Abstract
Kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which coexpress kisspeptins (Kps), neurokinin B (NKB), and dynorphin (Dyn), regulate gonadotropin secretion. The KNDy model proposes that NKB (a stimulator, through NK3R) and Dyn (an inhibitor, through κ-opioid receptor) shape Kp secretion onto GnRH neurons. However, some aspects of this paradigm remain ill defined. Here we aimed to characterize the following: 1) the effects of NKB signaling on FSH secretion and 2) the role of Dyn in gonadotropin secretion after NK3R activation; 3) additionally, we explored the roles of other tachykinin receptors, NK1R and NK2R, on gonadotropin release. Thus, the effects of the NK3R agonist, senktide, on FSH release were explored across postnatal development in male and female rats; gonadotropin responses to agonists of NK1R substance P and NK2R [neurokinin A (NKA)] were also monitored. Moreover, the effects of senktide on gonadotropin secretion were assessed after antagonizing Dyn actions by nor-binaltorphimine didydrochloride. Before puberty, rats of both sexes showed increased FSH secretion to senktide (and Kp-10). Conversely, adult female rats were irresponsive to senktide in terms of FSH, despite proven LH responses, whereas the adult males did not display FSH or LH responses to senktide, even at high doses. In turn, substance P and NKA stimulated gonadotropin secretion in prepubertal rats, whereas in adults modest gonadotropin responses to NKA were detected. By pretreatment with a Dyn antagonist, adult males became responsive to senktide in terms of LH secretion and displayed elevated basal LH and FSH levels; nor-binaltorphimine didydrochloride treatment uncovered FSH responses to senktide in adult females. Furthermore, the expression of Pdyn and Opkr1 (encoding Dyn and κ-opioid receptor, respectively) in the mediobasal hypothalamus was greater in males than in females at prepubertal ages. Overall, our data contribute to refining our understanding on how the elements of the KNDy node and related factors (ie, other tachykinins) differentially participate in the control of gonadotropins at different stages of rat postnatal maturation.
Our understanding of the neuroendocrine events underlying the control of GnRH release has significantly evolved since the identification of kisspeptins as the main elicitor of GnRH release. Indeed, hypothalamic neurons that produce kisspeptins (Kiss1 neurons) have been recognized as pivotal elements for the stimulatory control of GnRH neurons and a key relay in the transmission of the effects of numerous regulators of the reproductive axis (1). However, our knowledge of the overall mechanisms governing GnRH release remains incomplete. In fact, the pattern of GnRH release responds to a precise swing between stimulatory and inhibitory episodes that translates into GnRH pulses, which are mandatory for reproductive viability. This process also unveils the need for inhibitory signals on GnRH release (2), which are not yet fully defined.
Recently neurokinin B (NKB; encoded by the Tac2 gene in rodents and TAC3 in humans) and dynorphin-A (Dyn; encoded by the Pdyn gene) have been identified as cotransmitters of kisspeptins in a subpopulation of neurons in the arcuate nucleus (ARC), so-called KNDy (kisspeptin/neurokinin B/dynorphin) neurons (3–6). Pharmacological and genetic data have recently highlighted the relevance of this KNDy signaling in the control of the reproductive axis. On the one hand, NKB has been proposed as a predominant stimulatory signal (5–12). Importantly, KNDy neurons also express NKB receptors (NK3R, encoded by Tacr3) and are profusely interconnected, which suggests potential (auto-)regulatory loops of stimulatory nature (4, 13). Indeed, through this network of collateral projections and contacts, NKB signaling from KNDy neurons has been suggested to stimulate and synchronize kisspeptin secretion (14–16). In fact, NKB-dependent stimulation of gonadotropin release seems to be primarily mediated by the activation of Kiss1 neurons in the ARC, although additional, subtler, regulation of GnRH release at a different level, ie, kisspeptin-independent action, cannot be excluded (8, 9, 17–19).
Of note, NKB belongs to a family of related peptides, globally termed tachykinins (TKs), which includes also substance P (SP) and neurokinin A (NKA), among others (20). However, the action of these other TKs in the control of GnRH and gonadotropin release remains ill defined, as is their potential interplay with NKB and kisspeptins. Yet a recent electrophysiological study has demonstrated the ability of SP and NKA to increase the firing of ARC Kiss1 neurons in mice (21). Moreover, it was demonstrated cross-reactivity between NK3R and the receptors for SP (NK1R) and NKA (NK2R), which seems to be critical for the full action of NKB (21). Yet to our knowledge the roles of these TKs and their receptors in the control of gonadotropin secretion in vivo, at different developmental periods, remain largely unexplored.
On the other hand, Dyn has been demonstrated to inhibit gonadotropin release after binding the κ-opioid receptor (KOR) (22); however, contrary to NK3R, KOR is expressed at low levels in KNDy neurons (5, 19). Of note, as is the case for Kiss1, NKB and Dyn are subjected to the inhibitory negative feedback of sex steroids in the ARC (5, 6, 19, 23).
Altogether the above findings led relatively recently to the proposition of a new model for the precise control of GnRH pulsatility based on the dynamic interplay between KNDy peptides, in which the coordinated interaction of NKB and Dyn within ARC Kiss1 neurons would account for the alternation of stimulatory and inhibitory events that ultimately lead to the generation of kisspeptin (and hence GnRH) pulses in a process that is dependent on the sex steroid milieu (5, 6, 24). Nonetheless, the mode of action of NKB and Dyn to regulate kisspeptin release remains poorly defined. To note, despite the predominantly kisspeptin-mediated action of NKB on the reproductive axis, the stimulatory effect of NKB on LH release is less robust than that of kisspeptin, and null or even inhibitory actions have also been documented, depending on the species and the sex steroid levels (9, 12, 25). For instance, predominant inhibitory effects of the selective NK3R agonist, senktide, have been reported in rodents with null or low sex steroids levels, even though kisspeptins are known to stimulate gonadotropin secretion in these conditions. On the contrary, NKB induced significantly stimulatory LH responses in adult female rats and mice under physiological levels of sex steroids, whereas only adult male mice (but not rats) displayed LH responses to the same challenge (12, 19). These observations illustrate the complexity and the yet-incomplete characterization of the effects of NKB on the gonadotropic axis. In this context, the characterization of the effects of NKB stimulation on FSH release has consistently lagged behind, and only a modest stimulatory effect has been reported in adult male mice (19).
Additionally, the role of Dyn in the above-mentioned model of the KNDy control of GnRH pulses has received little attention recently; in fact, although its general inhibitory action on gonadotropin release has been described, the particular role of Dyn in the fine-tuning of KNDy neuronal function requires more in-depth characterization. In this context, we report here a series of functional studies to assess the specific role of NKB signaling in the control of FSH secretion in males and females at different developmental stages, as compared with its effects on LH release and the roles of kisspeptins and the other TKs, SP and NKA, on gonadotropin secretion. In addition, we explored the contribution of Dyn signaling in modulating the outcome of NKB-kisspeptin interactions in the control of gonadotropin release.
Materials and Methods
Animals and drugs
Wistar rats bred in the vivarium of the University of Córdoba, Spain, were used. The day the litters were born was considered as day 1 of age. The animals were maintained under constant conditions of light (14 h of lights, lights on at 7:00 am) and temperature (22°C). They were weaned at 21 days postpartum, when they were housed in groups of five rats per cage and with free access to standard rat chow and water ad libitum. For hormonal tests involving intracerebroventricular (icv) administration of drugs, the rats were caged individually from the day before cannulae implantation until termination of experiments. Correct positioning of the cannulae was checked by visual inspection (to exclude animals showing obvious displacement or detachment) and confirmed at necropsy. Experimental procedures were approved by the University of Córdoba Ethical Committee for Animal Experimentation and were conducted in accordance with the European Union norms for the care and use of experimental animals.
The NK3R agonist, senktide, was purchased from Sigma Chemical Co.. The agonists for NK1R (GR73632) and NK2R (GR64349) were purchased from Tocris Biosciences. The dose of each agonist, 600 pmol, was selected on the basis of previous references as fully effective in inducing gonadotropin responses by senktide in the rat (12, 25, 26). Additionally, in specific experiments, the effects of a dose of 3 nmol of senktide were also explored as a means to test the effect of a high dose of the NK3R agonist on FSH (and LH) release in both prepubertal and adult males. Rat/mouse kisspeptin-10 (Kp-10) was obtained from Phoenix Pharmaceuticals. An effective dose (ED) of Kp-10, 1 nmol, was selected based on previously published data showing its efficacy to elicit LH and FSH secretion in adult male and female rats (27–29). The antagonist of κ-opioid receptor, nor-binaltorphimine didydrochloride (nor-BNI), was purchased from Tocris Bioscience. The dose of 2 nmol icv was selected on the basis of previous references (30). All drugs were reconstituted with physiological saline, 0.9% NaCl. The compounds were diluted to their working concentrations immediately before the experiment and injected icv in a final volume of 5 μL per rat. As a general procedure, blood samples (250 μL) were obtained by jugular venipuncture, in keeping with standard procedures routinely running in our laboratory (8, 10, 12, 27–29), before and at different time points after administration of the various drugs. Animals injected with vehicle (0.9% NaCl) served as controls. Blood was centrifuged to isolate the serum, which was stored at −20°C until hormone measurements.
For experiments involving adult female rats, adult virgin animals were daily monitored by vaginal cytology to confirm the occurrence of regular estrous cycles; only animals showing at least two consecutive 4-day estrous cycles were used. In addition, experiments in gonadectomized (GNX) animals were conducted. For studies involving ovariectomy (OVX), regularly cycling, adult virgin female rats were subjected to bilateral OVX, via abdominal incision; hormonal tests or sampling was performed 2 weeks after surgery. Likewise, groups of adult male rats were orchidectomized (ORX), through a single midline abdominal incision, as previously reported (12).
Experimental design
Effects of senktide on FSH release along postnatal development
In experiment 1, functional tests were conducted to evaluate the effects of central administration of the NK3R agonist, senktide, on FSH secretion across postnatal development in male and female rats. To this end, male and female rats were icv injected with senktide, at the ED of 600 pmol, during the infantile (10 d), juvenile (25 d), pubertal (36 d in females and 45 d in males), and adult (>60 d) stages (n = 8–13/group); in adult female rats, injections were applied in the morning of diestrus 1 (D1) and proestrus. To allow delivery of senktide or vehicle into the lateral cerebral ventricle, animals were implanted with icv cannulae lowered to a depth of 2 (infantile), 3 (prepubertal) or 4 mm (adult) beneath the surface of the skull; the insert point was placed 1 mm posterior and 1.2 mm lateral to bregma. Hormonal tests were conducted at least 1–2 days after implantation of cannulae, a time point when animals were fully recovered. Blood samples were obtained by jugular venipuncture 20 minutes after senktide/vehicle injection, as described in detail elsewhere (12). In additional groups of juvenile (25 d) and adult (>60 d) male rats, the gonadotropin-releasing effects of a higher dose of senktide were also monitored. To this end, 3 nmol of senktide was icv injected, and blood samples were obtained by jugular puncture after 20 and 60 minutes.
FSH responses to senktide in adult intact and GNX rats
Based on the results from experiment 1, showing lack of FSH responses to senktide in pubertal and adult rats of both sexes, despite the proven ability of the NKB agonist to elicit LH release in adult females rats (12), in the next series of experiments, we aimed to assess the FSH-releasing effects of senktide in adult animals with different sex steroid backgrounds. Thus, in experiment 2, we evaluated the effects of bolus icv injection of senktide (600 pmol) or Kp-10 (1 nmol) on FSH release in adult intact, cyclic female (at D1) or sex steroid-depleted (OVX) rats (n = 10–12 rats/group). Additionally, a group of OVX animals (n = 10/group) was supplemented with 17β-estradiol (E2) immediately after OVX. Capsules filled with E2 were implanted sc via a small midscapular incision at the base of the neck; wound clips were used to close the incision. Sham-operated animals served as OVX controls. SILASTIC brand tubing (Dow Corning Corp; 20 mm long, 0.62 mm inner diameter, 1.25 mm outer diameter) was used for capsule preparation. Dilutions of crystalline E2 at a low dose (100 μg/mL, in olive oil) were used for capsule filling; this dose was selected to achieve moderate levels of circulating E2, as previously described (9). Blood samples were obtained by jugular venipuncture before (0 min) and at 15, 60, and 120 minutes after icv injection, following procedures as per experiment 1. Alike, in experiment 3, adult ORX or sham ORX male rats were subjected to a similar protocol of senktide (600 pmol) or Kp-10 (1 nmol) icv administration, and serial blood sampling was conducted at the time points indicated above.
Effects of NK1R and NK2R agonists on LH and FSH release in prepubertal and adult rats
Given recent evidence suggesting a cooperative role of other TKs in the central control of GnRH, and thereby gonadotropin secretion (21), in experiment 4, functional tests were conducted to evaluate in vivo the LH- and FSH-releasing effects of the central (icv) administration of the NK1R (GR73632) or NK2R (GR64349) agonists, in prepubertal (25 d) and intact adult (>60 d) male and female rats; adult female rats were tested on D1. To allow the delivery of each agonist or vehicle into the lateral cerebral ventricle, animals were implanted with icv cannulae as described above. Blood samples were obtained by jugular venipuncture 20 and 60 minutes after the agonist/vehicle injection in prepubertal rats and after 20, 60, and 120 minutes in adult rats (n = 8–10/group).
Effect of chronic Dyn blockade on gonadotropin responses to senktide
In an additional series of experiments, we intended to assess the role of Dyn in the control of adult gonadotropin secretion and in the modulation of responses to NKB. To this end, LH and FSH responses to senktide were evaluated after blockade of the effects of endogenous Dyn in female (experiment 5) and male (experiment 6) rats. For this purpose, adult male and female rats (females at D1) pretreated with the KOR antagonist, nor-BNI, or vehicle 90 minutes prior to the icv injection of senktide (600 pmol). Blood samples were collected before nor-BNI injection (−90 min) and at 0, 20, and 60 minutes after senktide injection. Animals treated with vehicle and nor-BNI alone (ie, not injected with senktide) served as control to monitor long-term responses to the Dyn antagonist.
Analysis of Pdyn and Opkr1 expression in rat hypothalamus along postnatal maturation
To gain further insight into the inhibitory actions of Dyn in the control of gonadotropin responses to NKB in male and female rats at different developmental stages, in experiment 7, we assessed the expression levels of the genes encoding Dyn (Pdyn) and its receptor, KOR (Opkr1), in hypothalamic samples (n = 5/group) from infantile (postnatal d 15), prepubertal (males: d 30; females: d 25), and pubertal (males: d 45; females: d 36) rats; ages were selected to match the maturational stage because female puberty occurs earlier than male puberty. The different groups of animals were euthanized, and the hypothalami were excised according to the coordinates indicated in the Paxinos and Watson Rat Brain Atlas (31). In keeping with our previous studies (32), each hypothalamic sample was dissected and divided into two fragments, containing the preoptic area (POA) or the mediobasal hypothalamus (MBH); the latter contains the ARC in which the KNDy population is located (1).
Real-time PCR
Hypothalamic samples were processed for total RNA isolation using TRIsure reagent (BIOLINE), following the instructions of the manufacturer. Two micrograms of each RNA sample were used for retrotranscriptase reaction (Bio-Rad Laboratories), following previously published protocols. Real-time RT-PCR was performed using the iCycler iQ real-time PCR detection system (Bio-Rad Laboratories). Hypothalamic Pdyn and Opkr1 expression was measured using specific primers-pairs and annealing conditions (see Table 1). As internal control for reverse transcription and reaction efficiency, amplification of a 240-bp fragment of S11 ribosomal protein, Rps11, mRNA was carried out in parallel in each sample. Calculation of relative expression levels of the targets was conducted based on the cycle threshold (CT) method. The CT for each sample was calculated using the iCycler iQ real-time PCR detection system software with an automatic fluorescence threshold setting. Fold expression of target mRNA over reference values was calculated by the equation 1+e-ΔΔCT, where ΔCT is determined by subtracting the corresponding RP-S11 CT value from the specific CT of the target (Pdyn or Oprk1), and δδCT was obtained by subtracting the δCT of each experimental sample from that of the reference sample. No significant differences in CT values were observed for Rps11 within each study groups.
Table 1.
Primer Pairs and Conditions Used for Quantitative PCR Amplification of the Indicated Targets
| Target (NCBI Reference) | Oligo Primers | Expected Size, bp | Annealing Temperature, ºC |
|---|---|---|---|
| Pdyn (NM_019374.3) | Sense (5′-GAT CGG CCA TCC TAT CAC CTG-3′) | 146 | 59 |
| AS (5′-GGA CCA CGC CAT TCT GTATCA C-3′) | |||
| Oprk1 (NM_017167.2) | Sense (5′-ATC ACC GCT GTC TACTCT GTG-3′) | 166 | 59 |
| AS (5′-CACTCT GGA AGG GCATAGTGG-3′) | |||
| Rps11 (NM_031110.1) | Sense (5′-CAT TCA GAC GGA GCG TGC TTA C-3′) | 240 | 58 |
| AS (5′-TGC ATC TTC ATC TTC GTC AC-3′) |
Abbreviations: AS, antisense; NCBI, National Center for Biotechnology Information.
Hormone measurements
Serum LH and FSH levels were measured using RIA kits supplied by the National Institutes of Health (Dr A. F. Parlow, National Hormone and Peptide Program, Torrance, CA). Rat LH-I-10 and FSH-I-9 were labeled with 125I using iodo-gen tubes, following the instructions of the manufacturer (Pierce Chemical Co). Hormone concentrations were expressed using reference preparations LH-RP-3 and FSH-RP-2 as standards. Intra- and interassay coefficients of variation were less than 8% and 10% for LH and 6% and 9% for FSH, respectively. The sensitivity of the assay was 5 pg/tube for LH and 20 pg/tube for FSH. Accuracy of hormone determinations was confirmed by assessment of rat serum samples of known concentrations (used as external controls).
Presentation of data and statistical analysis
Hormonal determinations were conducted in duplicate, with a minimal total number of 10 samples/group. All data are expressed as the mean ± SEM for each group. Results were analyzed for statistically significant differences using Student t tests or one-way or two-way ANOVA followed by the corresponding post hoc tests, when appropriate. P ≤ .05 was considered significant. All analyses were performed with Graph-Pad Prism version 5.0 (GraphPad Software, Inc).
Results
FSH responses to NKB agonist, senktide, in male and female rats along postnatal maturation
Senktide (600 pmol) was injected centrally (icv) to male and female rats at different stages of postnatal maturation. Of note, for adult cyclic females, hormonal tests were conducted in the morning (10:00 am) of D1 or proestrus, as representative phases of low and high circulating levels of estradiol, respectively. Serum was collected 20 minutes after icv injection of senktide as described above. This time was selected based on previous reports as the point at which maximal effects on LH release are achieved after central administration of senktide (12, 25, 26). Unambiguous FSH responses were detected in female rats only at postnatal days (PND) −10 and −25 (see Figure 1A; ***, P ≤ .0001); in contrast, no FSH responses to senktide were detected from PND25 onward. In males, FSH responses to the NKB agonist were detected only at PND10 (see Figure 1B; ***, P ≤ .0001), yet because male rats at this age had lower basal LH level than females, relative responses to senktide in PND10 males were higher (>3-fold increase) that in females of the same age. Of note, FSH responses were not detected in PND25 males injected with a higher, 3-nmol dose of senktide (Supplemental Figure 1A). Likewise, FSH (or LH) responses were not observed in adult males injected with 3 nmol senktide (Supplemental Figure 1B), indicating that the lack of FSH responses to NK3R activation in males at both ages is genuine and not due to low/insufficient stimulation.
Figure 1.
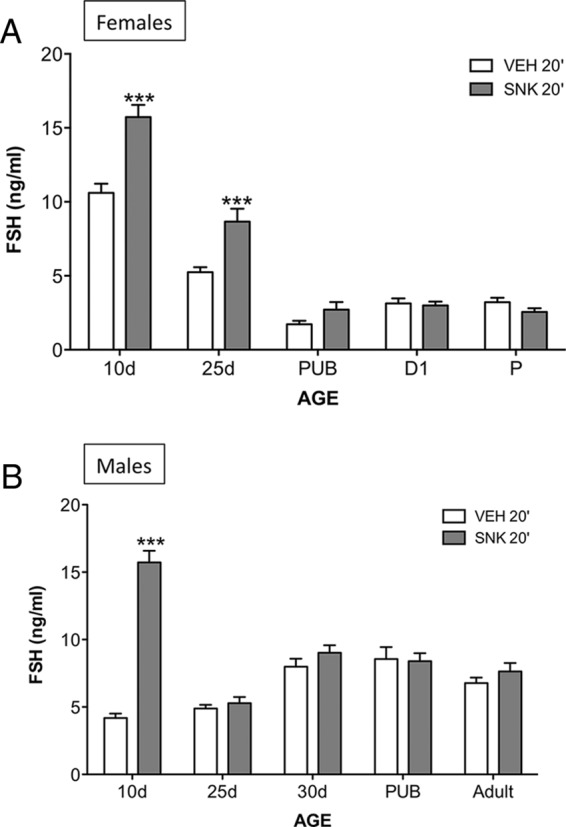
FSH responses to the NK3R agonist, senktide, in male and female rats at different stages of postnatal maturation. Female (A) and male (B) rats, at different postnatal ages (indicated on x-axis), were icv injected with an ED (600 pmol) of senktide or vehicle, and blood samples were obtained 20 minutes later for FSH determinations. Pubertal animals were 36 days old for females and 45 days old for males. Hormone values are means ± SEM. ***, P ≤ .0001 vs corresponding vehicle-injected groups; differences were analyzed for each age and sex using unpaired Student's t tests. P, proestrus; PUB, pubertal; SNK, senktide; VEH, vehicle.
Comparison of FSH responses after kisspeptin vs senktide treatment in adult rats
Our initial experiments documented the lack of FSH responses 20 minutes after central senktide treatment in adult rats. Reportedly, FSH may require a longer time to rise compared with LH (27). Therefore, to test whether FSH responses may take place at later time points after exposure to senktide, the effect of central injection of the NKB agonist on FSH release was monitored at 20, 60, and 120 minutes after the administration in intact adult male and female rats. Additionally, for comparative purposes, the FSH responses to the icv injection of Kp-10 were assessed at 15, 60, and 120 minutes; the earlier time point (15 min) was selected because this is reported to coincide with the peak release of LH after central Kp-10 administration (27–29). In both sexes, central administration of a single bolus of 600 pmol senktide was unable to stimulate FSH secretion over a period of 120 minutes. In contrast, prominent FSH increases were detected after an icv injection of 1 nmol Kp-10, which, in the case of females, peaked at 15 minutes in keeping with previous reports (27). The dynamics of FSH responses, though, were sexually dimorphic so that in females FSH levels had decreased to basal levels at 120 minutes (Figure 2A), whereas in males it steadily increased during the first hour after injection and remained at maximum levels at 120 minutes continued to increase in males (Figure 3A). Notably, our previous study using a similar experimental setting had documented that icv injection of 600 pmol senktide induces robust LH responses in adult cyclic female rats, whereas adult males were nonresponsive (12).
Figure 2.
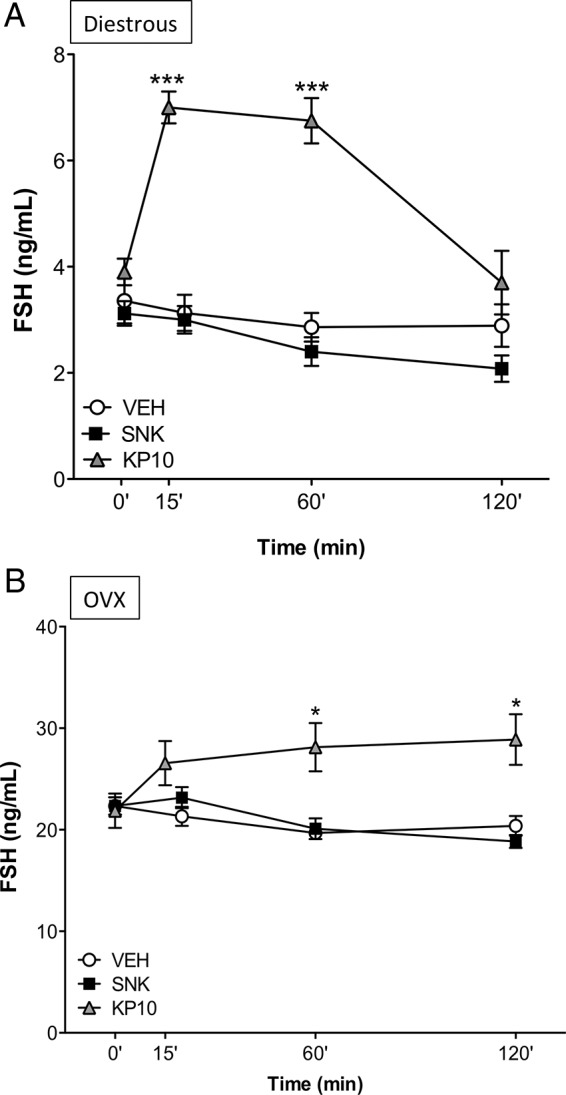
FSH responses to senktide or Kp-10 in adult, gonadal-intact, and OVX female rats. Effects of acute icv administration of senktide (SNK) or Kp-10 upon serum FSH release in cyclic female rats at diestrus (A) or OVX (B) female rats. Senktide (600 pmol) or Kp-10 (1 nmol) were acutely icv injected and blood samples for hormone determinations were obtained before (0 min) and 15/20 minutes (for Kp-10 or SNK, respectively), 60 and 120 minutes after injection of the compounds. *, P ≤ .05, ***, P ≤ .001 vs corresponding basal levels (0 min), as estimated by a two-way ANOVA followed by a Tukey post hoc test.
Figure 3.
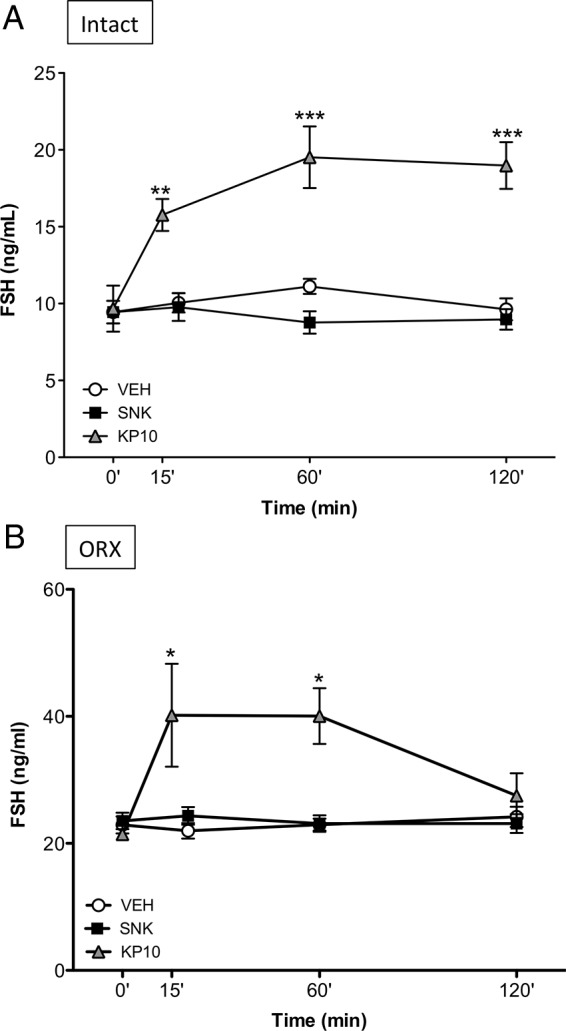
FSH responses to senktide or Kp-10 in adult, gonadal-intact, and ORX male rats. Effects of acute icv administration of senktide (SNK) or Kp-10 on FSH release in intact (A) or ORX (B) male rats. Senktide (600 pmol) or Kp-10 (1 nmol) were acutely icv injected and blood samples for hormone determination were obtained before (0 min) and 15/20 (for Kp-10 or SNK, respectively), 60 and 120 minutes after injection of the compounds. *, P ≤ .05, **, P ≤ .01, and ***, P ≤ .001 vs the corresponding basal levels (0 min), as estimated by a two-way ANOVA followed by a Tukey post hoc test.
Because of the different FSH responses to Kp-10 and the NKB agonist between males and females, we aimed to assess whether the actual circulating levels of gonadal steroids in both sexes would affect such dimorphism. Experiments in females involved testing of the effects of senktide and Kp-10 in OVX animals, subjected or not to replacement with (physiological) doses of E2. Central injection of senktide was unable to modify FSH levels, neither in OVX animals (see Figure 2B) nor in OVX+E2 rats (0 min = 18.45 ± 1.00; 20 min = 20.09 ± 0.75; 60 min = 19.22 ± 1.37; 120 min = 18.18 ± 0.79 ng/mL) at any time point studied. In contrast, FSH release was significantly augmented at 60 and 120 minutes after Kp-10 administration in OVX rats (Figure 2B). Similarly, OVX+E2 rats displayed significantly elevated FSH levels at 120 minutes after Kp-10 injection (0 min = 16.32 ± 0.76; 120 min = 22.44 ± 1.02; P ≤ .05). Effectiveness of sex steroid replacement in our models was demonstrated by the lowering of basal preinjection LH levels in OVX animals, compared with the circulating LH concentrations in OVX rats without E2 replacement (data not shown).
Experiments in male rats involved testing of the effects of senktide and Kp-10 in ORX animals. Similar to OVX females, adult castrated males failed to show any response of FSH to central senktide administration at any time point studied. In clear contrast, icv injection of Kp-10 induced robust increases in circulating FSH levels, at 15 and 60 minutes after the Kp-10 bolus (Figure 3B).
Responses to NK1R and NK2R agonists in male and female rats at prepubertal and adult stages
Considering the results of experiments 1–3 and the recently proposed role of TKs other than NKB in the control of GnRH secretion (21), hormonal analyses were applied to evaluate LH and FSH responses to central activation of other two well-known TK systems, SP/NK1R and NKA/NK2R, in both male and female rats at two representative developmental ages: prepubertal (25 d) and adult (>60 d). Of note, although electrophysiological recordings had documented the ability of SP and NKA to activate GnRH neurons in brain slices from adult mice ex vivo (21), the in vivo effects of these compounds remained scarcely evaluated.
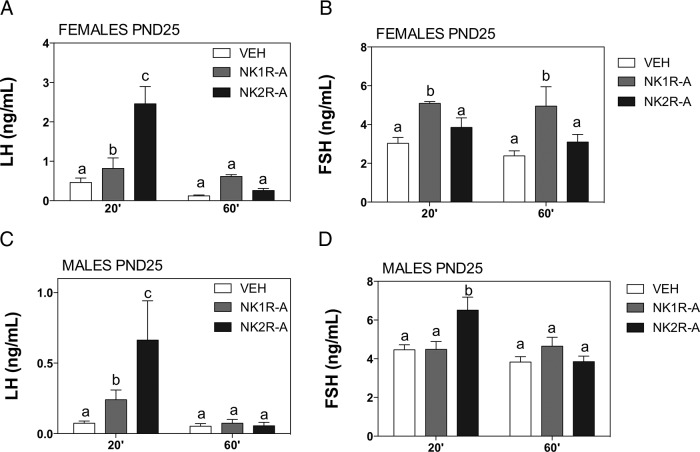
Our neuroendocrine tests revealed that the agonists of NK1R and NK2R were able to significantly increase LH release at 20 minutes after administration in prepubertal animals of both sexes (Figure 4, A and C), yet the stimulation induced by the NK2R agonist in immature animals was significantly greater than that evoked by NK1R agonist in both males and females. Interestingly, this effect was no longer present at 60 minutes after the injection (Figure 4, A and C). In terms of FSH release, a sex difference was noted in prepubertal rats: whereas the NK1R agonist induced a slight, but significant, elevation on FSH release at 20 minutes and 60 minutes only in females (Figure 4B), NK2R agonist stimulated FSH 20 minute after injection specifically in males (Figure 4D). Concerning adult rats, the specific NK1R agonist was devoid of any stimulatory effects on LH or FSH secretion in either males or females, at all the time points studied (Figure 5). In contrast, the specific agonist of NK2R induced a significant but transient (at 20 min) increase in LH levels in both sexes. In addition, persistently elevated FSH levels were detected in adult female rats at 60 and 120 minutes after NK2R-A injection (Figure 5).
Figure 4.
LH and FSH responses to agonists of NK1R (GR73632) and NK2R (GR64349) in prepubertal rats. Effects of acute icv administration of the agonists of tachykinin receptors, NK1R (NK1R-A, GR73632) and NK2R (NK2R-A, GR64349) on LH and FSH release in prepubertal (25 day old) female (A and B) and male (C and D) rats. NK1R-A (600 pmol) or NK2R-A (600 pmol) were acutely icv injected and blood samples for hormone determination were taken at 20 and 60 minutes after injection of the compounds; animals injected with vehicle (VEH) served as controls. Groups with different superscript letters are statistically different (P ≤ .05), as estimated by a two-way ANOVA followed by a Tukey post hoc test.
Figure 5.
LH and FSH responses to agonists of NK1R (GR73632) and NK2R (GR64349) in adult rats. Effects of acute icv administration of the agonists of tachykinin receptors, NK1R (NK1R-A, GR73632) and NK2R (NK2R-A, GR64349), on LH and FSH release in adult female (in D1; A and B) and male (C and D) rats. NK1R-A (600 pmol) or NK2R-A (600 pmol) were acutely icv injected and blood samples for hormone determination were taken before (0 minute) and at 20, 60, and 120 minutes after injection of the compounds; animals injected with vehicle (VEH) served as controls. Groups with different superscript letters are statistically different (P ≤ .05), as estimated by a two-way ANOVA followed by a Tukey post hoc test.
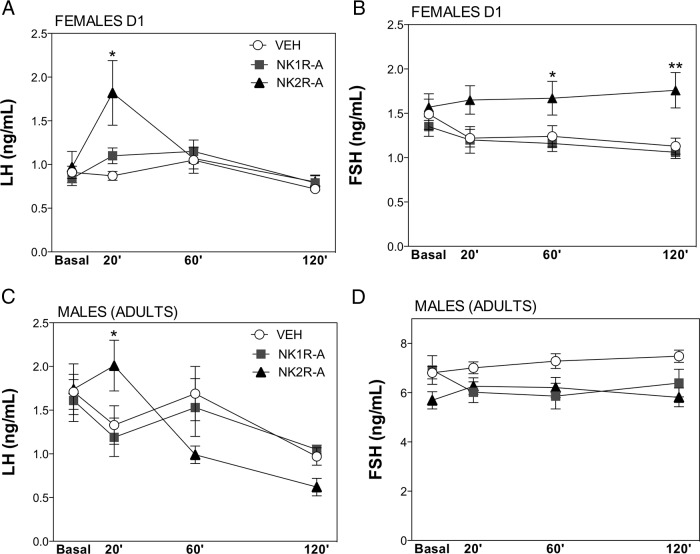
Effect of Dyn antagonism on LH responses to senktide in adult rats
As indicated above, we documented previously a marked sexual dimorphism in LH secretion after central administration of senktide in adult rats so that only females displayed clear-cut LH responses (12); FSH responses remained unexplored. For this reason, we investigated whether the endogenous tone of Dyn may play a role in such a divergent pattern and, by extension, in basal gonadotropin levels in adulthood. Adult rats were treated with vehicle or the Dyn receptor (KOR) antagonist, nor-BNI, 90 minutes prior to a central injection of senktide (600 pmol). Time-course analyses confirmed the patterns of LH response to central senktide injection in gonadal-intact, adult female and male rats (12). In animals with preserved Dyn signaling (ie, treated with vehicle), females, but not males, showed a robust increase of LH secretory mass, which was detectable at 20 minutes after senktide treatment and remained significantly elevated at 60 minutes after the injection (Figure 6, A and B).
Figure 6.
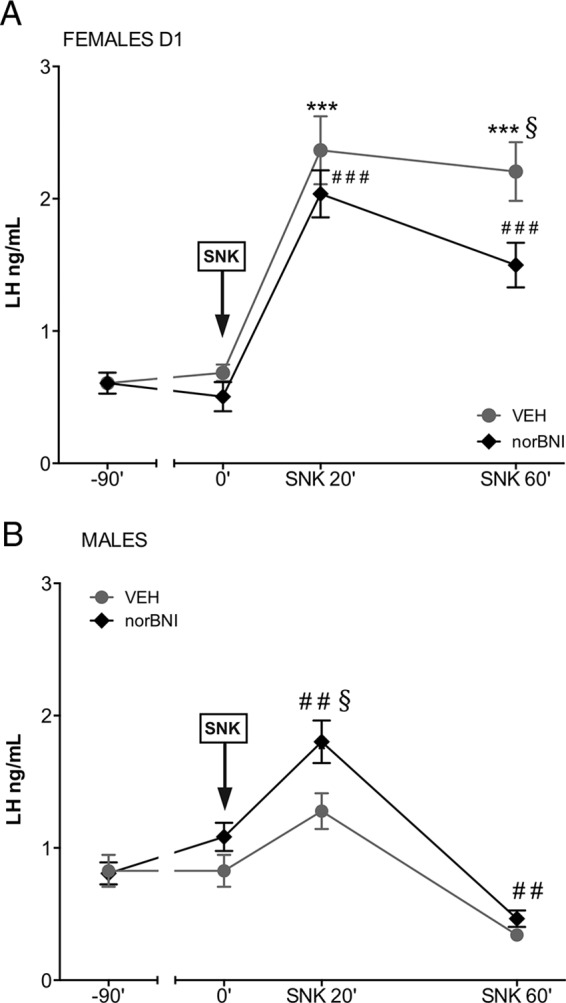
Effect of Dyn blockade on LH responses to senktide in male and female rats. Serum LH levels are shown from adult female (A) and male (B) rats, pretreated with a single dose of vehicle or the KOR antagonist, nor-BNI, 90 minutes before central senktide (SNK) injection. Blood samples for LH determinations were obtained prior to vehicle or nor-BNI administration (−90 min) and before (at 0 min) and 20 and 60 minutes after senktide administration. Panel A: ***, P ≤ .001, ###, P ≤ .001 vs corresponding basal values (ANOVA followed by the Student-Newman-Keuls multiple range test.); §, P ≤ .05 vs corresponding values in the treated group at the same time point (Student's t test). Panel B: ##, P ≤ .01 vs corresponding basal values (ANOVA followed by the Student-Newman-Keuls multiple range test.); §, P ≤ .05 vs corresponding values in the treated group at the same time point (Student's t test).
Strikingly, despite the inability of intact adult males to respond to senktide, pretreatment with nor-BNI was able to reinstate LH responsiveness to senktide so that LH levels at 20 minutes after the injection were similar to those in females (Figure 6B). Of note also, pretreatment with nor-BNI was sufficient to cause a rise in basal LH levels selectively in adult male rats. Importantly, however, the possibility that the observed rescue of LH responsiveness to senktide in adult male rats after Dyn blockade might be due to a sustained and progressive elevation of LH levels after nor-BNI administration can be ruled out by the results of our complementary study, in which adult rats were treated with nor-BNI at −90 minutes and subsequently injected with vehicle and serial blood sampling conducted at 0, 20, and 60 minutes after vehicle injection. As shown in Supplemental Figure 2, nor-BNI alone caused an elevation of basal LH levels 90 minutes after administration in males but failed to induce a further rise in circulating LH thereafter. This confirms that the effect of senktide in males pretreated with nor-BNI (as displayed in Figure 6B) is genuinely due to the effects of NK3R activation in the absence of Dyn actions and not because of Dyn blockade per se.
Effect of Dyn antagonism on FSH responses to senktide in adult rats
Basal FSH levels and responses to senktide were also explored after a blockade of endogenous Dyn actions. Of note, pretreatment of female rats with nor-BNI unmasked a significant stimulatory action of central senktide administration on FSH release, 20 minutes after icv injection (Figure 7A). Furthermore, in male rats, basal FSH levels were elevated by nor-BNI administration, and FSH concentrations were higher after senktide injection in males with blockade of endogenous Dyn actions by pretreatment with nor-BNI (Figure 7B). Again, experiments involving pretreatment with nor-BNI without subsequent administration of senktide ruled out the possibility that the above FSH responses to senktide in male and female rats preinjected with the Dyn antagonist might be due to the effect of nor-BNI alone (data not shown).
Figure 7.
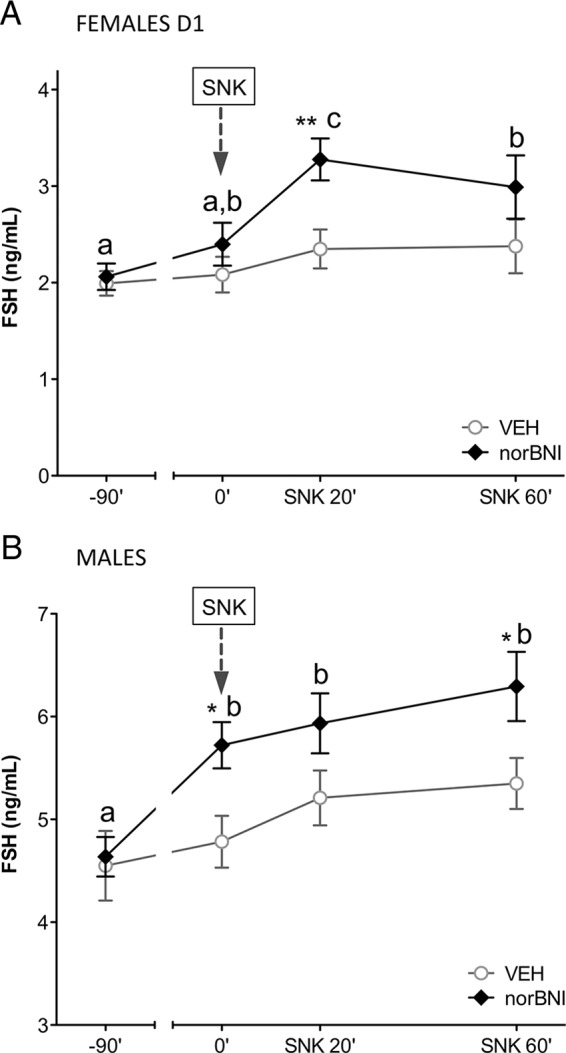
Effect of Dyn blockade on FSH responses to senktide in male and female rats. Serum FSH levels are shown from adult female (A) and male (B) rats, pretreated with a single dose of vehicle (VEH) or the KOR antagonist, nor-BNI, 90 minutes before central senktide (SNK) injection (denoted by an arrow). Blood samples for FSH determinations were obtained prior to vehicle or nor-BNI administration (−90 min) and before (at 0 min) and 20 and 60 minutes after senktide administration. *, P ≤ .05, **, P ≤ .01 vs corresponding vehicle-injected groups (Student t tests). For time-course analysis within VEH or nor-BNI groups, values with different letters are significantly different (ANOVA followed by Student-Newman-Keuls multiple range test).
Expression of Pdyn and Opkr1 in the hypothalamus of male and female rats
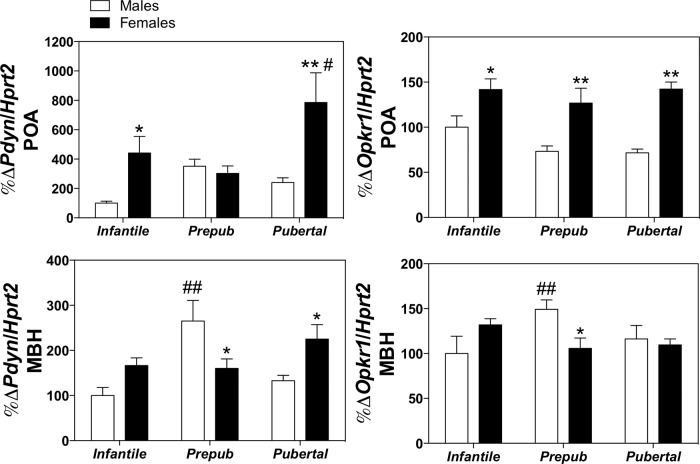
To shed light into the potential basis for the reported effects of nor-BNI treatment of the patterns of gonadotropin responses to senktide, expression analyses of the genes encoding Dyn (Pdyn) and KOR (Opkr1) were applied by quantitative RT-PCR in hypothalamic fragments of adult male and female rats dissected into POA or MBH areas; for comparative purposes, relative expression levels of these targets were assayed in infantile, prepubertal, and pubertal rats. Our expression analyses documented a marked sexual and anatomical differentiation in the expression pattern of Pdyn and Opkr1 in the POA and MBH (see Figure 8). Interestingly, the expression of both genes was significantly higher in the POA of females compared with males at almost all age points assessed; only Pdyn expression in the prepubertal period was similar in both sexes. In contrast, however, the expression of both Pdyn and Opkr1 in the MBH, which increased before puberty in both male and female rats, was significantly greater in the male during the prepubertal period (Figure 8).
Figure 8.
Hypothalamic expression patterns of Pdyn and Opkr1 in the MBH and POA in male and female rats. Relative expression levels of the mRNAs encoding Dyn (Pdyn) and its canonical receptor, KOR (Opkr1), were assayed in male and female rats at different stages of postnatal maturation: infantile, (pre-)prepubertal, and pubertal ages. To allow regional resolution, hypothalamic fragments were dissected and divided into two fragments, containing the POA or the MBH. Values of the specific targets were measured by quantitative PCR, normalized to the expression levels S11 ribosomal protein mRNA, and expressed as percentage change over the expression values in the MBH in infantile (15 d old) male rats. *, P ≤ .05, **, P ≤ .01 in females vs corresponding levels in males (Student t tests). For age-related changes, #, P ≤ .05, ##, P ≤ .01 vs expression values in corresponding infantile (15 d old) groups (two way ANOVA followed by Tukey post hoc test).
Discussion
The recent identification of inactivating mutations in TAC3 and TACR3 genes in humans (encoding NKB and NK3R, respectively) associated with hypogonadotropic hypogonadism (33, 34) unveiled a critical role of the NKB system in the neuroendocrine control of reproductive function. Although this severe phenotype observed in humans has not been fully recapitulated in Tacr3 null mice (35), perhaps due to compensatory effects in rodents, mice with defective NKB signaling do exhibit a significant impairment of their reproductive axis, therefore supporting a relevant, conserved role of NKB across species. Studies by our group and others have documented the coexpression of NKB with Dyn and kisspeptin in a distinct population of arcuate neurons, ie, KNDy neurons, in a number of species (3–6, 14, 15, 19), and offered compelling evidence for a predominantly stimulatory effect of NKB upon LH release in a process seemingly mediated by kisspeptin release (1, 4, 8, 13). These data, together with the inhibitory action of Dyn on gonadotropin release (1, 4, 8, 13), led to the proposal of a reciprocal role of NKB and Dyn in the dynamic (auto-)regulation of kisspeptin pulses by KNDy neurons and thereby, the control of GnRH secretion, thus, defining the so-called KNDy paradigm (4, 5, 13).
However, several aspects of the underlying mechanism governing NKB, and Dyn, action on kisspeptin/GnRH release remain elusive. Remarkably, important differences between NKB and kisspeptins in terms of stimulation of gonadotropin release have been documented. Thus, the LH-secreting potency of NKB (or its agonist, senktide) is significantly lower than that of kisspeptins (9, 11, 12, 28, 36). Furthermore, the stimulatory effect of NKB depends on the sex steroid milieu, with the inhibitory actions being detected in conditions of low or absent gonadal steroid levels (7, 9, 17, 37) and a clear sexual dimorphism in adults, with null stimulatory responses in males (12), at least in the rat. Unlike NKB, kisspeptins retain the full stimulatory action on LH release in both sexes, at all physiological stages and ages assessed to date (1). These discrepancies argue against a role of NKB as a mere switch-on signal for the release of kisspeptin pulses and highlight the tenable interplay of NKB with other regulatory signals produced or received by KNDy neurons.
In this context, it is notable that, despite the recent characterization of LH responses to NKB in various species (1, 4, 13) and the proven effects of kisspeptin on FSH release (27), the actions of NKB on FSH secretion have received less attention to date, with only subtle stimulatory responses being observed in male mice (19). Our current data are the first to document the ability of the NKB agonist, senktide, to stimulate FSH secretion in rats in a strikingly developmental-dependent manner. Thus, senktide induced clear FSH responses in infantile/juvenile rats but not in pubertal or adult rats, despite the robust FSH responses to kisspeptins documented throughout postnatal development (27). Of note, in adult rats, FSH secretion remained unresponsive to senktide, regardless of the steroid milieu. This is in contrast to the sex-dependent effects of the NKB agonist on LH secretion in adult rats: although adult male rats did not respond to senktide in terms of LH secretion (12), in adult females, senktide stimulated LH secretion in gonadal-intact but inhibited it in OVX rats (9, 17, 37). It is unlikely that the lack of FSH responses to senktide in adult rats could be due to the 600-pmol dose used because similar doses elicited clear-cut responses in immature animals, and this dose was sufficient to elicit already maximal LH responses, which could not be further enhanced by doses 5-fold higher than the one tested here (12). All in all, the above observations are difficult to reconcile under the prism of the classical KNDy paradigm and pose the question on why are gonadotropin responses to central NKB vs kisspeptin stimulation so different in several conditions in the rat.
Reasonably, part of the above divergence observed between NKB and kisspeptin action may be due to the mode of action of these molecules in the control of GnRH/gonadotropin secretion. Kisspeptins can trigger the activation of GnRH neurons directly (38) and hence would consistently elicit GnRH release and thereby LH and FSH secretion. In contrast, NKB would operate upstream of KNDy neurons, modifying the kisspeptin output to GnRH neurons. It is therefore tenable that, depending on different variables, such as sex, developmental age, and gonadal hormone milieu, the effect of NKB may result in variable patterns/levels of kisspeptin release, thus explaining the variable responses summarized above. The fact that, at least in the rat, NKB seems able to preferentially, if not exclusively, induce the secretion of LH vs FSH might be explained by the induction of kisspeptin/GnRH secretory patterns prone to preferentially activate pituitary LH secretion. Likewise, the sexual dimorphism in terms of the capacity of NKB to induce LH or FSH responses in females vs males may involve a preferential action/expression of NKB/NK3R in one sex over the other at certain developmental stages.
Another contributing factor for the differential responses between kisspeptins and NKB is that other tachykinins, as SP and NKA, acting via other receptors (such as NK1R and NK2R), might also play a role in controlling gonadotropin release so that it would not be NKB alone but rather NKB in conjunction with these other TKs what critically modulates kisspeptin release. This possibility has been recently put forward on the basis of electrophysiological analyses in mice (21). Yet despite some initial studies in the area (39, 40), the effects of TKs on gonadotropin secretion in vivo, at different developmental periods, remains largely unexplored. To our knowledge, our present data are the first to document that during the prepubertal period, just as is the case for senktide, activation of SP and NKA receptors can evoke significant gonadotropin responses, which in the case of LH are of greater amplitude for NKA/NK2R. In fact, transient but detectable LH responses to NKA were detected in adult male and females, whereas adult females appear to be responsive to NKA also in terms of FSH secretion. Although in general such effects were of modest magnitude, they illustrate interesting differences vs the profiles of LH and FSH responses to NK3R activation in males and females at different stages of maturation, as documented also here and in our previous study (12). Admittedly, however, how other TKs integrate in the KNDy paradigm is yet to be solved by additional functional and neuroanatomical studies.
Another element putatively involved in the generation of divergent responses to NKB vs kisspeptins for both gonadotropins is that related to the presumable action of NKB to modulate not only kisspeptin output but also the secretion of other KNDy products, including Dyn. In fact, studies in models of null or low sex steroid levels recently revealed that the inhibitory effects of senktide on LH secretion in female rats would require intact Dyn signaling (37), and Dyn decreases the ability of KNDy neurons to respond to senktide (41). Therefore, it is possible that the variable ability of the NKB agonist to stimulate LH and FSH secretion as function of sex and developmental stage in the rat may derive from a differential capacity of senktide to modulate Dyn secretion or actions, and/or fluctuations in the endogenous Dyn tone, defined by the expression of Dyn itself or its canonical receptor, KOR. Indeed, our functional data strongly suggest that the latter is a tenable possibility. Thus, in adult female rats, which display clear LH responses to senktide, blockade of Dyn signaling failed to alter basal gonadotropin levels as well as LH responses to the NKB agonist. In contrast, in male rats, which are unresponsive to senktide in terms of LH or FSH, pretreatment with the Dyn antagonist, nor-BNI, elevated the circulating levels of both gonadotropins and surfaced LH and FSH responses to senktide. Admittedly, nor-BNI pretreatment permitted also the manifestation of FSH responses to the NKB agonist in females. Altogether, these findings would suggest a higher Dyn-inhibitory tone in males so that its pharmacological blockade would result in the emergence of clear stimulatory effects in terms of basal gonadotropin concentrations and responses to senktide.
In line with the above argumentation, our expression studies have documented a significantly higher expression of both Pdyn and Okpr1 genes in the MBH of males vs females. Of note, this area is comprised mainly by the ARC, where KNDy neurons are located and therefore likely holds the component of the Dyn/KOR system, which is involved in KNDy regulation and the control of puberty onset and the reproductive axis (10, 13, 42). In fact, Dyn/KOR signaling in the brain has been proposed to play a role in the timing of puberty onset in rats (43). Of note, however, the higher expression levels in males reported here were detected selectively prepubertally but not during the pubertal transition to adulthood. Hence, although this phenomenon might be mechanistically relevant for the actual timing of puberty (delayed in males vs females) and in the sex differences in gonadotropin responsiveness to NK3R activation, ie, only males lose their capacity to mount LH responses to senktide during puberty, other mechanisms or sources of Dyn outside the MBH are likely to contribute also to the above sex divergence in the gonadotropic responses to senktide during and after puberty. In this context, it is interesting to note that Pdyn and Okpr1 gene expression was consistently higher in the POA of females during postnatal maturation. Although the POA is an area in which most of the GnRH neurons are situated (44), the functional relevance of such sex-dependent difference in the expression of the genes encoding Dyn and KOR awaits further investigation.
In summary, we present herein a series of functional and expression studies that expand on the characterization of key elements of the KNDy paradigm, namely NKB and Dyn, as well as of the putative roles of other TKs, such as SP and NKA, in the central regulation of GnRH/gonadotropin secretion in rats. Specifically our data unveil differences in FSH vs LH secretory responses to NKB (and kisspeptin) stimulation as a function of sex, stage of development, and prevailing gonadal hormone levels. In addition, we provide compelling evidence for the roles of Dyn in the dynamic control of basal gonadotropin secretion and responses to NKB stimulation in male and female rats. Altogether our results support and refine the predicted roles of KNDy neuropeptides as potential pacemakers of kisspeptin/GnRH pulses; rather than mere switch-on and -off signals of Kiss1 neurons in all conditions, NKB and Dyn seem to operate as reciprocal interplayers, subjected to the precise regulation of sex and developmental signals, to finely and differentially modulate the generation of kisspeptin pulses and thereby GnRH/gonadotropin secretion. All in all, these findings, together with recent developments on the putative gonadotropic roles of other tachykinins (21), as also documented here using our in vivo setting, will help to reshape our understanding of the KNDy paradigm and how the neuropeptide products of KNDy neurons variably participate in the precise control of gonadotropin secretion in both sexes at different developmental and functional stages of the reproductive axis.
Acknowledgments
We are indebted to Dr Enrique Aguilar for helpful comments and suggestions during the preparation of this manuscript. The superb technical assistance of Ana Belén Rodríguez and Antonia Sánchez-Arroyo is cordially appreciated.
This work was supported by Grants BFU2011–025021 from the Ministerio de Economía y Competitividad, Spain; cofunded with European Union funds from the Fondo Social Europeo of the European Community Program; Project Award P08-CVI-03788 from the Junta de Andalucía, Spain; European Union Research Contract DEER FP7-ENV-2007–1; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health Grant K99 HD071970; and the Charles H. Hood Foundation Child Health Research Award (to V.M.N.). Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III, Ministerio de Sanidad, Spain.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- CT
- cycle threshold
- D1
- diestrus 1
- Dyn
- dynorphin
- E2
- 17β-estradiol
- ED
- effective dose
- GNX
- gonadectomized
- icv
- intracerebroventricular
- KNDy
- kisspeptin/neurokinin B/dynorphin
- KOR
- κ-opioid receptor
- Kp
- kisspeptin
- MBH
- mediobasal hypothalamus
- NKA
- neurokinin A
- NKB
- neurokinin B
- nor-BNI
- nor-binaltorphimine didydrochloride
- ORX
- orchidectomized
- OVX
- ovariectomy
- PND
- postnatal day
- POA
- preoptic area
- SP
- substance P
- TK
- tachykinin.
References
- 1. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. [DOI] [PubMed] [Google Scholar]
- 2. Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207:1371–1373. [DOI] [PubMed] [Google Scholar]
- 3. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 4. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Billings HJ, Connors JM, Altman SN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 2010;151:3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 2012;153:316–328. [DOI] [PubMed] [Google Scholar]
- 9. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 2012;32:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010;151:4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruiz-Pino F, Navarro VM, Bentsen AH, et al. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology 2012;153:4818–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2012;8:40–53. [DOI] [PubMed] [Google Scholar]
- 14. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. [DOI] [PubMed] [Google Scholar]
- 15. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. [DOI] [PubMed] [Google Scholar]
- 16. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153:307–315. [DOI] [PubMed] [Google Scholar]
- 18. Grachev P, Li XF, Lin YS, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7:e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011;152:4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32:1972–1978. [DOI] [PubMed] [Google Scholar]
- 21. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013;154:2750–2760. [DOI] [PubMed] [Google Scholar]
- 22. Grachev P, Millar RP, O'Byrne KT. The role of neurokinin B signalling in reproductive neuroendocrinology. Neuroendocrinology. 2014;99:7–17. [DOI] [PubMed] [Google Scholar]
- 23. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 1994;60:337–345. [DOI] [PubMed] [Google Scholar]
- 24. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandoval-Guzman T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. [DOI] [PubMed] [Google Scholar]
- 26. Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 2012;153(1):316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navarro VM, Castellano JM, Fernandez-Fernandez R, et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 2005;146:1689–1697. [DOI] [PubMed] [Google Scholar]
- 28. Navarro VM, Castellano JM, Fernandez-Fernandez R, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. [DOI] [PubMed] [Google Scholar]
- 29. Roa J, Vigo E, Castellano JM, et al. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology. 2006;147:2864–2878. [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Galiano D, Pineda R, Roa J, et al. Differential modulation of gonadotropin responses to kisspeptin by aminoacidergic, peptidergic, and nitric oxide neurotransmission. Am J Physiol Endocrinol Metab. 2012;303:E1252–E1263. [DOI] [PubMed] [Google Scholar]
- 31. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set. San Diego, CA: Elsevier Science; 2004. [Google Scholar]
- 32. Sangiao-Alvarellos S, Manfredi-Lozano M, Ruiz-Pino F, et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013;154:942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 2009;41:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 2010;95:2287–2295. [DOI] [PubMed] [Google Scholar]
- 35. Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 2012;153(3):1498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153:4894–4904. [DOI] [PubMed] [Google Scholar]
- 38. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalra PS, Sahu A, Bonavera JJ, Kalra SP. Diverse effects of tachykinins on luteinizing hormone release in male rats: mechanism of action. Endocrinology. 1992;131:1195–1201. [DOI] [PubMed] [Google Scholar]
- 40. Sahu A, Kalra SP. Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology. 1992;130:1571–1577. [DOI] [PubMed] [Google Scholar]
- 41. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gill JC, Navarro VM, Kwong C, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology 2012;153:4883–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakahara T, Uenoyama Y, Iwase A, et al. Chronic peripheral administration of κ-opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. J Reprod Dev. 2013;59:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, ed. The Physiology of Reproduction. San Diego: Academic Press/Elsevier; 2006:1415–1482. [Google Scholar]