Abstract
The neonatal stage is characterized by weak responses to various infections and vaccines, thus the development of efficient formulas to improve vaccine effectiveness is of high priority. The glycolipid alpha galactosylceramide (αGalCer) is known as a potent immune modulator due mainly to natural killer (NK) T cell activation. Using a mouse tetanus toxoid (TT) immunization model, we observed that neonatal mice given αGalCer at the time of primary immunization on postnatal day (pnd) 17 had a significantly higher TT-specific immunoglobulin (Ig)M response as well as a memory IgG response, while αGalCer given on pnd 7 resulted in only marginal boosting. Consistently, immunostaining of the spleen sections from αGalCer-treated pnd 17 immunized neonates showed a higher number of Ki67+ cells in the splenic germinal centre area, suggesting a stronger response after immunization. In-vitro kinetic studies revealed that spleen cells from newborn to pnd 7 neonates did not respond to αGalCer stimulation, whereas cell proliferation was increased markedly by αGalCer after pnd 7, and became dramatic around neonatal pnd 17–18, which was accompanied by increased B, T and NK T cell populations in the spleen. In addition, in pnd 17 spleen cells, αGalCer significantly stimulated the production of NK T cytokines, interleukin (IL)-4 and interferon (IFN)-γ, and promoted the proliferation of CD23+ B cells, a subset of B cells enriched in germinal centres. These data suggest that αGalCer is an effective immune stimulus in the late neonatal stage, and thus may be useful in translational studies to test as a potential adjuvant to achieve a more efficient response to immunization.
Keywords: alpha-galactosylceramide, follicular B cells, germinal centre B cells, immunization, tetanus toxoid
Introduction
The immune response of neonates is often characterized by a lack or weakness of memory response, slow adaptive response and T helper type 2 (Th2) polarization, which not only represents the immaturity or suppression of the immune system, but also serves as a protective strategy in order to cope with new environmental stimuli 1–4. As a result, the reduced generation of Th1 responses renders the neonates highly susceptible to infections and therefore results in poor vaccine responses in early life 5–7. Numerous studies have focused on the development of efficient formulae to improve the response to immunization and offer long-lasting vaccine protection for newborns and infants 8,9. Among these, most have focused upon finding proper adjuvants that stimulate the innate immune system and shape the adaptive immune response, such as the Toll-like receptor ligands that target antigen-presenting cells and in turn activate B and T cell functions at this time, to achieve protective immunity 10–13.
Recently, the glycolipid alpha-galactosylceramide (αGalCer) has attracted attention as an immune modulator 14,15. Animal and clinical trials have shown αGalCer to be a promising antigen that facilitates anti-infective, anti-tumour and immune regulatory activities 16–18. The underlying mechanisms by which αGalCer works are centred mainly on the rapid activation of CD1d-restricted invariant natural killer (NK) T cells (iNK T) 19 which, upon stimulation, produce significant amounts of both interferon (IFN)-γ and interleukin (IL)-4 which then, in turn, influence the differentiation of dendritic cells, stimulation of macrophages to produce IL-12 and activation of T and B cells 20–22. Previously, we have shown that αGalCer is a strong stimulator for B cells of the adult mouse 23,24. αGalCer induced B cell proliferation and differentiation to increase the number of antibody-secreting cells in vitro and increased the production of tetanus toxoid (TT)-specific antibodies in vivo. Despite these encouraging results in adult animals, little is known about the development of the ability of the immune system to respond to this immunomodulator, and whether or not the neonatal vaccine response could be augmented by αGalCer. Here, our objective was to evaluate the ability of αGalCer to amend the compromised immune response during early life, which could help to augment the vaccination response for improved protection.
Materials and methods
Animals and experimental design
Animal protocols were approved by the Institutional Animal Use and Care Committee of Pennsylvania State University. BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) were bred under standard housing conditions and fed a chow diet. T cell receptor (TCR)-β knock-out mice from the Jackson Laboratory (Bar Harbor, ME, USA) were used to eliminate iNK T cell development. Spleens from mice of different ages, from postnatal day (pnd) 0 to 8-week adult, were collected to quantify in-vitro cell proliferation and for cell population analysis using flow cytometry.
Immunization protocol
Neonatal mice at pnd 7 and 17 were injected subcutaneously with TT [3 μg/mouse in 100 μl phosphate-buffered saline (PBS)] with or without αGalCer (0·7 μg/mouse; Enzo Life Sciences, Farmingdale, NY, USA) injected simultaneously with antigen. Blood was collected 10 days later. Some groups of mice were immunized again 3 weeks after the primary immunization and blood was collected 7 days later. Plasma was used to analyse the specific anti-TT immunoglobulin (Ig)M level (primary immunization) and IgG (secondary immunization) using a TT-specific enzyme-linked immunosorbent assay (ELISA), as described previously 25. Mouse spleens were embedded in optimal cutting temperature compound (OCT; Fisher Scientific, Pittsburgh, PA, USA) and rapidly frozen for cryosectioning and immunofluorescent antibody staining.
Cell culture and [3H]-thymidine incorporation
Splenocytes isolated by Ficoll-Hypaque® (Sigma, St Louis, MO, USA) centrifugation were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 5 × 10−5 M β-mercaptoethanol (Invitrogen, Carlsbad, CA, USA). For cell proliferation activity, cells were stimulated with αGalCer (100 ng/ml) for 3 days, then 0·1 μCi of [3H]-thymidine was added for the last 6 h of culture and the cells were harvested for determination of [3H]-thymidine incorporation.
Flow cytometry analysis
Cells (105 per well) were incubated with 0·1 μg of fluorescently labelled antibody for 1 h at room temperature using antibodies to CD3, TCR Vβ8, IgD and CD19 (BD Biosciences, San Jose, CA, USA) and IgM, Ki67, CD49b, CD23 and CD21 (eBiosciences, Inc., San Diego, CA, USA) 26. CD1d tetramer-Alexa 647 was provided by the NIH Tetramer Facility (Emory University), along with an unloaded tetramer control. Live cells were defined by propidium iodide staining, and unstained and isotype-control antibody-stained cells were used as negative controls to set up the gates; single stainings were used for compensation to ensure correct positive staining.
Carboxyfluorescein succinimidyl ester (CFSE) dye was also used to monitor cell proliferation (Life Technologies–Molecular Probes, Grand Island, NY, USA). Splenocytes were incubated with CFSE (10 nM in PBS) for 30 min, and then washed with culture medium containing 10% fetal bovine serum (FBS). Labelled cells were subjected to culture for 4 days followed by antibody staining and flow cytometry analysis.
Immunostaining of tissue
Spleen sections (10 μm) were stained with fluorescein isothiocyanate (FITC)-conjugated anti-IgM and phycoerythrin (PE)-conjugated anti-Ki67 antibodies and analysed by fluorescence microscopy, using isotype control antibodies as negative controls.
Anti-TT ELISA
Plasma anti-TT IgM and IgG levels were determined by ELISA 25 and the antibody levels were calculated based on a standard curve on each plate.
Statistical analysis
Data are the mean ± standard error of the mean (s.e.m.). Results were analysed by t-test or one- or two-way analysis of variance followed by Tukey's post-hoc test, using Prism version 6 software (GraphPad Software, Inc., San Diego, CA, USA). P < 0·05 was considered significant.
Results
αGalCer regulates the TT-specific antibody response in neonatal mice
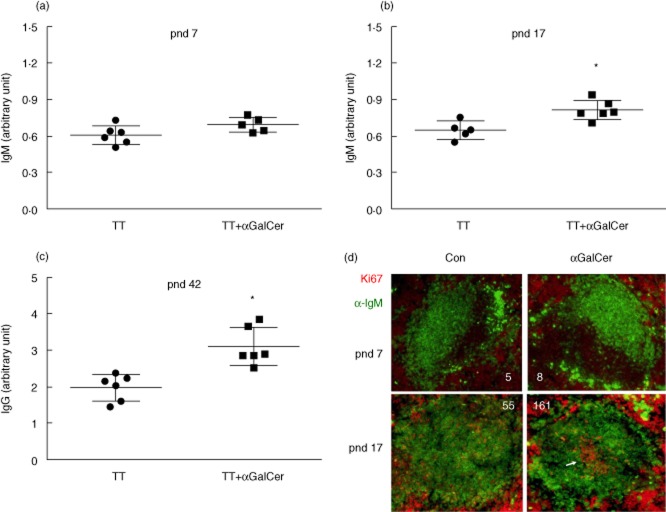
Using a neonatal immunization model adapted from our previous studies with adult mice 23, TT was administered at pnd 7 or 17, with and without αGalCer. The inclusion of αGalCer with TT-immunization on pnd 7 did not alter the specific anti-TT IgM level measured 10 days later (Fig. 1a) compared to the response of control mice; however, αGalCer given with TT on pnd 17 (Fig. 1b) resulted in significantly increased IgM production. Three weeks after the primary immunization mice were re-immunized and plasma IgG was determined 7 days later (Fig. 1c). In mice given αGalCer at primary immunization on pnd 17 the secondary TT-specific IgG level was also increased, whereas no significant increase was observed in pnd 7 mice (data not shown). Because mice from pnd 0 to 14 are considered as newborn 27, our result suggests that the response to αGalCer is developmentally regulated, but not apparent at the newborn/early neonatal age, due probably to the developmental insufficiency.
Figure 1.
Alpha-galactosylceramide (αGalCer) increases anti-tetanus toxoid (TT)-specific antibody production in postnatal day (pnd) 17 neonates. Pnd 7 (a) and pnd 17 (b) neonates were immunized with TT given with and without co-treatment with αGalCer. Plasma TT-specific immunoglobulin (Ig)M levels (enzyme-linked immunosorbent assay) were determined 10 days later. (c) TT-specific IgG for pnd 17 neonates 7 days after secondary immunization. Results represent two independent experiments each with five or six mice per group. Differences from control (TT alone) were determined by Student's t-test, P < 0·05. (d). Representative images of spleen sections (10 μm) after primary immunization stained with anti-IgM-fluorescein isothiocyanate (FITC) (green) and anti-Ki67-phycoerythrin (PE) (red). IgM outlined the follicles and Ki67 stained germinal centre activity (arrow). The number of red cells within the follicle region is noted on each of the representative images.
Splenic architecture is also regulated developmentally. Thus, follicle and germinal centre activity was examined using anti-IgM and anti-Ki67 immunostaining 24,28,29. Figure 1d shows that there were only a few sporadic Ki67 staining cells within the follicles from pnd 7 as well as pnd 17-immunized pups in the absence of αGalCer treatment. In contrast, spleens from pnd 17 neonates that received αGalCer with TT immunization showed an obvious segregation of Ki67-stained cells, a characteristic of splenic germinal centre activity 29, which is temporally consistent with the plasma anti-TT ELISA and suggests a mechanism for the increased responsiveness of the antibody response after co-stimulation with αGalCer.
αGalCer-stimulated mouse spleen cell proliferation increases gradually during postnatal growth and development
To further test the responsiveness of spleen cells to αGalCer, a 3-day culture assay previously established in adult mice 24,26 was used, in which total spleen cells were prepared from neonatal mice of various ages and treated with αGalCer in vitro, after which their proliferative potential was measured. Figure 2a shows that spleen cells were not responsive to αGalCer in the first postnatal week; however, in the second week αGalCer began to stimulate cell proliferation. Cell proliferation became significant around pnd 17–18, which correlated with the immunization results shown in Fig. 1. The responsiveness to αGalCer increased steadily during growth after birth and reached more than 10 times higher at adulthood (8 weeks) compared to early neonatal age.
Figure 2.
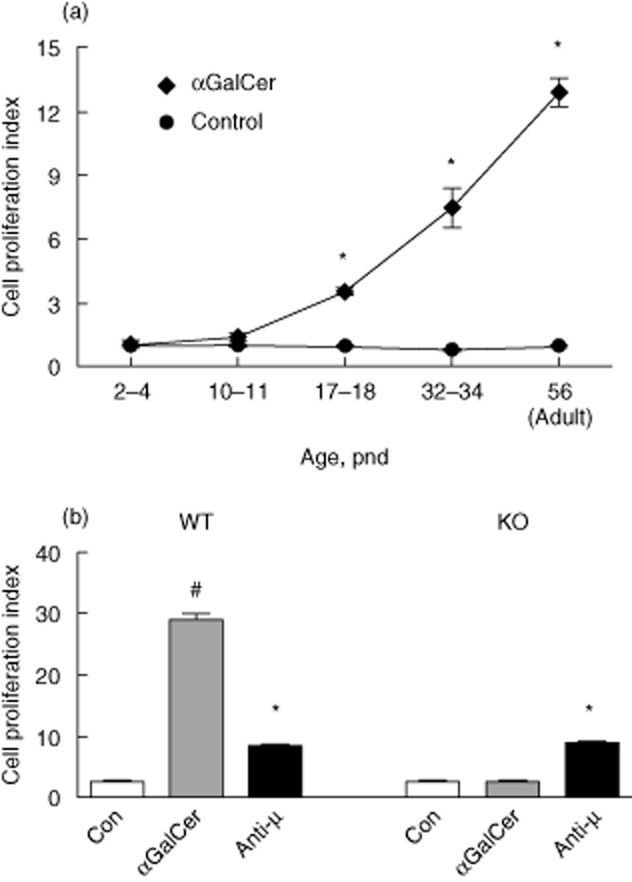
Alpha-galactosylceramide (αGalCer) increases spleen cell proliferation time-dependently after birth. (a). Kinetic study showed the effect of αGalCer in the stimulation of spleen cell proliferation. Spleen cells were isolated at each age and cultured for 3 days with and without αGalCer (100 ng/ml). [3H]-Thymidine incorporation was performed to determine the cell proliferation activity shown as proliferation index calculated as the fold change over the control unstimulated cultures. (b). αGalCer-mediated spleen cell proliferation is natural killer (NK) T cell-dependent. Splenocytes were isolated from wild-type (WT) and NK T-deficient mice (knock-out) and were stimulated in the presence and absence of αGalCer as described in (a); # and * = control (Con) group different from αGalCer-treated or anti-μ-stimulated group, P < 0·05.
To examine the role of αGalCer-mediated NK T activation in the regulation of spleen proliferation, NK T-deficient mice were used to perform the proliferation assay. As shown in Fig. 2b, spleen cell proliferation stimulated by αGalCer was blocked significantly in the absence of NK T cells, while the response to anti-μ, which causes B cell receptor (BCR) ligation, was not affected. These data suggest that germline ablation of NK T cells affects the development of the spleen's response to stimulation with αGalCer.
Splenic lymphocyte populations are gradually enriched after birth and αGalCer stimulates lymphocyte activation and differentiation
We then analysed the major cell population of the spleen, including CD3+ T cells, IgD+ B cells and CD11b+ myeloid cells, which are responsible for antigen presentation and mounting effective immune responses. The B cell population, marked by IgD+ cells, started to increase within the second week after birth, and continued to climb until adulthood (Fig. 3a). A similar trend was observed when CD19 was used to detect the B cell population (Fig. 3b). The T cell population was higher in the third week, increasing significantly until young adulthood (pnd 32–36) (Fig. 3a,b). Concomitantly, the proportion of CD11b+ myeloid cells, including monocytes, macrophages and dendritic cells, remained similar over time (Fig. 3a). The increase in B and T cells after the first postnatal week may explain the emergence of responsiveness to αGalCer at this time, manifested by increased in-vitro cell proliferation (Fig. 2) and in-vivo antibody production (Fig. 1) at the later neonatal age.
Figure 3.
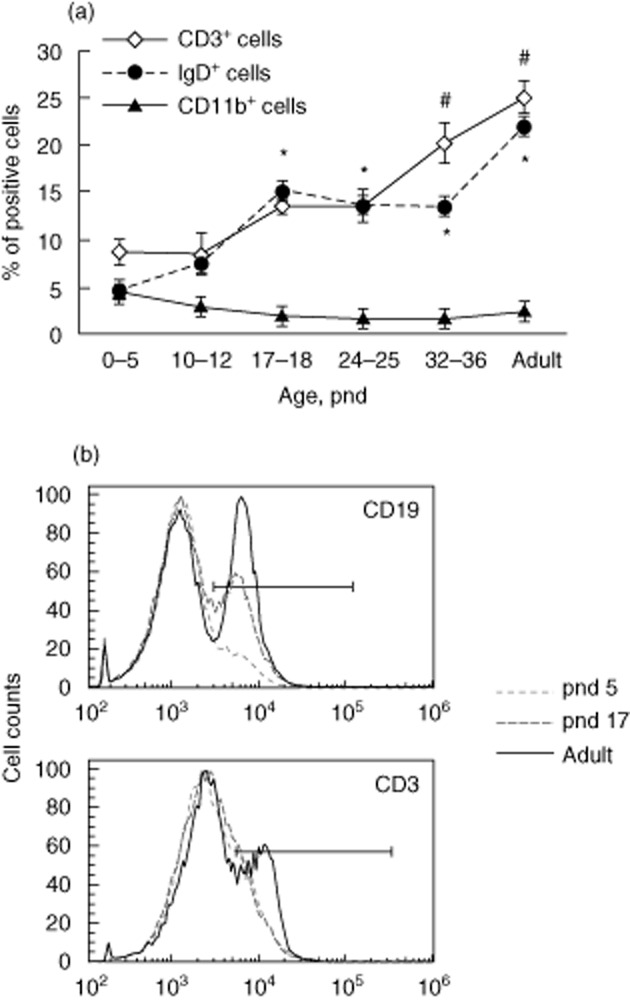
Splenic lymphocyte populations increase steadily after birth. Spleen cells were isolated and subjected to flow cytometry analysis. CD3, immunoglobulin (Ig)D and CD11b were used to identify T and B lymphocytes and myeloid cells, respectively. (a) T, B and myeloid cell populations at various times after birth, six mice per group. *,# = P < 0·05 compared to the same cell population on postnatal day 0–5. (b) Representative histograms of CD19 and CD3 staining of splenocytes from mice at three different ages.
The NK T cell population was examined early (pnd 7) and later (pnd 17) in the postnatal period to determine the NK T cell phenotypes at these times. As shown in Table 1, most NK T cells in the thymus, ∼35%, were either CD8-positive or CD4, CD8 double-positive cells at pnd 7. At pnd 17, although the percentage of double-positive cells remained the same, the proportion of cells expressing CD8+ declined while those expressing CD4+ increased, reaching approximately 12 and 16% of total cells, respectively. In contrast, splenic NK T cells were mainly CD8-positive, although the CD4-positive NK T cell population increased at pnd 17.
Table 1.
CD4 and CD8 distribution of CD1d tetramer-positive cells isolated from spleen and thymus of postnatal day (pnd) 7 and 17 mice
| CD8+ | CD4– | DP | |
|---|---|---|---|
| D 7 spleen | 39·7 | 1·5 | 5·5 |
| D 17 spleen | 20·3 | 9·6 | 5·3 |
| D 7 thymus | 34·4 | 4·1 | 35·0 |
| D 17 thymus | 12·2 | 16·8 | 34·4 |
αGalCer compared to lipopolysaccharide (LPS) differentially expands T and B cell populations in spleen cells of late-stage neonatal mice versus adult mice
To analyse splenocyte proliferation potential in response to αGalCer in detail, spleen cells were labelled with CFSE to trace cell proliferation activity during a 4-day culture, and cell surface markers were used to identify cells that responded to αGalCer stimulation. LPS, a strong mitogen for antigen-presenting cells including B cells, was used as positive control 30,31. Figure 4 shows that αGalCer increased T cell (CD3+), B cell (CD19+) and NK T cell (CD3dimCD11b+CD27+CD49b+) proliferation in pnd 17 and adult mice. Especially for T and B cells, the response to αGalCer became more dramatic as the mice grew older, whereas LPS significantly stimulated cell proliferation in the first postnatal week.
Figure 4.
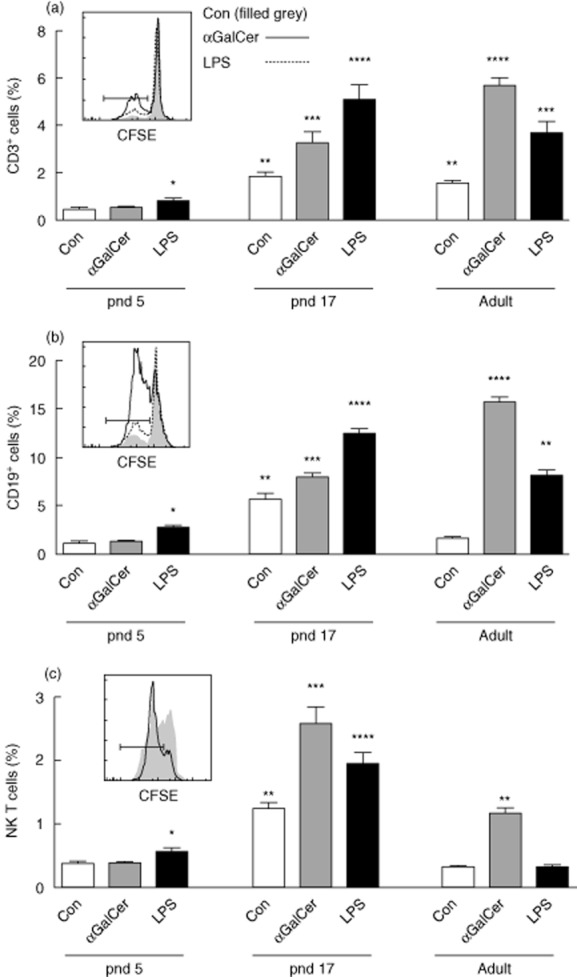
Alpha-galactosylceramide (αGalCer) stimulates splenic T cell, B cell and natural killer (NK) T cell proliferation differentially in comparison with lipopolysaccharide (LPS). Isolated splenocytes were labelled with carboxyfluorescein succinimidyl ester (CFSE) and cultured for 4 days with and without αGalCer (100 ng/ml) and LPS (100 ng/ml), then subjected to flow cytometry analysis. Proliferation of CD3+ T cells (a), CD19+B cell (b), and NK T cells (CD11b+CD3dimCD27+CD49b+) (c) was defined by analysing CFSE dye dilution. Inserts show representative CFSE dye patterns for each cell population [only control and αGalCer are shown in the inset to (c), as cell distributions from LPS and αGalCer treatments were similar]. The gates shown by bars define cells having undergone CFSE dye dilution. Graphs represent triplicates for two independent experiments. Groups with different numbers of * differed from each other by two-way analysis of variance (anova) and post-hoc test, P < 0·05.
As αGalCer is well known for its prominent stimulation of NK T cells, the NK T cell population was evaluated further. NK T cells were identified with both TCR Vβ8 and CD1d-tetramer staining, shown as the insert in Fig. 5a. Consistent with the data in Fig. 4c, αGalCer increased the NK T cell population significantly on pnd 17, but not on pnd 7. In addition, NK T cells from pnd 17 mice contained markedly higher levels of IL-4 and IFN-γ after stimulation with αGalCer, suggesting the activation of NK T cells by treatment. It is worth mention that the cytokine-producing cells, such as IFN-γ+ cells, belonged mainly to the proliferating cells which were stained as IFN-γ+ and CFSE diluted cells shown by the gate in the insert of Fig. 5c.
Figure 5.
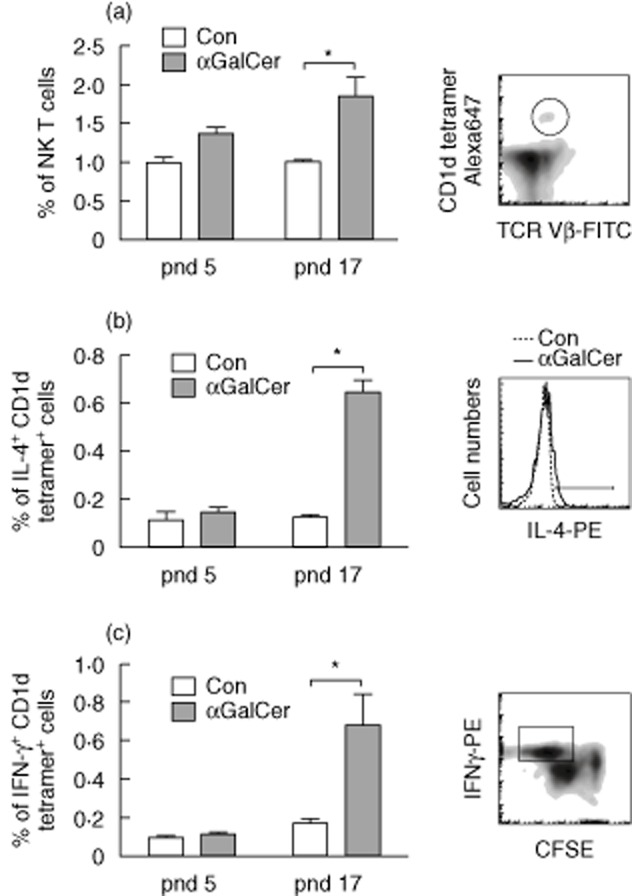
Alpha-galactosylceramide (αGalCer) stimulation enriches natural killer (NK) T cells and increases interleukin (IL)-4 and interferon (IFN)-γ production by NK T cells. Splenocytes were cultured in the presence and absence of αGalCer for 4 days. Cells were stained with T cell receptor (TCR) Vβ8 and CD1d tetramer, and then subjected to intracellular IL-4 or IFN-γ staining. (a) αGalCer increased NK T cell population from postnatal day (pnd) 17 mice. A representative flow cytometric graph was shown to indicate the NK T cell gate (TCR Vβ8+ CD1d tetramer+). αGalCer increased IL-4 (b) and IFN-γ (c) expression in NK T cells from pnd 17 mice. Splenocytes were first gated on TCR Vβ8+ CD1d tetramer+ cells, then cytokine levels were determined. *Treated compared to untreated control (Con) cells, P < 0·05.
Further analysis of B cells was also conducted using staining for CD23 and CD21, which discriminate B cells typical of the follicular (FO) region and marginal zone (MZ) surrounding the follicle 32,33. Results shown in Fig. 6 demonstrate that αGalCer stimulated proliferation of the CD23hiCD21lo B cell subset, a phenotype of follicular B cells, while LPS mainly stimulated the proliferation of B cells of the CD23loCD21hi subset, characteristic of MZ B cells. This was observed in both in pnd 17 and adult mice. The activation of the lymphocytes and especially the increase in specialized B cells may explain the effect of αGalCer in assisting the antibody response to immunization with a T cell-dependent protein antigen.
Figure 6.
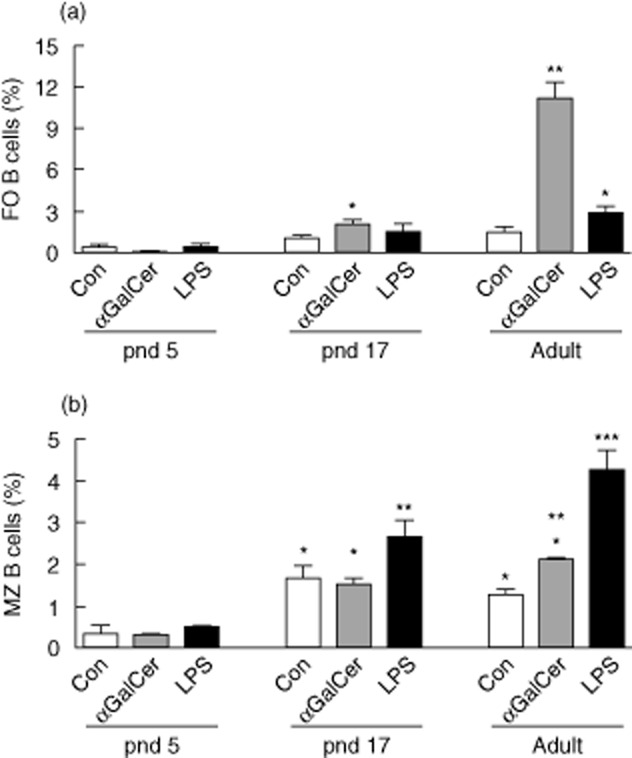
Alpha-galactosylceramide (αGalCer) increases proliferation of follicular B cells. Carboxyfluorescein succinimidyl ester (CFSE)-labelled splenocytes were cultured as in Fig. 4 and stained with anti-CD19, CD21 and CD23. (a) αGalCer stimulated proliferation of follicular (FO) B cells (CD19+CD23hiCD21lo) from pnd 17 neonatal and adult mice. (b) αGalCer compared to lipopolysaccharide (LPS) was less stimulatory for marginal zone (MZ) B cells (CD19+CD23loCD21hi). Groups with different numbers of * differed by two-way analysis of variance (anova) and post-hoc test, P < 0·05.
Discussion
The immune system at the neonatal stage is frequently deficient in its response to various immune challenges, as both innate and adaptive immune components are either suppressed or developmentally immature at this stage 3,34,35. Therefore, novel strategies are being sought, and finding safe and effective adjuvants is of importance for achieving protective vaccine responses. The glycolipid αGalCer has been shown as an effective adjuvant in adult animals for vaccination with protein antigens, as co-treatment with αGalCer at the time of immunization was well tolerated and resulted in increases in antibody production and antibody-secreting cell numbers and memory B cell generation 23,24,36. The underlying mechanisms may be due to the ability of αGalCer to stimulate iNK T cells, considered a major target 22, as well as B cells, which also expressed the surface receptor for αGalCer, the major histocompatibility complex (MHC) class I-like molecule CD1d 24,37. Here, we tested the possibility that αGalCer may be effective in the regulation of the neonatal immune system. Our results have shown, first, that αGalCer increased spleen cell proliferation and increased antibody production when administered after the second week (pnd 17–18). Secondly, the response to αGalCer was correlated with increases in lymphocyte populations, suggesting that the developmental maturation of the immune system is critical for the stimulatory effect of αGalCer, which is mediated by the CD1d-restricted iNK T and B cell activation 38–40. Thirdly, detailed studies confirmed that αGalCer stimulated the proliferation of spleen lymphocytes including T, B and NK T cells and NK T cell-associated cytokines. The stimulation was significant in pnd 17 mice and became more dramatic in adults. In this study, LPS was used as a positive control to evaluate splenocyte proliferation, as Toll-like receptor (TLR)-4 is a functional receptor in early as well as adult life 41. It is worth mention that LPS stimulated cell proliferation at a very early age (pnd 5), which was earlier than αGalCer (pnd 17), suggesting the importance of the TLR-4 response at the early neonatal time, while the response to αGalCer, mediated through the CD1d pathway, may take a longer time to develop.
It is interesting to note that among the B cell population, αGalCer especially stimulated the proliferation of cells with characteristics of follicular B cells (CD23hiCD21lo) while, on the contrary, LPS produced a stronger effect on the proliferation of B cells with the phenotype of MZ B cells (CD23loCD21hi). As the follicles are adjacent to T cell zones that facilitate the communication between activated follicular B cells and T helper cells in the immune response to T cell-dependent protein antigens 32, the results suggest that αGalCer may stimulate antibody production, as observed in vivo, through the stimulation of germinal centre activity. These observations also suggest that LPS serves as a critical innate signal in early life to stimulate the marginal zone response, as the ligand for the pattern recognition receptor TLR-4 is highly expressed on MZ B cells, as well as dendritic cells and macrophages 42, This could provide important surveillance for blood-borne pathogens 41,43. It has been reported that when LPS and αGalCer are co-administered they can synergize to each other to enhance LPS-mediated nitric oxide production and αGalCer-mediated IFN-γ production in splenocytes 44.
In conclusion, our results indicate both the limitations and the potential for αGalCer to stimulate the vaccine response according to postnatal age, as αGalCer was not effective in the early neonatal stage but became effective by the late neonatal stage, and continued to be effective in the adult. These results suggest that αGalCer may represent a new strategy for increasing the rate of effective immunization if used in the later neonatal to adult period.
Acknowledgments
This work was supported by a grant from NIH, DK-41479. The following tetramers were obtained through the NIH Tetramer Facility: Alexa 647-labelled mouse CD1d αGalCer-loaded and unloaded control tetramers.
Disclosures
The authors have no conflict of interest to disclose.
Author contributions
Q. C. designed and performed experiments, and wrote the manuscript; A. C. R. designed the experiments and wrote the manuscript.
References
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Wood N, Siegrist CA. Neonatal immunization: where do we stand? Curr Opin Infect Dis. 2011;24:190–195. doi: 10.1097/QCO.0b013e328345d563. [DOI] [PubMed] [Google Scholar]
- Belderbos ME, Levy O, Meyaard L, Bont L. Plasma-mediated immune suppression: a neonatal perspective. Pediatr Allergy Immunol. 2013;24:102–113. doi: 10.1111/pai.12023. [DOI] [PubMed] [Google Scholar]
- Ghazal P, Dickinson P, Smith CL. Early life response to infection. Curr Opin Infect Dis. 2013;26:213–218. doi: 10.1097/QCO.0b013e32835fb8bf. [DOI] [PubMed] [Google Scholar]
- Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, Shewen PE. Vaccination of neonates: problem and issues. Vaccine. 2012;30:1541–1559. doi: 10.1016/j.vaccine.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl. 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Renz H. Development and regulation of immune responses in pre- and postnatal life. Clin Biochem. 2011;44:495. doi: 10.1016/j.clinbiochem.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Kovarik J, Siegrist CA. The search for novel adjuvants for early life vaccinations: can ‘danger’ motifs show us the way? Arch Immunol Ther Exp (Warsz) 2001;49:209–215. [PubMed] [Google Scholar]
- Mastelic B, Kamath AT, Fontannaz P. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189:5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- Bjarnarson SP, Adarna BC, Benonisson H, Del Giudice G, Jonsdottir I. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. J Immunol. 2012;189:1265–1273. doi: 10.4049/jimmunol.1200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling DJ, Tan Z, Prokopowicz ZM. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLOS ONE. 2013;8:e58164. doi: 10.1371/journal.pone.0058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–231. doi: 10.1016/s1471-4914(02)02325-0. [DOI] [PubMed] [Google Scholar]
- Van Kaer L. Alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Rossjohn J. New ways to turn on NKT cells. J Exp Med. 2011;208:1121–1125. doi: 10.1084/jem.20110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y, Kohyama-Koganeya A, Hirabayashi Y. New insights on glucosylated lipids: metabolism and functions. Biochim Biophys Acta. 2013;1831:1475–1485. doi: 10.1016/j.bbalip.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Okamoto Y. NKT cells as an ideal anti-tumor immunotherapeutic. Front Immunol. 2013;4:409. doi: 10.3389/fimmu.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8– T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallevaey T, Selvanantham T. Strategy of lipid recognition by invariant natural killer T cells: ‘one for all and all for one’. Immunology. 2012;136:273–282. doi: 10.1111/j.1365-2567.2012.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- Chen Q, Mosovsky KL, Ross AC. Retinoic acid and alpha-galactosylceramide differentially regulate B cell activation in vitro and augment antibody production in vivo. Clin Vaccine Immunol. 2011;18:1015–1020. doi: 10.1128/CVI.00004-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Mosovsky KL, Ross AC. Retinoic acid and alpha-galactosylceramide regulate the expression of costimulatory receptors and transcription factors responsible for B cell activation and differentiation. Immunobiology. 2013;218:1477–1487. doi: 10.1016/j.imbio.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ross AC. Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci USA. 2005;102:14142–14149. doi: 10.1073/pnas.0505018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon MR, Oppenheimer K, Bonney EA. Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen. Int J Biol Sci. 2012;8:719–730. doi: 10.7150/ijbs.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz JD, Wang JH, Xie S, Hsu HC. Cytokine regulation of B-cell migratory behavior favors formation of germinal centers in autoimmune disease. Discov Med. 2011;11:76–85. [PMC free article] [PubMed] [Google Scholar]
- Daridon C, Loddenkemper C, Spieckermann S. Splenic proliferative lymphoid nodules distinct from germinal centers are sites of autoantigen stimulation in immune thrombocytopenia. Blood. 2012;120:5021–5031. doi: 10.1182/blood-2012-04-424648. [DOI] [PubMed] [Google Scholar]
- Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)–STAT3 axis. Cell Signal. 2012;24:1185–1194. doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Hebeis BJ, Vigorito E, Turner M. The p110delta subunit of phosphoinositide 3-kinase is required for the lipopolysaccharide response of mouse B cells. Biochem Soc Trans. 2004;32:789–791. doi: 10.1042/BST0320789. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- Zandvoort, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk J, Westerlaken GH, van Uden NO, Belderbos ME, Meyaard L, Bont LJ. Inhibitory receptor expression on neonatal immune cells. Clin Exp Immunol. 2012;169:164–171. doi: 10.1111/j.1365-2249.2012.04599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119:116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Deng S, Reboulet R. Natural killer T (NKT)–B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc Natl Acad Sci USA. 2013;110:16097–16102. doi: 10.1073/pnas.1303218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiai T, Watanabe H, Seki S. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992;77:556–563. [PMC free article] [PubMed] [Google Scholar]
- Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8– and CD4–8– subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994;180:699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Fortier A, Tighe M. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2012;13:44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Crabtree J, Rein-Weston A. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Santamaria R, Xu W. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Ito H, Ohtaki H, Seishima M. Toll-like receptor agonists and alpha-galactosylceramide synergistically enhance the production of interferon-gamma in murine splenocytes. Sci Rep. 2013;3:2559. doi: 10.1038/srep02559. [DOI] [PMC free article] [PubMed] [Google Scholar]