Abstract
The peptide oxytocin (OT) is secreted by hypothalamic neurons and exerts numerous actions related to reproduction. OT stimulation of prolactin secretion in female rats is important during the estrous cycle, pregnancy, and lactation. Here we report that OT also stimulates transients of intracellular Ca2+ concentration in somatotrophs and gonadotrophs as well as the release of GH and LH in a dose-dependent manner with EC50 values that closely correspond to the ligand affinity of the OT receptor (OTR). Remarkably, the hormone-releasing effect of OT in these two cell types is 2 orders of magnitude more sensitive than that in lactotrophs. The specific OTR agonist [Thr4,Gly7]-oxytocin acutely stimulated the release of LH, GH, and prolactin from female rat pituitary cells in primary culture and increased intracellular Ca2+ concentration in gonadotrophs, somatotrophs, and lactotrophs. In these three cell types, the effects on hormone release and intracellular Ca2+ of both OT and [Thr4,Gly7]oxytocin were abolished by the specific OT receptor antagonist desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT but not by the highly selective vasopressin V1a receptor antagonist, d(CH2)5[Tyr(Me)2,Dab5]AVP. Furthermore, 10 nM arginine vasopressin stimulated LH and GH release comparably with a dose of OT that was at least 10 times lower. Finally, the presence of the OTR-like immunoreactivity could be observed in all three cell types. Taken together, these results show that OT directly stimulates gonadotrophs, somatotrophs, and lactotrophs through OT receptors and suggest that OT signaling may serve to coordinate the release of different pituitary hormones during specific physiological conditions.
A wealth of anatomic, pharmacological, and functional evidence suggests a role for oxytocin (OT) in the regulation of anterior pituitary function (1). The oxytocinergic nerve terminals of magnocellular neurons in the neurohypophysis (2) and paraventricular parvicellular neurons in the external layer of the median eminence (3) release the neuropeptide in amounts sufficient to reach the anterior pituitary (4). In this gland, specific binding sites for OT were described (5, 6), and the expression of its receptor was demonstrated both at the mRNA (7, 8) and protein (8, 9) level. OT has been shown to stimulate prolactin (PRL) release in vivo in both male (10) and female (11) rats. The subsequent observation of direct stimulatory effects of OT on PRL secretion and intracellular Ca2+ concentration ([Ca2+]i) in lactotroph cells in vitro (12, 13) along with the presence of the oxytocin receptor (OTR) mRNA in lactotroph cells (7) quickly established a role for OT on the control of PRL secretion.
In contrast, the potential for neuroendocrine actions of OT on the secretion of gonadotropins and GH at the anterior pituitary has remained largely uncharacterized. OT has been shown to influence in vivo release of LH (14), contribute to the control of the gonadotrophin axis function (15–17), and stimulate LH synthesis and secretion in vitro (18, 19). However, the failure to demonstrate acute stimulatory effects in vivo on LH release (10, 14) as well as the contradictory evidence for direct effects of OT on gonadotrophs (20, 21) has cast doubt on the physiological significance of these OT effects on gonadotrophs. Likewise, effects of OT on GH secretion in vivo (22, 23) and in vitro (24) have been reported, but direct effects of OT at physiological doses on somatotrophs have not been established.
The present study seeks to clarify the direct effects of OT on cultured gonadotrophs and somatotrophs, using measurements of [Ca2+]i and hormone secretion and compare their responses with those of lactotrophs. We observed [Ca2+]i responses and secretion profiles consistent with a direct action of OT in both cell types through OTR. Finally, we showed that gonadotrophs, somatotrophs, and lactotrophs all exhibit OTR-like immunoreactivity.
Materials and Methods
Chemicals
Oxytocin and its rat-specific, OTR-selective analog (Thr4,Gly7)oxytocin (TGOT) (25) were obtained from Bachem Bioscience Inc. The selective OTR antagonist, desGly-NH2-d(CH2)5[D-Tyr2,Thr4,Orn8]vasotocin (26), was obtained from GenScript Corp. The highly selective vasopressin V1a antagonist, d(CH2)5[Tyr(Me)2,Dab5]arginine vasopressin (27), and the rat-specific vasopressin V1b-selective agonist, d[Leu4,Lys8]vasopressin (28), were gifts from Dr Maurice Manning (University of Toledo, Toledo, Ohio). All other compounds were from Sigma Chemical Co. unless otherwise stated.
Animals
Adult female Sprague Dawley rats (>60 d of age) weighing 250–300 g (Charles River Laboratories) were kept in standard rat cages under a 12-hour light, 12-hour dark cycle (lights on at 6:00 am) with water and rat chow available ad libitum. The stage of the estrous cycle was determined by daily vaginal smears, and only animals exhibiting at least two regular 4-day cycles were used; experiments were performed using cells obtained from rats on proestrus except for the dose-response studies that pooled rats from various stages of the cycle. Rats were euthanized under CO2 exposure followed by decapitation at 4:00 pm. All animal procedures were approved by the Florida State University Animal Care and Use Committee.
Cell dispersion and culture
Pituitary cell dispersion was conducted using the papain-deoxyribonuclease procedure as previously described (13), with the following modifications. Pituitary fragments were subjected to enzymatic digestion in a glass vial containing papain (3.55 U per gland; Worthington) and deoxyribonuclease I (25 U per gland; Worthington) in Hanks' balance salt solution for 45 minutes at 37°C, with a shaking rate of 30 rpm. Dispersed cells were resuspended in Medium 199 (Invitrogen) containing Earle's salts, 0.7 mM glutamine, sodium bicarbonate, 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin and counted using a Neubauer chamber. Viability of cells, determined by trypan blue exclusion, was always 92% or greater. Anterior pituitary cells were cultured as mixed cells and used for single-cell Ca2+ imaging, immunocytochemistry, and cell perfusion experiments the following day. The cells are cultured in vitro for a short period of time (≤24 h) and are known to retain their in vivo-imprinted physiological status (29–31).
Measurements of hormone release from perifused pituitary cells
Hormone secretion was monitored using cell column perifusion experiments. Briefly, 4 × 106 anterior pituitary cells were incubated with preswollen cytodex-3 beads in 35-mm petri dishes for 18 hours. The beads were then transferred to 0.5 mL chambers and perifused with HEPES-buffered saline solution (HBS) containing 25 mM HEPES, 138 mM NaCl, 5 mM KCl, 10 mM α-D-glucose, 0.7 mM sodium phosphate, 1 mM MgCl, 2 mM CaCl2, 0.1% BSA, 100 U/mL penicillin, and 100 μg/mL streptomycin for 2.5 hours at a flow rate of 0.5 mL/min and at 37°C to establish stable basal secretion. Fractions were collected in 1-minute intervals, stored at −20°C and later assayed for hormonal content by RIA. For experiments measuring FSH release, perfusions were conducted with the following changes: 2 × 107 cells were perifused at a flow rate of 0.12 mL/min, with fractions collected every 4 minutes.
Radioimmunoassays
GH, LH, FSH, and PRL were measured using standards and antiserum supplied by Dr Albert Parlow through the National Hormone and Pituitary Program (NHPP). 125I was purchased from PerkinElmer Life Sciences and used to prepare radiolabeled tracers of all four hormones by the chloramine T method. Hormone levels are expressed in terms of reference preparation RP-2 (for GH and FSH) and RP-3 (for LH and PRL). The lower limits of detection (in nanograms per milliliter) were 0.05, 0.6, 5.0, and 1.6 for PRL, LH, FSH, and GH, respectively. The intraassay and interassay coefficients of variation were 4.5% and 11.2% for PRL, 4.7% and 9.3% for LH, 3.9% and 8.6% for FSH, and 4.3% and 9.0% for GH.
Measurements of [Ca2+]i in single anterior pituitary cells
Cells were plated in glass bottom 10-mm microwell dishes (MatTek Corp) and cultured for 24 hours at a cell density of 105 cells/dish. Cells were rinsed once with HBS (without BSA) and then incubated in HBS containing 2 μM of fura-2-AM (Molecular Probes) for 45 minutes at room temperature. Cells were rinsed with HBS, transferred to the stage of an inverted microscope, and continuously perfused with HBS at room temperature for 15 minutes. Cells were then illuminated every 2 seconds with 340-nm and 380-nm light beams (50 msec exposure each) from a 175-W Xenon light source (DG4; Sutter Instrument). Light intensity was decreased by 90% before reaching the cells. Light focusing and imaging was through a ×10, 0.7 NA objective (Nikon Instruments). Emitted fluorescence light passed through an emission filter centered around 510 nm (Chroma Technology), and images were acquired with a 12-bit charge-coupled device camera set to 4 × 4 binning, controlled by TI Workbench software developed by T. Inoue. Regions of interest (ROIs) were drawn around selected cells and one background ROI was drawn in an empty area. For each ROI, a ratio r was calculated by averaging pixel values within each ROI for each excitation wavelength and dividing the values obtained after background subtraction:
Oxytocin stimuli were bath applied for periods of 2 minutes. Cells were identified by their responses to different secretagogues: cells responding to GnRH (1 nM) or a cocktail of GHRH (100 nM) plus ghrelin (10 nM) or TRH (100 nM) were deemed as putative gonadotrophs, somatotrophs, and lactotrophs, respectively.
Characterization of anti-OTR antibodies
Five different polyclonal antibodies, of which four were affinity purified, were used to investigate the presence of OTR-like immunoreactivity in anterior pituitary cells (see Table 1), each giving similar results. We had previously used one of these antibodies (goat anti-OTR antibody sc-8102) to demonstrate a single protein band in Western blot analysis of OTR expression in anterior pituitary tissue (9). The protein band corresponded to an estimated molecular size of 66 kDa in agreement with size estimates in other rat tissues (32, 33). Specificity of each antibody was validated by at least three of the following methods: 1) immunostaining of brain tissue slices demonstrated OTR-like immunoreactivity in the paraventricular nucleus as found by others (8) but not in neighboring areas; 2) no immunostaining was observed when the primary antibodies were omitted; 3) no immunostaining was observed when primary antibodies were substituted with nonimmune serum; 4) preadsorption of primary antibody with the immunizing peptide eliminated the staining almost completely and the reminder was regarded as negative, and 5) use of the somatolactotroph cell line GH4C1 as an additional control because we have observed that this cell line does not respond to OT and is derived from GH3 cells that do not express OTR (7, 34).
Table 1.
Antibodies Used in the Study
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Concentration or Dilution Used |
|---|---|---|---|---|---|
| Rat OTR (C terminal) | TFVLSRRSSSQRSCSQPSSA | Antirat OTR antibody | Alpha Diagnostics (OTR11-A) | Rabbit polyclonal | 10 μg/mL |
| Rat/human OTR (C terminal) | RRLGETSASKKSN | Anti-OTR antibody | Abcam (ab87312) | Goat polyclonal | 6.7 μg/mL |
| Human/rat OTR (C terminal) | Synthetic peptide corresponding to amino acids 350–389 of human OTR | OTR antibody (C-20) | Santa Cruz Biotechnology (sc-8102) | Goat polyclonal | 4 μg/mL |
| Human/rat OTR (N terminal) | Synthetic peptide corresponding to amino acids 21-42 of human OTR | OTR antibody | MBL International (MC-244) | Rabbit polyclonal | 5 μg/mL |
| Rat OTR (third intracellular loop) | WQNLRLKTAAAA | OTR antibody | Gloria Hoffman (JV3579) | Rabbit polyclonal | 1:10 000 |
Immunofluorescence of OTR
Cells were cultured on 20-mm glass coverslips at a cell density of 4 × 105 cells/coverslip and used the next day. Cells were rinsed three times in PBS 0.01 M (pH 7.3) with 1 mM CaCl2 and 1 mM MgCl2 and then fixed for 10 minutes with paraformaldehyde 4% in PBS 0.05 M (pH 7.0). The fixative was then removed and washed with PBS. After cells were treated with 0.3 M glycine (10 min), rinsed in PBS, and permeabilized with 0.4% Tween 20 (10 min), they were incubated in blocking solution containing 10% normal donkey serum, 0.3 M glycine and 0.1% Tween 20 for 2 hours. Cells were then washed in PBS and incubated 63 hours at 4°C with rabbit or goat primary antibodies to OTR (see Table 1). To enhance the sensitivity of immunofluorescence and use the primary antibodies at their maximum possible dilution, biotinylated tyramine-streptavidin amplification was performed. Cells were thoroughly washed and treated with 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity. For experiments using a goat primary antibody, cells were incubated with horseradish peroxidase-conjugated donkey antigoat secondary antibody (1:8000; Jackson ImmunoResearch Laboratories) for 90 minutes. For experiments using a rabbit primary antibody, an horseradish peroxidase-conjugated donkey antirabbit secondary antibody (1:8000; Pierce Biotechnology) was used. After thorough washes, a freshly prepared solution containing 1% of biotinylated tyramine plus 0.005% hydrogen peroxide (in 0.05 M PBS) was applied to the cells for 20 minutes. Cells were rinsed over 40 minutes and were incubated in the dark with fluorophore-conjugated streptavidin (Cy3 or Cy5 conjugates were used at 1:200; Life Technologies) at 37°C for 2.5 hours.
Immunofluorescence of gonadotrophs, somatotrophs, and lactotrophs
Cells were subsequently immunostained with primary antibodies against rat LH, GH, and PRL to identify gonadotrophs, somatotrophs, and lactotrophs. For immunofluorescence staining experiments using rabbit anti-OTR antibodies, cells were incubated simultaneously overnight with a goat polyclonal anti-PRL (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and guinea pig polyclonal anti-LH (1:30 000, AFP 1132093GP; NHPP) or guinea pig polyclonal anti-GH (1:1000; NHPP). Alternatively, for immunofluorescence staining experiments using the goat anti-OTR antibodies, cells were incubated with a guinea pig polyclonal anti-LH (1:30 000, AFP 1132093GP; NHPP) and rabbit polyclonal anti-GH (1:20 000, AFP5641801; NHPP) or rabbit polyclonal anti-PRL (1:30 000, AFP425_10_91; NHPP). Afterwards, cells were rinsed and incubated for 90 minutes (at 37°C, in darkness) with the following fluorophore-conjugated donkey secondary antibodies (as appropriate): Alexa 647-conjugated donkey antigoat, Alexa 647-conjugated donkey antiguinea pig (1:500; Jackson Immunoresearch Laboratories), AlexaFluor488-conjugated donkey antirabbit (1:500; Molecular Probes). After secondary antibodies were removed and washed, cells were incubated with 0.3% Sudan Black B (in 70% ethanol) for 10 minutes to quench cell autofluorescence, quickly rinsed in PBS several times, mounted, and coverslipped with mounting media (Aqua Polymount; Polysciences). The images were acquired using a Leica microscope attached to a cooled charge-coupled device camera (Andor Technology USA) and analyzed using Nikon analysis software (NIS Elements AR 3.2).
Calculations and statistical analyses
Hormone secretion data were plotted as representative traces from at least four independent experiments. Absolute hormone concentrations (in nanograms per milliliter) were plotted as a function of time (minutes). For the dose-response curve and comparative analysis, secretion data were expressed as the area under the curve (AUC) means ± SEM, and all concentrations were independently tested at least six times. The AUC measured the first 5 minutes of the secretion response and is defined as follows:
where Ni is the concentration of hormone in fraction i and mB is the mean basal value during the five fractions that preceded the time of stimulation. The amplitude of [Ca2+]i responses was measured for individual cells as the percentage increase over basal as follows:
where CB and COT are the mean value of the ratio r during 3 minutes prior to and 5 minutes following OT application, respectively.
Concentration-dependent effects of OT on hormone release were analyzed by nonlinear regression analysis, and dose-response curves were fitted to four-parameter sigmoidal curves with ALLFIT 2.6 and GraphPad Prism 4 software, each producing comparable results. The curve that gave the highest regression coefficient with the lowest residual SD was selected. The concentration at which the agonist displayed half maximal effect (EC50) was computed along with its 95% confidence interval (CI).
One-tailed comparisons of agonist-induced secretion responses vs basal conditions and of agonist secretory responses in the absence and presence of antagonist were performed by the nonparametric Mann-Whitney test for two independent samples. For comparisons of the percentage of responding cells and amplitude of [Ca2+]i responses to agonists pretreated or not with antagonists, the one-tail paired Wilcoxon signed-rank test was used. Data are thus presented as box plots showing median, interquartile range, and full range. For all statistical comparisons, P < .05 was considered significant; exact P values are provided when appropriate.
Results
OT stimulates hormone release and increases [Ca2+]i in gonadotrophs and somatotrophs
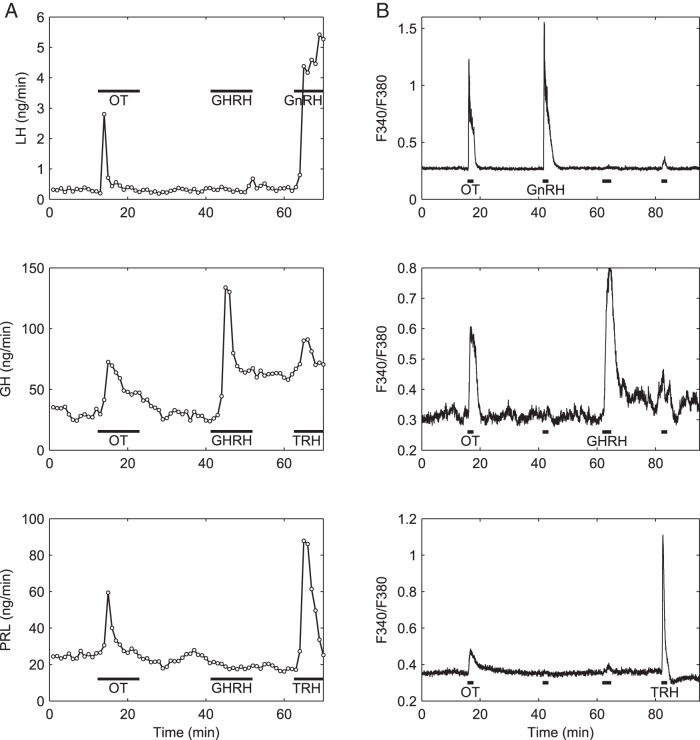
To first establish the secretory and intracellular Ca2+ responses of anterior pituitary cells to OT, cell perfusion and Ca2+ imaging experiments were conducted. At 10 nM, the nonapeptide-stimulated release of LH (Figure 1A, top panel), FSH (data not shown), and GH (Figure 1A, center panel) as well as an increase of Cai2+ in GnRH-responsive gonadotrophs (Figure 1B, top panel) and GHRH-responsive somatotrophs (Figure 1B, center panel). As previously reported by our laboratory (12, 13) and others (10, 34, 35), OT stimulated the release of PRL and elicited a transient of [Ca2+]i in lactotrophs (Figure 1, bottom panels).
Figure 1.
OT stimulates hormone secretion and evokes a rise in [Ca2+]i in rat gonadotrophs, somatotrophs, and lactotrophs. Effects of OT (10 nM, horizontal bar), GHRH (100 nM), TRH (100 nM), and GnRH (5 nM) on hormone release (A) and [Ca2+]i (B) in perifused pituitary cells of proestrus rats. In the Ca2+ imaging experiments, concentrations were the same except for GnRH (1 nM); the average traces of only those cells that responded specifically to each secretagogue and not others are shown. Top panels, Gonadotroph responses to OT and GnRH; center panels, somatotroph responses to OT and GHRH; bottom panels, lactotroph responses to OT and TRH. Representative traces of at least five independent experiments are shown. For this and the following figure, horizontal bars indicate duration of drug applications.
The response to OT is mediated by OT receptors in gonadotrophs and somatotrophs
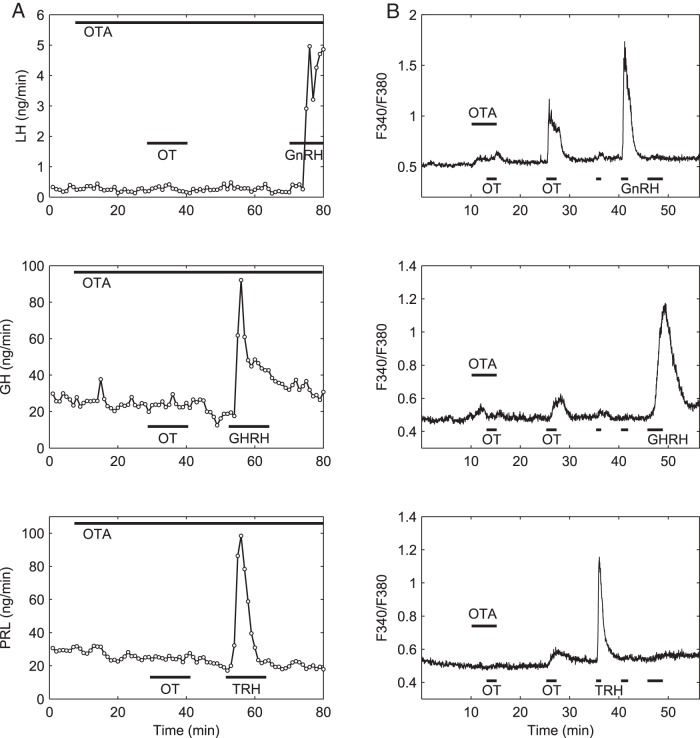
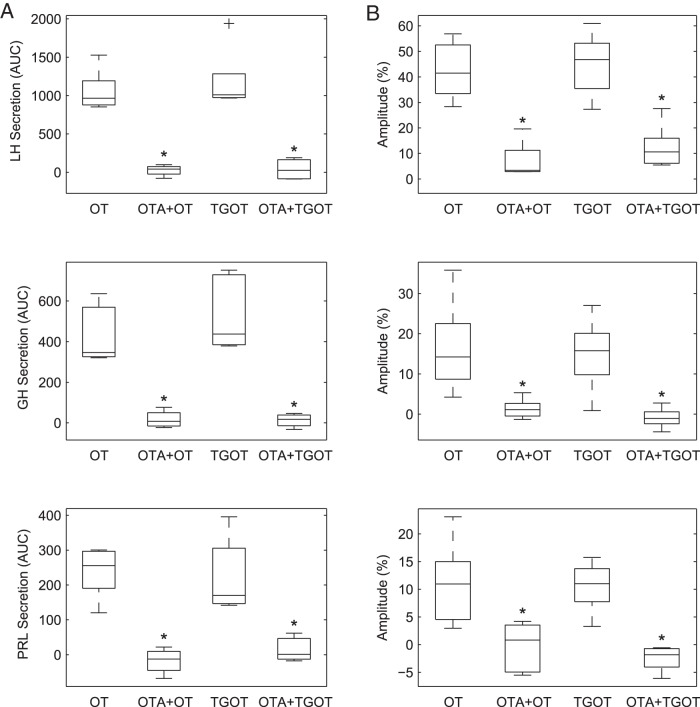
To confirm the specificity of these OT responses, we evaluated the effect of the selective OTR antagonist desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT (OTA) (26), which we have used previously in vivo (9, 36, 37) and in vitro (13). Indeed, OTA selectively blocked OT-induced responses in [Ca2+]i and secretion; OTA did not affect responses to cell-specific secretagogues and its effect was reversed upon removal (Figure 2). OTA (100 nM) inhibited the OT-stimulated release of LH, GH, and PRL (OTA + OT vs OT, P = .0079 for each hormone, Mann-Whitney test, n = 4) (Figure 3A). Likewise, OTA nearly abolished the [Ca2+]i response to OT in all three cell types (P = .0310 for each cell type, one tail paired Wilkinson signed rank test, n = 5) (Figure 3B). The inhibitory effect of OTA was due to a decrease of both the amplitude of individual responses and the number of responsive cells (not shown).
Figure 2.
OT-induced stimulation of hormone secretion and [Ca2+]i transients is blocked by selective antagonism to OTR. A, The selective OTR antagonist OTA eliminates OT-induced hormone secretion in perifused gonadotrophs (top panel), somatotrophs (center panel), and lactotrophs (bottom panel). The OTA (100 nM) was applied to perfusion media 20 minutes before cells were challenged with OT (10 nM) and remained throughout the experiment. Although OTA blocked the response to OT, it did not block the response to the cell secretagogues GnRH (5 nM), GHRH (100 nM), or TRH (100 nM) applied at the end of the experiment. B, The selective OTR antagonist OTA blocked OT-induced [Ca2+]i responses in gonadotrophs (top panel), somatotrophs (center panel), and lactotrophs (bottom panel). The OTA (100 nM) was bath applied for 5 minutes and OT (10 nM) was added during the final 2 minutes of the OTA incubation; both agents were then washed out before the application of a second pulse of OT, which elicited a response that was similar to that of cells untreated with the antagonist. A brief pulse of GnRH (1 nM), GHRH (100 nM) + ghrelin (10 nM), and TRH (100 nM) were applied at the end of the experiment to identify gonadotrophs, somatotrophs, and lactotrophs, and the average traces for all cells of each type are shown. Representative traces of at least four independent experiments are shown.
Figure 3.
The effects of both OT and the OTR-selective agonist TGOT on hormone secretion and [Ca2+]i transients are blocked by the selective OTR antagonist OTA. Perfused pituitary cells were pretreated or not with OTA (100 nM) and then challenged with OT or TGOT (10 nM) as shown in Figure 2. A, Agonist-induced LH (top panel), GH (center panel), and PRL (bottom panel) release. Hormone release was normalized relative to basal secretion and summed over 5 minutes (AUC). Both OT and TGOT significantly stimulated the release of all three hormones (P = .0008, Mann-Whitney test, n = 5); OTA-pretreatment almost completely blocked these effects. *, P = .0079, OT vs OTA+OT and TGOT vs OTA+TGOT. B, Average [Ca2+]i increase to agonist stimulation in gonadotrophs (top panel), somatotrophs (center panel), and lactotrophs (bottom panel). The mean increase relative to baseline, expressed as a percentage, was computed for each cell and then averaged over all GnRH-, GHRH-, or TRH-responsive cells in a given experiment. The OT- and TGOT-stimulated [Ca2+]i increase in all three identified cell types was significantly reduced by OTA pretreatment. *, P = .0310, Wilcoxon signed rank test (n = 5). Box plots show the median (middle bar), interquartile range (box), range (whiskers), and outliers (+).
To further confirm that the observed OT effects on hormone release and [Ca2+]i responses are mediated by OTR in each cell type, we conducted parallel studies using the species-specific, highly selective OTR agonist, TGOT (25), known to have a selectivity for OTR of at least 4 orders of magnitude over the V1b (38) and V1a (26) receptors. TGOT (10 nM) stimulated hormone secretion and elicited [Ca2+]i responses in gonadotrophs (Figure 3, top panels), somatotrophs (center panel), and lactotrophs (bottom panel), in a manner similar to that exerted by OT. The hormone-releasing effect of TGOT was significantly different from basal (P = .0008, Mann-Whitney test, n = 5) but not from that produced by OT in these three cell types (Figure 3A). Similarly, TGOT evoked [Ca2+]i responses of mean amplitudes comparable with those stimulated by OT (Figure 3B). Consistent with the inhibitory effects of OTA on OT-induced responses, the stimulatory effect of TGOT (10 nM) on the release of LH, GH, and PRL was inhibited by OTA by 96% ± 7.2%, 94.6% ± 2.6% and 90.5% ± 10.8%, respectively (n = 4) (Figure 3A). OTA also inhibited the mean amplitude of TGOT-stimulated [Ca2+]i responses in all three cell types (Figure 3B) (P = .0310 for each cell type, paired Wilcoxon signed rank test, n = 5).
In additional experiments we used the highly selective antagonist of vasopressin V1a receptors d(CH2)5[Tyr(Me)2, Dab5]AVP (27) and the V1b receptor-selective agonist d[Leu4,Lys8]VP (28). When preincubated in the presence of d(CH2)5[Tyr(Me)2,Dab5]AVP, both OT and TGOT induced responses in hormone secretion in gonadotrophs and somatotrophs that were comparable with those induced without the V1a receptor antagonist. For example, the AUC values of the hormone-releasing effect of OT, alone or in combination with the V1a antagonist, were as follows: for LH, 1062.4 ± 122.7 (n = 5) and 1463.9 ± 234.0 (n = 3), respectively (P > .05); and for GH, 435.1 ± 65.3 (n = 5) and 232.8 ± 53.8 (n = 3), respectively (P > .05). Furthermore, the V1b-selective agonist d[Leu4,Lys8]VP (10 nM) failed to elicit LH release but produced a weak stimulatory effect on GH release that was significantly lower than that triggered by OT or TGOT (V1b selective agonist: 183.8 ± 21.6, n = 4 vs OT: 435.1 ± 65.4, n = 5, P = .0079, Mann-Whitney test). Finally, AVP (10 nM) stimulated hormone release in both cell types in a manner comparable with that elicited by a dose of OT that was at least 1 order of magnitude lower (data not shown). Taken together, these results suggest that the OT-induced responses in gonadotrophs, somatotrophs, and lactotrophs are mediated by OTRs.
Comparison of OT responses in gonadotrophs, somatotrophs, and lactotrophs
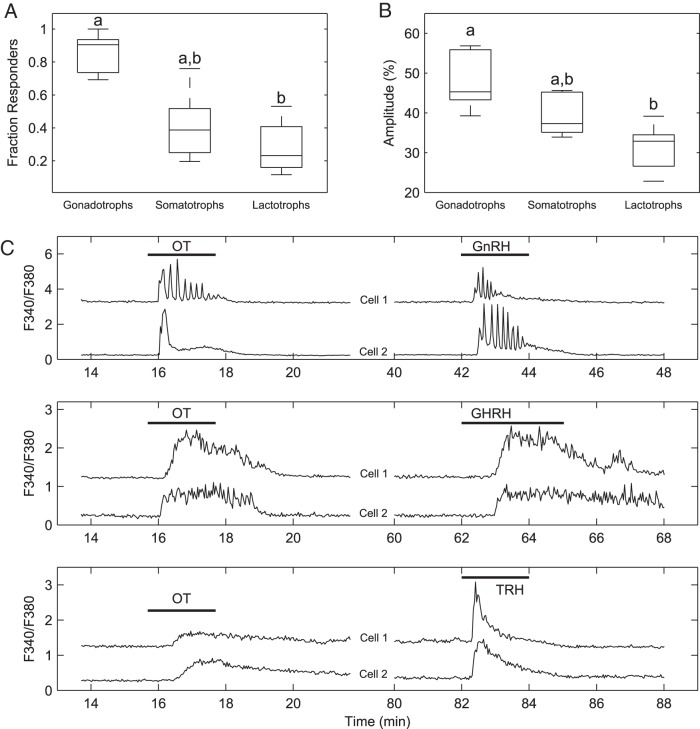
To compare the effects of OT on [Ca2+]i in the different cell types, we quantified the number of gonadotrophs, somatotrophs, and lactotrophs that responded to 10 nM OT as well as the amplitude of [Ca2+]i transients in OT-responsive cells. Although this concentration of OT is expected to be 10-fold less than the amount required to saturate all OTR-specific binding sites in the anterior pituitary (5), it was chosen to assess the population size of each cell type that would be sensitive to physiological doses of the nonapeptide (4). We found that a large majority of gonadotrophs (85.23% ± 5.68%, n = 5) were stimulated by OT (10 nM) (Figure 4A), whereas less than half of somatotrophs (40.97% ± 9.74%, n = 5) were sensitive to the nonapeptide at the same concentration. A significant fraction of lactotrophs responded to OT as well (28.36% ± 7.44%, n = 5). A similar profile was obtained when the amplitude of [Ca2+]i responses was compared, with gonadotrophs exhibiting significantly larger responses than lactotrophs (Figure 4B). In addition, we found that the pattern of [Ca2+]i dynamics stimulated by OT varied among cell types in a manner consistent with their intrinsic Ca2+-handling properties (39) and resembled those elicited by cell-specific secretagogues (Figure 4C).
Figure 4.
Analysis of single-cell Ca2+ responses in OT-responsive cells. A, The fraction of gonadotrophs, somatotrophs, or lactotrophs that responded to OT (in the absence of OTA) differs among cell types, with gonadotrophs showing a significantly higher fraction than lactotrophs (P = .0124, Kruskal-Wallis test, Tukey's multiple comparison test). B, Amplitude of responses in OT-responsive identified cells. Gonadotrophs showed significantly higher amplitude than lactotrophs (P = .0118, Kruskal-Wallis test, Tukey's multiple comparison test). C, Pairs of representative OT responses in gonadotrophs (top panel), somatotrophs (middle panel), and lactotrophs (bottom panel). Different lowercase letters indicate means that are significantly different from each other.
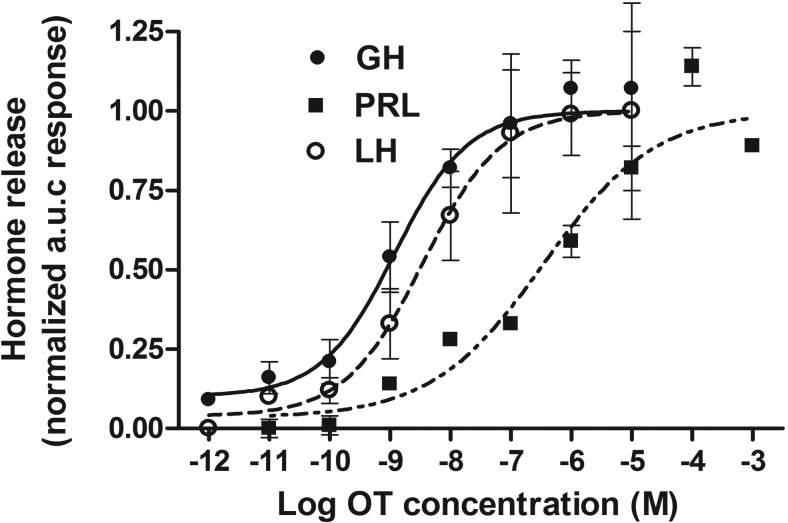
To further characterize the nature of OT-induced responses in gonadotrophs and somatotrophs, dose-response studies of the hormone-releasing effect of OT were performed. PRL release data obtained previously (13) were also included for comparative purposes. OT stimulated hormone release in a dose-dependent manner in all three cell types, but the dose-response curves of LH and GH release were located to the left of that for PRL (Figure 5). A nonlinear regression analysis showed that the EC50 of OT-induced hormone secretion in gonadotrophs and somatotrophs were 3.5 and 1.2 nM, respectively, which were in the range of the ligand affinity for anterior pituitary OT receptors from estrogen-treated female rats (5). Both EC50 values were 2 orders of magnitude lower compared with that of the PRL-releasing effect of OT in lactotrophs (0.47 μM) (13, 35), indicating that gonadotrophs and somatotrophs were more sensitive to OT than lactotrophs in our in vitro conditions.
Figure 5.
Dose-response curves of the hormone-releasing effect of OT in perifused anterior pituitary cells from female cycling rats. For each concentration of agonist, the AUC values are calculated for the release of GH (closed circles), LH (open circles), and PRL (closed squares). Results are shown as normalized AUC means ± SEM from at least six independent experiments. Continuous lines represent the best fit curve for each hormonal response. Regression curves were calculated and EC50 values determined as described in Materials and Methods. For LH release the following was found: EC50 3.5 nM (95% CI 2.2–5.4 nM); GH release: EC50 1.2 nM (95% CI 0.5–2.7 nM); and for PRL release: EC50 0.47 μM (95% CI 0.09–2.03 μM). Mean basal values were 0.35 ± 0.03, 26.6 ± 2.4, and 30.1 ± 2.4 ng/min for LH, GH, and PRL, respectively. LH and GH data were obtained from pituitary cells of cycling rats at different stages; PRL data were obtained from cells of proestrous rats and reported elsewhere (13).
Immunocytochemical staining of OTR-like immunoreactivity in gonadotrophs and somatotrophs
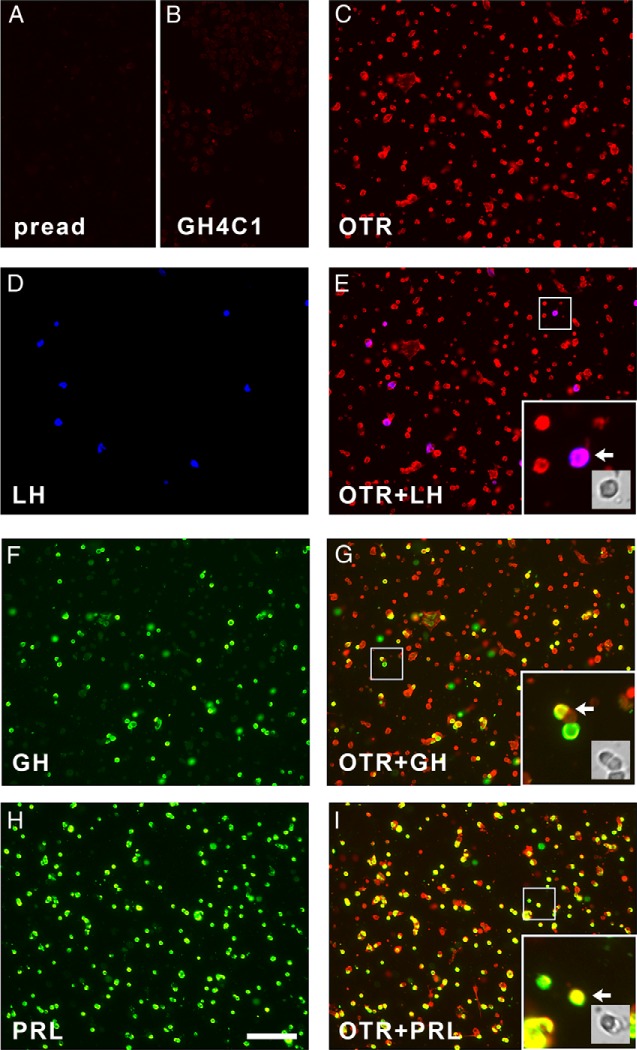
Collectively our results provided strong evidence supporting the presence of OTR activity in gonadotrophs and somatotrophs in addition to lactotrophs. Therefore, we sought to determine the presence of OTR-like immunoreactivity in these cell types by triple immunofluorescence using cultured anterior pituitary cells and various antibodies raised against different regions of the receptor (listed in Table 1). Preadsorption of primary antibody with the immunizing peptide removed OTR-like immunopositive staining from anterior pituitary cells (Figure 6A). Immunostaining of the OT-unresponsive rat pituitary cell line GH4C1 resulted in a faint fluorescence staining (Figure 6B), and its intensity distribution was used as a reference to quantify the percentage of pituitary cells with OTR-like immunoreactivity. Anterior pituitary cells were deemed OTR-positive if their intensity of OTR-like staining was greater than 3 SD above the mean intensity of GH4C1 cells. Regardless of the anti-OTR antibody used, OTR-like immunoreactivity was not restricted to lactotrophs (Figure 6, H and I) but was widespread in anterior pituitary cells and also could be found in gonadotrophs (Figure 6, D and E) and somatotrophs (Figure 6, F and G). Using the criterion stated above, 95% of gonadotrophs, 85% of somatotrophs, and 85% of lactotrophs were OTR positive (mean of four independent experiments).
Figure 6.
Immunofluorescence staining of cultured rat anterior pituitary cells for OTR and pituitary hormones. Images from a representative triple immunofluorescence experiment are shown. In all panels, OTR-like immunoreactivity is shown in red, and images were acquired using the same exposure time (18 msec). A, Preadsorption of anti-OTR primary antibody with the immunizing peptide for the OTR removed immunopositive staining of anterior pituitary cells. pread, preadsorption. B, Immunostaining of GH4C1 cells (which do not respond to OT) with anti-OTR antibody resulted in almost negligible staining. C, Immunostaining of anterior pituitary cells with anti-OTR antibody resulted in widespread positive staining with varying intensity. D, Cells stained for LH, shown in blue. E, Merged image showing double-labeling of OTR and LH. F, Cells stained for GH, shown in green. G, Merged image showing double labeling of OTR and GH. H, Cells stained for PRL, shown in green. I, Merged image showing double labeling of OTR and PRL. Insets in E, G, and I, High-magnification detail of the indicated area, illustrating receptor/hormone colocalization for each of the three cell types. Arrows indicate a double-labeled cell whose bright field image is also shown. Images in panels C–G belong to the same dish; only double-labeled cell images are shown for clarity. For the experiment shown here, the affinity-purified goat anti-OTR from Abcam (ab87312, 6.7 μg/mL) was used; the results obtained with other primary antibodies (listed in Table 1) gave comparable results. Scale bar, 100 μm in all panels.
Discussion
Since the demonstration of the hormone-releasing effects of OT and AVP on PRL and ACTH, respectively, the hypothesis that anterior pituitary hormone secretion may also be controlled by neurohypophysial hormones emerged. Here, using a perfusion system that allowed the measurements of acute, parallel responses in [Ca2+]i and secretion while minimizing the possibility of indirect effects due to cell-to-cell interactions, we provide evidence that gonadotrophs and somatotrophs, in addition to lactotrophs, are sensitive to OT stimulation and respond with transient elevations of [Ca2+]i and hormone release in a dose-dependent manner (Figures 1 and 5). These responses were also evoked by an OTR-selective agonist and abolished by an OTR-selective antagonist (Figures 2 and 3), suggesting that the OT-induced effects are mediated by OTRs in all three cell types. Together with the observation that OT-induced [Ca2+]i responses mimic those of cell type-specific secretagogues (Figure 4) and our immunofluorescence studies showing positive OTR-like immunoreactivity present in all three cell types (Figure 6), our results suggest that OT may act directly on different anterior pituitary cell types to coordinate hormone release.
OT and AVP differ in only two amino acids (40), raising the possibility that the effects of OT that we observed might have been mediated in part through anterior pituitary vasopressin V1a and V1b receptors (38, 41). However, several lines of evidence suggest that the effects of OT on gonadotrophs and somatotrophs are direct and mediated by homologous receptors, as in lactotrophs. First, OT and the selective OTR agonist TGOT (25) elicited comparable responses in [Ca2+]i and secretion in all three cell types at a concentration (10 nM) that is 10-fold higher than their affinity values for pituitary OTR (5, 6) and at least 1–3 orders of magnitude lower than those for V1 receptor subtypes (26). At this concentration, OT displaced the binding of 3H-OT and 3H-AVP to rat pituitary membranes by approximately 80% and 10%, respectively (5), indicating that only a very small fraction of vasopressin receptors might be bound to OT.
Second, an equivalent concentration of AVP resulted in weak LH and GH secretory responses that compared with those obtained with a dose of OT that was at least 10 times lower, consistent with the observation that the oxytocic activity of AVP in rat bioassays was several times lower than that of OT (26). Similarly, the V1b receptor-selective agonist d[Leu4,Lys8]VP (28) had no effect on LH release and a weak effect on GH secretion.
Third, the selective OTR antagonist OTA abolished OT- and TGOT-induced Ca2+ and secretion responses in all three cell types at a concentration (100 nM) that is almost 2 orders of magnitude higher and at least 1 order of magnitude lower than its affinity for OTR and V1 receptors, respectively (26). Fourth, the stimulatory effects of both OT and TGOT in gonadotrophs and somatotrophs could still be observed in the presence of the highly selective antagonist of vasopressin V1a receptors d(CH2)5[Tyr(Me)2,Dab5]AVP (27), which has been shown to lack OTR agonism and antagonism activities (26, 42). Taken together, these results strongly suggest that the OT-elicited actions in the three cell types are predominantly mediated by OTRs.
The observed stimulatory effect of OT on LH release is consistent with previous studies (19, 43), but we did not find inhibitory effects on GH secretion at high OT concentrations as reported previously (24). This discrepancy may stem from differences in the experimental conditions, such as the gender of rats, age of the cell culture, incubation times, and mode of incubation. In particular, the long (4 h) static incubations used elsewhere (24) occlude discrimination between the effects on hormone release or synthesis and may bring about indirect effects through paracrine interactions. Also, because we have observed desensitization of responses to continuous OT exposure in all three cell types, it is likely that desensitization may occur during long incubations with OT.
[Ca2+]i imaging experiments using physiological OT concentrations demonstrated that the nonapeptide stimulated a large majority of gonadotrophs and nearly half of the somatotrophs. The expression of OTRs in anterior pituitary cells other than lactotrophs was further confirmed by our immunocytochemical results, which indicated that the large majority of these three cell types express OTR-like immunoreactivity. Because a previous in situ hybridization study detected OTR mRNA only in lactotrophs (7), it is possible that its half-life is significantly lower in other cell types. Moreover, this receptor is known to be present in very limited numbers in this gland (6), although OTR-mediated effects could be demonstrated in lactotrophs from cycling female rats (13, 35). Membrane preparations of anterior pituitaries from randomly cyclic female rats or ovariectomized rats exhibited no appreciable specific binding of an OTR-selective radioligand, and those from estrogen-treated rats increased this binding to a maximum capacity that was still 30-fold lower than that measured in uterine membrane preparations (6). Given this low expression, we used tyramine-streptavidin amplification to increase the signal to detect cells positive for OTR-like immunoreactivity in the anterior pituitary of intact proestrus rats. Despite the relatively low level of expression, we observed robust responses in terms of hormone secretion and [Ca2+]i transients. OTR expression might not be limited to the three cell types we studied because it has been shown that mouse corticotrophs also express this receptor (44).
The anterior pituitary expresses the uterine-type OTR shown in other tissues to mediate OT-induced mobilization of [Ca2+]i through the Gq/11 protein-mediated stimulation of phospholipase C (PLC)-β (40). PLC-β activation results in the generation of inositol 1,4,5-trisphosphate and 1,2-diacylglycerol; the former triggers the release of Ca2+ from the endoplasmic reticulum and the latter stimulates protein kinase C. The Gq/11-PLC-Ca2+ mobilization pathway is likely operative in this gland as OT-induced Ca2+ mobilization was observed in a gonadotroph cell line (45), corticotrophs (46, 47), and lactotrophs (12, 13). Here we establish that OT can evoke transients of [Ca2+]i in primary cultured gonadotrophs and somatotrophs and further show that the pattern of [Ca2+]i dynamics stimulated by OT is cell type specific, perhaps most strikingly demonstrated by the oscillations seen in gonadotrophs. This is consistent with the finding that OT stimulated oscillations of outward current in voltage-clamped gonadotrophs from castrated male rats (20).
We found that gonadotrophs and somatotrophs are much more sensitive to OT in vitro than are lactotrophs, possibly reflecting differences in the coupling of OTR to [Ca2+]i signaling pathways among cell types. The observed low sensitivity of lactotrophs to OT agrees with previous in vitro reports (34, 35) and rules out the possibility that this cell type mediates OT effects on gonadotrophs and somatotrophs through a paracrine mechanism. The lower sensitivity of lactotrophs to OT in vitro may not be explained in terms of differences in receptor number as OTR-like immunoreactivity was equally represented among the three cell types. Alternatively, it might be related to the absence in the perfusion medium of hypothalamic neurohormones (dopamine, in particular) and/or peripheral factors normally present in vivo that may be required for effective coupling of the OTR to secretory pathways in lactotrophs. Hypothalamic neurohormones and peripheral factors are known to alter (either positively or negatively) the response of the gland to secretagogues (48–50). A similar argument may explain the observation that OT did not affect LH and GH in vivo in animals that did exhibit a transient increase of PRL in plasma after OT administration (10), despite the high sensitivity of gonadotrophs and somatotrophs to OT in vitro. In this case, the lack of LH and GH acute responses to OT in vivo might be due to the inhibitory influence of physiological modulators that are not present in our in vitro conditions.
Our results indicate that gonadotrophs and somatotrophs have the potential to release their hormones in response to OT concentrations found in portal blood (51), suggesting that OT could elicit secretory responses in both cell types in vivo. Interestingly, several observations that established a physiologically relevant role for OT on the regulation of PRL secretion apply to LH release as well. The concentration of OT in rat pituitary portal blood peaks at the onset of the PRL and LH surges in the afternoon of proestrus (51) and antagonism of this endogenous OT increase inhibits both proestrous surges (52). Furthermore, exogenous administration of OT advanced both the gonadotrophin (14) and PRL (53) proestrous surges in rats as well as the midcycle LH surge in healthy women (54). OT-induced facilitation of the LH surge might occur through synergism between OT and GnRH to elicit augmented LH release at the anterior pituitary (55, 56). Our results also suggest that OT can contribute to the control of GH release and energy metabolism through a direct action on somatotrophs. This observation is of potential physiological relevance as blunted GH responses have been observed in Prader-Willi syndrome patients, a multisystemic disorder associated with a selective loss of OT neurons in the paraventricular nucleus (57).
OT might act in concert with other factors to coordinate secretion of PRL, GH, and LH. Thus, its effects on the release of these hormones may depend on the physiological context. For example, during suckling, a time when OT secretion is increased both centrally and systemically (58) with an accompanying elevation of PRL levels, increased GH secretion (59) as well as a depletion of pituitary GH content occurs (60, 61), whereas basal LH levels decrease (62). Likewise, a dramatic increase in GH levels occurs in parallel to a reciprocal decrease of PRL at the onset of parturition (59) when a remarkable increase of OTR expression is observed in the uterus, hypothalamus, and anterior pituitary (40).
In summary, this work shows that OT can stimulate hormone release and evoke [Ca2+]i transients through OTRs in gonadotrophs and somatotrophs in addition to lactotrophs. Given that OT can also act in corticotrophs (38, 44) to modulate ACTH release (47, 63) and may have an inhibitory effect on thyrotrophs (64), the nonapeptide may modulate anterior pituitary hormone release through direct actions on possibly all endocrine cell types of the gland.
Acknowledgments
We thank Dr G. Hoffman for helpful tips on immunocytochemistry and generously donating the rabbit polyclonal antirat OTR antiserum (JV3579) as well as Dr M. Manning for providing the selective V1a antagonist and V1b agonist. We also thank Dr T. Inoue for providing and supporting the software TI Workbench for acquisition and analysis of the intracellular calcium signals. We also thank Mr Charles Badland for photo art assistance and A. Stathopoulos for helpful comments and discussion.
This work was supported by National Institutes of Health Grant DK-43200.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- AVP
- arginine vasopressin
- [Ca2+]i
- intracellular Ca2+ concentration
- CI
- confidence interval
- HBS
- HEPES-buffered saline solution
- NHPP
- National Hormone and Pituitary Program
- OT
- oxytocin
- OTA
- OTR antagonist desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT
- OTR
- oxytocin receptor
- PLC
- phospholipase C
- PRL
- prolactin
- ROI
- region of interest
- TGOT
- (Thr4,Gly7)oxytocin
- VP
- vasopressin.
References
- 1. Samson WK, Schell DA. Oxytocin and the anterior pituitary gland. Adv Exp Med Biol. 1995;395:355–364. [PubMed] [Google Scholar]
- 2. Vandesande F, Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretroy system of the rat. Cell Tissue Res. 1975;164:153–162. [DOI] [PubMed] [Google Scholar]
- 3. Silverman AJ. Ultrastructural studies on the localization of neurohypophyseal hormones and their carrier proteins. J Histochem Cytochem. 1976;24:816–827. [DOI] [PubMed] [Google Scholar]
- 4. Gibbs DM. High concentrations of oxytocin in hypophysial portal plasma. Endocrinology. 1984;114:1216–1218. [DOI] [PubMed] [Google Scholar]
- 5. Antoni FA. Oxytocin receptors in rat adenohypophysis: evidence from radioligand binding studies. Endocrinology. 1986;119:2393–2395. [DOI] [PubMed] [Google Scholar]
- 6. Chadio SE, Antoni FA. Characterization of oxytocin receptors in rat adenohypophysis using a radioiodinated receptor antagonist peptide. J Endocrinol. 1989;122:465–470. [DOI] [PubMed] [Google Scholar]
- 7. Breton C, Pechoux C, Morel G, Zingg HH. Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology. 1995;136:2928–2936. [DOI] [PubMed] [Google Scholar]
- 8. Adan RAH, Van Leeuwen FW, Sonnemans MAF, et al. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–4028. [DOI] [PubMed] [Google Scholar]
- 9. Kennett JE, Poletini MO, Fitch CA, Freeman ME. Antagonism of oxytocin prevents suckling- and estradiol-induced, but not progesterone-induced, secretion of prolactin. Endocrinology. 2009;150:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lumpkin MD, Samson WK, McCann SM. Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology. 1983;112:1711–1717. [DOI] [PubMed] [Google Scholar]
- 11. Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology. 1986;119:554–560. [DOI] [PubMed] [Google Scholar]
- 12. Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tabak J, Gonzalez-Iglesias AE, Toporikova N, Bertram R, Freeman ME. Variations in the response of pituitary lactotrophs to oxytocin during the rat estrous cycle. Endocrinology. 2010;151:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robinson G, Evans JJ. Oxytocin has a role in gonadotrophin regulation in rats. J Endocrinol. 1990;125:425–432. [DOI] [PubMed] [Google Scholar]
- 15. Shibusawa K, Saito S, Fukuda M, Kawai T, Yamada J, Tomozawa K. Neurosecretion of oxytocin stimulates the release of pituitary gonadotrophin. Endocrinol Jpn. 1955;2:183–187. [DOI] [PubMed] [Google Scholar]
- 16. Melin P. Gonadotrophic activity of the pituitary and urine of the male rabbit after oxytocin treatment. Acta Endocrinol. 1971;66:529–539. [DOI] [PubMed] [Google Scholar]
- 17. Robinson G, Evans JJ, Forster ME. Oxytocin can affect follicular development in the adult mouse. Acta Endocrinol. 1985;108:273–276. [DOI] [PubMed] [Google Scholar]
- 18. Robinson G, Evans JJ, Catt KJ. Oxytocin stimulates LH production by the anterior pituitary gland of the rat. J Endocrinol. 1992;132:277–283. [DOI] [PubMed] [Google Scholar]
- 19. Evans JJ, Robinson G, Catt KJ. Gonadotrophin-releasing activity of neurohypophysial hormones: I. Potential for modulation of pituitary hormone secretion in rats. J Endocrinol. 1989;122:99–106. [DOI] [PubMed] [Google Scholar]
- 20. Billiard J. Functional heterogeneity of pituitary gonadotropes in response to a variety of neuromodulators. Mol Cell Endocrinol. 1996;123:163–170. [DOI] [PubMed] [Google Scholar]
- 21. Samson WK, Alexander BD, Skala KD, Huang F-LS. Ricin-cytotoxin conjugate administration reveals a physiologically relevant role for oxytocin in the control of gonadotropin secretion. Ann NY Acad Sci. 1992;652:411–422. [DOI] [PubMed] [Google Scholar]
- 22. Björkstrand E, Hulting A-L, Uvnäs-Moberg K. Evidence for a dual function of oxytocin in the control of growth hormone secretion in rats. Regul Peptides. 1997;69:1–5. [DOI] [PubMed] [Google Scholar]
- 23. Rico M, Vidal S, Lorenzo MT, Moya L, De la Cruz LF. Effects of acute administration of growth hormone-releasing hormone (GHRH) and oxytocin on somatotroph cells in sheep. Morphometric study and growth hormone (GH) secretion. J Endocrinol Invest. 1995;18:442–449. [DOI] [PubMed] [Google Scholar]
- 24. Hulting A-L, Grenbäck E, Pineda J, et al. Effect of oxytocin on growth hormone release in vitro. Regul Pept. 1996;67:69–73. [DOI] [PubMed] [Google Scholar]
- 25. Lowbridge J, Manning M, Haldar J, Sawyer WH. Synthesis and some pharmacological properties of [4-threonine, 7-glycine]oxytocin, [1-(L-2-hydroxy-3-mercaptopropanoic acid), 4-threonine, 7-glycine]oxytocin (hydroxy[Thr4, Gly7]oxytocin), and [7-Glycine]oxytocin, peptides with high oxytocic-antidiuretic selectivity. J Med Chem. 1977;20:120–123. [DOI] [PubMed] [Google Scholar]
- 26. Manning M, Misicka A, Olma A, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan WY, Wo NC, Cheng LL, Manning M. Isosteric substitution of Asn5 in antagonists of oxytocin and vasopressin leads to highly selective and potent oxytocin and V1a receptor antagonists: new approaches for the design of potential tocolytics for preterm labor. J Pharmacol Exp Ther. 1996;277:999–1003. [PubMed] [Google Scholar]
- 28. Pena A, Murat B, Trueba M, et al. Pharmacological and physiological characterization of d[Leu4,Lys8]vasopressin, the first V1b-selective agonist for rat vasopressin/oxytocin receptors. Endocrinology. 2007;148:4136–4146. [DOI] [PubMed] [Google Scholar]
- 29. Tomic M, Cesnajaj M, Catt KJ, Stojilkovic SS. Developmental and physiological aspects of Ca2+ signaling in agonist-stimulated pituitary gonadotrophs. Endocrinology. 1994;135:1762–1771. [DOI] [PubMed] [Google Scholar]
- 30. Lacau-Mengido IM, Gonzalez-Iglesias AE, Lux-Lantos V, Libertun C, Becú-Villalobos D. Ontogenic and sexual differences in pituitary GnRH receptors and intracellular Ca2+ mobilization induced by GnRH. Endocrine. 1998;8:177–183. [DOI] [PubMed] [Google Scholar]
- 31. Close FT, Freeman ME. Effects of ovarian steroid hormones on dopamine-controlled prolactin secretory responses in vitro. Neuroendocrinology. 1997;65:430–435. [DOI] [PubMed] [Google Scholar]
- 32. Umscheid CA, Wu WX, Gordan P, Nathanielsz PW. Up-regulation of oxytocin receptor messenger ribonucleic acid and protein by E2 in the cervix of ovariectomized rat. Biol Reprod. 1998;59:1131–1138. [DOI] [PubMed] [Google Scholar]
- 33. Muller M, Soloff MS, Fahrenholz F. Photoaffinity labelling of the oxytocin receptor in plasma membranes from rat mammary gland. FEBS Lett. 1989;242:333–336. [DOI] [PubMed] [Google Scholar]
- 34. Liu JW, Ben-Jonathan N. Prolactin-releasing activity of neurohypophysial hormones: structure-function relationship. Endocrinology. 1994;134:114–118. [DOI] [PubMed] [Google Scholar]
- 35. Chadio SE, Antoni FA. Specific oxytocin agonist stimulates prolactin release but has no effect on inositol phosphate accumulation in isolated rat anterior pituitary cells. J Mol Endocrinol. 1993;10:107–114. [DOI] [PubMed] [Google Scholar]
- 36. McKee DT, Poletini MO, Bertram R, Freeman ME. Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology. 2007;148:4649–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helena CV, McKee DT, Bertram R, Walker AM, Freeman ME. The rhythmic secretion of mating-induced prolactin secretion is controlled by prolactin acting centrally. Endocrinology. 2009;150:3245–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schlosser SF, Almeida OFX, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. [DOI] [PubMed] [Google Scholar]
- 39. Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. [DOI] [PubMed] [Google Scholar]
- 41. Orcel H, Tobin VA, Alonso G, Rabié A. Immunocytochemical localization of vasopressin V1a receptors in the rat pituitary gonadotropes. Endocrinology. 2002;143:4385–4388. [DOI] [PubMed] [Google Scholar]
- 42. Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Progr Brain Res. 2008;170:473–512. [DOI] [PubMed] [Google Scholar]
- 43. Evans JJ, Pragg FL, Mason DR. Release of luteinizing hormone from the anterior pituitary gland in vitro can be concurrently regulated by at least three peptides: gonadotropin-releasing hormone, oxytocin and neuropeptide Y. Neuroendocrinology. 2001;73:408–416. [DOI] [PubMed] [Google Scholar]
- 44. Nakamura K, Fujiwara Y, Mizutani R, et al. Effects of vasopressin V1b receptor deficiency on adrenocorticotropin release from anterior pituitary cells in response to oxytocin administration. Endocrinology. 2008;149:4883–4891. [DOI] [PubMed] [Google Scholar]
- 45. Evans JJ, Forrest-Owen W, McArdle CA. Oxytocin receptor-mediated activation of phosphoinositidase C and elevation of cytosolic calcium in the gonadotrope-derived αT3–1 cell line. Endocrinology. 1997;138:2049–2055. [DOI] [PubMed] [Google Scholar]
- 46. Won JG, Oki Y, Orth DN. Roles of intracellular and extracellular calcium in the kinetic profile of adrenocorticotropin secretion by perifused rat anterior pituitary cells. II. Arginine vasopressin, oxytocin, and angiotensin-II stimulation. Endocrinology. 1990;126:858–868. [DOI] [PubMed] [Google Scholar]
- 47. Link H, Dayanithi G, Fohr KJ, Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992;130:2183–2191. [DOI] [PubMed] [Google Scholar]
- 48. Kanyicska B, Livingstone JD, Freeman ME. Long term exposure to dopamine reverses the inhibitory effect of endothelin-1 on prolactin secretion. Endocrinology. 1995;136:990–994. [DOI] [PubMed] [Google Scholar]
- 49. Yu WH, Kimura M, McCann SM. Effect of somatostatin on the release of gonadotropins in male rats. Proc Soc Exp Biol Med. 1997;214:83–86. [DOI] [PubMed] [Google Scholar]
- 50. Gonzalez-Iglesias AE, Freeman ME. Brain control over pituitary gland hormones. In: Pfaff DW, ed. Neuroscience in the 21st Century. New York: Springer-Verlag; 2012. [Google Scholar]
- 51. Sarkar DK, Gibbs DM. Cyclic variation of oxytocin in the blood of pituitary portal vessels of rats. Neuroendocrinology. 1984;39:481–483. [DOI] [PubMed] [Google Scholar]
- 52. Johnston CA, Negro-Vilar A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology. 1988;122:341–350. [DOI] [PubMed] [Google Scholar]
- 53. Gonzalez-Iglesias AE, Cristancho-Gordo R, Arias-Cristancho JA, Bertram R. Peripheral administration of oxytocin acutely increases prolactin systemic levels and advances the onset of the prolactin surge in proestrus. 42nd Annual Meeting of the Society for Neuroscience, New Orleans, Louisiana, 2012, 683.16. [Google Scholar]
- 54. Hull ML, Reid RA, Evans JJ, Benny PS, Aickin DR. Preovulatory oxytocin administration promotes the onset of the luteinizing hormone surge in human females. Hum Reprod. 1995;10:2266–2269. [DOI] [PubMed] [Google Scholar]
- 55. Evans JJ, Hurd SJ, Mason DR. Oxytocin modulates the luteinizing hormone response of the rat anterior pituitary to gonadotrophin-releasing hormone in vitro. J Endocrinol. 1995;145:113–119. [DOI] [PubMed] [Google Scholar]
- 56. Evans JJ, Tulloch S. Effects of administration of oxytocin in association with gonadotropin-releasing hormone on luteinizing hormone levels in rats in vivo. Peptides. 1995;16:145–150. [DOI] [PubMed] [Google Scholar]
- 57. Swaab F, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab. 1995;80:573–579. [DOI] [PubMed] [Google Scholar]
- 58. Kendrick K, Keverne E, Baldwin B, Sharman D. Cerebrospinal fluid levels of acethylcholinesterase, monoamines and oxytocin during labour, parturition, vagocervical stimulation, lamb separation and suckling in sheep. Neuroendocrinology. 1986;44:149–156. [DOI] [PubMed] [Google Scholar]
- 59. Saunders A, Terry LC, Audet J, Brazeau P, Martin JB. Dynamic studies of growth hormone and prolactin secretion in the female rat. Neuroendocrinology. 1976;21:193–203. [DOI] [PubMed] [Google Scholar]
- 60. Grosvenor CE, Krulich L, McCann SM. Depletion of pituitary concentration of growth hormone as a result of suckling in the lactating rat. Endocrinology. 1968;82:617–619. [DOI] [PubMed] [Google Scholar]
- 61. Sar M, Meites J. Effects of suckling on pituitary release of prolactin, GH, and TSH in postpartum lactating rats. Neuroendocrinology. 1969;4:25–31. [DOI] [PubMed] [Google Scholar]
- 62. Hammons J-A, Velasco M, Rothchild I. Effect of the sudden withdrawal or increase of suckling on serum LH levels in ovariectomized postparturient rats. Endocrinology. 1973;92:206–211. [DOI] [PubMed] [Google Scholar]
- 63. Oki Y, Peatman TW, Qu ZC, Orth DN. Effects of intracellular Ca2+ depletion and glucocorticoid on stimulated adrenocorticotropin release by rat anterior pituitary cells in a microperifusion system. Endocrinology. 1991;128:1589–1596. [DOI] [PubMed] [Google Scholar]
- 64. Frawley LS, Leong DA, Neill JD. Oxytocin attenuates TRH-induced TSH release from rat pituitary cells. Neuroendocrinology. 1985;40:201–204. [DOI] [PubMed] [Google Scholar]