Abstract
Chronic consumption by experimental animals of a typical Western diet high in saturated fats and cholesterol during postnatal life has been demonstrated to impair skeletal development. However, the underlying mechanism by which high-fat, energy-dense diets affect bone-forming cell phenotypes is poorly understood. Here, we show that male weanling rats fed a diet containing 45% fat and 0.5% cholesterol made with casein (HF-Cas) for 6 weeks displayed lower bone mineral density and strength compared with those of AIN-93G–fed dietary controls. Substitution of casein with soy protein isolate (SPI) in the high-fat diet (HF-SPI) prevented these effects. The bone-sparing effects of SPI were associated with prevention of HF-Cas–induced osteoblast senescence pathways through suppression of the p53/p21 signaling pathways. HF-Cas–fed rats had increased caveolin-1 and down-regulated Sirt1, leading to activations of peroxisome proliferator–activated receptor γ (PPARγ) and p53/p21, whereas rats fed HF-SPI suppressed caveolin-1 and activated Sirt1 to deacetylate PPARγ and p53 in bone. Treatment of osteoblastic cells with nonesterified free fatty acid (NEFA) increased cell senescence signaling pathways. Isoflavones significantly blocked activations of senescence-associated β-galactosidase and PPARγ/p53/p21 by NEFA. Finally, replicative senescent osteoblastic cells and bone marrow mesenchymal ST2 cells exhibited behavior similar to that of cells treated with NEFA and in vivo bone cells in rats fed the HF-Cas diet. These results suggest that (1) high concentrations of NEFA occurring with HF intake are mediators of osteoblast cell senescence leading to impairment of bone development and acquisition and (2) the molecular mechanisms underlying the SPI-protective effects involve isoflavone-induced inhibition of osteoblastic cell senescence to prevent HF-induced bone impairments.
Modeling and maturation of the skeletal system in the pediatric population are affected by nutritional status, dietary factors, body composition, and weight-bearing effects (1). Manipulations of nutritional intakes or dietary factors in early life may dramatically change the course of chronic diseases such as degenerative bone disorders and obesity development. In particular, excessive consumption of a Western diet (defined as having high saturated fat and cholesterol levels) is believed to be associated with development of obesity. Despite disagreement in the clinical literature regarding the effect of obesity on bone development (2, 3), feeding such a Western diet (high-fat diet [HFD]) to rodents has been shown to inhibit bone formation (4, 5). Moreover, impaired fetal skeletal development was also revealed in a HFD-induced maternal obesity rat model (6).
A variety of hormonal factors are altered in plasma of obese animals, including insulin, leptin, IGF-I and nonesterified free fatty acid (NEFA) (5, 7–9). Plasma circulating NEFAs are either directly derived from diet or secreted by adipose tissue. Our previous results (5) and numerous other studies have shown that NEFAs are able to activate peroxisome proliferator–activated receptor γ (PPARγ) and increase its transcription. Many fatty acid metabolites are considered as specific ligands for PPARγ (10, 11). The role of PPARγ in adipogenesis is well known; however, additional functions of PPARγ on cellular signal transduction in different cell types are being discovered. For example, it has been shown that overexpression or activation of PPARγ will in turn accelerate the senescence pathway by inducing p16 expression in a ligand-dependent manner (12) in human diploid fibroblasts. In this regard, PPARγ was suggested to be one such molecule linking exterior factors (such as diet) and interior factors (such as the p16 gene) to control cellular senescence.
Although the mechanisms are not well understood, both obesity and cellular senescence are significantly accompanied by inflammation at both the cellular and tissue levels (13). On the other hand, an interesting study reported that the reduction of fat mass was associated with increased longevity in mice (14). Increased longevity could result from suppression of cellular senescence pathways or decreased programmed cell death. This in turn suggests an interrelationship between increased fat mass in obesity and accelerated cellular senescence. Cellular senescence is usually monitored by increased senescence-associated β-galactosidase (SA-β-gal) activity in both cultured cells and in vivo tissues (15, 16). Overexpression of biomarkers such as p53/p21 and/or p16 is also commonly used for detecting senescent cells (17). Cellular senescence has been widely investigated as a potential mechanism of tumor suppression; however, its functional contribution to noncancer tissue pathology is poorly understood. It has been reported that a HFD induces senescence in the vascular system (18); we, therefore, hypothesize that feeding of a HFD may also be associated with senescence in the skeletal system.
Effective approaches for managing obesity are extremely limited. Medication, weight loss programs, and dietary interventions have been the most widely used. However, presently there are only 2 medications approved in the United States for long-term use, and they are associated with a variety of side effects (19). Weight loss programs have been successful; however, they are often accompanied by significant bone loss (20). Furthermore, obese children present a special problem, because appropriate interventions would need to improve body composition while simultaneously protecting against stunting of growth. Dietary intervention may be a more appropriate choice. In this regard, a soy protein isolate (SPI) diet has been investigated as a candidate for the prevention of metabolic syndrome in early development (21). The effects of soy diet on bone have been attributed to potential estrogenic actions related to its high content of phytoestrogens such as genistein and daidzein, which are isoflavones structurally similar to 17β-estradiol (22). However, we recently reported that the effects of an SPI diet and estrogens differed. The SPI diet, but not estradiol, was able to down-regulate caveolin-1 expression remarkably in bone (23) and prevent osteoblastic cell senescence (24). Caveolin-1 has been shown to be a main component of the caveolar plasma membranes found in most cell types. It is involved in vesicular trafficking, cholesterol homeostasis, cell adhesion, apoptosis, and senescence. The effect of an SPI diet on bone, caveolin-1 expression, and HFD-induced bone cellular senescence was therefore explored in the current study.
Materials and Methods
Animals and diets
Forty male Sprague-Dawley rats [genetic background Hsd:Sprague Dawley (CD)] purchased from Harlan Industries arrived on postnatal day (PND) 20 and on PND24 were randomly assigned to 1 of 4 groups (n = 10). Rats were fed 1 of 4 diets: (1) a standard low-fat (5%) AIN-93G diet formulated with casein as the sole protein (LF-Cas); (2) a low-fat diet made with the AIN-93G diet formula except that casein was replaced with soy protein isolate (LF-SPI); 3) a high-fat/high-cholesterol diet containing 18.8 MJ/kg energy, 195 g/kg casein protein, 483 g/kg carbohydrate, 210 g/kg anhydrous milk fat, 5 g/kg cholesterol, and 50 g/kg cellulose fiber (HF-Cas); and (4) the same HFD described in 3 above, except that casein was replaced by SPI (HF-SPI). Therefore, HFD-fed rats take 18.7% kcal from protein, 41.4% kcal from carbohydrate, and 39.9% kcal from fat vs 19% kcal from protein, 65.2% kcal from carbohydrate, and 16.3% kcal from fat for the AIN-93G–based diet. Thus, there were 2 low-fat diets, 1 with casein (LF-Cas) and 1 with SPI (LF-SPI), and there were 2 high-fat diets, 1 with casein (HF-Cas) and 1 with SPI (HF-SPI). Rats were fed their respective diets from PND24 to PND68. The HF-SPI group had ad libitum access to food, but the other 3 groups were pair-fed to match the food intake of the HF-SPI group. All rats had ad libitum access to water. Rats were housed in an Association for Assessment and Accreditation of Laboratory Animal Care–approved animal facility at the Arkansas Children's Hospital Research Institute with constant humidity and lights on from 6:00 am to 6:00 pm at 22°C. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences (Little Rock, Arkansas). Rat body weights were monitored weekly. At the completion of the experiment, rats were anesthetized by injection with 100 mg of Nembutal/kg body weight (Avent Laboratories), followed by decapitation; legs, vertebrae, and serum were collected.
Bone analyses
Peripheral quantitative computerized tomography (pQCT) was performed on formalin-fixed left tibia for bone mineral density (BMD) measurement using a method well established in our laboratory (25). A STRATEC XCT 960 M unit (XCT Research SA, Norland Medical Systems) specifically configured for small bone specimens was used. Software version 5.4 was used with thresholds of 570 mg/cm3 to distinguish cortical bone and 214 mg/cm3 to distinguish trabecular from cortical and subcortical bone. Tibial BMD and bone mineral content (BMC) were calculated. The position for pQCT scanning was defined at a distance from the proximal tibia 1 mm below the growth plate corresponding to 7% of the total length of the tibia. The distance between each scan was 1 mm; a total of 5 scans (5 slices) were performed. Data are expressed as the mean of 3 contiguous slices with the greatest trabecular bone density.
A compression test of the fourth lumbar vertebra was performed at room temperature using a custom-made, steel lower plate designed in the form of a cup. The processes of the vertebral body were removed carefully to ensure that the cortical shell was undamaged and therefore not mechanically compromised. The intervertebral discs were also removed. Vertebrae were thawed to room temperature before mechanical testing. The dimensions of the major and minor axes of the vertebral body were also determined by caliper. The vertebral body was placed with the cranial end facing upward in the lower cup of the testing machine (Instron 8874; Instron Corp.). The top plate, smaller in diameter than the cup, was lowered onto the vertebra to a compressive preload of 5 N, at which point the displacement was set at zero. Displacement was measured from the actuator displacement transducer of the testing machine. The mechanical properties including ultimate strength/stress and stiffness were recorded with a digital caliper.
Right tibia bone histology and immunostaining using p53 and p21 antibodies (Santa Cruz Biotechnology) on decalcified tibia sections were performed using the standard protocol from the VectaStain ABC kit (Vector Laboratories). For p53 and p21 immunostaining, sections were deparaffinized, blocked with normal goat serum in 2% BSA-PBS for 30 minutes and incubated with monoclonal antibody to p53 and polyclonal antibody to p21 for 60 minutes. After 3 washes with PBS, sections were incubated with biotinylated secondary antimouse antibody for p53, which was labeled with streptavidin-conjugated Oregon green tag, and antirabbit antibody for p21, which was labeled with streptavidin-conjugated Texas red tag (Molecular Probes), respectively, and then were counterstained with 4′,6-diamidino-2-phenylindole (Molecular Probes).
Cell culture
Bone marrow stromal cell line ST2 and osteoblastic cell line OB6 were used. Cells were cultured in α-MEM supplemented with 10% fetal bovine serum (HyClone), penicillin (100 units/mL), streptomycin (100 μg/mL), and glutamine (4 mM). Fresh ST2 or OB6 cells are cells taken out from liquid nitrogen, cultured, and passaged no more than twice. Fresh ST2 and OB6 cells were designated as early passage cells (passage 2 [p2]). In contrast, after 30 passages, ST2 or OB6 cells were defined as intrinsic (eg, replicative) senescent cells (p30). For different assay purposes (mRNA, cell staining, and proteins), different sizes of cell culture plates were used, and cells were treated in -the presence or absence of NEFA (2.5 mM palmitic acid, 0.5 mM stearic acid, 1.0 nM oleic acid, 1.5 mM linoleic acid, and 0.4 arachidonic acid) and isoflavone (1 μM genistein, 0.5 μM daidzein, and 0.1 μM glycetein) for 24 hours, 48 hours, or 3 days. Free fatty acids were dissolved in 95% ethanol at 60°C and then mixed with prewarmed BSA (10%) to yield a stock concentration of 10 mM. The concentrations and ratio of NEFA and isoflavones were adjusted to be identical to their appearance in serum from HFD-fed or SPI diet–fed rats (22, 24). Individual isoflavone was purchased from Plantec, and NEFA was purchased from Sigma-Aldrich. Cell culture medium ELISAs for the carboxy-terminal peptide fragment of type 1 collagen (C1CP), and osteocalcin levels were performed using enzyme immunoassay kits (TSZ Scientific). A nonradioactive cell proliferation assay was performed following the protocol provided by the manufacturer (Part TB169; Promega Corporation).
SA-β-gal activity and staining
The SA-β-gal activity assay was performed by using a β-galactosidase enzyme assay kit (Promega); the absorbance was measured at 420 nm according to the manufacturer's instructions. Bone section and cell β-galactosidase staining were also performed according to a method published previously (26). Senescent cells were identified as blue-stained cells by standard light microscopy.
Western blotting and coimmunoprecipitation
Right femur bone tissue and in vitro cellular proteins were extracted for Western immunoblot analysis using cell lysis buffer as described previously (26). In brief, after aspiration of bone marrow, smashed femur bone was homogenized in 0.5 mL of radioimmunoprecipitation assay buffer containing phenylmethylsulfonyl fluoride to a final concentration of 1 mM and protease inhibitor cocktail (catalog. no. BP-475; Boston BioProducts). After the supernatant was spun at 12 000 rpm for 15 minutes, at +4°C, it was diluted in 1% SDS (1:10). Western blot and coimmunoprecipitation (for acetylated protein pull-down studies) analyses were performed using standard protocols. The following primary antibodies were used: acetylated p53, rabbit polyclonal (catalog no. 2570, 1:1000 dilution; Cell Signaling, Technology); total-p53, rabbit polyclonal (catalog no. 9282, 1:2000 dilution; Cell Signaling Technology); caveolin-1, rabbit polyclonal (catalog no. sc894, 1:2000 dilution; Santa Cruz Biotechnology); Sirt1, rabbit polyclonal (catalog no. sc15404, 1:2000 dilution; Santa Cruz Biotechnology); β-actin, mouse monoclonal (catalog no. A1978, 1:5000 dilution; Sigma-Aldrich); p21, rabbit polyclonal (catalog no. 397, 1:1000 dilution; Santa Cruz Technology); and PPARγ, rabbit polyclonal (catalog no. ab5907, 1:2000 dilution; Abcam). This antibody information was also summarized in a Supplemental Table 1. Secondary antibodies were purchased from Santa Cruz Biotechnology. Blots were developed using chemiluminescence (Pierce Biotechnology) according to the manufacturer's recommendations.
Constructs for luciferase assay and chromatin immunoprecipitation (ChIP) assay
The PPARγ overexpression plasmid was purchased from Addgene (Addgene plasmid 1015). peroxisome proliferator–activated receptor response element (PPRE)-luciferase (luc) expression plasmids (27) were kindly provided by Dr B. Spiegelman (Harvard Medical School, Boston, Massachusetts). Luciferase reporter constructs were introduced into OB6 cells by transient transfection using Lipofectamine 2000 (Invitrogen). OB6 cells were plated in 48-well plates and transfected 24 hours later with a total of 0.4 μg of DNA. After transfection, cells were treated with either NEFA or isoflavone or their combination for 24 to 48 hours. Luciferase activity assays were performed as described previously (5). The ChIP assay was performed basically as described in the protocol of the ChIP assay kit from Active Motif (catalog nos. 53008 and 53009). NEFA- and/or isoflavone-treated OB6 cells were cross-linked with formaldehyde in PBS for 10 minutes and lysed in the lysis buffer provided in the kit. Then 50 μL of chromatin was incubated with anti-PPARγ antibody. Recovered immunoprecipitates were used as a template for PCR of p53 and p21 genes using the following mouse-specific primers of p53 and p21 designed based on their promoter regions (Table 1). The PCR conditions were as follows: an initial melt step at 94°C for 3 minutes, then 36 cycles of 94°C for 20 seconds, 59°C for 30 seconds, and 72°C for 30 seconds, and then a hold cycle at 10°C. The total volume of each PCR was 25 μL.
Table 1.
Real-Time RT-PCR Primer Sequences
| Forward Primer | Reverse Primer | |
|---|---|---|
| Mouse gene | ||
| p21 | CCTTCCTCACCTGTGTCGTCTT | TGGGATGCACTGGGTGTTCT |
| p53 | GGAGACATTTTCAGGCTTATGGA | GCCTTCAAAAAACTCCTCAACATC |
| Osterix | TGCAGCAAATTTGGCGGCTCTA | TCCATTGGTGCTTGAGAAGGGA |
| ALP | CCAATGTAGCCAAGAATGTCATCA | GCCCGTGTTGTGGTGTAGCT |
| GAPDH | GTATGACTCCACTCACGGCAAA | GGTCTCGCTCCTGGAAGATG |
| Mouse p21 promoter | ||
| Set 1 | TCTGGGCTTTTCCATCACATC | GATTCCCCCTTCAGGACTTACC |
| Set 2 | GCAGCAAAGCAGCAGACAAG | CCCACACTGGTCCCCATTT |
| Set 3 | TGGGTCCTTCCCTTGGTTAAT | GGGTCCCTCAGCTCCTTTTT |
| Set 4 | ACAGAAACCCTAAATGTGGCATTC | GGTCAGTGAGCTCCAGGCTCTA |
| Mouse P53 promotor | ||
| Set 1 | TGTCCCGGAGATTGCATTACA | GCTTCCATCAAGGTAGATCAGGAT |
| Set 2 | TCAACAGGTGACGCATGGA | TGGATTACGCCGAAATGGTAA |
| Set 3 | CCTTGCCCATCGTACTTACCA | TCTCGCAGGTACCCCGATAC |
| Set 4 | GCTGTGCAATTAAAGGCTGTGA | TGTTCTCCGAGATACTTGGTATCG |
RNA isolation and real-time RT-PCR
Cell RNA from isolated in vitro cultured cells were extracted using TRI Reagent (MRC Inc) according to the manufacturer's recommendations followed by DNase digestion and column cleanup using QIAGEN mini columns (25). Reverse transcription was carried out using an iScript cDNA synthesis kit from Bio-Rad. All primers for real-time PCR analysis used in this report were designed using Primer Express software 2.0.0 (Applied Biosystems).
Statistical analyses
Numerical variables were expressed as means ± SD. Comparisons between groups were performed with a two-factor ANOVA to test the null hypothesis of equal means across the 2 fat percentages and the 2 proteins and their interactions. The data were tested and found to be normally distributed, and there were no significant interactions between the HF diet and SPI” P = .9, when BMD was the outcome; and P = .8, when protein expression was the outcome. Pairwise comparisons using the Tukey-Kramer post hoc test were used to identify groups whose means differed significantly while the family-wise error rate was retained at 5%.
Results
SPI prevents HF-induced impairment of bone quality
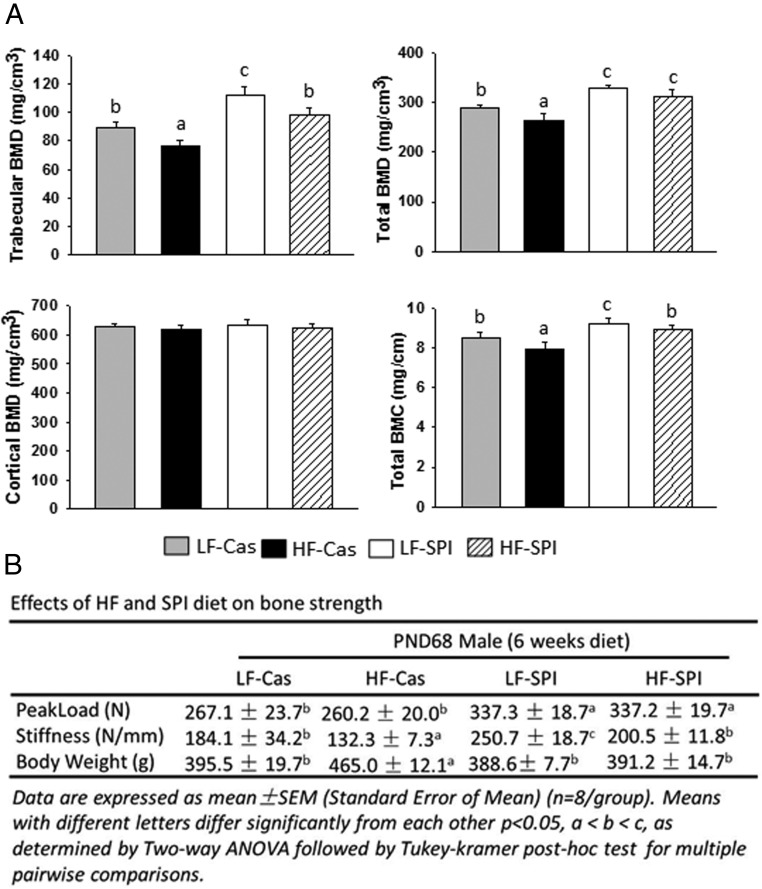
pQCT scanning of the proximal tibia revealed that both trabecular and total BMD were decreased in the HF-Cas group compared with those in the LF-Cas group (P < .05) (Figure 1A). Similarly, total BMC, but not cortical BMD, was also significantly lower in the HF-Cas group than in the LF-Cas group (Figure 1A). Total BMD and BMC along with trabecular BMD were all significantly increased in the LF-SPI diet group compared with those in the LF-Cas group, and trabecular BMD in the LF-SPI group was the highest among all groups. Total BMD was greater in the HF-SPI group than in the LF-Cas group (P < .05) (Figure 1A). Final body weights showed that the HF-Cas rats were heavier than the LF-Cas rats: 465.0 ± 12.1 g vs 395.5 ± 19.7 g, respectively (P = .026). LF-SPI rats had the lowest body weight and HF-SPI rats had body weights similar to those of the LF-Cas group (Figure 1B). We previously reported that HF-Cas rats had larger abdominal fat pads (1.61 ± 0.21%) and gonadal fat pads (1.39 ± 0.11%) than LF controls (0.85 ± 0.08% and 0.81 ± 0.0%, respectively) (P < .05), demonstrating that these HF-Cas rats were obese (28). Impaired bone acquisition in HF-Cas rats was consistent with our previous observations for which we used a rat total enteral nutrition model of HFD feeding, while keeping body weights equal between groups to demonstrate that low bone quality was associated with development of obesity (5). These results are also associated with bone strength data in vertebrae examined by a vertebrae compression test (Figure 1B). We found that both peak load and stiffness were highest in the LF-SPI group (Figure 1B). Stiffness, but not peak load, was significantly lower in the HF-Cas group compared with that in the LF-Cas control group (P < .05) (Figure 1B). Surprisingly, both peak load (P < .05) and stiffness were higher in the HF-SPI group than in the LF-Cas control group (Figure 1B).
Figure 1.
Cofeeding of an SPI diet prevents HFD-induced impairment of bone quality in rats. A, pQCT analysis of the proximal tibial in 4 different diet rats (n = 9 rats). Trabecular BMD (milligrams per cubic centimeter), total BMD, cortical BMD, and total BMC are presented. B, A fourth vertebra compression test was performed, and parameters of peak load and stiffness are presented along with final rat body weights. Data are expressed as means ± SD (n = 9/group). Means with different letters differ significantly from each other at P < .05 using the Tukey-Kramer post hoc test for pairwise comparisons.
Osteoblastic cell senescence
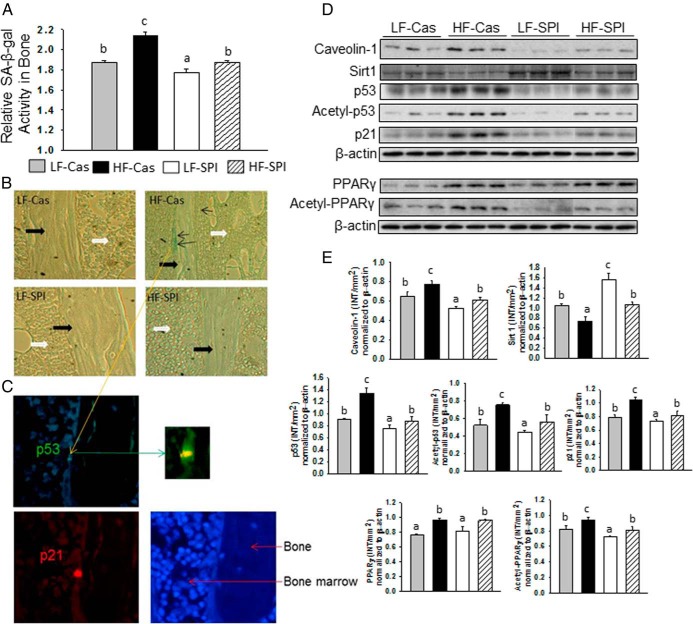
We previously hypothesized that diet-induced obesity may reduce osteoblast activity and drive mesenchymal stromal cell differentiation to favor an adipocyte lineage over an osteoblast lineage due possibly to increased cell senescence signaling (29). The potentials of premature senescent mesenchymal stromal cells and osteoblasts to differentiate toward mature osteoblasts or express and secrete collagen type 1 (Col1) were significantly decreased (30). We therefore examined whether there is a linkage between impaired bone development in HFD-induced obese rats and an increased senescence pathway in bone and osteoblasts. To do this, total protein was isolated from bone and SA-β-gal activity was measured using an enzymatic assay method we have published previously (26). SA-β-gal activity was significantly highest in the HF-Cas rats compared with that in all other groups (Figure 2A). Surprisingly, the SPI diet completely abolished the HFD effects on SA-β-gal activity (Figure 2A). To identify senescent osteoblastic cells in situ, we used SA-β-gal staining on tibial bone sections and found that clustering of SA-β-gal–positive blue osteoblastic cells on the surface of the trabecular bone spicules were only present in samples from HF-Cas rats (Figure 2B). The SA-β-gal–positive blue osteoblastic cell was further positively identified using coimmunostaining with p53 and p21 antibodies (Figure 2C). These senescence-associated markers have been causally linked to the senescence program; cells typically coexpressing multiple senescence markers are thought to have a decreased ability to proliferate. Results fully agreed with SA-β-gal activity measurement using proteins isolated from bone. Furthermore, Western blot analyses showed increases in the expression of caveolin-1 but decreases in Sirt1 expression in bone from the HF-Cas group (Figure 2, D and E). The expression of these proteins in the LF-SPI group were the opposite of those from the HF-Cas group (Figure 2, D and E). Substantial increases in protein expression of p53 and acetylated p53, known as the active form of p53 that binds DNA, were found in bone from HF-Cas rats compared with its expression in bone from LF-Cas rats (Figure 2, D and E). The SPI diet completely blocked HF-Cas diet–induced total p53 and acetylation of p53 in bone (Figure 2, D and E). p21, a known effector of p53, showed an expression pattern similar to that of p53: its protein expression in bone from HF-Cas rats was increased, and its expression was inhibited by the SPI diet when SPI was cofed with the HF diet (Figure 2, D and E). It was not surprising that PPARγ was activated in bone from HF-Cas rats (Figure 2, D and E). SPI itself had no effect on total PPARγ expression in bone; however, the SPI diet decreased acetylation of PPARγ and inhibited overexpression of this active form of PPARγ when SPI was cofed with the HF diet (Figure 2, D and E).
Figure 2.
Senescence activated pathway in osteoblastic cells and accumulation in bone. A, SA-β-gal activity analyses in total proteins isolated from bone after aspiration of bone marrow cells with 4 different diet groups was performed using an ELISA method. Data are expressed as means ± SD (n = 9 per group). Means with different letters differ significantly from each other at P < .05. B, SA-β-gal activity staining in long bone sections from 4 different diet groups. Pictures are illustrating typical bone surface clustered SA-β-gal–positive blue osteoblasts only in bone from HF-Cas rats in a sagittal section under ×20 magnifications. Thick black arrows indicate trabecular bone spicule and white arrows indicate bone marrow area; thin black arrows indicate positive senescent osteoblastic cells. C, SA-β-gal–positive blue osteoblasts from HF-Cas rat coexpress senescence markers p53 and p21. The photograph illustrates 1 double-positive cell with p21 red and p53 green and 4′,6-diamidino-2-phenylindole staining for nucleus. D, Western blots of caveolin-1, Sirt1, total p53, acetylated p53, p21, PPARγ, acetylated PPARγ, and β-actin are depicted for 3 samples from 4 different diet groups. Nine samples from each group were pooled to 3 samples per group. E, The bar graphs represent the ratio of intensity of the band of caveolin-1, Sirt1, total p53, acetylated p53, p21, PPARγ, acetylated PPARγ normalized to β-actin protein for each 3 samples from 4 different diet groups using Quantity One software (Bio-Rad). Data are expressed as means ± SD. Means with different letters differ significantly from each other at P < .05 by two-way ANOVA followed by the Tukey-Kramer post hoc test for pairwise comparisons.
Role of NEFA and isoflavones
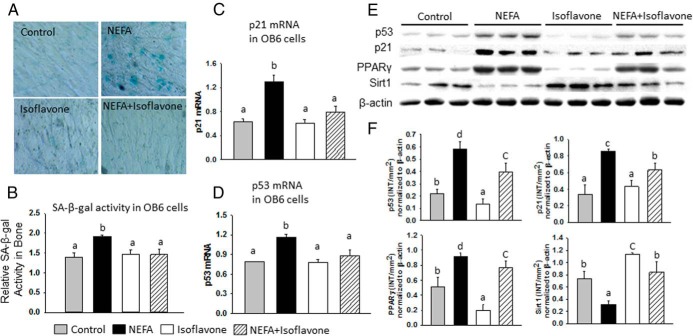
NEFA from increased fat stores was thought to be one of the most important factors affecting bone in diet-induced obese animal models (28). SPI consumption results in the appearance of many bioactive compounds in blood. To date, isoflavones, because of their structural similarity to estrogens, have been the most studied components associated with dietary soy and have been found to have beneficial effects on bone (24, 28). Therefore, to determine whether elevated NEFA levels in serum are one of the mechanisms of the effects of HF on bone and whether phytoestrogen isoflavones associated with the SPI diet are the basis by which SPI prevents HF effects in rats, we first identified isoflavone levels in serum associated with an SPI diet using a method published previously from our laboratory (31, 32). Similar to our previously published results (22), total isoflavone concentrations were in the range of 1 μM. Next, osteoblastic OB6 cells were treated with NEFA in the presence or absence of isoflavones. The concentration and ratios of individual NEFAs and isoflavones were similar to their appearance in the serum of rats fed HF and SPI diets, respectively. Five major NEFAs (palmitic, stearic, oleic, linoleic, and arachidonic acids in the ratio of 5:1:2:3:1, with palmitate as the most prominent NEFA) were found in rat serum and showed roughly 5-fold higher concentrations in HF-Cas rats than in LF-Cas rats. Three-day treatment of cells with the NEFA mixture produced a significantly activated senescence pathway, determined by SA-β-gal–positive blue staining (Figure 3A), significantly increased SA-β-gal protein activity (Figure 3B), and significantly increased p53 and p21 mRNA expression (Figure 3C) compared with those of control vehicle–treated cells. Isoflavone treatment alone did not change the basal level of expression of cell senescence markers, although it completely blocked NEFA-triggered activation of the osteoblast senescence pathway when it was cotreated with NEFA (Figure 3, A–F). Western blot analysis further confirmed activations of p53 and p21 (Figure 3E) and increased PPARγ but decreased Sirt1 expression by NEFA (Figure 3, E and F). Isoflavone abolished the effects of NEFA on these protein expressions in osteoblastic cells (Figure 3, E and F).
Figure 3.
NEFA plays a critical role on the activated senescence pathway in osteoblastic cells, whereas isoflavone prevented it. A, OB6 cells treated with vehicle (control), NEFA, isoflavone, and their combination were stained with SA-β-gal. NEFA-treated cells showed more SA-β-gal-positive blue cells. B, ELISA for SA-β-gal activity in total proteins isolated from OB6 cells with 4 different treatments. C, Real-time PCR analyses for p21 mRNA expression after OB6 cells were treated with NEFA, isoflavone, and their combination for 3 days. D, Real-time PCR analyses for p53 mRNA expression after OB6 cells treated with NEFA, isoflavone, and their combination for 3 days. E, Western blots for p53, p21, PPARγ, and Sirt1 protein expression in OB6 cells in triplicate after treatment with NEFA, isoflavone, or their combination for 3 days. F, The bar graphs represent the ratio of intensity of the band of p53, p21, PPARγ, and Sirt1 protein expression normalized to β-actin protein for 3 samples each from 4 different treatments using Quantity One software. Data are expressed as means ± SD. Means with different letters differ significantly from each other at P < .05 by two-way ANOVA followed by the Tukey-Kramer post hoc test for pairwise comparisons.
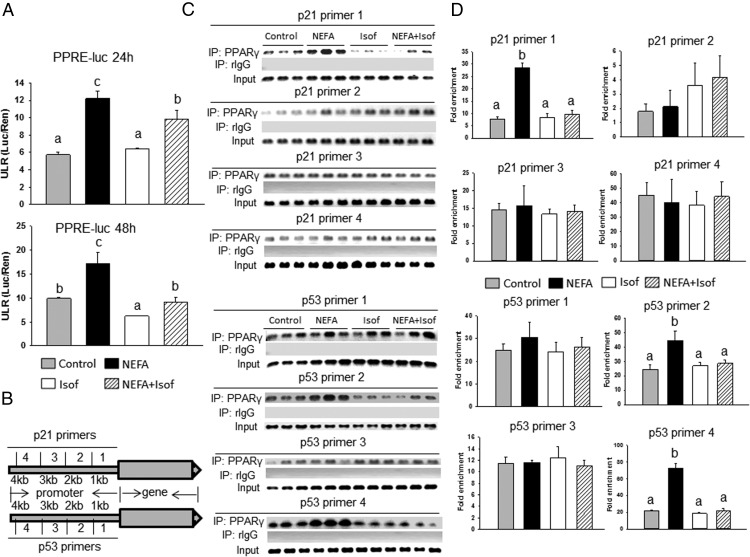
To obtain further mechanistic explanations for our observed results in which isoflavone inhibits the effects of NEFA on PPARγ, p53, and p21 expression, we next studied PPARγ promoter luciferase activity and performed a ChIP assay in osteoblastic cells treated with either NEFA or isoflavones or their combination. NEFA significantly increased PPARγ promoter activity at both 24 and 48 hours as determined by a PPRE-luciferase reporter assay in osteoblastic OB6 cells (Figure 4A). Isoflavones time dependently decreased PPARγ promoter activity and inhibited NEFA-induced activation of the PPARγ promoter (Figure 4A, top and bottom panels). Next, a ChIP assay using PPARγ antibody was performed in OB6 cells after 48 hours of treatment with or without NEFA and isoflavones. We designed 4 sets of primers (Figure 4B) from 4 different promoter regions of the p53 and p21 genes to examine whether PPARγ truly interacts with or binds to either the p53 or p21 gene and whether NEFA or isoflavone interferes with their binding. Subsequent PCR amplification of possible adjacent PPREs in the enhancer of the murine p21 and p53 (possible target genes for PPARγ) genes was performed. Interestingly, we found that there were pronounced increases in the binding of PPARγ to the p21 promoter using primer set 1 and to the p53 promoter using primer sets 2 and 4 in OB6 cells after treatment with NEFA (Figure 4, C and D). Clearly, isoflavones blocked NEFA-enhanced binding of PPARγ to both p21 and p53 at these sites, but it did not change the basal levels of the binding of PPARγ to either p21 or p53 (Figure 4, C and D). These data indicated that HFD-induced obesity or NEFA increases not only PPARγ expression but also its transcriptional activity to increase p21 and/or p53 expression and isoflavones antagonize these.
Figure 4.
NEFA facilitated PPARγ binding to p21/p53 promoter in OB6 cells, and isoflavone blocked it. A, Luciferase activity in OB6 cells transfected with a PPRE-luc reporter construct and treated with NEFA, isoflavone (Isof), and their combination for 24 and 48 hours. ULR, unit of luminescence to renilla; Luc, luciferase; Ren, Renilla. B, Schematic diagram of the p21 and p53 gene promoters and design for 4 sets of primers from each p21 and p53 gene promoter. C and D, ChIP (IP) of 4 sets of possible mouse p21 and p53 enhancer elements by specific anti-PPARγ antibody in OB6 cells after treatment with NEFA, isoflavones, and their combination for 24 hours. Bars represent means ± SD in triplicate. Means with different letters differ significantly from each other at P < .05.
Overexpression of PPARγ accelerates osteoblastic cell senescence
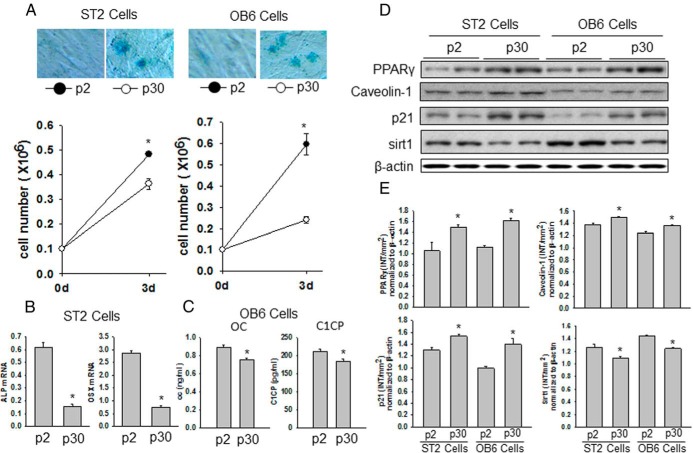
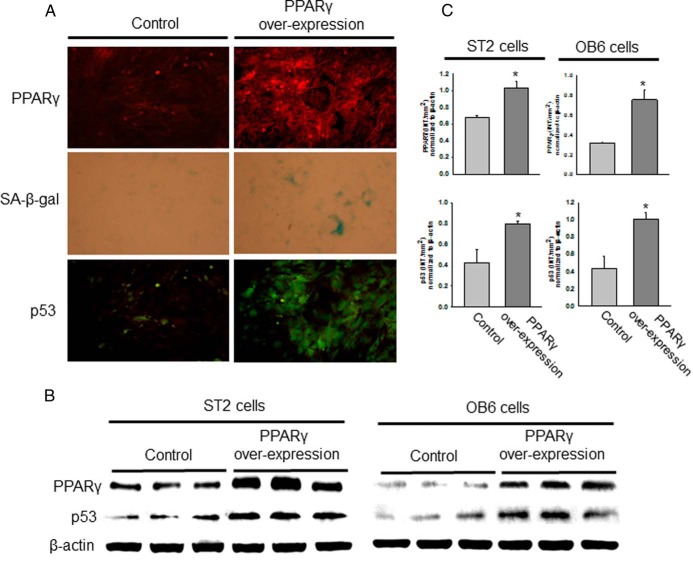
We have demonstrated that HF or NEFA exposure results in acceleration of the osteoblastic cell senescence pathways. To examine whether HFD- or NEFA-induced senescent osteoblastic cells have similar increased PPARγ expression in the future, we generated premature senescent osteoblastic cells in vitro by passaging OB6 and ST2 cells more than 30 times (p30). After multiple passages, these cells were called replicative senescent cells. Cells were passaged each time they became confluent after 5 to 6 days of culture. After 30 passages, both OB6 and ST2 cells displayed a senescent cell phenotype, as determined by SA-β-gal blue staining, compared with that of earlier passage cells (p2) (Figure 5A). Moreover, both OB6 and ST2 p30 cells had a significantly lower potential to proliferate compared with that of p2 cells (Figure 5A). In both OB6 and ST2 p30 cells, we found increased PPARγ, caveolin-1, and p21 expression but decreased Sirt1 expression compared with that of their respective p2 cells (Figure 5, D and E). These senescent p30 ST2 cells showed less alkaline phosphatase and osterix mRNA expression (Figure 5B), and senescent p30 OB6 cells showed a significantly lesser ability to secrete osteocalcin and C1CP, a de novo collagen synthesis marker measured in cell culture medium, than their respective p2 cells (Figure 5C). Finally, when we artificially overexpress PPARγ in ST2 cells (and similarly in OB6 cells; data not shown), these cells quickly showed increased SA-β-gal blue staining and p53 antibody staining (Figure 6A), indicating that these cells underwent senescent programming. This was confirmed using Western blots, which showed increased expression of PPARγ and p53 in both ST2 cells and OB6 cells (Figure 6, B and C). These data implied that the osteoblastic cell senescence pathway is controlled by overexpression of PPARγ.
Figure 5.
Senescent osteoblastic cells display increased PPARγ expression and decreased osteocalcin secretion. A, OB6 cells (right) and ST2 cells (left) were passaged either 2 (p2) or 30 times (p30); SA-β-gal–positive blue-stained senescent OB6 and ST2 cells were found in p30. Below the photographs, the graphs show significantly reduced proliferation of p30 OB6 and ST2 cells compared with that of p2 cells. B, mRNA was isolated from p2 and p30 ST2 cells. Osteoblastic cell differentiation markers alkaline phosphatase (ALP) and osterix (OSX) mRNA expression were determined using real-time PCR. C, Secreted osteocalcin (OC) and C1CP in culture medium were measured using ELISA after both p2 and p30 OB6 cells were cultured. D, Proteins were isolated from both p2 and p30 OB6 and ST2 cells. Western blots for PPARγ, caveolin-1, p21, and Sirt1 are shown. E, The bar graphs represent the ratio of intensity of the band of PPARγ, caveolin-1, p21, and Sirt1 protein expression normalized to β-actin protein for each 2 samples from p2 and p30 cells using Quantity One software (Bio-Rad). *, P < .05, p2 vs p30.
Figure 6.
PPARγ stimulates osteoblastic cell senescence. A, ST2 cells were transfected with a PPARγ overexpression construct or a control vector, and after 48 hours, PPARγ and p53 antibody immunostaining and SA-β-gal staining was performed. Blue cells represent positive senescent cells. PPARγ is shown in red and p53 expression in green. B, OB6 and ST2 cells were transfected with a PPARγ overexpression construct or a control vector, and after 48 hours, Western blots were performed for PPARγ and p53 expression. β-Actin expression was used as a control. C, The bar graphs represent the ratio of intensity of the band of PPARγ and p53 protein expression normalization to β-actin protein for 3 samples each from control and PPARγ overexpressed cells using Quantity One software (Bio-Rad). *, P < .05, control vs PPARγ overexpressed cells.
Discussion
The current study was designed to explore the potential link between impaired bone development in HFD-fed rats and osteoblastic cell senescence. Our data suggest that increased osteoblastic cell senescence pathways may be a central mediator of impairment of bone quality (bone density and strength) in HFD-induced obese rats. We further found that SPI-containing diets blocked the impairment of bone quality during early development, and this occurs with prevention of osteoblastic cell senescence. The current study for the first time provides evidence regarding the activation of cellular senescence pathways in bone that occur in HFD-induced obese animals. We proposed that significantly increased NEFA levels in the circulation may explain the activated senescence pathways in bone observed in HFD-fed rats. The protective effect on bone by SPI incorporated into the HFD appears to be due, at least in part, to elevated serum levels of isoflavones derived from SPI consumption. We showed that in vitro replicative senescent osteoblastic cells had significantly lower differentiation and proliferation activities. Cell senescent signaling pathways in these cells were similar to those observed in bone and osteoblastic cells from animals fed a HFD, indicating that the HFD may activate the common senescent signaling pathway in bone in vivo and osteoblastic cells.
Cellular senescence is a stable form of cell cycle arrest that may limit the proliferative potential of a cell and promotes aging (33). However, our understanding of how cellular senescence contributes to noncancer tissue pathophysiology is extremely limited (29). Accelerated premature cell senescence in young animals may result in an early aging process (34). Furthermore, senescent osteoblasts display a large flattened morphology and accumulated SA-β-gal activity that distinguishes them from most quiescent cells (29, 35) and sex steroid deficiency triggers bone matrix collagen degradation that in turn accelerates resident cells entering the activated senescence pathway (29). Although the stimuli may be different, ie, sex steroid deficiency in our previous study vs HFD feeding in the current report, the signaling pathways presented in osteoblastic cells were similar. In both HFD-induced obesity and sex steroid deficiency, increased bone and osteoblast senescence was associated with bone loss. In the current study, we have shown clear evidence of increased SA-β-gal activity, p53 acetylation, and p21 activation in osteoblastic cells after HFD feeding. These results suggest that increased bone cell senescence may play a central role in bone pathology, or osteoblastic cellular senescence may be a fundamental factor contributing to bone loss associated with, HFD-induced obesity, sex steroid deficiency, and other conditions, such as systemic insulin resistance and chronic alcohol abuse (14, 24, 26). To our knowledge, the current report is the first showing osteoblastic cell senescence in association with HFD-induced obesity. However, using an adipose tissue–specific insulin receptor knockout animal model, Blüher et al (14), demonstrated that a reduction in fat mass was associated with increased longevity, suggesting that an increase in fat mass may lead to decreased longevity, possibly through effects on insulin signaling. Increased longevity reflects either delayed cellular senescence or perhaps decreased cell apoptosis. We believe that our findings were in agreement with results demonstrated in genetically modified animal models. A recent study in Drosophila also indicated that fat cells or dietary nutrients may be key regulators of transitions in stem cell behavior (they either go to a senescent, less proliferative, stage or differentiate into tissue-specific cells [36]), which may have direct relevance to our findings.
Specifically, we presented evidence of a cell senescence signaling cascade in bone and osteoblastic cells in response to HFD feeding and NEFA treatments, respectively. Both treatments activated PPARγ in bone and osteoblastic cells. PPARγ is a well-known nuclear receptor superfamily that regulates the transcription of numerous target genes by binding to PPRE (a specific DNA-binding site) (37). PPARγ may bind to a PPRE in the p16 promoter and thereby regulate p16 expression and the cell senescence pathway (12). PPARγ was also able to bind to both the p21 and the p53 promoter, and the binding was facilitated by NEFA to regulate osteoblastic cell senescence pathways, perhaps because both p21 and p53 promoters also contain a repeat of the hexanucleotide TGGCGACTATCCA sequence for PPARγ (38, 39). Furthermore, we found that HFD-induced obesity or NEFA down-regulated Sirt1 expression. This finding is interesting because Sirt1 (sirtuin 1) is an important determinant of longevity that involves several cell signal transductions to regulate cellular senescence (40). These findings are consistent with a study showing that caloric restriction increases the lifespan of many organisms by increasing Sirt1 expression through repression of PPARγ activity (41). Interestingly, epigenetic regulation of both PPARγ and p53 was reported recently (42), and epigenetically suppressed tumor suppressor genes were suggested to be involved in the potential for cancer cell metabolism. There is no evidence of whether HFD-activated PPARγ and p53 are involved in bone cancer cell metabolism; our data indicated that HFD-induced cellular senescence may function differently on contributions of noncancer tissue pathology vs cancer cell metabolism.
Although the mechanisms for how HFD-induced impairment of bone development and the SPI diet promote bone formation are not well understood, our current study showed that substitution of casein with SPI in the HFD prevented such HFD-induced impairment of bone development. Our data suggest that the preventive effect of the SPI diet on bone was due to inhibition of osteoblastic cell senescence pathways, most likely through increased isoflavone and down-regulation of caveolin-1 expression. We have shown that senescent osteoblasts remarkably reduced their ability to secrete osteocalcin and C1CP, a collagen de novo synthesis marker, and were less responsive to stimuli of growth factors such as insulin and IGF-I (29).
Cellular senescence can be divided into 2 categories: replicative senescence and stress-induced premature senescence. Therefore, senescence not only occurs with aging but can also occur prematurely in response to a variety of stresses. Previous studies have shown that overexpression of caveolin-1 in mouse embryonic fibroblasts is sufficient to block these cells in the G0/G1 phase of the cell cycle through modulation of the p53/p21 pathway (43). This evidence suggests that caveolin-1 is at least involved in mediating one of the categories of cellular senescence. Indeed, we and others have previously shown that caveolin-1 expression is increased in replicative senescent mesenchymal stem cells (22, 44) and bone marrow stromal cells (45). In addition, old human diploid fibroblasts express higher levels of caveolin-1 than younger cells (46, 47), further supporting the important role of caveolin-1 in cellular senescence. We believe that the ability of SPI diet to prevent HFD-induced osteoblast senescence is due to the capability of SPI diet to suppress caveolin-1 expression and thereafter sequester Sirt1 to deacetylate PPARγ and p53. This action of the SPI diet is due at least in part to isoflavones. Because these SPI-associated isoflavones are structurally similar to 17-β-estradiol, a majority of the research has focused on their estrogenic effects on bone, although they may also exert nonestrogenic effects on bone (22, 23).
In conclusion, feeding young, prepubertal rats a diet composed of casein protein that is high in saturated fat and cholesterol produced osteoblastic cell senescence and compromised bone quality. Replacing casein protein in the HFD with SPI improved bone quality and prevented osteoblastic cell senescence, and this was associated with increased serum isoflavones. Isoflavones were shown in vitro to prevent NEFA-induced senescence and, therefore, are candidates for the bioactive components within SPI responsible for preventing HF-induced bone impairment in young rats.
Acknowledgments
This work was supported in part by the US Department of Agriculture/Agricultural Research Service (Grant 6251–51000-003 to the Arkansas Children's Nutrition Center).
This study was conceived and planned by J.R.C., T.M.B., and M.J.J.R.; the experimental work was done by J.R.C., O.P.L., and M.L.B.; the data analysis was performed by J.R.C.; the manuscript was written by J.R.C., T.M.B., M.J.J.R., and M.L.B.; all authors discussed the results and commented on the article; and J.R.C. supervised the entire project.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMC
- bone mineral content
- BMD
- bone mineral density
- Cas
- casein
- ChIP
- chromatin immunoprecipitation
- C1CP
- cross-linked C-telopeptide of type 1 collagen
- Col1
- collagen type 1
- HF
- high fat
- HFD
- high-fat diet
- LF
- low fat
- LF-Cas
- standard low-fat (5%) AIN-93G diet formulated with casein as the sole protein
- HF-Cas
- high-fat/high-cholesterol diet group
- HF-SPI
- high-fat/high-cholesterol diet except the casein was replaced by SPI
- LF-SPI
- a low-fat diet made with the AIN-93G diet formula except casein was replaced with soy protein isolate
- luc
- luciferase
- NEFA
- nonesterified free fatty acid
- PND
- postnatal day
- PPARγ
- peroxisome proliferator-activated receptor γ
- PPRE
- peroxisome proliferator–activated receptor response element
- pQCT
- peripheral quantitative computerized tomography
- SA-β-gal
- senescence-associated β-galactosidase
- SPI
- soy protein isolate.
References
- 1. Davies JH, Evans BA, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao LJ, Jiang H, Papasian CJ, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. [DOI] [PubMed] [Google Scholar]
- 4. Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. [DOI] [PubMed] [Google Scholar]
- 5. Chen JR, Lazarenko OP, Wu X, et al. Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS One. 2010;5:e13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen JR, Zhang J, Lazarenko OP, et al. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J. 2012;26:1131–1141. [DOI] [PubMed] [Google Scholar]
- 7. Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606. [DOI] [PubMed] [Google Scholar]
- 8. Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. [DOI] [PubMed] [Google Scholar]
- 9. He J, Rosen CJ, Adams DJ, Kream BE. Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone. 2006;38:826–835. [DOI] [PubMed] [Google Scholar]
- 10. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. [DOI] [PubMed] [Google Scholar]
- 11. Kahn CR, Chen L, Cohen SE. Unraveling the mechanism of action of thiazolidinediones. J Clin Invest. 2000;106:1305–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan Q, Huang J, Zhou R, et al. PPARγ accelerates cellular senescence by inducing p16INK4α expression in human diploid fibroblasts. J Cell Sci. 2008;121(Pt 13):2235–2245. [DOI] [PubMed] [Google Scholar]
- 13. Jimenez-Gomez Y, Mattison JA, Pearson KJ, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. [DOI] [PubMed] [Google Scholar]
- 15. West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989;184:138–147. [DOI] [PubMed] [Google Scholar]
- 16. Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onetti Y, Jiménez-Altayó F, Heras M, Vila E, Dantas AP. Western-type diet induces senescence, modifies vascular function in non-senescence mice and triggers adaptive mechanisms in senescent ones. Exp Gerontol. 2013;48:1410–1419. [DOI] [PubMed] [Google Scholar]
- 19. Baile CA, Yang JY, Rayalam S, et al. Effect of resveratrol on fat mobilization. Ann NY Acad Sci. 2011;1215:40–47. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012;27:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ronis MJ, Chen Y, Badeaux J, Badger TM. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J Nutr. 2009;139:1431–1438. [DOI] [PubMed] [Google Scholar]
- 22. Badger TM, Ronis MJJ, Wolff G, et al. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med (Maywood). 2008;233:1242–1254. [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Lazarenko OP, Wu X, et al. Differential effects of short term feeding of a soy protein isolate diet and estrogen treatment on bone in the pre-pubertal rat. PLoS One. 2012;7:e35736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Lazarenko OP, Blackburn ML, Badger TM, Ronis MJ, Chen JR. Soy protein isolate down-regulates caveolin-1 expression to suppress osteoblastic cell senescence pathways. FASEB J. 2014;28:3134–3145. [DOI] [PubMed] [Google Scholar]
- 25. Chen JR, Lazarenko OP, Blackburn ML, Badeaux JV, Badger TM, Ronis MJ. Infant formula promotes bone growth in neonatal piglets by enhancing osteoblastogenesis through bone morphogenic protein signaling. J Nutr. 2009;139:1839–1847. [DOI] [PubMed] [Google Scholar]
- 26. Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res. 2009;24:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. [DOI] [PubMed] [Google Scholar]
- 28. Chen JR, Zhang J, Lazarenko OP, et al. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J. 2013;27:3514–3523. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Lazarenko OP, Blackburn ML, Badger TM, Ronis MJ, Chen JR. Blueberry consumption prevents loss of collagen in bone matrix and inhibits senescence pathways in osteoblastic cells. Age (Dordr). 2013;35:807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Lazarenko OP, Blackburn ML, et al. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS One. 2011;6:e24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cimino CO, Shelnutt SR, Ronis MJ, Badger TM. An LC-MS method to determine concentrations of isoflavones and their sulfate and glucuronide conjugates in urine. Clin Chim Acta. 1999;287:69–82. [DOI] [PubMed] [Google Scholar]
- 32. Gu L, Laly M, Chang HC, et al. Isoflavone conjugates are underestimated in tissues using enzymatic hydrolysis. J Agric Food Chem. 2005;53:6858–6863. [DOI] [PubMed] [Google Scholar]
- 33. Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. [DOI] [PubMed] [Google Scholar]
- 36. Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu D, Han C, Wu T. 15-PGDH inhibits hepatocellular carcinoma growth through 15-keto-PGE2/PPARγ-mediated activation of p21WAF1/Cip1. Oncogene. 2014;33:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonofiglio D, Aquila S, Catalano S, et al. Peroxisome proliferator-activated receptor-gamma activates p53 gene promoter binding to the nuclear factor-kappaB sequence in human MCF7 breast cancer cells. Mol Endocrinol. 2006;20:3083–3092. [DOI] [PubMed] [Google Scholar]
- 40. Satoh A, Brace CS, Rensing N, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kawano Y, Nasu K, Hijiya N, et al. CCAAT/enhancer-binding protein α is epigenetically silenced by histone deacetylation in endometriosis and promotes the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2013;98:E1474–82. [DOI] [PubMed] [Google Scholar]
- 43. Galbiati F, Volonté D, Liu J, et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent mechanism. Mol Biol Cell. 2001;12:2229–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park JS, Kim HY, Kim HW, et al. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells. Mech Ageing Dev. 2005;126:551–559. [DOI] [PubMed] [Google Scholar]
- 45. Sun C, Wang N, Huang J, et al. Inhibition of phosphatidylcholine-specific phospholipase C prevents bone marrow stromal cell senescence in vitro. J Cell Biochem. 2009;108:519–528. [DOI] [PubMed] [Google Scholar]
- 46. Park WY, Park JS, Cho KA, et al. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847–20852. [DOI] [PubMed] [Google Scholar]
- 47. Wheaton K, Sampsel K, Boisvert FM, Davy A, Robbins S, Riabowol K. Loss of functional caveolae during senescence of human fibroblasts. J Cell Physiol. 2001;187:226–235. [DOI] [PubMed] [Google Scholar]