Abstract
Tachykinins are comprised of the family of related peptides, substance P (SP), neurokinin A (NKA), and neurokinin B (NKB). NKB has emerged as regulator of kisspeptin release in the arcuate nucleus (ARC), whereas the roles of SP and NKA in reproduction remain unknown. This work explores the roles of SP and NKA in the central regulation of GnRH release. First, central infusion of specific agonists for the receptors of SP (neurokinin receptor 1, NK1R), NKA (NK2R) and NKB (NK3R) each induced gonadotropin release in adult male and ovariectomized, estradiol-replaced female mice, which was absent in Kiss1r−/− mice, indicating a kisspeptin-dependent action. The NK2R agonist, however, decreased LH release in ovariectomized-sham replaced females, as documented for NK3R agonists but in contrast to the NK1R agonist, which further increased LH release. Second, Tac1 (encoding SP and NKA) expression in the ARC and ventromedial nucleus was inhibited by circulating estradiol but did not colocalize with Kiss1 mRNA. Third, about half of isolated ARC Kiss1 neurons expressed Tacr1 (NK1R) and 100% Tacr3 (NK3R); for anteroventral-periventricular Kiss1 neurons and GnRH neurons, approximately one-fourth expressed Tacr1 and one-tenth Tacr3; Tacr2 (NK2R) expression was absent in all cases. Overall, these results identify a potent regulation of gonadotropin release by the SP/NK1R and NKA/NK2R systems in the presence of kisspeptin-Kiss1r signaling, indicating that they may, along with NKB/NK3R, control GnRH release, at least in part through actions on Kiss1 neurons.
Understanding the central and peripheral mechanisms that control kisspeptin release has become a major avenue of research in reproductive endocrinology (1). However, the precise neuroendocrine events that determine the action of Kiss1 neurons and translate their message into congruent GnRH secretion remain largely unknown. Recently, Kiss1 neurons in the arcuate nucleus (ARC) have been described to coexpress neurokinin B (NKB) and dynorphin A, thereafter renamed KNDy neurons (2). A number of studies have since emerged to document a predominantly stimulatory action of NKB on gonadotropin release in multiple mammalian species in a process sensitive to the circulating levels of sex steroids (3–6), consistent with the hypogonadotropic hypogonadism observed in humans and mice with deficient NKB signaling (7–10). Moreover, compelling evidence suggests that NKB exerts this action in a kisspeptin-dependent manner by acting directly on KNDy neurons through autosynaptic loops (11, 12). Nonetheless, although these studies represent an important step forward in the understanding of the mechanisms governing GnRH release, further research is needed to fully decipher the complex hierarchy of neuronal factors that participates in the control of kisspeptin/GnRH release.
Interestingly, NKB, encoded by the Tac2 gene in rodents, belongs to a family of closely related peptides termed tachykinins, which also includes substance P (SP) and neurokinin A (NKA), both encoded by Tac1 (13). However, the action of these additional tachykinins in the control of GnRH and gonadotropin release has not been defined. Over the past 3 decades, numerous studies have associated SP with nociceptive and inflammatory processes in the brain (14), as well as with psychiatric disorders (15), but only a few reports have demonstrated a stimulatory action of SP (and NKA) in the central control of reproductive function in rodents and men (16–20). Importantly, an elegant study by de Croft et al (21) has recently documented the ability of SP and NKA to activate the firing of Kiss1 neurons in the ARC, placing these tachykinins in the spotlight as possible neuromodulators of kisspeptin release. Moreover, they demonstrated cross-reactivity between the receptor for NKB (neurokinin receptor 3, NK3R) and those for SP (neurokinin receptor 1, NK1R) and NKA (neurokinin receptor 2, NK2R), which appears critical for the full action of NKB, in line with previous experiments in rats indicating the involvement of the 3 tachykinin receptors in the compensatory rise of LH after gonadectomy (GDX) (22).
The primary goal of this study was to assess the effects of specific activation of the receptors for SP and NKA in the central control of reproductive function in vivo, as well as to determine the expression and regulation of Tac1 mRNA in the hypothalamus and localization of the tachykinin receptors, through a series of genetic, functional and histological studies in the mouse.
Materials and Methods
Mice
Adult wild-type (WT) male and female C57Bl6 mice were purchased from Charles River Laboratories International, Inc. All experiments were approved by the Harvard Medical Area Standing Committee on Animals in the Harvard Medical School Center for Animal Resources and Comparative Medicine. Mice were maintained in a 12-hour light, 12-hour dark cycle and were fed a standard rodent diet. Free-hand intracerebroventricular (icv) drug administration was performed in these animals as described previously (23).
Kiss1r-deficient (Kiss1r−/−) mice and WT littermates were generated as described previously (11) and bred in the vivarium of the University of Córdoba, Spain. The mice were maintained under constant conditions of light (14 h of light, from 7 am) and temperature (22°C) and were weaned at age postnatal day 21, when they were housed in groups of 5 mice per cage and with free access to standard mouse chow and water ad libitum. For hormonal tests involving icv cannulation, mice were caged individually from the day before cannula implantation until termination of experiments. Correct positioning of the cannulae was checked by visual inspection, in order to exclude animals showing obvious displacement or detachment, and was confirmed at necropsy. Experimental procedures were approved by the University of Córdoba Ethical Committee for animal experimentation and were conducted in accordance with the European Union normative for care and use of experimental animals.
Kiss1-CreGFP (C57BL6/J and S129 background) mice were produced by Steiner and coworkers at the University of Washington (24) and Gnrh1-GFP (CBB6) mice were produced by Dr Suzanne Moenter, currently at the University of Michigan (25), and housed under constant temperature and light in a 12-hour light, 12-hour dark cycle with lights on from 6 am to 6 pm at the Oregon Health and Science University. Transgenic Kiss1-CreGFP mice were maintained as heterozygous by breeding with WT C57BL6/J mice. Gnrh1-GFP mice were maintained as homozygous and used as such. Food and water were provided ad libitum. All animal treatments at Oregon Health and Science University are in accordance with institutional guidelines based on National Institute of Health standards and were performed with institutional Animal Care and Use Committee approval.
Drugs
The NK1R agonist (GR73632), NK2R agonist (GR64349), and NK3R agonist (senktide) were purchased from Tocris, and 17β-estradiol (E2) was purchased from Sigma Chemical. The doses of GR73632, GR64349, and senktide, 600 pmol in 5 μL of physiological saline, 0.9% NaCl, were selected on the basis of previous references as maximally effective for senktide in inducing gonadotropin responses in the rat (26). Doses of the NK1R and NK2R agonists were selected to span from low (600 pmol) to high (3 nmol) in order to identify secretory responses on gonadotropin release. For experiments involving adult intact WT female mice, adult virgin female mice were monitored daily by vaginal cytology to confirm the occurrence of regular estrous cycles; only mice with at least 2 consecutive regular estrous cycles were subsequently used for hormonal and molecular analyses. In addition, for experiments involving ovariectomy (OVX) and steroid replacement, groups of adult female mice were subjected to bilateral GDX via abdominal incision under light isofluorane anesthesia 1 week before hormonal tests or tissue sampling. Immediately after GDX, capsules filled with E2 or vehicle (sesame seed oil) were implanted sc via a small midscapular incision at the base of the neck; wound clips were used to close the incision. Silastic tubing (15 mm long, 0.078 in inner diameter, 0.125 in outer diameter; Dow Corning) was used for capsule preparation. Dilutions of crystalline E2 at a low dose (20 μg/mL, in sesame oil) were used to fill capsules; this dose was selected to achieve moderate levels of circulating E2, as previously described (4, 27, 28).
Experimental design
LH and FSH responses to the selective agonists of NK1R, NK2R, and NK3R in adult male mice (experiment 1)
NKB and the specific agonists were administered centrally through icv injections into the lateral cerebral ventricle (600 pmol/5 μL); the site of the injection was 1 mm posterior and 1.2 mm lateral to bregma. Blood samples were collected 25 minutes after senktide injection. Animals injected with vehicle (physiological saline, 0.9% NaCl) served as controls (n = 5–8/group).
LH responses to NK1R, NK2R, and NK3R agonists in adult OVX+sham/E2-treated female mice (experiment 2)
Adult (8 wk) female mice were bilaterally OVX and sham or E2 replaced for 1 week (n = 5/group). Central icv administration of the specific agonists was performed as described above. A group treated with senktide (600 pmol/5 μL) was included as a positive control based on the previously reported sex-steroid-dependent effect of senktide on gonadotropin release. NK1R and NK2R agonists where injected at 2 doses: 600 pmol/5 μL (based on the maximally ED of senktide) and 3 nmol/5 μL, to determine the ability of the system to respond to a higher concentration. Blood samples were collected 25 minutes after injection. Animals injected with vehicle (physiological saline, 0.9% NaCl) served as controls.
LH responses to NK1R, NK2R, and NK3R agonists in adult male and female kisspeptin receptor (Kiss1r) null mice (experiment 3)
Each agonist was injected icv (600 pmol/5 μL) as described above. Blood samples were collected 30 minutes after injection. Animals injected with vehicle (physiological saline, 0.9% NaCl) served as controls (n = 8–10/group).
Mapping of Tac1 expression and regulation by E2 in the brain of female mice (experiments 4 and 5)
In experiments 4 and 5, we aimed to map the expression of Tac1 in the brain of female mice and compare the effects of E2 on the expression of Tac1 in the positive hypothalamic nuclei identified in experiment 4, by comparing expression in OVX female mice, replaced with E2 or vehicle (n = 5/group). One week after surgery, animals were decapitated in the morning (10 am), and trunk blood and brains were collected, frozen on dry ice, and stored at −80°C for in situ hybridization (ISH). Plasma levels of LH were measured to determine the efficacy of the hormone replacement.
Colocalization of Tac1 and Kiss1 mRNA in the ARC and the anteroventral periventricular and periventricular nuclei (AVPV/PeN) of female mice (experiment 6)
To determine the presence of coexpression between Tac1 and Kiss1 mRNA in key areas (anterior hypothalamus, AVPV/PeN; and posterior hypothalamus, ARC) double-labeled ISH was performed in additional tissue samples collected in experiment 4. Of note, OVX+sham and OVX+E2 animals were used to determine Kiss1/Tac1 coexpression in the ARC and AVPV/PeN, respectively, in order to maximize the expression of Kiss1 mRNA in each nucleus.
Tacr1, Tacr2, and Tacr3 mRNA expression in Kiss1 and GnRH neurons (experiment 7)
Single cell transcriptomes of Kiss1 and GnRH neurons were isolated from female Kiss1-CreGFP and Gnrh1-GFP mice between 3 and 10 months of age. The Kiss1-CreGFP mice were OVX bilaterally. The mice used to collect Kiss1 arcuate neurons were killed on days 6–7 after OVX. Mice used to collect Kiss1 AVPV/PeN neurons were treated with 2 doses of estrogen benzoate (1 μg on d 5 and 2 μg on d 6) before experimentation on day 7 after OVX. Intact diestrous Gnrh1-GFP mice were used to collect GnRH neurons. See below for single neuronal harvesting and PCR details.
Tissue preparation
In selected experiments (see experiments 4–6), brains were removed for ISH, frozen on dry ice, and then stored at −80°C until sectioned. Five sets of 20-μm sections in the coronal plane were cut on a cryostat, from the diagonal band of Broca to the mammillary bodies, thaw mounted onto SuperFrost Plus slides (VWR Scientific), and stored at −80°C. A single set was used for each ISH experiment (adjacent sections 100 μm apart).
Hormone measurements
Serum LH and FSH levels in experiments 1, 2, and 4 were measured using a Milliplex MAP immunoassay (Mouse Pituitary panel; Millipore) in the Luminex 200 (29, 30). The minimum detectable concentration (pg/mL) for LH was 1.9 and for FSH was 9.5. The intraassay coefficient of variation was less than 15%. LH levels in experiment 3 were measured in 50-μL samples using a double-antibody method and RIA kits supplied by the National Institutes of Health (Dr A.F. Parlow, National Hormone and Peptide Program). Rat LH-I-10 was labeled with 125I with the use of Iodo-gen tubes, following the manufacturer's instructions (Pierce). Hormone concentrations were measured compared with the reference preparation LH-RP-3 as a standard. Intra- and interassay coefficients of variation were less than 8% and 10%, respectively.
Detection of Tac1 mRNA
Total RNA was extracted from mouse brain using an RNAqueous kit (Ambion). RNA was reverse transcribed into cDNA for subsequent PCR. Primers were designed based on the published sequence of the mouse Tac1 gene (GenBank accession number NM_009311.2) with forward primers starting at 101 bp and reverse primers starting at 453 bp. Primers were custom synthesized (Invitrogen). PCRs contained the following in a volume of 25 μL: 2 μL of reverse transcriptase reaction product, 0.2 μm of each primer, 12.5 μL of RediTaq polymerase (Sigma-Aldrich), and 8.5 μL of water. Clamp polymerase sequences for T7 or T3 polymerase were added for the final primer product sequence and transcribed for ISH.
Detection of Kiss1 mRNA
The Kiss1 probe used for detection of Kiss1 mRNA was described previously (23). The Kiss1-specific sequence of the probe spans bases 76–486 of the mouse cDNA sequence (GenBank accession number AF472576). The procedure for ISH is outlined below.
Single-label ISH of Tac1 mRNA
Tac1 mRNA sense and antisense probes were transcribed with T7 or T3 polymerase (Fermentas), as described previously (31). Briefly, radiolabeled probes were synthesized in vitro by inclusion of the next ingredients in a volume of 20 μL: 250 Ci [33P] uridine triphosphate (PerkinElmer Life and Analytical Sciences), 1 μg of PCR product, 0.5mM each ATP, CTP, and GTP, and 40 U of polymerase. Residual DNA was digested with 4 U of deoxyribonuclease (Ambion), and the deoxyribonuclease reaction was terminated by addition of 2 μL of 0.5M EDTA (pH 8.0). The riboprobes were separated from unincorporated nucleotides with ProbeQuant G-50 Micro Columns (GE Healthcare). Slides with mice hypothalamic sections from the different experimental groups were processed as reported previously (4).
Double-label ISH of Kiss1/Tac1 mRNA
Antisense mouse Kiss1 probe was transcribed from linearized pAMP1 plasmid as described previously (23). The cDNA template for the Tac1 riboprobe was generated by PCR with primers that were designed to contain promoters for T7 RNA polymerase in the antisense direction and T3 RNA polymerase in the sense direction. Radiolabeled riboprobe for Tac1 was synthesized as described above. Digoxigenin-labeled Kiss1 antisense riboprobe was synthesized with T7 RNA polymerase and digoxigenin labeling mix (Roche) according to the instructions of the manufacturer. Slides were processed for double-labeled ISH as described previously (32). Slides were stored at 4°C and developed 7 days later.
Quantification and analysis of Tac1 mRNA
Brain sections were analyzed unilaterally. Slides from all of the animals were read under dark-field illumination and analyzed using ImageJ software to count the total number of cells. Data are presented depicting the number of cells within the coronal sections containing the hypothalamic nucleus studied for each set, not the total mRNA in this specific nucleus. The starting and ending point of quantification was determined according to the atlas of Paxinos and Franklin (33).
Quantification and analysis of Kiss1 and Tac1 mRNAs double labeling
The brain sections were analyzed unilaterally. Kiss1 mRNA-containing cells were visualized under fluorescent illumination, and ImageJ software was used to count the number of silver clusters over each Kiss1 cell. The starting and ending point of quantification was determined according to Paxinos and Franklin (33).
Tacr1, Tacr2, and Tacr3 expression in Kiss1 and GnRH neurons
Individual Kiss1 and GnRH neurons were harvested from dispersed hypothalamic slice preparations as previously described (34). Briefly, the microdissected slice was incubated in protease and then washed in low Ca2+ artificial cerebrospinal fluid (aCSF) and then in aCSF. The digested slice was triturated, and the effluent containing the dispersed cells was plated on a glass bottom dish. Under a constant flow of oxygenated aCSF, individual neurons were identified, patched, and harvested into a standard glass pipette with gentle suction using the XenoWorks microinjector system (Sutter Instruments). The contents of the pipette were expelled into a siliconized 0.5-mL tube containing a solution of 1× Invitrogen Superscript III buffer, 15 U of ribonuclease inhibitor (Promega), 10mM of dithiothreitol, and diethylpyrocarbonate-treated water in a total of 5 μL and immediately frozen on dry ice. Each harvested cell and controls (aCSF near the cells, tissue RNA and cells with and without reverse transcriptase) were reverse transcribed as previously described (34), and the cDNA was stored at −20°C.
Primers were designed to span at least one intron-exon boundary using the Clone Manager software program (Scientific and Educational software). Stringent PCR conditions were tested to determine the optimal primer concentration, magnesium concentration and annealing temperature to produce a single clear band. The primer sequences are as follows: mouse Tacr1 (accession number NM_009313, 148-bp product) forward primer 1720–1738 nt, reverse primer 1849–1867 nt; mouse Tacr2 (accession number NM_009314, 152-bp product) forward primer 1197–1217 nt, reverse primer 1330–1348 nt; mouse Tacr3 (accession number NM_021382, 175-bp product) forward primer 906–925 nt, reverse primer 1059–1080 nt. PCR was performed on 3 μL of cDNA in a 30-μL final volume containing 1× Go Taq Flexi buffer (Promega), MgCl2 (2.5mM Tacr1, 1.5mM Tacr2, and 2.0mM Tacr3), 0.33mM deoxynucleoside triphosphate, 0.33μM forward and reverse primers, 2-U Go Taq and 0.22-μg TaqStart antibody (Clontech) for 50 cycles of amplification with specific annealing temperatures (Tacr1, 57°C; Tacr2, 61°C; and Tacr3, 63.5°C). PCR products were visualized with ethidium bromide on a 2% agarose gel.
Single cell PCR data analysis
For determination of neuronal expression of a particular transcript, 12–28 cells/animal were harvested from 3–5 animals, with totals of 36–126 cells. The number of arcuate or AVPV/PeN Kiss1 neurons or preoptic area (POA) GnRH neurons expressing each transcript was counted for each animal and the mean number of neurons/animal was determined and used for further analysis of mean, SEM, and percentage expression.
Statistical analysis
All data are expressed as the mean ± SEM for each group. One- or two-way ANOVA followed by Bonferroni's post hoc test were used to assess variation among experimental groups. Significance level was set at P < .05. All analyses were performed with GraphPad Prism Software, Inc.
Results
LH and FSH responses to selective agonists of NK1R, NK2R, and NK3R in adult male mice
The ability of NKB and selective agonists for NK1R (GR73762), NK2R (GR64349), and NK3R (senktide) to acutely modify LH and FSH secretion in adult (8 wk) intact male mice (n = 5–8 mice/group) was explored. These compounds (600 pmol) were injected centrally in adult male mice. Significant increases in LH and FSH were detected 25 minutes after icv injection of NKB and of each of the selective agonists (P ≤ .05) (Figure 1). Of note, although all 4 compounds exerted similar stimulatory effects on FSH release and all increased LH release, the selective agonists showed a trend towards inducing greater LH release than NKB, which reached significance in the NK2R agonist-treated group.
Figure 1.
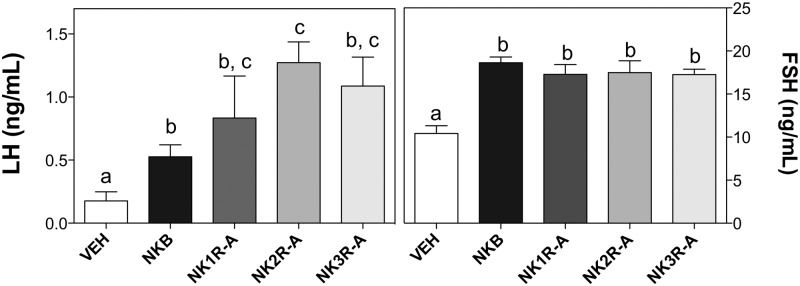
Serum LH (left panel) and FSH (right panel) values of adult male mice 25 minutes after central injection of 600 pmol NKB, GR73632 (NK1R-A), GR64349 (NK2R-A), and senktide (NK3R-A). Statistical analysis was performed using one-way ANOVA with Newman-Keuls post hoc test. Different letters indicate significant differences between groups (P < .05).
LH responses to NK1R, NK2R, and NK3R selective agonists in adult OVX+sham/E2 replaced female mice
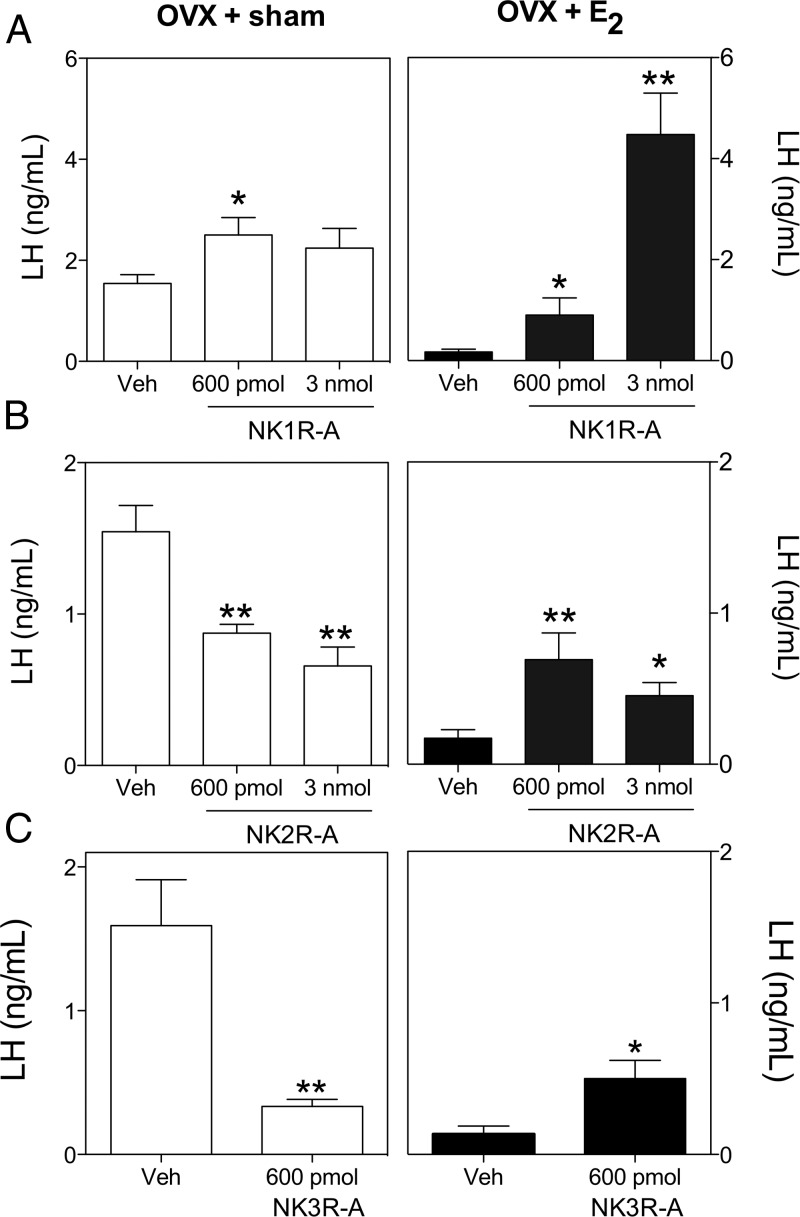
Experiment 1 showed significant stimulatory actions of all 3 tachykinin receptor agonists on gonadotropin secretion in the male mouse. In the female, previous studies have demonstrated either stimulatory or inhibitory responses to senktide, depending on the sex steroid milieu (3–5). Therefore, we hypothesized that the gonadotropic responses to NK1R and NK2R activation would similarly be subjected to regulation by circulating sex steroids. The aims of this experiment were to 1) determine whether females also respond to NK1R and NK2R stimulation with changes in gonadotropin secretion and, if so, 2) determine whether the effect on gonadotropin secretion is E2 dependent. Interestingly, the NK1R agonist did not reduce LH release, and 600 pmol NK1R agonist was even able to further increase the release of LH in OVX+sham-treated mice. In OVX+E2-treated animals, the NK1R agonist induced a robust stimulation of LH release, by approximately 20-fold in the group treated with 3 nmol NK1R agonist, compared with vehicle-treated controls (Figure 2A). The NK2R agonist, however, displayed a senktide-like action in terms of LH release, showing inhibition of LH release in OVX+sham-treated animals but clear stimulation in OVX+E2-treated animals (Figure 2B). In both cases for the NK2R agonist, 600 pmol and 3 nmol had similar effects, resembling previous reports for senktide (26). Indeed, this previously documented action of senktide (4) was replicated in an additional group of animals, which significantly reduced plasma LH levels in sham-treated OVX mice but had a clear stimulatory effect in E2-treated OVX mice after central senktide administration (Figure 2C).
Figure 2.
Serum LH levels of adult OVX+sham and OVX+E2 female mice 25 minutes after central injection of 600 pmol or 3 nmol of GR73632 (NK1R-A) and GR64349 (NK2R-A) or 600 pmol of senktide (NK3R-A). One-way ANOVA + Newman-Keuls post hoc test or t test (NK3R-A). *, P < .05; **, P < .001 compared with vehicle-treated controls.
LH responses to NK1R, NK2R, and NK3R agonists in adult male and female kisspeptin receptor (Kiss1r) knockout mice
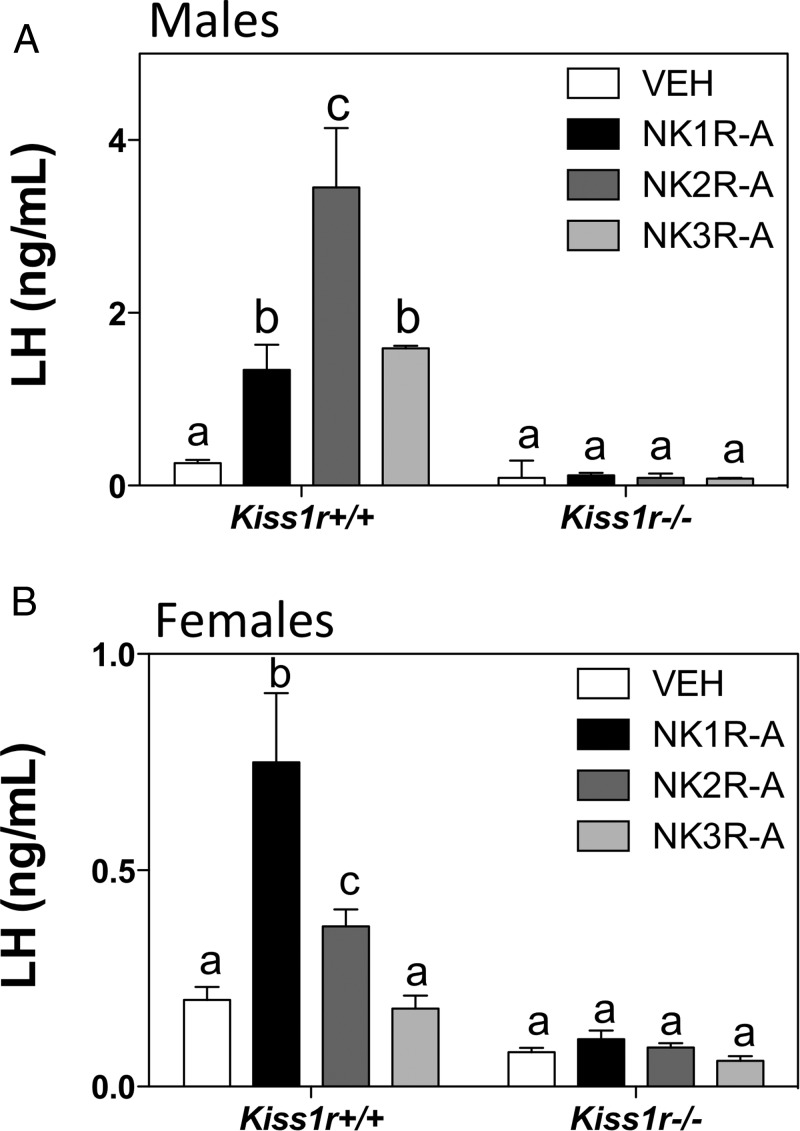
Previous studies have demonstrated a kisspeptin-dependent action of NKB/senktide to induce gonadotropin release (11, 12). In order to test whether stimulation of gonadotropin release by NK1R and NK2R agonists is kisspeptin-dependent, adult intact WT (Kiss1r+/+) males and females (in diestrus) and Kiss1r−/− littermates were studied. Male and female Kiss1r null mice were GnRH primed before the experiments, as described previously (11). The effect of central icv administration of selective tachykinin receptor agonists (600 pmol) was performed in parallel with adult age-matched WT males and females. Control females were monitored for regular estrous cycles and selected for the experiment on the morning of diestrus. Unlike WT mice, both male and female Kiss1r−/− mice showed complete absence of a stimulatory effect of any of the tachykinin receptor agonists on LH secretion (Figure 3, A and B). Interestingly, the ability to induce LH release after central administration of the agonists was greatest for the NK2R agonist (Figure 3A) in the males but greatest for the NK1R agonist in the females (Figure 3B).
Figure 3.
Serum LH levels in WT (Kiss1r+/+) and Kiss1r−/− adult (A) male and (B) diestrous female mice 20 minutes after central injection of 600 pmol GR73632 (NK1R-A), GR64349 (NK2R-A), or senktide (NK3R-A). Two-way ANOVA + Bonferroni's post hoc test. Different letters indicate significant differences between groups (P < .05).
Mapping of Tac1 expression and regulation by E2 in the brain of female mice
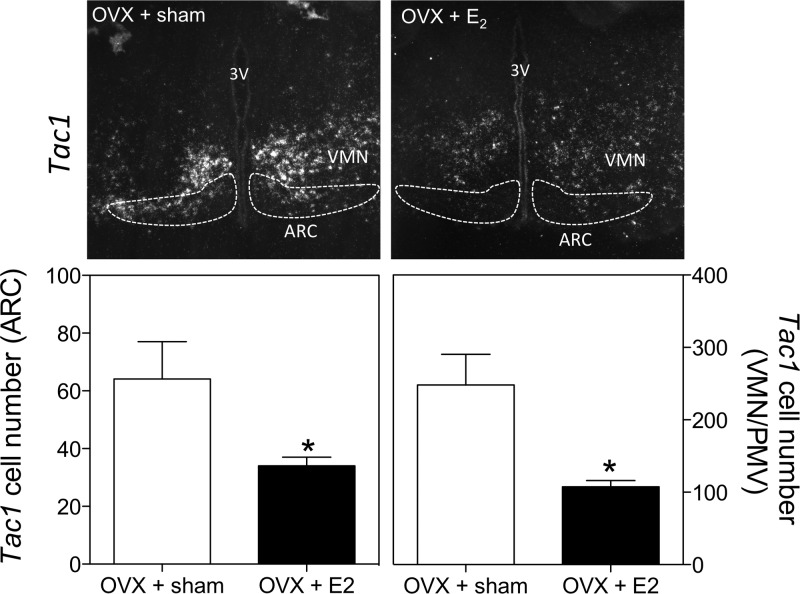
Tac1 mRNA (encoding SP and NKA) has been identified previously in the hypothalamus of several species, including rodents and humans. However, a detailed description of the distribution of Tac1 mRNA in the mouse is lacking (33). Here, we aimed to map the expression of Tac1 in the brain of female mice by ISH in OVX+sham-treated and OVX+E2 mice. Tac1 mRNA was expressed in the cerebral cortex, caudate putamen, horizontal limb of the diagonal band, olfactory tubercule, paraventricular hypothalamic nucleus (posterior part), ARC, ventromedial nucleus, central amygdaloid nucleus, medial forebrain bundle, parasubthalamic nucleus, basomedial amygdaloid nucleus, ventral part of the premammillary nucleus (PMV), retromammillary decussation, and ventral tegmental area (Figure 4). Within the hypothalamus, expression was found to be concentrated mainly in 2 regions: the ARC and the ventromedial nucleus (VMN). All known cotransmitters present in ARC Kiss1 neurons (Kiss1, NKB, and dynorphin) are inhibited by sex steroids as part of their hypothesized role in the negative feedback of sex steroids upon GnRH release. To test whether E2 regulates Tac1 expression in these nuclei, we compared OVX animals treated with sc implanted empty (sham) capsules or E2-filled capsules (OVX+sham treated vs OVX+E2 treated) and found that the number of Tac1-expressing neurons in the ARC and VMN (and the PMV) were significantly reduced by E2 treatment in OVX mice (P < .05) (Figure 5). No apparent differences in Tac1 expression in response to E2 treatment were found in the rest of the nuclei described above (Supplemental Figure 1).
Figure 4.
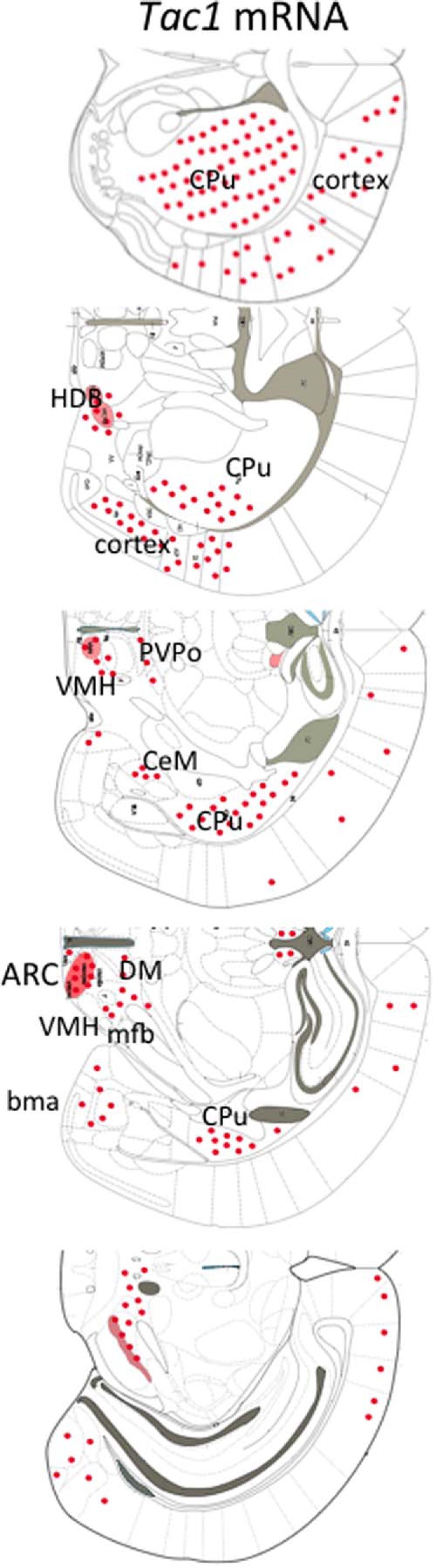
Schematic representation of the neuroanatomical distribution of Tac1 mRNA in adult OVX female mice as assessed by ISH. Red dots indicate areas where Tac1 mRNA neurons are detected. Red shading depicts a higher concentration of Tac1 mRNA. bma, basomedial amygdaloid nucleus, anterior part; CPu, caudate putamen; DM, dorsomedial nucleus; CeM, central amygdaloid nucleus; HDB, nucleus of the horizontal limb of the diagonal band; PVPo, paraventricular thalamic nucleus, posterior part; VMH, ventromedial nucleus.
Figure 5.
Representative microphotographs of Tac1 expression in the hypothalamus of OVX and OVX+E2 adult female mice (upper left and right panels, respectively) and number of Tac1-positive cells in the ARC (lower left panel) and VMN/PMV (lower right panel) of adult OVX and OVX+E2 female mice. *, P < .05 compared with OVX+sham, by t test. 3V, third ventricle.
Colocalization of Tac1 and Kiss1 mRNA in the ARC and the AVPV/PeN of female mice
Tac2 is known to be coexpressed with Kiss1 in the ARC of the mouse (31). Based on the kisspeptin dependence of gonadotropic stimulation by NK1R and NK2R agonists, we hypothesized that Tac1 might be similarly coexpressed with Kiss1. Coexpression of Tac1 and Kiss1 was assessed in ARC and AVPV/PeN of adult female mice. In order to maximize Kiss1 and Tac1 mRNA expression in the ARC and the AVPV, OVX+sham-treated, and OVX+E2-treated animals were used, respectively (27, 28, 35). Interestingly, there was no detectable Tac1 expression in the AVPV/PeN. Furthermore, in the ARC, the Tac1-positive neurons detected were near, but did not colocalize with, Kiss1-positive neurons in OVX mice (Figure 6).
Figure 6.
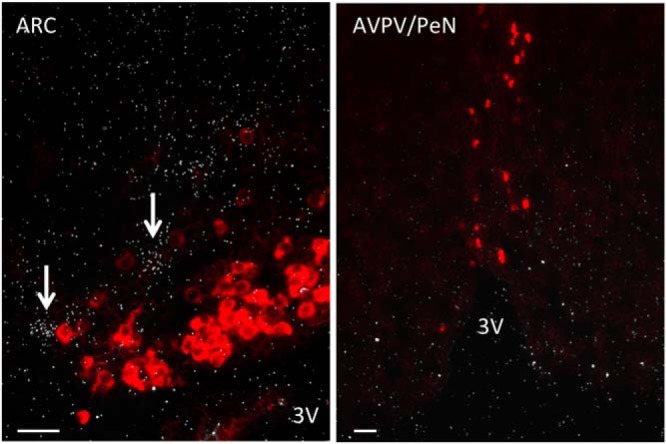
Representative microphotograph of a double label ISH depicting absence of colocalization between Kiss1-expressing neurons (red cells) and Tac1-expressing neurons (silver grains, indicated by white arrows) in the ARC (left panel) and AVPV/PeN (right panel) areas of adult OVX and OVX+E2 mice, respectively. 3V, third ventricle.
Tacr1, Tacr2, and Tacr3 mRNA expression in Kiss1 and GnRH neurons
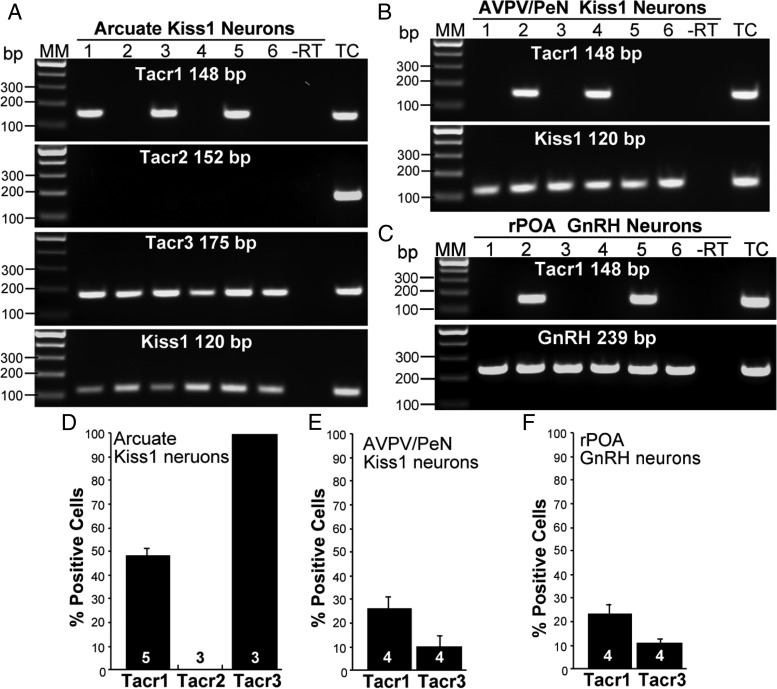
Single cell RT-PCR analysis of the expression of all 3 tachykinin receptors (Tacr1, Tacr2, and Tacr3 mRNA) in Kiss1 (ARC and AVPV/PeN) and GnRH neurons showed that almost half (48.9 ± 3.0%, n = 5) of Kiss1 neurons in the ARC (126 neurons assessed from 5 animals) and over one-fourth (26.8 ± 5.5%, n = 4) of Kiss1 neurons in the AVPV/PeN (90 neurons assessed from 4 animals) express Tacr1 mRNA, which is also present in a subset of GnRH neurons (22.9 ± 5.1%, n = 4) (100 neurons assessed from 4 animals) (Figure 7). Tacr2, however, was absent from both populations of Kiss1 neurons and GnRH neurons, but present in basal hypothalamic RNA, used as a positive control. Finally, Tacr3 was confirmed to be present in all (100%) ARC Kiss1 neurons (36 neurons assessed from 3 animals) (Figure 7) but minimally present (10.0 ± 4.9%, n = 4) in AVPV/PeN Kiss1 neurons (81 neurons assessed from 4 animals), as previously described (31). Of note, Tacr3 mRNA was also detected in a small subset of GnRH neurons (11.0 ± 1.1%, n = 4) (100 neurons assessed from 4 animals).
Figure 7.
Representative gels illustrating the expression of Tacr1, Tacr2, Tacr3, and Kiss1 in dispersed and harvested Kiss1-GFP neurons in the ARC (A) and AVPV/PeN (B) nuclei and Gnrh1-GFP neurons in the rostral POA (rPOA) (C). Expected sizes for the PCR products are 148 bp for Tacr1, 152 bp for Tacr2, 175 bp for Tacr3, and 120 bp for Kiss1. Summary bar graphs of the mean ± SEM percentage expression of Tacr1, Tacr2, and Tacr3 of ARC Kiss1 neurons (D), AVPV/PeN Kiss1 neurons (E), and GnRH neurons (F) expressing each of the transcripts per animal (n = 3–5 animals; 12–28 neurons/animal). MM, molecular markers. TC, tissue control for ARC Kiss1 neurons was ARC RNA for Tacr1, Tacr3 and Kiss1, and basal hypothalamic RNA for Tacr2. TC for AVPV and GnRH neurons was POA RNA. Note: Tacr2 expression in AVPV/PeN Kiss1 and GnRH neurons was also not detected.
Discussion
The identification of a growing number of regulators of kisspeptin release is adding to the complexity of the central mechanisms governing reproduction, increasing the need for further investigation. Mounting studies are expanding on the action of the kisspeptin cotransmitter, the tachykinin NKB, on the control of GnRH release (36). However, the tachykinin family includes 2 additional neuropeptides, SP and NKA (13), whose potential actions in the control of reproduction have not been thoroughly explored. Early studies documented a robust stimulatory action of LH release by SP in rats, rabbits and humans (16–20, 37) and recent electrophysiological studies have described potent stimulatory actions of SP and NKA on ARC Kiss1 neurons in the mouse (21). Of note, this study showed that, in vitro, the action of NKB requires not only the presence of its putative receptor, NK3R, but also the receptors for SP and NKA (NK1R and NK2R, respectively), in line with previous studies in vivo indicating that blockade of all 3 tachykinin receptors (but not each one of them individually) suppressed the compensatory rise of LH after GDX in rats (22). These data extended the previously described cross-reactivity between tachykinins and their receptors (38–40). In these studies, the affinities or EC50 values of each tachykinin for NK1R, NK2R, and NK3R, respectively, were reported as follows: SP = 2nM, 2200nM, and 18000nM; NKA = 16nM, 3nM, and 1300nM; and NKB = 70nM, 25nM, and 4nM (41). These data suggest a likely interaction of NKA with NK1R as well as NK2R, and of NKB with all 3 receptors, at relatively low concentrations. Therefore, in this study, we used specific agonists to characterize specifically the effects of activation of the putative receptors of SP (NK1R-A: GR73632; EC50 NK1R = 4nM; NK2R = 960nM; and NK3R > 1000nM), NKA (NK2R-A: GR64349; EC50 NK1R > 1000nM, NK2R = 3.7nM, and NK3R > 1000nM), and NKB (NK3R-A: senktide; EC50 NK1R > 10 000nM, NK2R > 10 000nM, and NK3R = 18nM) (41, 42).
Central administration of NK1R and NK2R agonists to intact adult male mice induced clear stimulatory gonadotropin responses, similar in magnitude to the responses evoked by senktide. In the female, however, previous studies described a dual inhibitory and stimulatory action of senktide on LH release depending on the absence or presence of physiological levels of sex steroids, respectively (3–5). Intriguingly, we show that central activation of NK2R recapitulates this dual effect, whereas the activation of NK1R induces LH release in OVX animals and an even greater stimulation at higher doses in OVX+E2-treated mice, reminiscent of kisspeptin's action (43). Of note, at low physiological levels of E2, such as during diestrus, the induction of LH release by NK1R agonists is similar to that evoked in intact males (Figure 3, A and B). The present data indicate that NK2R and NK3R may converge on a common pathway to regulate GnRH release in a sex steroid dependent manner, consistent with previous reports in the rat indicating inhibition of LH release after NK2R stimulation (18, 20). In this vein, the present data showed lack of senktide-induced LH release in diestrous females, supporting the contention of highly sensitive responsiveness of NK2R and NK3R activation to the circulating levels of E2. In contrast, NK1R appears to act through different regulatory mechanisms. It is possible that the additional stimulatory action of NK1R-A on LH release comes from the action of SP on both populations of Kiss1 neurons, because we have observed that a fraction of AVPV/PeN Kiss1 neurons expresses Tacr1 but not Tacr2 and virtually no Tacr3 mRNA. Moreover, recent studies indicated that the inhibitory action of NKB on LH release is opioid mediated (3), similar to what was previously suggested for the inhibitory action of NKA on LH in the rat (20), further suggesting common regulatory pathways for NKA and NKB in the control of gonadotropin release.
A critical aspect for the action of NKB on gonadotropin secretion is its dependence on kisspeptin release (11, 12). The activation of Kiss1 neurons by SP and NKA (21) suggests that all 3 tachykinins may require Kiss1 neurons as mediators for their reproductive role. However, the study by de Croft et al (21) did not address whether SP and NKA may also act on GnRH neurons or, perhaps, through alternative kisspeptin-independent mechanisms. These possibilities are addressed in our studies using Kiss1r null mice. These mice showed a conspicuous absence of LH responses to any of the tachykinin receptor agonists, which therefore limits their effect to kisspeptin/kiss1r-dependent mechanisms, either on or upstream Kiss1 neurons or, potentially, on GnRH neurons in the presence of kisspeptin-Kiss1r signaling, because we have observed a subset of GnRH neurons that express SP and NKB receptors. Intriguingly, control animals included in this study showed a clear sexual dimorphism in terms of the effects of NK1R and NK2R activation, with NK1R agonists being more potent than NK2R agonists to stimulate LH release in females (supporting the potential additional action of SP on AVPV/PeN Kiss1 neurons), and vice versa in males. The action of NKA, however, remains mysterious, because Tacr2 has been identified in neither Kiss1 nor GnRH neurons, while showing a kisspeptin-dependent action, thus suggesting the presence of unidentified intermediate neurons upstream of Kiss1 neurons. Of note, our present data are in keeping with the overall expression of tachykinin receptors in the ARC of mice, where Tacr1 expression is approximately half that of Tacr3 and Tacr2 is almost undetectable (44).
Based on the above documented action of SP and NKA receptors on gonadotropin release, deciphering the specific role(s) of these neurotransmitters in the control of reproduction is crucial to understand reproductive physiology. Thus, sex steroids play a key role in the control of GnRH release, acting on Kiss1 neurons, and may even shift the biological action of specific ligands, as we observed for NK2R (present data) and NK3R agonists (3–5). Of note, the mRNA expression of all neuropeptides coexpressed in Kiss1 neurons (kisspeptin, NKB, and dynorphin) is significantly inhibited by sex steroids, suggesting their involvement in the negative feedback of sex steroids upon GnRH release (31). Given the similarities in action between NKB, SP, and NKA on gonadotropin release, we hypothesized that Tac1 (encoding SP and NKA) would be also expressed in the ARC of the mouse and likely inhibited by estradiol. Indeed, we observed a wide distribution of Tac1 mRNA throughout the brain, which was particularly intense in the VMN/PMV and, to a lesser extent, in the ARC, in keeping with previous reports of SP immunoreactivity in rats, monkeys, and humans (45–50). Both populations were sensitive to the inhibitory action of circulating E2. This suggests that SP and NKA may participate in the negative feedback actions of sex steroids on GnRH and is consistent with previous studies of increased SP mRNA in the hypothalamus of postmenopausal women (46) and variations in the content of SP in the ARC of rats along the estrous cycle (50). However, previous studies in OVX rats (45) did not show the increase in gene expression observed in humans (46) and mice (present data), possibly depicting species differences. These previous studies in rats documented lack of colocalization between SP and NKB in the ARC (45). Our studies have confirmed that Tac1 and Kiss1 are not colocalized in either the ARC or the AVPV/PeN. Nonetheless, the population of Tac1 neurons in the ARC appeared to be in close contact with Kiss1 neurons (at least in OVX females), possibly facilitating the interaction between both neurons to regulate kisspeptin release. Admittedly, human studies have indicated that a subset of Kiss1 neurons coexpress SP, which supports potential differences in the tachykinin systems across species (51).
In summary, this work presents a series of experiments that expand on the roles of tachykinins in the central control of GnRH release through SP and NKA, which activate Kiss1 (and possibly GnRH) neurons from an additional population of neurons (Tac1 neurons) located in the ARC and/or the VMN. These findings are in agreement with a number of studies that revealed a dense plexus of SP fibers in the median eminence (47, 52–54), with a predominant origin in the VMN (49), thus supporting a likely action of, at least, SP from this area in the mediobasal hypothalamus.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research grants from the National Institute of Health and by National Institutes of Health (NIH) Grant R01 HD019938 (to U.B.K.); NIH Grant K99 HD071970, Charles H. Hood Foundation for Child Health Research Program and the Microgrant Program from The Biomedical Research Institute and the Center for Faculty Development and Diversity's Office for Research Careers at the Brigham and Women's Hospital (V.M.N.); by The Scientific and Technological Research Council of Turkey Grant 2219 (to S.S.); and by NIH Grants R01 NS043330 and R01 DK068098 (to O.K.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- ARC
- arcuate nucleus
- AVPV/PeN
- anteroventral periventricular and periventricular nuclei
- E2
- 17β-estradiol
- GDX
- gonadectomy
- icv
- intracerebroventricular
- ISH
- in situ hybridization
- KNDy
- presence of kiss1, NKB and dynorphin in the same neuron
- NKA
- neurokinin A
- NKB
- neurokinin B
- NK1R
- neurokinin receptor 1
- NK2R
- neurokinin receptor 2
- NK3R
- neurokinin receptor 3
- OVX
- ovariectomy
- PMV
- premammillary nucleus
- POA
- preoptic area
- SP
- substance P
- VMN
- ventromedial nucleus
- WT
- wild type.
References
- 1. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1316. [DOI] [PubMed] [Google Scholar]
- 2. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. [DOI] [PubMed] [Google Scholar]
- 4. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026(2):307–312. [DOI] [PubMed] [Google Scholar]
- 6. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295. [DOI] [PubMed] [Google Scholar]
- 11. García-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. [DOI] [PubMed] [Google Scholar]
- 12. Grachev P, Li XF, Lin YS, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7(9):e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32(9):1972–1978. [DOI] [PubMed] [Google Scholar]
- 14. De Felipe C, Herrero JF, O'Brien JA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. [DOI] [PubMed] [Google Scholar]
- 15. Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–272. [DOI] [PubMed] [Google Scholar]
- 16. Arisawa M, De Palatis L, Ho R, et al. Stimulatory role of substance P on gonadotropin release in ovariectomized rats. Neuroendocrinology. 1990;51(5):523–529. [DOI] [PubMed] [Google Scholar]
- 17. Coiro V, Volpi R, Capretti L, et al. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism. 1992;41(7):689–691. [DOI] [PubMed] [Google Scholar]
- 18. Sahu A, Kalra SP. Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology. 1992;130(3):1571–1577. [DOI] [PubMed] [Google Scholar]
- 19. Vijayan E, McCann SM. In vivo and in vitro effects of substance P and neurotensin on gonadotropin and prolactin release. Endocrinology. 1979;105(1):64–68. [DOI] [PubMed] [Google Scholar]
- 20. Kalra PS, Sahu A, Bonavera JJ, Kalra SP. Diverse effects of tachykinins on luteinizing hormone release in male rats: mechanism of action. Endocrinology. 1992;131(3):1195–1201. [DOI] [PubMed] [Google Scholar]
- 21. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 22. Noritake K, Matsuoka T, Ohsawa T, et al. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409–415. [DOI] [PubMed] [Google Scholar]
- 23. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 24. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–419. [DOI] [PubMed] [Google Scholar]
- 26. Ruiz-Pino F, Navarro VM, Bentsen AH, et al. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology. 2012;153(10):4818–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gill JC, Navarro VM, Kwong C, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012;153(10):4883–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5(7):e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh SP, Wolfe A, Ng Y, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor α (ESR1). Biol Reprod. 2009;81(3):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin C, Navarro VM, Simavli S, et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J Neurosci. 2014;34(17):6047–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. [DOI] [PubMed] [Google Scholar]
- 33. Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. 4th ed San Diego, CA: Academic Press; 2013. [Google Scholar]
- 34. Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol. 2013;367(1–2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 36. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Traczyk WZ, Pau KY, Kaynard AH, Spies HG. Modulatory role of substance P on gonadotropin and prolactin secretion in the rabbit. J Physiol Pharmacol. 1992;43(3):279–297. [PubMed] [Google Scholar]
- 38. Beaujouan JC, Saffroy M, Torrens Y, Glowinski J. Different subtypes of tachykinin NK(1) receptor binding sites are present in the rat brain. J Neurochem. 2000;75(3):1015–1026. [DOI] [PubMed] [Google Scholar]
- 39. Cascieri MA, Huang RR, Fong TM, et al. Determination of the amino acid residues in substance P conferring selectivity and specificity for the rat neurokinin receptors. Mol Pharmacol. 1992;41(6):1096–1099. [PubMed] [Google Scholar]
- 40. Gether U, Johansen TE, Schwartz TW. Chimeric NK1 (substance P)/NK3 (neurokinin B) receptors. Identification of domains determining the binding specificity of tachykinin agonists. J Biol Chem. 1993;268(11):7893–7898. [PubMed] [Google Scholar]
- 41. Seabrook GR, Bowery BJ, Hill RG. Pharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slices. Eur J Pharmacol. 1995;273(1–2):113–119. [DOI] [PubMed] [Google Scholar]
- 42. Deal MJ, Hagan RM, Ireland SJ, et al. Conformationally constrained tachykinin analogues: potent and highly selective neurokinin NK-2 receptor agonists. J Med Chem. 1992;35(22):4195–4204. [DOI] [PubMed] [Google Scholar]
- 43. Roa J, Vigo E, Castellano JM, et al. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147(6):2864–2878. [DOI] [PubMed] [Google Scholar]
- 44. Ronnekleiv OK, Fang Y, Zhang C, Nestor CC, Mao P, Kelly MJ. Research resource: gene profiling of G proteincoupled receptors in the arcuate nucleus of the female. Mol Endocrinol. 2014:me20141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–345. [DOI] [PubMed] [Google Scholar]
- 46. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 47. RØnnekleiv OK, Kelly MJ, Eskay RL. Distribution of immunoreactive substance P neurons in the hypothalamus and pituitary of the rhesus monkey. J Comp Neurol. 1984;224(1):51–59. [DOI] [PubMed] [Google Scholar]
- 48. Harlan RE, Garcia MM, Krause JE. Cellular localization of substance P- and neurokinin A-encoding preprotachykinin mRNA in the female rat brain. J Comp Neurol. 1989;287(2):179–212. [DOI] [PubMed] [Google Scholar]
- 49. Yamano M, Inagaki S, Kito S, Tohyama M. A substance P-containing pathway from the hypothalamic ventromedial nucleus to the medial preoptic area of the rat: an immunohistochemical analysis. Neuroscience. 1986;18(2):395–402. [DOI] [PubMed] [Google Scholar]
- 50. Tsuruo Y, Hisano S, Okamura Y, Tsukamoto N, Daikoku S. Hypothalamic substance P-containing neurons. Sex-dependent topographical differences and ultrastructural transformations associated with stages of the estrous cycle. Brain Res. 1984;305(2):331–341. [DOI] [PubMed] [Google Scholar]
- 51. Hrabovszky E, Borsay BA, Racz K, et al. Substance p immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2013;8(8):e72369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hökfelt T, Pernow B, Nilsson G, Wetterberg L, Goldstein M, Jeffcoate SL. Dense plexus of substance P immunoreactive nerve terminals in eminentia medialis of the primate hypothalamus. Proc Natl Acad Sci USA. 1978;75(2):1013–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Makara GB, Kakucska I, Lenoir V, Kerdelhue B, Palkovits M. A substance P-containing hypothalamic neuronal system projects to the median eminence. Brain Res. 1986;374(2):399–401. [DOI] [PubMed] [Google Scholar]
- 54. Tsuruo Y, Kawano H, Hisano S, et al. Substance P-containing neurons innervating LHRH-containing neurons in the septo-preoptic area of rats. Neuroendocrinology. 1991;53(3):236–245. [DOI] [PubMed] [Google Scholar]