer HEPES (KRH) buffer (pH 7.4; 115mM NaCl, 5mM KCl, 2.5mM CaCl2, 1mM MgCl2, 24mM NaHCO3, 25mM HEPES, and 1-mg/mL BSA) containing 5mM glucose and were incubated for 3 hours at 37°C. The islets were then transferred to KRH buffer containing 2.8mM glucose and were incubated for 1 hour. Subsequently, the islets were divided into 2 groups (n = 10 each); one group was incubated for 1 hour in KRH buffer containing 2.8mM glucose, and the other group was placed in KRH buffer containing 16.8mM glucose. For rescue experiments with 5-HT treatment, islets were incubated for 1 hour in KRH buffer containing 2.8mM or 11mM glucose with or without 10nM 5-HT (Sigma). Subsequently, the islets were handpicked and sonicated in 0.5-mL acid-ethanol (1.5-mL HCl in 100 mL of 70% ethanol). The supernatant from each group was collected for the insulin ELISA assay and stored at −80°C. Insulin secretion was calculated by dividing the secreted insulin content by the insulin content extracted from the islets. To measure the pancreatic insulin content, pancreata were placed in acid-ethanol (1.5-mL HCl in 100 mL of 70% ethanol), homogenized, and incubated at −20°C for 8 hours. The homogenate was centrifuged for 15 minutes at 2000 rpm at 4°C, and the aqueous phase was transferred to a new tube. Then, 100 μL of the extract were neutralized with 100 μL of 1M Tris-Cl (pH 7.5). The pancreatic insulin content was calculated by dividing the total insulin content by the pancreas weight. The pancreas perfusion experiments were performed as described previously (11). The perfusate was introduced into the celiac artery at a rate of 1 mL/min, and the effluent was collected at 1-minute intervals for 40 minutes from the portal vein after stimulation with 16.7mM glucose (40 samples/mouse perfusion experiment). The insulin concentrations were measured using an insulin ELISA kit (ALPCO).
Plasma 5-HT concentration measurement
To determine the plasma 5-HT levels, blood was obtained by cardiac puncture and collected in EDTA-treated sample tubes (Sarstedt). The samples were then centrifuged for 15 minutes at 2000g at 4°C. The 5-HT concentrations were measured as described in the 5-HT ELISA kit (LDN).
Real-time PCR analysis
Mouse pancreatic islets were isolated by collagenase digestion as described above. Total RNA from the islets was purified using TRIzol (Invitrogen). cDNA was synthesized using Superscript III Reverse Transcriptase (Invitrogen). Gene expression was analyzed using the next primers: Tph1 forward primer (ACCATGATTGAAGACAACAAGGAG), Tph1 reverse primer (TCAACTGTTCTCGGCTGATG), Tph2 For (GCCATGCAGCCCGCAATGATGATG), Tph2 Rev (CTACCGCTGTCTTGCTGCTC), Htr3a For (AAATCAGGGCGAGTGGGAGCTG), Htr3a Rev (GACACGATGATGAGGAAGACTG), Htr2b For (AAATAAGCCACCTCAACGCCT), Htr2b Rev (TCCCGAAATGTCTTATTGAAGAG), Actin For (CAGCTTCTTTGCAGCTCCTT), and Actin Rev (CTTCTCCATGTCGTCCCAGT). Real-time PCR was performed using Power SYBR Green PCR Master Mix (Invitrogen), and the data were analyzed on a Viia7 system (Invitrogen).
Statistical analysis
All data are presented as the mean ± SEM. Statistical significance was determined by a standard Student's t test or by ANOVA with Tukey's honest significant difference post hoc analysis for multiple group comparisons.
Results
Htr3a KO mice showed glucose intolerance on an HFD
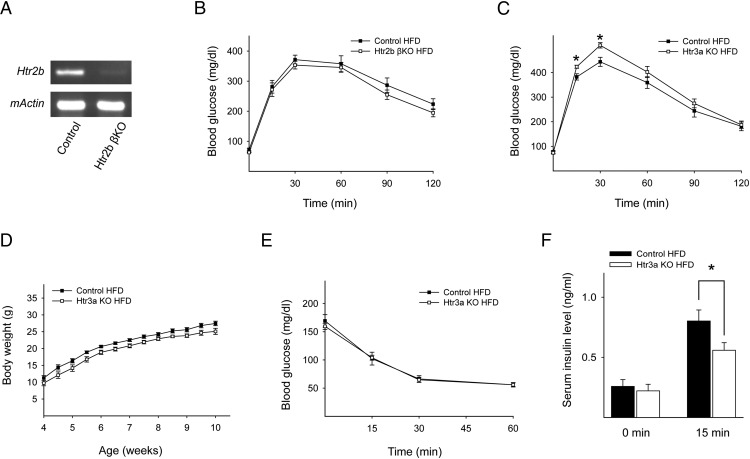
During pregnancy, 5-HT increases β-cell proliferation and GSIS through Htr2b and Htr3, respectively. Conventional Htr2b KO mice and Htr3a KO mice exhibit glucose intolerance during pregnancy, although they have normal glucose tolerance in the nonpregnant state (10, 11). During the progression of type 2 diabetes, β-cells experience similar changes to compensate for insulin resistance. However, the involvement of 5-HT in this compensatory mechanism has not been tested. This prompted us to characterize the metabolic phenotype of Htr2b βKO mice and Htr3a KO mice under metabolic stress conditions. To induce metabolic stress, mice were fed an HFD for 6 weeks, beginning at 4 weeks of age. Cre-mediated disruption of Htr2b expression in β-cells was confirmed by RT-PCR of isolated islets from Htr2b βKO mice (Figure 1A). On an HFD, there was no difference in the glucose levels during an ip glucose tolerance test in Htr2b βKO mice compared with control mice (Figure 1B). However, Htr3a KO mice exhibited worse glucose tolerance than control mice on an HFD (Figure 1C), although Htr3a KO mice had normal growth and weight gain on an HFD (Figure 1D) (11). The insulin tolerance test revealed no significant difference between control and Htr3a KO mice (Figure 1E), but the serum insulin concentration after glucose stimulation was reduced in the Htr3a KO mice fed an HFD (Figure 1F). Taken together, these results suggested that defective insulin secretion induced glucose intolerance in Htr3a KO mice after being on an HFD.
Figure 1.
Htr3a KO mice exhibited glucose intolerance on an HFD. A, Representative RT-PCR data of Htr2b gene expression in islets from control and Htr2b βKO mice at 10 weeks of age. β-actin was used as an internal control. B and C, Blood glucose levels were measured after the ip injection of glucose (2 g/kg body weight) in mice at 10 weeks of age. Mice were fed an HFD from 4 to 10 weeks of age. B, Control (black squares) and Htr2b βKO (white squares). MIP-CreER;Htr2b fl/fl mice were injected with corn oil (control) or tamoxifen (Htr2b βKO) at 3 weeks of age. C, WT littermate control (black squares) and Htr3a KO (white squares). D, Body weight curves for WT littermate control mice on an HFD (black squares) and Htr3a KO mice on an HFD (white squares). E, Insulin tolerance test (0.75 U/kg body weight) in WT littermate control mice on an HFD (black squares) and in Htr3a KO mice on an HFD (white squares). F, Serum insulin levels in WT littermate control mice on an HFD (black squares) and in Htr3a KO mice on an HFD (white squares) before and 15 minutes after a glucose (2 g/kg body weight) injection. The data are presented as the mean ± SEM of at least 5 independent experiments. *, P < .05.
Impaired GSIS from islets of HFD-fed Htr3a KO mice
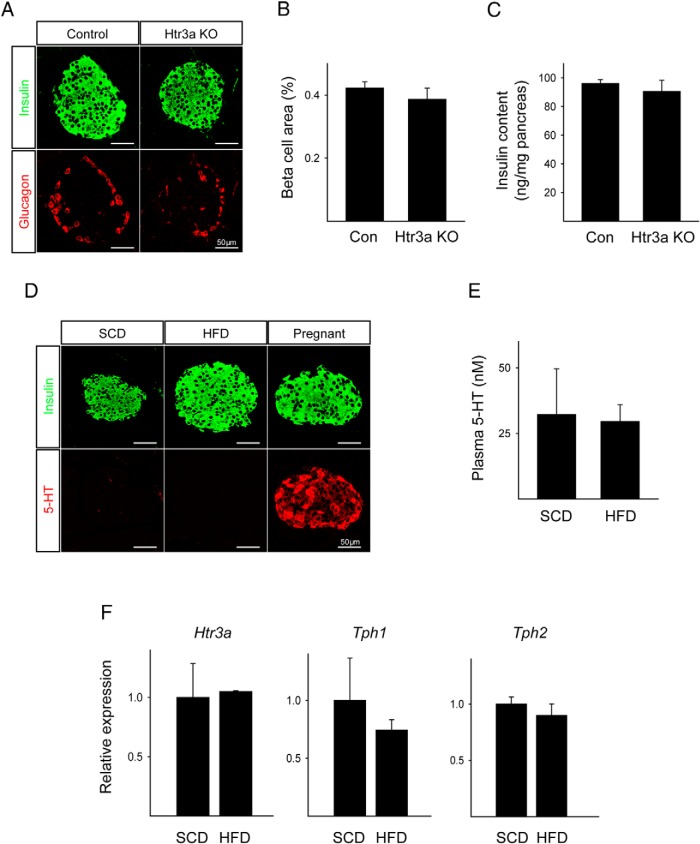
To ascertain the effect of an HFD on the β-cell mass in Htr3a KO mice, we performed immunostaining. Htr3a KO mice on an HFD showed normal islet morphology, β-cell mass, and pancreatic insulin content (Figure 2, A–C). 5-HT was undetectable in the islets of mice fed an HFD or in the islets of those fed an SCD (Figure 2D), and the plasma 5-HT concentration did not change (Figure 2E). The gene expression of Htr3a, Tph1, and Tph2 was not altered in the pancreatic islets in response to the HFD (Figure 2F).
Figure 2.
5-HT did not increase in response to an HFD. A, Pancreatic sections from HFD-fed WT littermate control and HFD-fed Htr3a KO mice stained for insulin (green) and glucagon (red). B, The calculated β-cell area from HFD-fed WT littermate control and HFD-fed Htr3a KO mice. C, Pancreatic insulin content per total pancreas. D, Pancreatic sections from SCD-fed and HFD-fed WT mice stained for insulin (green) and 5-HT (red). Pancreatic sections from pregnant mice were used as positive controls for 5-HT. E, Plasma 5-HT concentrations as measured by ELISA. F, mRNA expression of Htr3a, Tph1, and Tph2 as measured by real-time PCR from RNA from islets of SCD-fed and HFD-fed WT mice. The data are presented as the mean ± SEM of at least 4 independent experiments.
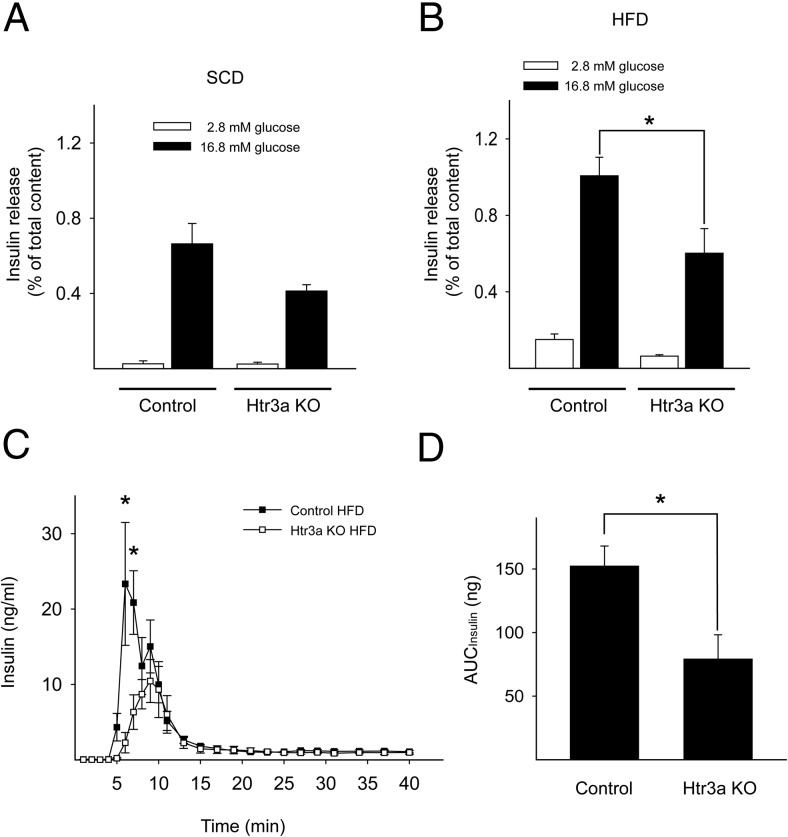
To determine the insulin secretory function of pancreatic islets in HFD-fed Htr3a KO mice, we tested GSIS in vitro. In batch culture, GSIS did not change significantly in SCD-fed Htr3a KO islets (Figure 3A). However, GSIS decreased significantly in HFD-fed Htr3a KO islets (Figure 3B). To further confirm the defective GSIS in HFD-fed mice, we performed pancreas perfusion experiments. In a previous report, GSIS did not decrease in the perfused pancreata of Htr3a KO mice fed an SCD (11). In contrast, in response to an HFD, Htr3a KO mice showed significantly decreased GSIS in perfused pancreata (Figure 3, C and D). In particular, first-phase insulin secretion decreased in HFD-fed Htr3a KO mice. These data indicated that Htr3 is important in pancreatic β-cells for insulin secretion in response to glucose, which becomes more critical under metabolic stress conditions. In addition, these data suggested that the severe glucose intolerance in HFD-fed Htr3a KO mice is not caused by abnormal islet morphology and insufficient β-cell mass but by defective insulin secretion.
Figure 3.
Islets from Htr3a KO mice showed impaired insulin secretion. After SCD-fed (A) and HFD-fed (B) mouse islets at 10 weeks of age were stimulated with 2.8mM or 16.8mM glucose, insulin secretion was measured and normalized by the amount of insulin extracted from the islets. C and D, Insulin secretion was measured from perfused WT littermate control and Htr3a KO pancreata as the glucose concentration was shifted from 2.8mM to 16.7mM, and the area under the curve (AUC) is shown in D. The data are presented as the mean ± SEM of at least 4 independent experiments. *, P < .05.
Tph1 βKO mice showed impaired insulin secretion on an HFD
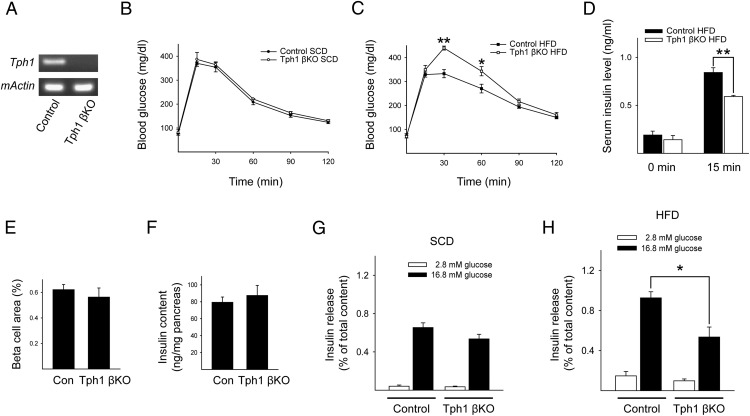
Although 5-HT was undetectable by immunostaining in islets from both SCD-fed and HFD-fed mice, there is substantial evidence from different methodologies supporting the presence of 5-HT in pancreatic islets, and 5-HT has been associated with the regulation of blood glucose levels (10, 21–23). In addition, our data suggested the possibility that the small amount of 5-HT synthesized by β-cells contributes to insulin secretion through Htr3 in autocrine/paracrine manner. This small contribution can usually be ignored because of the compensatory capacity of β-cells, but it becomes evident under metabolic stress conditions, such as being on an HFD. To confirm this hypothesis, we blocked 5-HT synthesis in β-cells by knocking out Tph1 in adult β-cells. Cre-mediated disruption of Tph1 expression was confirmed by RT-PCR of isolated islets from Tph1 βKO mice (Figure 4A). As shown in Figure 4B, Tph1 βKO mice showed normal glucose tolerance on an SCD, which is in contrast to the glucose intolerance in conventional Tph1 KO mice (21). Considering the insulin resistance as well as the defective insulin secretion in conventional Tph1 KO mice, our data suggested that Tph1 βKO mice maintain normal glucose tolerance due to the absence of insulin resistance and that Tph1 βKO mice could develop glucose intolerance in response to metabolic stress because of insufficient compensatory insulin secretion from β-cells. Indeed, Tph1 βKO mice exhibited glucose intolerance on an HFD (Figure 4C). Serum insulin levels after glucose challenge were reduced in HFD-fed Tph1 βKO mice (Figure 4D). However, β-cell mass and pancreatic insulin content were not affected in Tph1 βKO mice (Figure 4, E and F).
Figure 4.
Tph1 βKO mice exhibited impaired insulin secretion on an HFD. A, Representative RT-PCR data of Tph1 gene expression in islets from control and Tph1 βKO mice at 10 weeks of age. β-actin was used as an internal control. B and C, Blood glucose levels were measured after the ip injection of glucose (2 g/kg body weight) in 10-week-old mice. Mice were fed an SCD (B) or an HFD (C) from 4 to 10 weeks of age. MIP-CreER;Tph1 fl/fl mice were injected with corn oil (control, black circles or squares) or tamoxifen (Tph1 βKO, white circles or squares) at 3 weeks of age. D, Serum insulin levels in control mice on an HFD (black squares) and in Tph1 βKO mice on an HFD (white squares) before and 15 minutes after a glucose (2 g/kg body weight) injection. E, The calculated β-cell area from HFD-fed control and HFD-fed Tph1 βKO mice. F, Pancreatic insulin content per total pancreas. G and H, After islets from SCD-fed (G) and HFD-fed (H) Tph1 βKO mice at 10 weeks of age were stimulated with 2.8mM or 16.8mM glucose, insulin secretion was measured and normalized by the amount of insulin extracted from the islets. The data are presented as the mean ± SEM of at least 5 independent experiments. *, P < .05; **, P < .01.
In addition, we measured GSIS in vitro to determine the insulin secretory function of pancreatic islets from HFD-fed Tph1 βKO mice. Islets isolated from SCD-fed Tph1 βKO mice showed normal GSIS compared with control (Figure 4G), but GSIS was significantly decreased in islets isolated from HFD-fed Tph1 βKO mice (Figure 4H). These results are consistent with the GSIS observed in islets isolated from Htr3a KO mice (Figure 3, A and B). These data suggested that basal levels of 5-HT are important for regulating insulin secretion from pancreatic β-cells and that this action becomes more important under metabolic stress conditions.
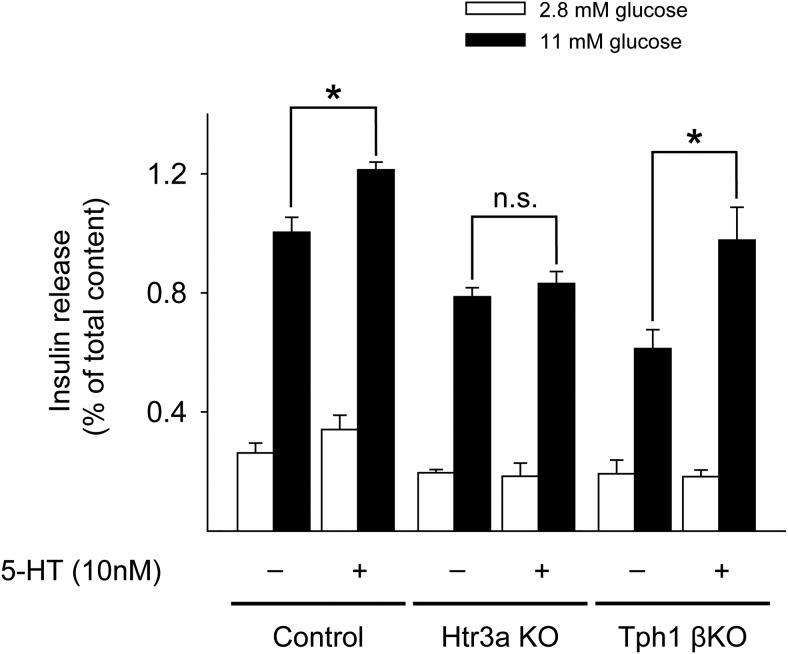
To test the direct involvement of 5-HT in GSIS, we performed rescue experiments by treating islets with 5-HT (10nM) in vitro. 5-HT could increase GSIS in both control and Tph1 βKO islets but not in Htr3a KO islets (Figure 5). These data indicated that 5-HT derived from β-cells regulates insulin secretion via activation of Htr3 in pancreatic β-cells.
Figure 5.
5-HT treatment improved GSIS in islets. Islets from HFD-fed Htr3a KO and HFD-fed Tph1 βKO mice at 10 weeks of age were stimulated with 2.8mM or 11mM glucose alone or in combination with 10nM 5-HT, and insulin secretion was measured and normalized by the amount of insulin extracted from the islets. The data are presented as the mean ± SEM of at least 5 independent experiments. *, P < .05.
Discussion
It has been known for more than 3 decades that 5-HT is present in pancreatic β-cells, but the physiological role of 5-HT in regulating blood glucose levels is not clear yet (22, 23). Recent reports suggested that intracellular serotonylation might regulate insulin exocytosis (21). More recently, 5-HT has been implicated in the adaptation of β-cells to pregnancy (9–11). Upon lactogenic stimulation, 5-HT production from β-cells increases during pregnancy, and 5-HT increases β-cell proliferation and GSIS through Htr2b and Htr3, respectively. This compensatory function of 5-HT during pregnancy raises the question of whether 5-HT could play a similar role in an insulin-resistant state, because the compensatory mechanism for insulin resistance is similar to that for pregnancy. To directly address this question, we have characterized the metabolic phenotype of Htr2b βKO, Htr3a KO, and Tph1 βKO mice on an HFD. Because 5-HT is present in the developing pancreas and potentially influences β-cell development, we induced the knockout of Htr2b and Tph1 at 3 weeks of age by injecting tamoxifen into MIP-CreER;Htr2b fl/fl and MIP-CreER;Tph1 fl/fl mice. Htr2b βKO mice showed normal glucose tolerance under both SCD and HFD conditions. However, both Htr3a KO mice and Tph1 βKO mice showed impaired glucose tolerance in an HFD-induced insulin-resistant state. Pancreas perfusion experiment revealed a defect in first-phase insulin secretion from HFD-fed Htr3a KO mice. In contrast to pregnancy, in which 5-HT synthesis in islets dramatically increases, the HFD did not increase intraislet 5-HT synthesis or blood 5-HT levels. Although Tph1 βKO mice did not show glucose intolerance on an SCD, Tph1 βKO mice developed glucose intolerance after being on an HFD, as did Htr3a KO mice. In addition, islets from HFD-fed Tph1 βKO mice exhibited impaired insulin secretion. Rescue experiments confirmed that 5-HT regulates insulin secretion via activation of Htr3 in pancreatic β-cells. Taken together, these data demonstrated that 5-HT regulates insulin secretory function in β-cells through Htr3 and that this action becomes more critical under HFD-induced metabolic stress condition.
During pregnancy, increased 5-HT activates Htr3, which induces a depolarizing shift in the resting membrane potential of β-cells, thereby promoting depolarization and insulin secretion (11). We did not observe an increase in 5-HT levels in the pancreatic islets or plasma of HFD-fed mice. Nevertheless, on an HFD, Htr3a KO mice and Tph1 βKO mice exhibited glucose intolerance. Under normal metabolic conditions, not all β-cells are necessary for maintaining normal blood glucose levels. However, metabolic stress, such as insulin resistance, increases the metabolic demand for insulin secretion (24). Here, the role of Htr3 in β-cells becomes more critical, because these cells need to work harder to keep up with the insulin demand. Therefore, Htr3a KO mice displayed a metabolic phenotype on an HFD without an accompanying increase in 5-HT, which is consistent with the metabolic phenotype of Tph1 βKO mice on an HFD.
Paulmann et al (21) demonstrated the importance of serotonylation in β-cells for insulin secretion. In this paper, the authors showed that Ca2+ activated the serotonylation of Rab3a and Rab27a, which is crucial for insulin exocytosis. Thus, Tph1 KO mice exhibited glucose intolerance. The authors also claimed that extracellular 5-HT inhibited insulin secretion by activating Htr1a. Although their finding was interesting, there are several caveats that could explain why Tph1 βKO mice only showed glucose intolerance on an HFD in our study. First, the authors did not provide clear evidence for the inhibition of insulin secretion by extracellular 5-HT. Mouse β-cells do not express Htr1a (10, 25). Further, systemic administration of 5-HT impacts multiple tissues involved in glucose metabolism, including liver, where it increases gluconeogenesis, resulting in a net increase in blood glucose levels (26). Second, the authors employed whole-cell patch-clamp experiments in fresh pancreas slices to monitor insulin secretion and showed decreased insulin secretion in the Tph1 KO mouse pancreas. However, this system cannot exclude the effects of extracellular 5-HT secreted from neighboring cells. Third, the authors did not consider the increase in insulin resistance that occurs in Tph1 KO mice. Taken together, mice in which the Tph1 gene is deleted in the germ line lack Tph1 protein in all tissues and have both defective insulin secretion and insulin resistance. Thus, the metabolic phenotype of germ line Tph1 KO mice is more similar to that of Tph1 βKO mice on an HFD than to Tph1 βKO on an SCD because of the HFD-induced insulin resistance.
In conclusion, we determined that 5-HT is necessary to maintain normal GSIS from β-cells through Htr3 and that this mechanism becomes more important in the HFD-induced insulin-resistant state. Our findings suggest that 5-HT systems play an important role in β-cell function in the HFD-induced insulin-resistant state and that defects in 5-HT systems may contribute to the development of type 2 diabetes. Therefore, additional studies will provide greater insight into the pathophysiology and treatment of type 2 diabetes.
Acknowledgments
We thank Hee-Saeng Jung and Taechang Yang of Korea Advanced Institute of Science and Technology for their technical support and Chul-Ho Lee of Korea Research Institute Bioscience and Biotechnology for mouse breeding and maintenance.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (Grant HI11C1964) (to H.K.); the Bio & Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science, ICT and Future Planning (Grant NRF-2012M3A9B2027974) (to H.K.); National Institutes of Health Grants P30 DK020595 (to L.H.P.), R01 DK092616 (to M.W.R.), and P30 DK063720 (to M.S.G.); Juvenile Diabetes Research Foundation Grants 16–2007-428 (to M.S.G.) and 10–2010-553 (to H.K.); and the Korean Diabetes Association, 2013 (C.O.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- GSIS
- glucose-stimulated insulin secretion
- HFD
- high-fat diet
- 5-HT
- 5-hydroxytryptamine
- Htr2b
- Gαq-coupled 5-HT2b receptor
- Htr3
- 5-HT3 receptor
- KO
- knock-out
- KRH
- Krebs-Ringer HEPES
- SCD
- standard chow diet
- Tph1
- tryptophan hydroxylase 1.
References
- 1. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. [DOI] [PubMed] [Google Scholar]
- 2. Chen C, Hosokawa H, Bumbalo LM, Leahy JL. Mechanism of compensatory hyperinsulinemia in normoglycemic insulin-resistant spontaneously hypertensive rats. Augmented enzymatic activity of glucokinase in β-cells. J Clin Invest. 1994;94(1):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jetton TL, Lausier J, LaRock K, et al. Mechanisms of compensatory β-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54(8):2294–2304. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez A, Merino B, Marroquí L, et al. Insulin hypersecretion in islets from diet-induced hyperinsulinemic obese female mice is associated with several functional adaptations in individual β-cells. Endocrinology. 2013;154(10):3515–3524. [DOI] [PubMed] [Google Scholar]
- 5. Sachdeva MM, Stoffers DA. Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal β-cell mass expansion. Mol Endocrinol. 2009;23(6):747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115(3):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29(6):301–307. [DOI] [PubMed] [Google Scholar]
- 8. Rieck S, White P, Schug J, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23(10):1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schraenen A, Lemaire K, de Faudeur G, et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse β cells during pregnancy. Diabetologia. 2010;53(12):2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nature medicine. 2010;16(7):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohara-Imaizumi M, Kim H, Yoshida M, et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc Natl Acad Sci USA. 2013;110(48):19420–19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339(6227):706–709. [DOI] [PubMed] [Google Scholar]
- 13. Davies PA, Pistis M, Hanna MC, et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397(6717):359–363. [DOI] [PubMed] [Google Scholar]
- 14. Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006;12(28):3615–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor–the relationship between structure and function. Neuropharmacology. 2009;56(1):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drews G, Krippeit-Drews P, Düfer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–163. [DOI] [PubMed] [Google Scholar]
- 17. Zeitz KP, Guy N, Malmberg AB, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22(3):1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamarina NA, Roe MW, Philipson L. Characterization of mice expressing Ins1 gene promoter driven CreERT recombinase for conditional gene deletion in pancreatic β-cells. Islets. 2014;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szot GL, Koudria P, Bluestone JA. Murine pancreatic islet isolation. J Vis Exp. 2007(7):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paulmann N, Grohmann M, Voigt JP, et al. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLoS Biol. 2009;7(10):e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gershon MD, Ross LL. Location of sites of 5-hydroxytryptamine storage and metabolism by radioautography. J Physiol. 1966;186(2):477–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekholm R, Ericson LE, Lundquist I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7(5):339–348. [DOI] [PubMed] [Google Scholar]
- 24. Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest. 2000;106(3):329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohta Y, Kosaka Y, Kishimoto N, et al. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes. 2011;60(12):3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16(5):588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]