Graphical abstract
Keywords: Calcium phosphate cement, Gelatine, Soybean, Bone regeneration, Rabbit model
Abstract
Major limitations of calcium phosphate cements (CPCs) are their relatively slow degradation rate and the lack of macropores allowing the ingrowth of bone tissue. The development of self-setting cement foams has been proposed as a suitable strategy to overcome these limitations. In previous work we developed a gelatine-based hydroxyapatite foam (G-foam), which exhibited good injectability and cohesion, interconnected porosity and good biocompatibility in vitro. In the present study we evaluated the in vivo performance of the G-foam. Furthermore, we investigated whether enrichment of the foam with soybean extract (SG-foam) increased its bioactivity. G-foam, SG-foam and non-foamed CPC were implanted in a critical-size bone defect in the distal femoral condyle of New Zealand white rabbits. Bone formation and degradation of the materials were investigated after 4, 12 and 20 weeks using histological and biomechanical methods. The foams maintained their macroporosity after injection and setting in vivo. Compared to non-foamed CPC, cellular degradation of the foams was considerably increased and accompanied by new bone formation. The additional functionalization with soybean extract in the SG-foam slightly reduced the degradation rate and positively influenced bone formation in the defect. Furthermore, both foams exhibited excellent biocompatibility, implying that these novel materials may be promising for clinical application in non-loaded bone defects.
1. Introduction
Calcium phosphate ceramics are frequently used as bone substitutes. In general, they are biocompatible, bioactive and integrate well in bony host tissue [1]. They are used as granules, blocks or cements either to reconstruct bone defects after trauma or to augment weak bone prior to implant placement. The advantages of using moldable calcium phosphate cements (CPCs) instead of pre-formed blocks or granules are the option to apply them using a minimally invasive surgical procedure and better adaptability to the defect geometry.
However, a major disadvantage of CPC is the lack of macropores to allow cell colonization and vessel formation. This hinders cell-mediated material resorption, which is particularly required for apatite cements given their low physicochemical solubility [2]. The development of self-setting cement foams has been proposed as a suitable strategy to overcome this limitation. Mechanical foaming of the cement paste by incorporating a biocompatible foaming agent has been demonstrated to be a promising approach [2]. Our group was the first to use albumen (i.e. egg white) as a foaming agent [3]. We confirmed the suitability of the resulting macroporous self-setting foam as a bone filler in vivo in rabbits, clearly demonstrating that the macroporous structure was maintained after implantation and that the foam resorbed significantly more rapidly compared to non-foamed cements [4]. Although the albumen foam was replaced by newly formed bone, the albumen did, however, evoke a moderate immunogenic reaction [4]. More recently, our group demonstrated that gelatine could also be used efficiently as a foaming agent for CPC [2,5]. Gelatine is denatured collagen, the main protein in bone extracellular matrix, and has been shown to be biocompatible [6]. Perut et al. demonstrated that novel calcium phosphate foams containing gelatine exhibited good osteogenic properties in vitro [7]. Therefore, one aim of the present study was to evaluate the performance of this novel foam under in vivo conditions.
Taking a further developmental step, we also combined gelatine-based foams with soybean-derived compounds [7]. The rationales behind the application of soybean extract were its good foaming capabilities and the improvement of injectability of the cements [7]. Even more important is that soybean extract contains the isoflavones genistin and daidzin, which in contact with plasma are activated to genistein and daidzein [8,9]. These activated isoflavones, so-called phytoestrogens, are considered to provoke a beneficial effect on bone metabolism by acting similarly to estrogen [10]. They also elicit anti-inflammatory effects, including the inhibition of the T- and B-cell responses and natural killer cell cytotoxic activity [10,11]. These effects could potentially decrease the inflammatory response to an implant and stimulate bone formation. Santin et al. confirmed this by demonstrating decreased osteoclast formation and activity, and increased osteoblast differentiation after stimulating these cells with soybean-based biomaterial granules [11]. In agreement with these results, our group demonstrated that the enrichment of the novel hydroxyapatite/gelatine foam with soybean extract favored osteoblast activity and differentiation in vitro [7]. It was also demonstrated in a rabbit model that polymeric hydrogels which were functionalized with soybean extract showed good biocompatibility and high bone regeneration potential [12,13].
Based on our previous work, the aim of this study was to evaluate the in vivo performance of injectable self-setting hydroxyapatite/gelatine foams with and without soybean-extract enrichment. Using a critical-size defect in the rabbit femur, we investigated the time course of material degradation and new bone formation in comparison with non-foamed CPC.
2. Materials and method
2.1. Preparation of materials
We synthesized three types of material: non-foamed CPC, hydroxyapatite/gelatine foam (G-foam) and soybean-enriched hydroxyapatite/gelatine foam (SG-foam) as we described previously in detail [7].
The calcium phosphate powder, which was the basis for all the materials, consisted of 98 wt.% of α-tricalcium phosphate (α-TCP) and 2 wt.% of precipitated hydroxyapatite (pHA, Merck, Darmstadt, Germany). The α-TCP was prepared by heat treatment of a stoichiometric mixture of CaCO3 and CaHPO4 (both Sigma-Aldrich, Gillingham, UK) at 1400 °C, followed by quenching in air, to avoid the unwanted beta phase, and finally milled to obtain the powder.
The liquid phase was a 2.5 wt.% Na2HPO4 water solution (Merck, Darmstadt, Germany). To prepare the G- and SG-foam, the liquid phase was prepared by dissolving respectively 15 and 5 wt.% of bovine type B gelatine (Bloom 250, Rousselot, Courbevoie, France) in the 2.5 wt.% Na2HPO4 (Merck, Germany) water solution at 50 °C in a water bath. The liquid phase of the SG-foam was additionally enriched with 20 wt.% of soybean extract, which was obtained from soybean flour (Infinity Foods, Brighton, UK) using a co-solvent defatting system at 50 °C according to a previously described process [7].
For the in vivo test, the cement powder and the Na2HPO4 salt were sterilized using 25 kGy gamma radiation, while the polymeric components of the liquid phase were sterilized as dried powders using 8 kGy gamma radiation to avoid their denaturation. To prepare the sterile liquid phase, all the sterile components were dissolved under a sterile hood in previously autoclaved distilled water. The cement foaming process was performed under sterile conditions in the operating theater using a water bath at 50 °C. First, 2 ml of the liquid phase were foamed for 1 min at 11,000 rpm using a customized hand mixer. Second, after foaming, the cement powder was incorporated in the liquid foam and further mixed using a sterile spatula, preventing foam disruption, until complete homogenization of the paste.
The liquid to powder (L/P) ratio for the preparation of the G- and SG-foam was adjusted to 0.75 ml g−1 and 0.55 ml g−1, respectively. For the non-foamed CPC, the liquid phase consisted of a 2.5 wt.% Na2HPO4 water solution without any polymer incorporation and was mixed by spatula with the cement powder at an L/P ratio of 0.50 ml g−1. At these L/P ratios, all three pastes presented complete injectability and good cohesion in distilled water.
Finally, for orthotopic implantation, the pastes were filled into sterile syringes and manually injected into the bone defect. In all cases, the time elapsed from the liquid-phase foaming until the implantation by injection was <5 min. In contrast, for subcutaneous implantation the pastes were injected in Teflon molds and allowed to set for 12 days in Ringer’s solution (0.9% NaCl) at 37 °C to obtain disks of 5 mm diameter and 3 mm thickness. Prior to implantation, the pre-set disks were sterilized using 25 kGy gamma radiation.
2.2. Animal study design and surgery
The experimental procedures were performed in accordance with the international regulations for the care and use of laboratory animals, and were approved by the German government (Regierungspräsidium Tübingen, No. 837). 60 skeletally mature female New Zealand white rabbits (age: 28 weeks; mean weight: 3.8 ± 0.5 kg) were randomly divided into three groups (n = 20 per group), corresponding to the three tested materials: CPC, G-foam and SG-foam. Each material was implanted in both the left and right femur.
After premedication with a subcutaneous injection of atropine sulfate (Atropinsulfat Braun 0.5 mg®, B.Braun, Melsungen, Germany), the rabbits were anesthetized using an intravenous injection of a mixture of ketamine hydrochloride (Ketamin 10%®, WDT, Garbsen, Germany) and xylazine hydrochloride (Rompun®, 2%, Bayer Health Care, Grenzach, Germany). The lateral condyle of the left and right femur was exposed and a cylindrical defect of 5 mm diameter and 10 mm depth was drilled. Bone debris were removed from the drill hole by washing with sterile saline solution prior to injection of the materials. The muscle and the subcutaneous soft tissue were sutured. A pre-set cement disk (5 mm diameter, 3 mm thickness) of the same material composition was placed subcutaneously before closing the skin. Immediately after surgery, the animals were allowed full weight bearing and freedom of movement.
Six animals were euthanized after 4 weeks and seven animals after 12 and 20 weeks, respectively, per material group. Both femurs as well as the subcutaneous samples were recovered for analysis. One femur was evaluated histologically and the other by biomechanical testing as described below.
2.3. Assessment of the foam-setting reaction in vivo
The progress of the setting reaction in vivo was assessed by determining the crystalline phases present in the foams 1 month after orthotopic implantation. The samples, embedded in methyl methacrylate resin (Technovit VLC 7200®, Heraeus Kulzer GmbH, Wehrheim, Germany), were analyzed using X-ray diffraction (XRD; PANalytical, X’Pert PRO Alpha-1, Almelo, Netherlands) by scanning in Bragg–Brentano geometry using copper Kα radiation. The experimental conditions were: scan step 0.033°, scan interval 4-100°, counting time 200 s per point, voltage 45 kV and intensity 40 mA.
2.4. Histological evaluation
For histomorphometry, the distal part of the femur and the corresponding subcutaneous implants were explanted immediately after euthanasia of the rabbits. The samples were fixed in buffered 4% formalin, dehydrated using increasing alcohol concentrations and embedded in methyl methacrylate. Then, 80 μm sections were prepared as described previously [14]. These bone sections were stained using Paragon (Paragon C&C; New York, USA) or Giemsa while the subcutaneous samples were stained using Giemsa. Histological slices were examined using light microscopy (Axiophot®; Zeiss, Oberkochen, Germany). For quantitative histological analysis, the point-counting method (⩾1000 points per sample) was performed at 100-fold magnification [15]. The relative amounts of the remaining material, newly formed bone and soft tissue were determined. As control, the intact bone was evaluated in the same manner. Histological evaluation was performed in a blinded manner.
2.5. Biomechanical evaluation
To investigate the mechanical competence of the treated defect region, we performed a compression test. Immediately after explantation, a 3 mm slice was cut out of the distal part of the left femur in the middle and perpendicular to the axis of the cylindrical bone defect. A compression load was applied to the defect region in a material-testing machine (Zwick Z010, Zwick, Ulm, Germany) using a steel indenter of 4 mm diameter at a constant velocity of 2 mm min–1 and a preload of 0.5 N. The force was recorded continuously using a 500 N load cell (KAF-TC, AST Zwick, Ulm, Germany). The test was stopped automatically at a maximum force of 400 N or maximum indentation of 1.5 mm. The stiffness of the sample was analyzed using the software program of the testing machine (testXpert®, Zwick, Ulm, Germany). From the linear region of the load (F)–deflection (d) curve, the stiffness S was calculated (S = F/d). For comparison, the intact bone was evaluated in the same manner. Additionally, we determined the compression stiffness of non-implanted cements, which were injected in Teflon molds having the same size as the bone defect, in the same way after in vitro setting for 12 days in Ringer’s solution.
2.6. Statistics
Data analysis was performed using the statistic-analyzing program JMP® (Version 5.0.1.2, SAS Institute, Cary, NC, USA). The Wilcoxon Rank Sum test was performed to determine significant differences between the material groups. To determine significant differences within a material group over time, the Kruskal–Wallis test was performed. The level of significance was set at p < 0.05.
3. Results
3.1. Material handling during surgery
All surgeries were performed without any complications. Both of the foams and the non-foamed cement could be rapidly (<5 min) prepared under sterile conditions in the operating theater without the need for special equipment. All the pastes were injectable as described previously for the laboratory conditions [7] and exhibited good cohesion in vivo. None of the materials disintegrated after contact with blood.
3.2. Setting reaction in vivo
According to the XRD analysis (Fig. 1), α-TCP had entirely hydrolyzed into hydroxyapatite 4 weeks after implantation in the SG-foam, whereas in the non-foamed CPC traces of unreacted α-TCP were still visible. The broad and overlapped nature of the peaks observed for both samples, the unfoamed CPC and the SG-foam, accounted for the small size of the hydroxyapatite crystals. This effect was even more prominent in the intact bone sample, which exhibited a smaller crystallinity, associated to the nanometric size of apatite crystals in bone.
Fig. 1.
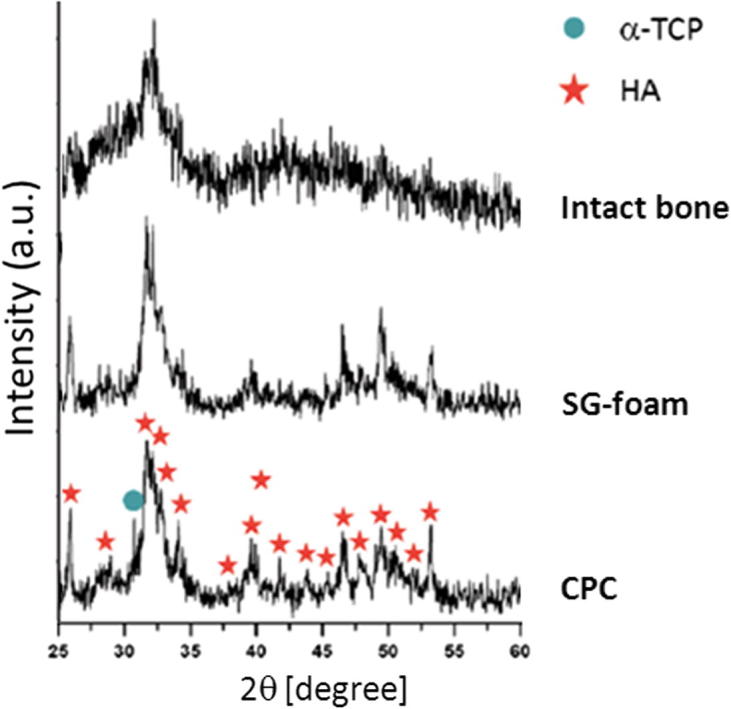
XRD patterns of the non-foamed CPC and the SG-foam implants 4 weeks after implantation compared with the intact bone.
3.3. Histological evaluation
3.3.1. Non-foamed CPC
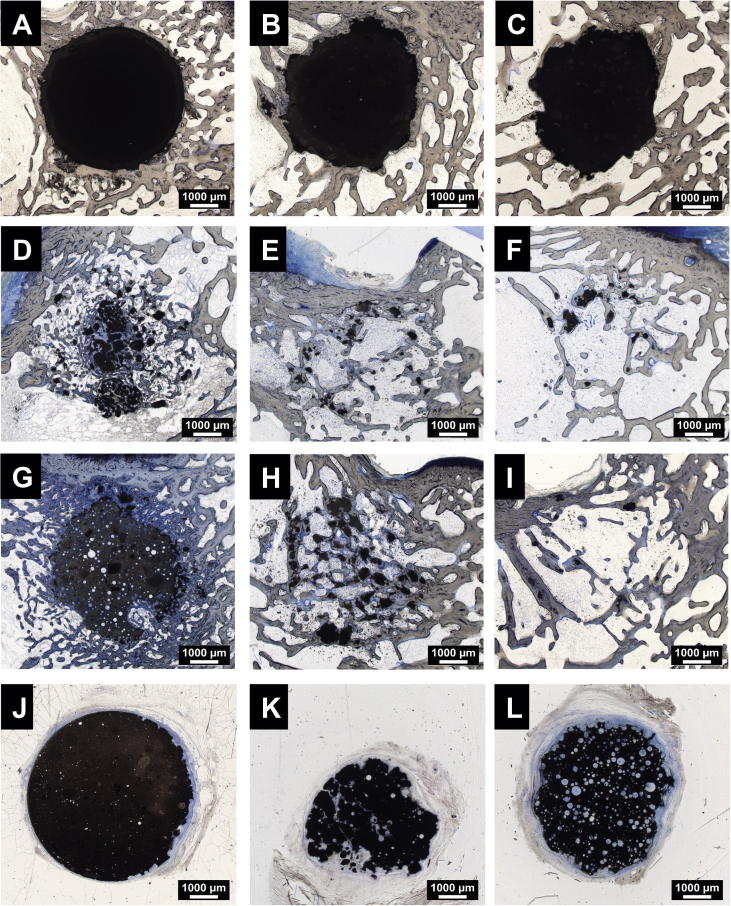
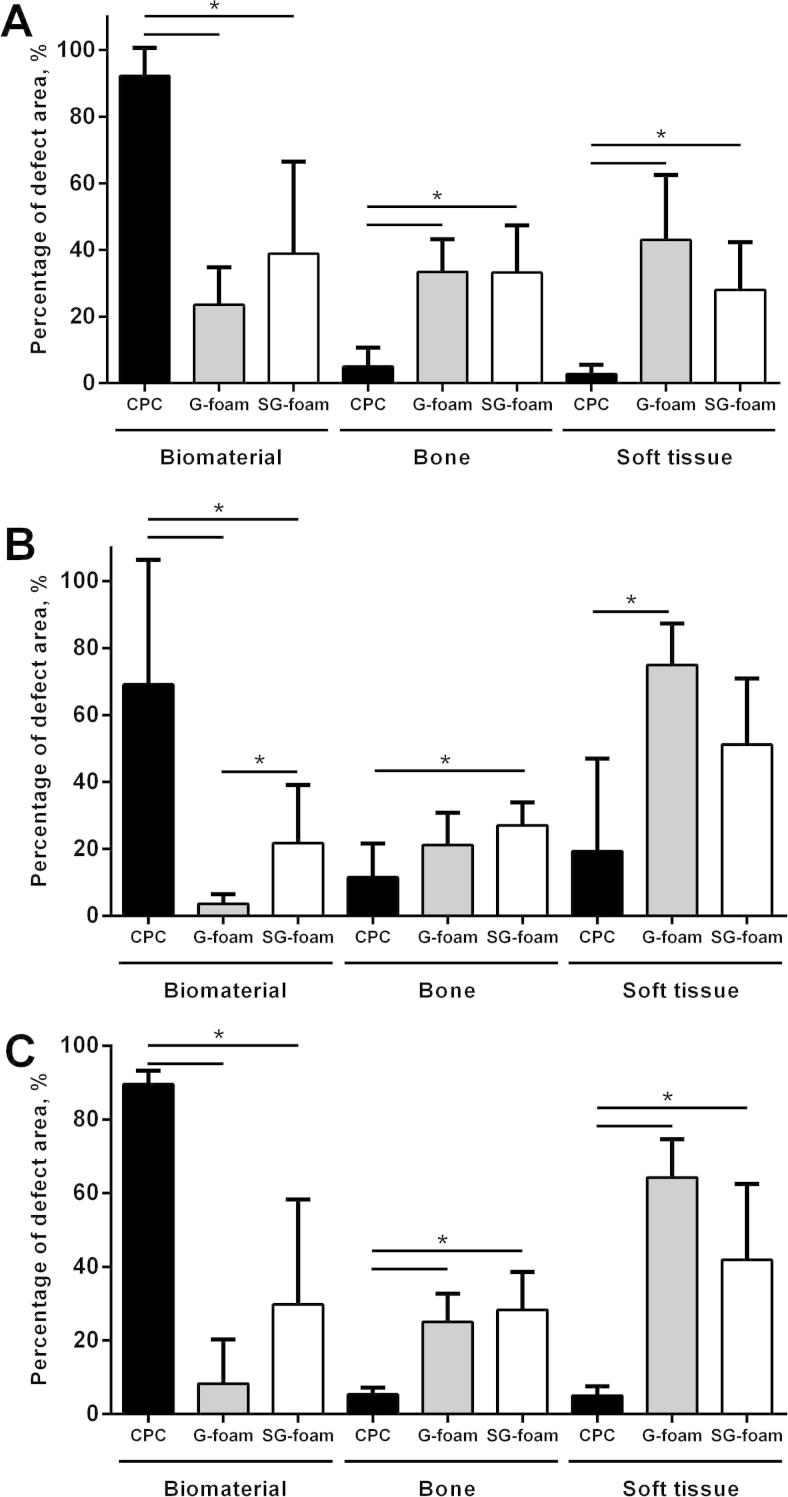
The non-foamed CPC did not significantly degrade during the 20-week implantation period (Figs. 2A–C and 4), with >90% of the defect region still filled with the CPC (Figs. 2C and 4C). Only at the implant edge did minor degradation occur, and a dense layer of newly formed bone completely covered the cement surface, indicating good biocompatibility and excellent osteointegration (Fig. 3A). As expected, only a few small pores were visible in the cement.
Fig. 2.
Histological images of bony implants. Upper row: non-foam CPC after 4 (A), 12 (B) and 20 (C) weeks; second row: G-foam after 4 (D), 12 (E) and 20 (F) weeks; third row: SG-foam after 4 (G), 12 (H) and 20 (I) weeks; bottom row: subcutaneous implants after 20 weeks (J: CPC; K: G-foam; L: SG-foam). Paragon staining. Bars: 1000 μm.
Fig. 4.
Quantification of the remaining material and of the tissue in the defect area after 4 (A), 12 (B) and 20 (C) weeks of implantation. All the data are presented as mean ± SD (n = 6-7, ∗p < 0.05).
Fig. 3.
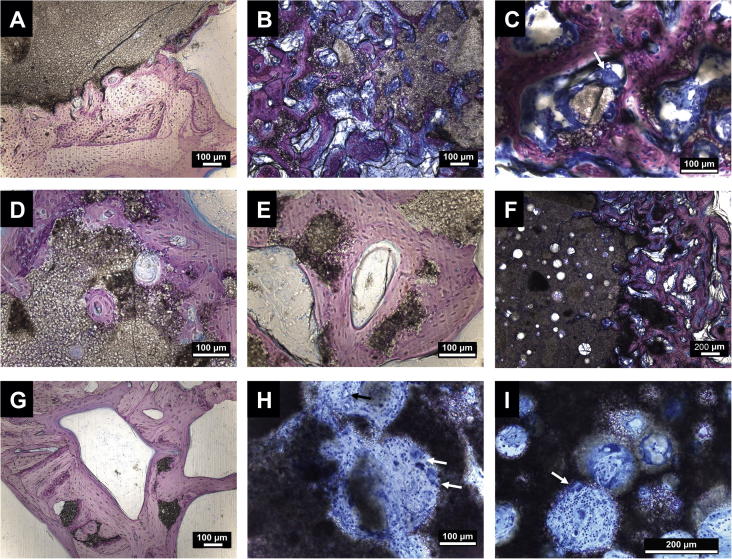
Histological images at higher magnifications of bony (A–G, Giemsa staining) and subcutaneous implants (H–I; Giemsa staining). (A) CPC after 12 weeks; (B) G-foam after 4 weeks; (C) osteoclast on foam particles (white arrow) in G-foam; (D) G-foam after 12 weeks; (E) G-foam after 20 weeks; (F) SG-foam after 4 weeks; (G) SG-foam after 20 weeks (remaining foam particles are embedded in the new bone); (H) subcutaneous G-foam implant (vessel formation is present (black arrow) and macrophages are on the cement (white arrows)); (I) subcutaneous SG-foam implant (macrophage on the foam is marked with a white arrow). Bars: 100 μm (A–E, G and H) or 200 μm (F and I).
3.3.2. G-foam
In contrast, the G-foam was rapidly degraded, starting from the edges of the implant (Figs. 2D–F and 4). Within 4 weeks, only 24 ± 11% of the defect area were filled with the material (Fig. 4A). Many pores were visible in the remaining material, indicating that its macroporous structure was maintained after injection and setting (Fig. 3B). The degraded G-foam was completely replaced by trabecular bone, which filled 33 ± 10% of the entire defect region after 4 weeks (Fig. 4A). The remaining G-foam was located in the center of the defect area and its outer pores were widely invaded by bone tissue, thereby forming a dense bone–material transition zone (Figs. 2D and 3B). We frequently observed osteoclast-like cells degrading the material near the cement surface (Fig. 3C). The newly formed bone contained much osteoid, indicating high metabolic activity.
Few material remnants remained at 12 and 20 weeks after implantation in the center of the defect, and these were completely embedded in new bone trabeculae without any soft tissue layer at the interface (Figs. 2E and F and 3D and E). After 12 and 20 weeks, the relative amount of bone in the defect area was 21 ± 10% and 25 ± 8%, respectively, thereby attaining the physiological trabecular bone density of intact bone at the same location (23 ± 9%). The main part of the defect was filled with soft tissue, representing normal bone marrow between the newly formed trabeculae (Fig. 4B and C). No inflammatory reaction was observed at any time point, indicating good biocompatibility (Fig. 3D and E). Very rarely were single macrophages and foreign body giant cells found.
3.3.3. SG-foam
The SG-foam tended to exhibit a slower degradation rate compared to the G-foam, with a significantly lower degradation being observed at 12 weeks (Figs. 2G–I and 4A–C). After 4 weeks, 39 ± 28% of the defect region were filled with material, with no significant difference compared to the G-foam (Fig. 4A), while after 12 and 20 weeks it was still 22 ± 17% and 30 ± 29%, respectively (Fig. 4B and C). Again, we found no significant inflammatory reaction and only rarely macrophages or foreign body giant cells, indicating excellent biocompatibility (Fig. 3F and G).
The new trabecular bone in the surrounding of the SG-foam appeared to be denser compared to the G-foam (Figs. 2G and H and 3F). The amount of newly formed bone in the defect region was not significantly different from the defects treated with the G-foam, whereas the soft tissue fraction, representing the bone marrow between the bone trabeculae, was decreased, although not significantly, confirming the observation of a higher bone density (Fig. 4). As in the G-foam-treated defects, the outer pores of the SG-foam were invaded by bone tissue, thereby forming a dense bone–material transition zone (Fig. 3F and G).
3.3.4. Subcutaneous implants
The subcutaneously implanted, pre-set cements were surrounded by a soft tissue layer (Fig. 2J–L). Again, no inflammatory reaction was observed. After 4 and 12 weeks, we observed no significant degradation of any of the materials. While the G-foams were slightly degraded after 20 weeks (Fig. 2K), the SG-foams did not significantly resorb during this time (Fig. 2L). In contrast, in both foams many pores were visible. The pores were filled with connective tissue containing blood vessels and a few macrophages and foreign body giant cells (Fig. 3H and I). No ectopic bone formation was observed in any of the subcutaneous implants at any time point.
3.4. Biomechanical results
The stiffness of non-foamed CPC did not significantly change during implantation and was considerably higher than that of the intact trabecular bone at the same region (Fig. 5).
Fig. 5.
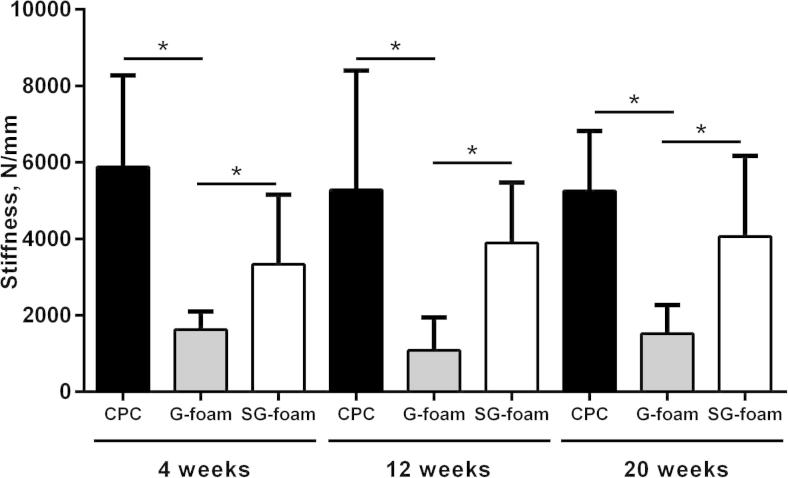
Stiffness of the defect zone in the different material groups 4, 12 and 20 weeks after implantation. All the data are presented as mean ± SD (n = 6-7, ∗p < 0.05).
Because the new bone formed a composite with the residual materials, both the bone and the materials could have contributed to the mechanical performance in the defect area. After 4 weeks, at which time point ∼75% of the G-foam was degraded, the stiffness in the defect region was 1623 ± 478 N mm−1 and in the range of the physiological bone of the same region (1181 ± 504 N mm−1) (Fig. 5). The stiffness did not significantly change over the implantation period, when the G-foam was nearly completely replaced by new bone (Fig. 5). At all implantation time points, the stiffness in the defect region of the SG-foam-treated group was significantly greater compared to the G-foam-treated group and also significantly greater than that of the intact bone (3338 ± 1816, 3896 ± 1578 and 4058 ± 2112 N mm−1 4, 12 and 20 weeks after implantation, respectively). The stiffness of the SG-foam-treated defects did not significantly change during implantation (Fig. 5).
The stiffness of the non-foamed CPC cement measured in vitro after 12 days in Ringer’s solution was 1574 ± 545 N mm−1, and of the G- and SG-foam 25 ± 6 N mm−1 and 469 ± 110 N mm−1, respectively.
4. Discussion
This study demonstrated that injectable macroporous gelatine-based calcium phosphate foams could be used successfully for the treatment of critical-size bone defects in rabbits. The foams maintained their macroporosity after injection and setting in vivo. Compared to non-foamed CPC, cellular degradation was considerably increased and accompanied by new bone formation. The additional functionalization with soybean extract slightly reduced the degradation rate of the foam and significantly increased the mechanical performance in the defect area. Furthermore, both foams exhibited excellent biocompatibility, implying that the novel materials may be promising for clinical application in non-loaded bone defects.
To introduce macroporosity and increase bioactivity, we used gelatine and soybean extracts as additives in an α-TCP cement, which converts into calcium-deficient hydroxyapatite (CDHA) during the setting reaction [16]. A great advantage of gelatine application over other foaming agents is its osteostimulative properties. The addition of gelatine improved the attachment and spreading of osteoblastic cells on calcium phosphate in vitro [17,18] and increased proliferation and osteogenic differentiation [19,20].
Our preceding in vitro studies demonstrated that the addition of gelatine and soybean extract did not affect the conversion of α-TCP to CDHA [7,21]. The XRD analysis in the present in vivo study revealed that the α-TCP in the foams hydrolyzed almost entirely to CDHA 1 month after implantation. Interestingly, the reaction proceeded further in the foamed than in the unfoamed cements, probably due to a higher exposure to the body fluids as a result of the higher porosity. This suggests that the foams also successfully hardened in the in vivo environment. In our previous studies, we were able to demonstrate that the addition of gelatine and soybean extracts considerably improved the cohesion and injectability of the cement paste, and that the final porosity, pore interconnectivity and pore size could be modulated by the gelatine and soybean extract concentrations [7,21]. According to our previous work [7], the size and spherical shape of the macropores obtained were equivalent in the G- and SG-foam, being in the range of 10 to 20 μm. Furthermore, the foams were similar in terms of the open macroporosity available for new bone ingrowth. The total open porosity determined using mercury intrusion porosimetry was 65.0% and 62.4%, respectively, for the G- and SG-foam. Moreover, the open macroporosity was 23.2% and 32.0%, respectively, with a mean connection size of ∼20 μm for both foam types [7]. In contrast, the non-foamed CPC had no open macroporosity, with the 45.7% porosity corresponding to pores <5 μm. Because of methodological reasons, we were unable to determine the porosity quantitatively after explantation of samples in the present study. However, we observed many pores in the remaining foams in the histological slides 4 weeks after implantation, implying that the foams maintained their macroporous structure after injection and setting under in vivo conditions without disintegration or pore collapse.
The introduction of macropores significantly increased the degradation rate of the CPC. Within 4 weeks, ∼76% and 61%, respectively, of the G- and SG-foam were degraded, whereas a minimal amount of non-foamed CPC was resorbed even 20 weeks after implantation. The higher degradation rate of the foams may result from the greater accessibility of the material for degrading cells, including osteoclasts, and blood vessels. Other authors developed composites of CPC and gelatine microspheres, where the macroporosity was achieved by passive dissolution of the gelatine in situ [22,23]. However, because of the low diffusion of physiological fluids inside the cement, the macropores were formed only in the periphery of the implant and bone ingrowth was limited to that site [22]. In contrast, the macroporosity produced by the mechanical foaming, as was the case of the foams tested in this study, is widely available for cell colonization immediately from the time of implantation. Indeed, we found newly formed bone deeply penetrating the pores from the outside; however, the pores within the center of the foams were not completely invaded after 4 weeks. This suggests that the outer and inner macropores were not completely interconnected or that the pore size was not sufficiently large to be entirely penetrated by tissue. However, we do not consider this to be a disadvantage, because the timing of material degradation and new bone formation was effectively synchronized in both the G- and SG-foam.
Degradation of calcium-deficient hydroxyapatite occurs mainly by active cellular resorption and to a lesser extent through physical dissolution [24]. Notably, the pre-set foams, which were subcutaneously implanted, degraded slowly compared to the orthotopic implants, even when the pores were invaded by soft tissue, suggesting that the degradation observed after orthotopic implantation was performed predominantly by bone-specific osteoclasts, which could be observed near the foams. Osteoclasts are able to resorb not only natural bone but also synthetic hydroxyapatite by secreting H+ from their subcellular compartment, thus decreasing the local pH [25,26]. In the subcutaneously implanted foams, we only occasionally found macrophages and a few foreign body giant cells degrading the material. The slower cellular degradation of the SG-foam after orthotopic and ectopic implantation may be due to the suppressive effect of isoflavones on monocytes/macrophages and osteoclasts [11]. This implies that soybean extracts could be used to reduce the immune response and to modulate the cellular degradation of biomaterials.
Both foams exhibited excellent biocompatibility and were completely replaced by bone. The new bone invaded the pores, thus building a dense composite, and there was intimate contact to the inner and outer surfaces of the foams. Other authors observed poor osseointegration of pre-formed gelatin–hydroxyapatite composites after implantation in the mandible of monkeys possibly due to the less tight contact of the implant and host tissue by using a pre-formed material instead of cement [27]. Liao et al. reported even inflammatory reactions towards injectable calcium phosphate cements containing gelatin microspheres after implantation of femoral condyles of rabbits [28]. However, the positive effect of gelatine observed in the present study was confirmed by other in vivo studies demonstrating excellent osteointegration and bone ingrowth in either cement [29] or pre-formed implants [30].
We expected that the enrichment of the foam with soybean extract would stimulate bone formation, because phytoestrogens are known to stimulate osteoblast proliferation and differentiation [7,10,11]. The quantitative histological analysis showed that the relative amount of newly formed bone in the defects treated with the SG-foam was not significantly different compared to the G-foam, although we did observe that the bone was denser in the surrounding of the SG-foam. This observation was further supported by the lower relative amount of bone marrow between the bone trabeculae, which, however, did not reach statistical significance when compared to the G-foam. Nevertheless, these results suggest an osteostimulative effect of soybean enrichment. These results confirm a previous paper of Giavaresi et al., who demonstrated that soybean-based hydrogels promote bone formation in a rabbit bone defect model [12]. Therefore, it can be suggested that the functionalization with soybean extracts increases the bioactivity of bone fillers, thus being an alternative to osteoinductive growth factors.
The synchronized timing of bone formation and the degradation of the foams led to an excellent mechanical performance in the defect area. The compression tests demonstrated that the stiffness of the G-foam-treated defects was already in the physiological range of intact bone 4 weeks after implantation and was maintained until the material was completely degraded. The stiffness was significantly less compared to non-foamed CPC. This may be an advantage in some clinical applications, including the treatment of subchondral bone defects. There, the high stiffness of solid cements is considered to negatively influence the subchondral bone and the cartilage, thus increasing the risk of osteoarthritis [31]. Interestingly, the SG-foam-treated defects exhibited a significantly higher stiffness compared to those of the G-foam. This may result from the higher bone density observed in the histological evaluation and, therefore, further supported our suggestion that the addition of soybean extract may have an osteostimulative effect. Because the new bone formed a composite with the remaining foam, the foam could also influence the mechanical properties in the defect area. The evaluation of samples hardened in vitro showed that the initial stiffness of the foams was much lower compared to non-foamed CPC, indicating that the foams may not significantly contribute to the mechanical performance of the bone–material composite in the defect region, even if the mechanical properties of the SG-foam are increased compared to the G-foam [7]. This also suggests that the foams could only be used in a non-loaded environment.
In a previous in vivo study, we used albumen as a foaming agent for CPC and tested the resulting foam in a similar rabbit model [4]. Albumen resulted in a ⩽75% porosity, and bone growth and neovascularization were observed within the material pores. After 12 weeks of implantation, the residual material fraction in the bone defect was ∼35% of the initial value, being within the range of the SG-foam used in the present study. However, we observed more macrophages and multinucleated cells, indicating a slight immunogenic reaction, probably provoked by the albumen, and the resorption rate was slightly more rapid than the bone ingrowth [4]. The biological performance and the osteostimulation of the gelatine-based foams used in the present study were clearly superior compared to our previously developed albumen-based foams.
5. Conclusions
The results of the present study suggest that the macroporous gelatine-based CPC foams, which were previously developed by our group [7,21], exhibited excellent behavior when applied in vivo in a rabbit model. They could be easily and rapidly prepared in the operating theater and retained good injectability and cohesion as determined in vitro [7], and maintained their macroporosity under in vivo conditions. In addition, they were biocompatible, degraded considerably more rapidly compared to non-foam CPC and were synchronically replaced by newly formed bone. The enrichment of the foam with soybean extracts appeared to support bone formation, resulting in denser bone and increased mechanical performance. Both foams may be suitable as injectable cements for the treatment of non-loaded bone defects, but could be also used in a pre-set form as porous scaffolds for tissue engineering.
Acknowledgements
The authors wish to thank the European Community for funding this study (NMP3-CT-2005-013912). E.B.M. acknowledges the PhD scholarship from the Mexican Council for Science and Technology (CONACyT). Support for the research of M.P.G. was received through the ICREA Academia prize for excellence in research, funded by the Generalitat de Catalunya.
Appendix A. Figures with essential colour discrimination
Certain figures in this article, particularly Fig. 1 is difficult to interpret in black and white. The full colour images can be found in the on-line version, at http://dx.doi.org/10.1016/j.actbio.2014.10.034.
References
- 1.Dorozhkin S.V., Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed. 2002;41:3130–3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Ginebra M.P., Espanol M., Montufar E.B., Perez R.A., Mestres G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010;6:2863–2873. doi: 10.1016/j.actbio.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Ginebra M.P., Delgado J.A., Harr I., Almirall A., Del Valle S., Planell J.A. Factors affecting the structure and properties of an injectable self-setting calcium phosphate foam. J Biomed Mater Res A. 2007;80:351–361. doi: 10.1002/jbm.a.30886. [DOI] [PubMed] [Google Scholar]
- 4.Del Valle S., Mino N., Munoz F., Gonzalez A., Planell J.A., Ginebra M.P. In vivo evaluation of an injectable macroporous calcium phosphate cement. J Mater Sci Mater Med. 2007;18:353–361. doi: 10.1007/s10856-006-0700-y. [DOI] [PubMed] [Google Scholar]
- 5.Montufar E.B., Traykova T., Planell J.A., Ginebra M.P. Comparison of a low molecular weight and a macromolecular surfactant as foaming agents for injectable self setting hydroxyapatite foams: polysorbate 80 versus gelatin. Mater Sci Eng. 2011;31:1498–1504. [Google Scholar]
- 6.Djagny V.B., Wang Z., Xu S. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci Nutr. 2001;41:481–492. doi: 10.1080/20014091091904. [DOI] [PubMed] [Google Scholar]
- 7.Perut F., Montufar E.B., Ciapetti G., Santin M., Salvage J., Traykova T. Novel soybean/gelatine-based bioactive and injectable hydroxyapatite foam: material properties and cell response. Acta Biomater. 2011;7:1780–1787. doi: 10.1016/j.actbio.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Murkies A.L., Wilcox G., Davis S.R. Clinical review 92: phytoestrogens. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 9.Rickard D.J., Monroe D.G., Ruesink T.J., Khosla S., Riggs B.I., Spelsberg T.C. Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors alpha and beta. J Cell Biochem. 2003;89:633–646. doi: 10.1002/jcb.10539. [DOI] [PubMed] [Google Scholar]
- 10.Middleton E., Jr., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 11.Santin M., Morris C., Standen G., Nicolais L., Ambrosio L. A new class of bioactive and biodegradable soybean-based bone fillers. Biomacromolecules. 2007;8:2706–2711. doi: 10.1021/bm0703362. [DOI] [PubMed] [Google Scholar]
- 12.Giavaresi G., Fini M., Salvage J., NicoliAldini N., Giardino R., Ambrosio L. Bone regeneration potential of a soybean-based filler: experimental study in a rabbit cancellous bone defects. J Mater Sci Mater Med. 2010;21:615–626. doi: 10.1007/s10856-009-3870-6. [DOI] [PubMed] [Google Scholar]
- 13.Santin M., Ambrosio L. Soybean-based biomaterials: preparation, properties and tissue regeneration potential. Expert Rev Med Devices. 2008;5:349–358. doi: 10.1586/17434440.5.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Kanter B., Geffers M., Ignatius A., Gbureck U. Control of in vivo mineral bone cement degradation. Acta Biomater. 2014;10:3279–3287. doi: 10.1016/j.actbio.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Ignatius A.A., Betz O., Augat P., Claes L.E. In vivo investigations on composites made of resorbable ceramics and poly(lactide) used as bone graft substitutes. J Biomed Mater Res A. 2001;58:701–709. doi: 10.1002/jbm.10024. [DOI] [PubMed] [Google Scholar]
- 16.Ginebra M.P., Fernandez E., De Maeyer E.A., Verbeeck R.M., Boltong M.G., Ginebra J. Setting reaction and hardening of an apatitic calcium phosphate cement. J Dent Res. 1997;76:905–912. doi: 10.1177/00220345970760041201. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues C.V., Serricella P., Linhares A.B., Guerdes R.M., Borojevic R., Rossi M.A. Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials. 2003;24:4987–4997. doi: 10.1016/s0142-9612(03)00410-1. [DOI] [PubMed] [Google Scholar]
- 18.Rohanizadeh R., Swain M.V., Mason R.S. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med. 2008;19:1173–1182. doi: 10.1007/s10856-007-3154-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X., Cai Q., Liu H., Zhang S., Wei Y., Yang X. Calcium ion release and osteoblastic behavior of gelatin/beta-tricalcium phosphate composite nanofibers fabricated by electrospinning. Mater Lett. 2012;73:172–175. [Google Scholar]
- 20.Huang Y., Yan Y., Pang X., Ding Q., Han S. Bioactivity and corrosion properties of gelatin-containing and strontium-doped calcium phosphate composite coating. Appl Surf Sci. 2013;282:583–589. [Google Scholar]
- 21.Montufar E.B., Traykova T., Schacht E., Ambrosio L., Santin M., Planell J.A. Self-hardening calcium deficient hydroxyapatite/gelatine foams for bone regeneration. J Mater Sci Mater Med. 2010;21:863–869. doi: 10.1007/s10856-009-3918-7. [DOI] [PubMed] [Google Scholar]
- 22.Link D.P., van den Dolder J., van den Beucken J.J., Habraken W., Soede A., Boerman O.C. Evaluation of an orthotopically implanted calcium phosphate cement containing gelatin microparticles. J Biomed Mater Res. 2009;90:372–379. doi: 10.1002/jbm.a.32091. [DOI] [PubMed] [Google Scholar]
- 23.Habraken W.J.E.M., Wolke J.G.C., Mikos A.G., Jansen J.A. Porcine gelatin microsphere/calcium phosphate cement composites: An in vitro degradation study. J Biomed Mater Res B Appl Biomater. 2009;91:555–561. doi: 10.1002/jbm.b.31429. [DOI] [PubMed] [Google Scholar]
- 24.Bourgeois B., Laboux O., Obadia L., Gauthier O., Betti E., Aguado E. Calcium-deficient apatite: a first in vivo study concerning bone ingrowth. J Biomed Mater Res A. 2003;65:402–408. doi: 10.1002/jbm.a.10518. [DOI] [PubMed] [Google Scholar]
- 25.Keller J., Brink S., Busse B., Schilling A.F., Schinke T., Amling M. Divergent resorbability and effects on osteoclast formation of commonly used bone substitutes in a human in vitro-assay. PLoS ONE. 2012;7:e46757. doi: 10.1371/journal.pone.0046757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Egaña J.T., Reckhenrich A.K., Schenck T.L., Lohmeyer J.A., Schantz J.T. Cell-based resorption assays for bone graft substitutes. Acta Biomater. 2012;8:13–19. doi: 10.1016/j.actbio.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Asahina I., Watanabe M., Sakurai N., Mori M., Enomoto S. Repair of bone defect in primate mandible using a bone morphogenetic protein (BMP)-hydroxyapatite-collagen composite. J Med Dent Sci. 1997;44:63–70. [PubMed] [Google Scholar]
- 28.Liao H., Walboomers X.F., Habraken W.J., Zhang Z., Li Y., Grijpma D.W. Injectable calcium phosphate cement with PLGA, gelatin and PTMC microspheres in a rabbit femoral defect. Acta Biomater. 2011;7:1752–1759. doi: 10.1016/j.actbio.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto G., Sugita Y., Kubo K., Yoshida W., Ikada Y., Sobajima S. Gelatin powders accelerate the resorption of calcium phosphate cement and improve healing in the alveolar ridge. J Biomater Appl. 2014;28:1316–1324. doi: 10.1177/0885328213507299. [DOI] [PubMed] [Google Scholar]
- 30.Gil-Albarova J., Vila M., Badiola-Vargas J., Sánchez-Salcedo S., Herrera A., Vallet-Regi M. In vivo osteointegration of three-dimensional crosslinked gelatin-coated hydroxyapatite foams. Acta Biomater. 2012;8:3777–3783. doi: 10.1016/j.actbio.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Schlichting K., Schell H., Kleemann R.U., Schill A., Weiler A., Duda G.N. Influence of scaffold stiffness on subchondral bone and subsequent cartilage regeneration in an ovine model of osteochondral defect healing. Am J Sports Med. 2008;36:2379–2391. doi: 10.1177/0363546508322899. [DOI] [PubMed] [Google Scholar]