Abstract
Recombinant human binding immunoglobulin protein (BiP) has previously demonstrated anti-inflammatory properties in multiple models of inflammatory arthritis. We investigated whether these immunoregulatory properties could be exploited using gene therapy techniques. A single intraperitoneal injection of lentiviral vector containing the murine BiP (Lenti-mBiP) or green fluorescent protein (Lenti-GFP) transgene was administered in low- or high-dose studies during early arthritis. Disease activity was assessed by visual scoring, histology, serum cytokine and antibody production measured by cell enzyme-linked immunosorbent assay (ELISA) and ELISA, respectively. Lentiviral vector treatment caused significant induction of interferon (IFN)-γ responses regardless of the transgene; however, further specific effects were directly attributable to the BiP transgene. In both studies Lenti-mBiP suppressed clinical arthritis significantly. Histological examination showed that low-dose Lenti-mBiP suppressed inflammatory cell infiltration, cartilage destruction and significantly reduced pathogenic anti-type II collagen (CII) antibodies. Lenti-mBiP treatment caused significant up-regulation of soluble cytotoxic T lymphocyte antigen-4 (sCTLA-4) serum levels and down-regulation of interleukin (IL)-17A production in response to CII cell restimulation. In-vitro studies confirmed that Lenti-mBiP spleen cells could significantly suppress the release of IL-17A from CII primed responder cells following CII restimulation in vitro, and this suppression was associated with increased IL-10 production. Neutralization of CTLA-4 in further co-culture experiments demonstrated inverse regulation of IL-17A production. In conclusion, these data demonstrate proof of principle for the therapeutic potential of systemic lentiviral vector delivery of the BiP transgene leading to immunoregulation of arthritis by induction of soluble CTLA-4 and suppression of IL-17A production.
Keywords: BiP, collagen-induced arthritis, gene therapy, lentiviral vector
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease characterized primarily by a destructive inflammatory polyarthritis leading to joint failure, deformity and disability 1. A growing armamentarium of biological therapies exist to treat ongoing disease and prevent RA progression; however, gene therapy has the potential to be superior to conventional pharmacological therapy for RA. Cis and trans acting regulatory elements may allow therapeutic gene expression to be placed under anatomical control or responsive to stress and inflammation, thus minimizing unwanted immunosuppression following systemic delivery 2–4. Using a variety of viral and non-viral vectors, gene therapy has already delivered promising results in disease models of inflammatory arthritis 5–7 and the rheumatology clinic 8.
Binding immunoglobulin protein (BiP/GRP78) is a highly conserved endoplasmic reticulum chaperone that displays potent extracellular anti-inflammatory and immunomodulatory activity in both mouse and man on release from stressed cells 9. Recombinant human BiP (rhuBiP) binds to an unidentified receptor expressed predominantly on the surface of human peripheral blood monocytes and induces an anti-inflammatory gene expression profile dominated by interleukin (IL)-10 secretion, attenuation of tumour necrosis factor (TNF)-α production and reduced CD86 and human leucocyte antigen D-related (HLA-DR) expression 10. Human monocyte-derived dendritic cells treated with rhuBiP during their differentiation fail to fully mature and induce cytotoxic T lymphocyte antigen-4 (CTLA-4)+ regulatory T cell development 11. Moreover, rhuBiP has demonstrated therapeutic efficacy in a number of animal models of RA, including murine collagen-induced arthritis (CIA) 12, rat adjuvant arthritis 13 and the xenogeneic model of rheumatoid synovial membrane transplanted into severe combined immunodeficient mice 14. Previous gene transfer experiments have shown BiP to have immunomodulatory and protective effects in a model of islet transplantation 15. Accordingly, we have proposed that BiP be classed within a family of stress proteins called the resolution-associated molecular patterns, molecules that are released from stressed and necrotic cells that act as molecular counterpoints to damage-associated molecular patterns, feeding pro-resolution signals into immunological networks 16.
In this study we investigated the therapeutic potential of in-vivo lentiviral vector delivery of the murine BiP transgene (Lenti-mBiP) compared with the control green fluorescent protein (GFP) transgene in the mouse model of CIA. Lenti-mBiP-treated mice showed reduced pathology, disease markers and inflammatory mediators when compared with Lenti-GFP-treated animals. Possible mechanisms of BiP-mediated suppression of arthritis were also investigated.
Materials and methods
Preparation and production of protein and lentiviral vectors
Murine BiP (mBiP) cDNA (NCBI Accession number: BC050927) was acquired from the Mammalian Gene Collection (RZPD, Berlin, Germany). Quikchange site-directed mutagenesis (Stratagene, Amsterdam, the Netherlands) was used to exclude the 3′-Lys-Asp-Glu-Leu (KDEL) endoplasmic reticulum targeting sequence facilitating exaggerated BiP secretion.
Lentiviral vectors
For gene therapy application the pRRLSIN18 third-generation, self-inactivating, HIV-1 lentiviral vector was used, as described previously 17, and contained either an enhanced GFP reporter gene or a KDEL– murine BiP cDNA under control of the hCMV promoter. Vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped recombinant lentiviral vector particles were produced by bulk transient transfection of human embryonic kidney (HEK) 293T cells, as described previously 18,19. Recombinant lentivirus was aliquoted and stored in OptiMEM (Invitogen, Paisley, UK) at −80°C. Titration of lentivirus was carried out by transduction of HEK293T cells in the presence of polybrene, as described previously 18,19. The titres of vector preparations without fluorescent transgenes were determined by quantitative polymerase chain reaction (PCR) relative to a preparation of lentivirus of known titre. In these cases, 100 ng of total genomic DNA was amplified using primers and probes directed against the lentiviral vector long terminal repeats (LTR) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal reference standard 19. Culture supernatants were harvested and tested for secreted mBiP by enzyme-linked immunosorbent assay (ELISA).
Validation of mBiP function
Peripheral blood mononuclear cells (PBMC) from healthy, consenting volunteers were separated by density gradient centrifugation using lymphoprep (Axis-Shield Diagnostics, Dundee, UK) and cultured in RPMI supplemented with 10% fetal calf serum (FCS); 1 × 106 cells were cultured with or without 20 μg/ml rhuBiP or mBiP. Supernatants were harvested after 24 h and IL-10 and TNF-α concentrations determined by ELISA. Human studies had the full approval of the Guy's Hospital Ethical Committee.
Collagen-induced arthritis model
CIA was induced in 8–12-week-old dilute brown non-Agouti/1 (DBA/1) mice (Harlan, Oxfordshire, UK) as described previously and clinical arthritis scored in a blinded fashion 12. Animals were allocated randomly to treatment or control groups. In the treatment groups, mice were given a single intraperitoneal injection with either 1 × 107 (low-dose study) or 2·5 × 107 (high-dose study) infectious lentiviral particles diluted in 100 μl phosphate-buffered saline (PBS) at arthritis onset. Arthritis onset was defined by a mean clinical score of 1 in the low-dose study (performed at Wichita State University) and a mean clinical score of 2·5 in the high-dose study (performed at King's College London). All animals were maintained in humane conditions, as dictated by local procedures and the US Animal Welfare Act 1968 and/or the King's College London Institutional Animal Care and Ethics Review Committee. All procedures were conducted in accordance with UK Home Office guidelines and UK Home Office project licence number PPL 70/6547.
Immunological analysis
Single-cell suspensions of spleen and inguinal lymph node cells were prepared as described previously 12. For stimulation assays, cells (2·5 × 106 cells/well) were cultured in 48-well plates (Appleton Woods, Birmingham, UK) in a final volume of 1 ml RPMI supplemented with 10% heat-inactivated FCS and stimulated with 2 μg/ml concanavalin A (ConA) or 40 μg/ml collagen type II (CII). Flow cytometry was used to determine the percentage of CD3+, CD4+, CD8+, B220+ and CD11b+ cells comprising each culture; no differences were observed between immune cell subpopulations and the cultures. In ex-vivo co-culture cytokine production experiments, 1·25 × 106 CIA responder splenocytes were co-cultured with 1·25 × 106 Lenti-BiP-, Lenti-GFP- or control-treated splenocytes and stimulated with 40 μg/ml CII. Cytokine secretion from cell cultures was determined at 72 h using the previously described carboxyethyl lysine ELISA (CelELISA) technique 20.
Primed animals for in-vitro co-culture experiments
DBA/1 mice were primed subcutaneously with either CII emulsified in complete Freund's adjuvant (CFA) (100 μg CII) or rhuBiP (100 μg rhuBiP) and left for 12 days. After 12 days, spleen and lymph nodes were dissected from euthanized mice and single-cell suspensions prepared. The cells were either cultured separately or at a 2:1 CII : BiP-primed cell ratio, respectively, and restimulated with CII (40 μg/ml) in the presence or absence of neutralizing antibody to CTLA-4 (1·5 μg/ml) (BD Pharmingen, Oxford, UK) or an isotype control (1·5 μg/ml) (eBioscience, Hatfield, UK). Cytokine secretion from cell cultures was determined at 72 h using the previously described CelELISA technique 20. Some cells were also removed and prepared for the flow-cytometric analysis of intracellular staining for CTLA-4.
Histological analysis
Mouse paws were removed and stored in 10% neutral buffered formalin solution (Surgipath, Richmond, IL, USA). Decalcification, sectioning, staining and histological analysis was performed independently (Professor Paul Wooley's laboratory, Wichita State University, KS, USA), as described previously 21. Sections were stained with haematoxylin and eosin to assess inflammation. Histological scores were confirmed by light microscopy using previously described parameters 21.
Serological analysis
Serum was collected at the beginning of CIA experiments and terminal exsanguination performed at the close of the CIA study. Total anti-CII immunoglobulin (Ig)G and anti-BiP IgG was determined by ELISA, as described previously 12,22.
Soluble CTLA-4 ELISA
ELISA plates (Nunc Maxisorp) were coated with anti-CTLA-4 capture antibody (2 μg/ml) (clone 9H10; Pharmingen, Oxford, UK) in carbonate buffer (0·1 M, pH 9·6) overnight at 4°C. Plates were washed once with Hanks's balanced salt solution (HBSS; PAA Laboratories, Yeovil, UK), blocked with 3% PBS–BSA for 1 h at 37°C then washed twice with HBSS. Serum samples (1:4 dilution in HBSS) were added and incubated overnight at 37°C in a humidified atmosphere. Plates were washed twice with PBS-Tween 20, before the addition of biotin-conjugated anti-sCTLA-4 (1 μg/ml) (clone UC10-4B9; eBioscience). Following incubation for 2 h at room temperature plates were washed with PBS-Tween 20. Extravidin–alkaline phosphatase (Sigma, Poole, UK) was added for 90 min at room temperature. Finally plates were washed with PBS-Tween 20 before the addition of phosphatase substrate (Sigma, Poole, UK). The plates were developed in the dark for 24–120 h. A culture control supernatant from a ConA-stimulated mouse cell culture was arbitrarily given the concentration of 1000 relative units (RU) and diluted to give a standard curve. Optical density (OD) was read at 410 nm.
Statistical analysis
Data were collected and analysed using both Microsoft Excel and GraphPad Prism versions 4 and 5. Statistical analysis between treatment groups was carried out using Kruskal–Wallis non-parametric analysis of variance (anova) (comparison of cohorts of mice treated in vivo with saline, Lenti-GFP or Lenti-BiP) and two-tailed unpaired Student's t-test (individual treatment groups in ex-vivo and in-vitro serological and immunological experiments). P-values of less than 0·05 were regarded as significant.
Results
Biological activity of mBiP gene product
Supernatants from cells transfected with the mBiP gene were collected during 96 h and showed clear accumulation of secreted mBiP, in comparison with untransfected cells and those transfected with the empty vector (Fig. 1a). Vector-derived mBiP demonstrated similar immunological characteristics as rhuBiP. Although rhuBiP induced significantly higher concentrations of IL-10 than mBiP at all serial dilutions, the pattern of cytokine induction, including the IL-10 : TNF-α ratios, were not significantly different between mBiP and rhuBiP or between any serial dilution of either proteins (Fig. 1b). mBiP and rhuBiP also stimulated equivalent concentrations of TNF-α and IL-10 from murine spleen and lymph node cells after 24 h incubation (Fig. 1c). Collectively, this was taken as evidence that vector produced secreted mBiP, with immunological properties similar to an established immunoregulatory protein (rhuBiP).
Figure 1.
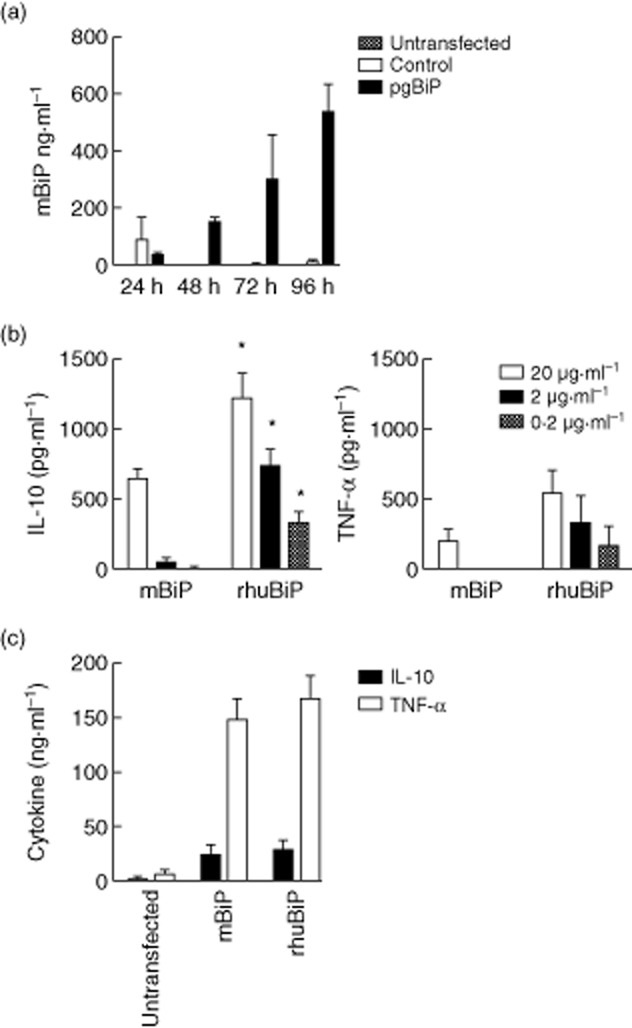
The binding immunoglobulin protein (BiP) transgene induced biologically active BiP protein production: (a) 5 × 104 human embryonic kidney (HEK) 293T cells were transfected with empty vector or pgBiP. Serial BiP concentration in culture supernatants were measured at 24, 48, 72 and 96 h after transfection by enzyme-linked immunosorbent assay (ELISA). (b) Serial dilutions of mBiP or recombinant human (rhu)BiP were used to stimulate 1 × 106 cultured human peripheral blood mononuclear cells (PBMCs). The production of cytokines, interleukin (IL)-10 and tumour necrosis factor (TNF)-α, induced by the addition of mBiP or recombinant human (rhu) BiP protein, was measured at 24 h. (c) Mixed splenocytes/lymph nodes (2·5 × 106) from dilute brown non-Agouti/1 (DBA/1) mice were stimulated with 20 μg of rhuBiP or mBiP. Induced IL-10 and TNF-α were measured at 24 h. Significance was determined by Student's unpaired t-test. *P < 0·05.
Systemic BiP gene therapy is therapeutic in CIA
Low-dose study
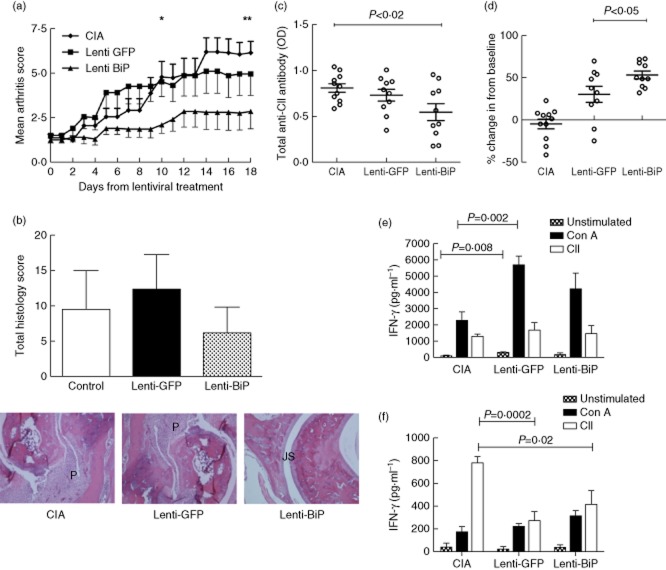
Treatment of diseased animals with Lenti-mBiP demonstrated significant therapeutic efficacy when compared with the untreated CIA control mice and those that received Lenti-GFP (Fig. 2a). Statistically significant suppression of arthritis scores was evident between the groups treated with vehicle and Lenti-mBiP on days 10 and 18. There was no significant difference between the Lenti-GFP and Lenti-mBiP groups. None the less, histological scores revealed clear differences in the severity of joint damage when comparing the paws from animals with median severity of clinical arthritis from each group (Fig. 2b). Joints from the CIA and Lenti-GFP control groups show pannus (P) invasion and cartilage destruction in contrast to those from Lenti-mBiP animals, where the joint space and cartilage remain relatively well preserved (Fig. 2b).
Figure 2.
Low-dose lentiviral vector containing the murine binding immunoglobulin protein (Lenti-mBiP) treatment improves histological and serological parameters of collagen-induced arthritis. Dilute brown non-Agouti/1 (DBA/1) mice (n = 10 or 11/group) were either untreated or treated with 1 × 107 infectious viral vector particles of either Lenti-green fluorescent protein (GFP) or Lenti-mBiP administered intraperitoneally at disease onset. (a) Clinical arthritis scores were assessed regularly up to day 18 post-treatment. Data represents the mean and standard error of the mean (*P < 0·03 Lenti-GFP versus Lenti-BiP; **P < 0·01 CIA versus Lenti-BiP; significance was determined using Kruskal–Wallis one-way analysis of variance) (b) Haematoxylin and eosin (H&E) staining (magnification ×100) of representative animals with median pathology in the hind paws from the different treatment groups. P = pannus, JS = normal joint space. A histogram shows the mean and standard deviation of the histological scores for each group of five animals. (c) At termination the serum level of total anti collagen type II (CII) immunoglobulin (Ig)G antibodies was measured by enzyme-linked immunosorbent assay (ELISA) and (d) the percentage increase in anti-BiP IgG levels from the individual baseline level at the start of the study was recorded. Mixed lymph node and splenocytes (2·5 × 106) were cultured and restimulated with 40 μg/ml CII or 2 μg/ml concanavalin A (ConA). Interferon (IFN)-γ (e) and interleukin (IL)-10 (f) concentrations in culture supernatants were determined at 72 h by carboxyethyl lysine ELISA (CelELISA) (n = 5/group). Statistical significance was analysed using GraphPad software and significance was determined using unpaired two-tailed Student's t-test. Significance was taken as <0·05 and the values are as shown.
Furthermore, analysis of serum antibodies to CII confirmed that treatment with Lenti-mBiP significantly lowered the antibody titre compared with CIA control mice (P < 0·02). Although there was a trend, there was no significant difference in antibody levels between the Lenti-GFP and the Lenti-mBiP groups (Fig. 2c).
Systemic lentiviral expression of a soluble transgene product in mice has previously generated serum concentrations in the pg/ml range 23. In our study, mBiP was not detectable in the pretreatment state or in any of the treatment groups (data not shown). This most probably represents the relatively poor sensitivity of the available ELISA for mBiP, which can detect only to a minimum ng/ml level 23. Additional evidence that the mBiP transgene was active was provided by the serum anti-BiP antibody levels, which were raised significantly in the Lenti-mBiP group compared with the CIA group (P < 0·05) after individual normalization of pretreatment to post-treatment levels of anti-BiP antibodies (Fig. 2d). This contrasted with a small but insignificant increase in the anti-BiP antibody titre in the Lenti-GFP mice when compared with the CIA group. Anti-BiP antibodies titres did not correlate with final clinical disease activity scores, suggesting that these effects were mainly a consequence of in-vivo cell transduction and transgene expression from the lentiviral vector.
Overall, this low-dose study demonstrated that the Lenti-mBiP transgene was active and had the potential to modulate clinical and immunological parameters of CIA. However, it was not possible to exclude a generic effect of the lentiviral vector on CIA development, as there were no significant differences in arthritis between the two lentiviral groups. Indeed, preliminary evidence suggested that treatment of CIA with a lentivirus, regardless of the expressed transgene, led to an exaggerated interferon (IFN)-γ response to mitogenic stimulation by ConA (Fig. 2e,f) (CIA: 2250 ± 553 pg/ml versus Lenti-GFP: 5704 ± 536 pg/ml versus Lenti-mBiP: 4225 ± 975; n = 5 in each group; P < 0·05 Lenti-GFP versus CIA) and a suppression of IL-10 responses to CII restimulation (CIA: 780·4 ± 54 pg/ml versus Lenti-GFP: 273 ± 171 pg/ml versus Lenti-mBiP 416 ± 263, n = 5 in each group; P < 0·05 Lenti-GFP versus CIA and Lenti-BiP versus CIA). Therefore, we sought evidence that the mBiP transgene had a specific therapeutic effect following treatment.
High-dose study
Animals were dosed when mild arthritis had developed (clinical score = 2·5). Treatment with Lenti-mBiP delayed the clinical progression of CIA from day 5 onwards compared to animals receiving the Lenti-GFP vector. Disease scores were reduced significantly compared to animals receiving the Lenti-GFP control after day 19 of the study (day 19, P = 0·03, day 21, P = 0·002) (Fig. 3a).
Figure 3.
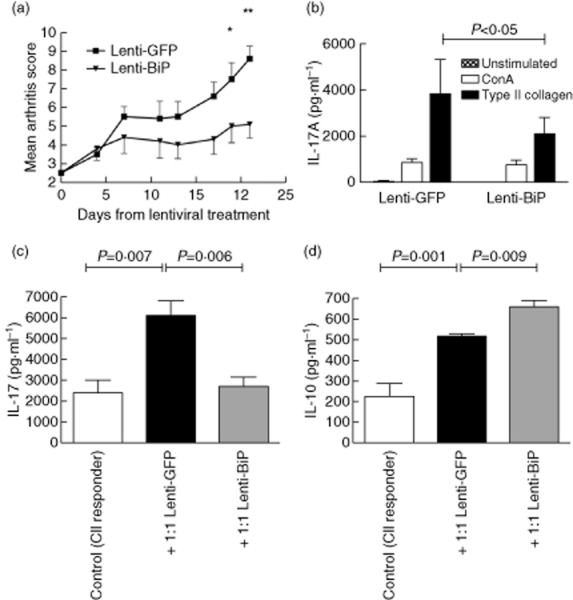
High-dose lentiviral vector containing the murine binding immunoglobulin protein (Lenti-mBiP) treatment ameliorates collagen-induced arthritis (CIA) and suppresses collagen-specific interleukin (IL)-17 responses: dilute brown non-Agouti/1 (DBA/1) mice (n = 10/group) were treated with 2·5 × 107 infectious viral particles of either Lenti-green fluorescent protein (GFP) (Lenti-GFP) or Lenti-mBiP administered intraperitoneally at onset of mild arthritis (clinical score 2). (a) Clinical arthritis scores were assessed regularly for a further 21 days after treatment. The mean ± standard error of the mean is shown. *P < 0·05 Lenti-GFP versus Lenti-BiP; **P < 0·005 Lenti-GFP versus Lenti-BiP (significance was determined using Kruskal–Wallis one-way analysis of variance). (b) Spleens and lymph nodes were dissected at termination and 2·5 × 106 cells were either unstimulated or stimulated with concanavalin A (ConA) (2 μg/ml) or type II collagen (CII)(40 μg/ml) as shown. Single-cell cultures of mixed splenocytes and lymph node cells from either control collagen-induced arthritis (CIA), Lenti-GFP or Lenti-mBiP individual mice were used in co-culture with CII primed cells (1·25·106). Cells were stimulated for 72 h with CII (40 μg/ml). (c) IL-17A and (d) IL-10 secretion was measured by carboxyethyl lysine enzyme-linked immunosorbent assay (CelELISA). The mean ± standard error of the mean is shown. (n = 4/group). Statistical significance was analysed using GraphPad software and significance was determined using the unpaired two-tailed Student's t-test. Significance was taken as <0·05 and the values are as shown.
Systemic lentiviral gene therapy with mBiP is associated with reduced IL-17A responses to type II collagen
IL-17A production was reduced significantly on CII restimulation of spleen and lymph node cells taken from animals treated with Lenti-BiP compared with those from Lenti-GFP-treated animals (Fig. 3b).
Further analysis showed the ability of Lenti-mBiP cells to regulate the production of IL-17A (Fig. 3b,c). On restimulation with CII, Lenti-mBiP cells produced significantly less IL-17A (P = 0·006), and in co-culture with CII primed responder cells production of IL-17A remained low (Fig. 3c). Furthermore, co-culturing Lenti-BiP-treated cells with CII primed responder cells was associated with increased IL-10 secretion (Fig. 3d). These effects appear unique and attributable to conditioning by the mBiP transgene. In both experiments, the co-culture of cells exposed to lentiviral vectors in vivo with CII-primed responder cells led to significant increases in the levels of both IL-10 and IL-17 produced ex vivo. This strongly suggests, as before, that exposure to the lentivirus itself alters the immunological parameters of the collagen-induced arthritis model and of lymphocytes derived from treated animals.
Human in-vitro studies have shown that BiP can induce CTLA-4+ regulatory T cells 11. We therefore investigated whether soluble CTLA-4 (sCTLA-4) was detectable in mouse serum at the termination of the study. Strikingly, the Lenti-mBiP group showed significantly higher levels of sCTLA-4 compared with the Lenti-GFP group (P = 0·015) (Fig. 4a).
Figure 4.
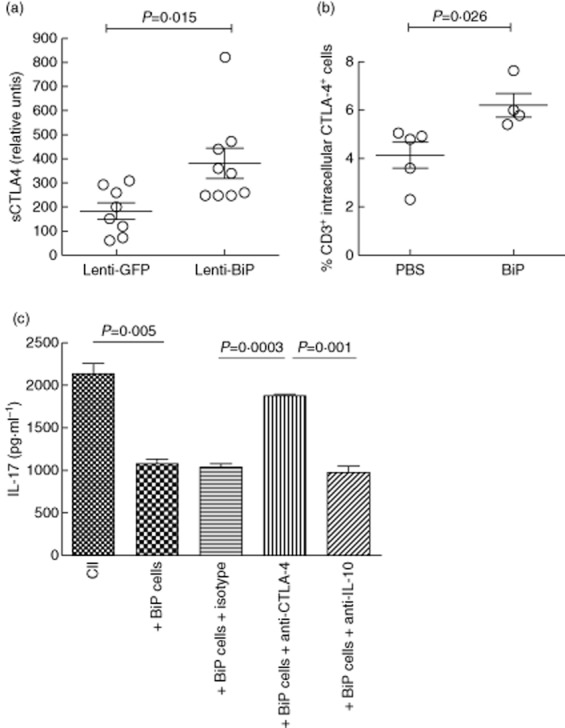
Neutralization of cytotoxic T lymphocyte antigen-4 (CTLA-4) and interleukin (IL)-10 affect interferon (IFN)-γ and IL-17 production, respectively. (a) The total soluble CTLA-4 serum concentration was determined for each mouse at termination of the high-dose study by ELISA (n = 6–9/group). Single-cell cultures of mixed splenocytes and lymph node cells from individual mice were used throughout the following experiments. Cells isolated from binding immunoglobulin protein (BiP)-primed animals, given a subcutaneous injection of BiP (200 μg/ml) and culled after 12 days, were stained for intracellular cytokine expression of (b) ex-vivo CTLA-4 on CD3+ T cells from spleens and lymph nodes. (c) Collagen type II (CII) primed cells were co-cultured with cells from BiP primed animals in the absence and presence of neutralizing anti-CTLA-4 (1·5 μg/ml), anti-IL-10 (1·5 μg/ml) and isotype control (1·5 μg/ml) and stimulated for 72 h with CII (40 μg/ml). Cytokine production was measured by carboxyethyl lysine enzyme-linked immunosorbent assay (CelELISA) (n = 5/group). The mean ± standard error of the mean is shown. Statistical significance was analysed using GraphPad software and significance was determined using the unpaired two-tailed Student's t-test. Significance was taken as <0·05 and the values are as shown.
Complementary experiments were undertaken to investigate whether the cytokine changes observed following Lenti-mBiP treatment could be recapitulated by direct priming of animals with rhuBiP in vivo. BiP-primed mice showed an elevated frequency of CD3+ T cells expressing intracellular CTLA-4 when compared with control animals (Fig. 4b).
Role of soluble CTLA-4 and IL-10 in BiP primed cell function
To determine the importance of sCTLA-4 and/or IL-10 to the system, mixed spleen and lymph node cells from animals primed with either CII or rhuBiP were co-cultured at a 2:1 ratio, respectively, and stimulated with CII in the absence and presence of neutralizing antibodies for CTLA-4 and IL-10. The addition of BiP-primed cells in co-culture with CII-primed cells significantly reduced production of IL-17A. The neutralization of CTLA-4 in these cultures increased the production of IL-17A to that of the CII primed cells alone (Fig. 4c). IL-17A inhibition was entirely consequent on CTLA-4 produced by the BiP-primed cells, as no effect on IL-17A production was observed following neutralization of CTLA-4 in cultures containing CII primed cells only (data not shown).
Discussion
This study demonstrates the therapeutic potential of systemic lentiviral delivery of an mBiP transgene in murine CIA. Striking similarities were observed between the properties of mBiP and rhuBiP in standardized and well-defined assays of BiP's immunological activity 24. Subtle differences were observed in the total concentration of IL-10 generated from human PBMC following rhuBiP and mBiP stimulation. These differences were limited to IL-10, and no significant differences were observed in the ratio of IL-10 to TNF-α. No significant differences were observed when rhuBiP or mBiP were used to stimulate murine cells. This is not surprising, as mBiP and rhuBiP share 99·4% homology at an amino acid level and suggest that the immunological activity of BiP may be conserved across phyla. Those differences observed may be due, in part, to the eukaryotic or prokaryotc cell source of the protein and the consequent differences in folding and glycosylation.
Important differences between the actions of intracellular and extracellular BiP should be considered with respect to the removal of the ER localizing KDEL sequence. The previously described immunological properties of extracellular BiP are consistent with extracellular BiP acting on a cell surface receptor 10,11. These properties are quite distinct from those displayed by intracellular, endoplasmic reticulum localized BiP, which regulates the unfolded protein response and induces cytoprotective and anti-apoptotic signals when cells are challenged by endoplasmic reticulum stress 25. Thus, intracellular BiP may augment chronic inflammation through anti-apoptotic protection of inflammatory cells, such as the rheumatoid fibroblast-like synoviocyte 26 yet, as an extracellular protein, regulate inflammation on secretion from stressed cells 16. A more complete dissection of the relative contributions of intracellular and extracellular BiP to the pathogenesis and resolution of chronic inflammation will require greater understanding of cells transduced by lentiviral vectors, the receptor or receptors to which BiP binds on the cell surface and the downstream signalling pathways employed. Nevertheless, we have demonstrated that removal of the KDEL facilitates BiP secretion from transfected cells in vitro; the raised concentrations of anti-BiP antibodies in Lenti-mBiP-treated animals provides indirect evidence of vector-derived BiP secretion in vivo. However, any increase in serum BiP below the detection range of the BiP ELISA (ng/ml) proved that lentiviral vectors rarely induce serum concentrations of secreted proteins in excess of pg/ml quantities 23. Previous studies have suggested that trafficking immune cells are transduced by intraperitoneal delivery of VSV-G pseudotyped lentivirus 23. With respect to this study, we propose that vector-derived secreted BiP conditions transduced leucocytes towards an anti-inflammatory phenotype.
Extracellular rhuBiP has both prophylactic and therapeutic action in CIA 13. In two independent experiments, Lenti-mBiP treatment was associated with amelioration of clinical, serological and immunological manifestations of disease, consistent with our previous work 13. The subtle differences in the severity of overall arthritis in each experiment are due probably to the well-reported interexperiment variability within the CIA model 27. A contributory factor to the lowered anti-CII levels observed in Lenti-mBiP-treated mice might be the relative lack of IL-17A in the Lenti-mBiP-treated mice 28. The close association between IL-17 and anti-CII antibody production was apparent when CIA was induced in IL-17-deficient mice 29, resulting in reduced production of anti-CII antibodies and milder disease. IL-17 30, especially IL-17A 31, but also IL-17B and IL-17C 32, are important pathogenic proinflammatory mediators in arthritis 33. Spleen and lymph node cells derived from the Lenti-mBiP-treated mice inhibited IL-17A production significantly when compared with cells from Lenti-GFP-treated animals. Concomitantly, these Lenti-mBiP treated cells up-regulated IL-10 in co-culture with CII primed cells. These data support the counter-regulation of IL-10 and T helper type 17 (Th17)/IL-17A, as reported in both CIA 6 and RA 34. Of note is the previously reported observation that rhuBiP-primed cells ameliorate CIA following adoptive transfer, suggesting that BiP-primed cells have intrinsic regulatory properties 12.
The negative co-stimulatory signal of CTLA-4 has already been successfully exploited experimentally, using local and systemic gene therapies in CIA 35,36, and clinically in RA 37. Both rhuBiP and Lenti-mBiP have shown potential to induce CTLA-4 production. Further proof of the regulatory importance of this was provided by the in-vitro neutralization of CTLA-4, which reversed the inhibitory effect of BiP on IL-17A production. This counter-regulation between IL-17A and sCTLA4 has been reported previously by Ying . 27 in BALB/c mice, where the blocking of the CTLA-4–CD80 interaction potentiated Th17 differentiation. In the current study the induction of CTLA-4 is dependent upon the mBiP transgene, rather than the lentiviral construct.
Although thought initially to be less immunogenic than other viral vectors (e.g. adenoviruses), there is a strong body of evidence suggesting that immune responses are mounted to recombinant lentiviral vectors in vivo 38,39. Our data demonstrate that varicella zoster virus (VZV)-G pseudotyped lentiviral vectors strongly induce IFN-γ secretion in CIA and suppress antigen-specific IL-10 secretion. The role of IFN-γ in the pathogenesis of CIA is complex; in sum, it is believed that early exposure to IFN-γ in the CIA model leads to exacerbation of disease pathogenesis 40, while later exposure to IFN-γ leads to amelioration of disease, possibly via induction of indoleamine-2,3-dioxygenase 41. Although IFN-γ is associated with inflammatory disease, the IFN-γ receptor knock-out mouse perversely develops more severe arthritis than the wild-type, similar to the IL-10 knock-out mouse 42 43. Both Lenti-GFP and Lenti-mBiP vectors induce IFN-γ: cells from Lenti-GFP-treated animals have significantly higher baseline and induced IFN-γ secretion in response to mitogenic stimulus, associated with a trend towards early worsening of disease activity between days 4 and 8 post-arthritis onset, but marginally improved arthritis scores compared to CIA controls at later time-points. Lenti-mBiP-treated animals show non-significant trends towards increased IFN-γ secretion, suggesting that the mBiP transgene is exerting an immunomodulatory effect over vector-induced IFN-γ secretion. The results from co-culture experiments with CII responder cells initially appear counterintuitive, but could be explained by fundamental changes in the expression of immunologically relevant cell surface molecules [e.g. CD80/CD86/major histocompatibility complex (MHC)] induced directly or indirectly by the lentiviral vector subsequently modulating cell–cell interactions and consequent cytokine profiles. The consequences of lentiviral vector-induced IFN-γ are complex and probably alter the immunological and clinical development of the CIA model, and we urge caution when attributing immunological mechanisms to transgenes contained within future lentiviral-based gene therapies.
In summary, this study extends previous observations of the immunoregulatory properties of extracellular BiP. We demonstrate that lentivirus-derived BiP reduces disease activity in CIA, with associated up-regulation of sCTLA-4 and suppression of IL-17. Moreover, we provide evidence that CTLA-4 plays an important mechanistic role in BiP immunoregulation using the clinically relevant rhuBiP molecule. Currently, translation of BiP into a gene therapy for rheumatic disease is premature, and further development would necessitate a more complete understanding of the delicate balance between intracellular and extracellular BiP and the development of a suitable, safe gene therapy vector for human use.
Acknowledgments
This work was funded by the Oliver Bird Trust of the Nuffield Foundation. We would like to thank the BRC for the use of equipment during these studies.
Disclosure
G. S. P. and V. M. C. are shareholders in a non-profit-making company which has the patent for the use of BiP in the treatment of rheumatoid arthritis. No other authors have any conflicts of interest.
Author contributions
A. M. S. performed the experiments (UK and USA), helped to design the study and wrote the paper, L. S. K. helped to design the study, provided reagents and reviewed the paper; M. A. helped to design the study; P. H. W. helped to design the study (USA); G. S. P. helped to design the study; H. L. C. helped to perform the animal experiments (UK) and design the study; S. J. T. helped to perform the animal experiments (UK) and design the study; V. M. C. performed some experiments, designed the study and wrote the paper.
References
- Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907–927. doi: 10.1016/j.berh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Dong D, Dubeau L, Bading J. Spontaneous and controllable activation of suicide gene expression driven by the stress-inducible grp78 promoter resulting in eradication of sizable human tumors. Hum Gene Ther. 2004;15:553–561. doi: 10.1089/104303404323142006. [DOI] [PubMed] [Google Scholar]
- Geurts J, Arntz OJ, Bennink MB, Joosten LA, van den Berg WB, van de Loo FAJ. Application of a disease-regulated promoter is a safer mode of local IL-4 gene therapy for arthritis. Gene Ther. 14:1632–1638. doi: 10.1038/sj.gt.3303022. [DOI] [PubMed] [Google Scholar]
- Miagkov A, Varley A. Endogenous regulation of a therapeutic transgene restores homeostasis in arthritic joints. J Clin Invest. 109:1223–1229. doi: 10.1172/JCI14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Thompson SJ, Wooley PH, Panayi G, Klavinskis LS. Immune modulation of collagen-induced arthritis by intranasal cytokine gene delivery: a model for the therapy of rheumatoid arthritis. Arthritis Rheum. 2007;52:3761–3771. doi: 10.1002/art.21473. [DOI] [PubMed] [Google Scholar]
- Henningsson L, Eneljung T, Jirholt P. Disease-dependent local IL-10 production ameliorates collagen induced arthritis in mice. PLOS ONE. 2002;7:e49731. doi: 10.1371/journal.pone.0049731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalon Z, Bruckheimer EM, Lustig KH, Burstein H. Long-term suppression of experimental arthritis following intramuscular administration of a pseudotyped AAV2/1-TNFR: Fc Vector. Mol Ther. 2012;15:264–269. doi: 10.1038/sj.mt.6300043. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Wei N, Fudman EJ. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J Rheumatol. 37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- Shields AM, Thompson SJ, Panayi GS, Corrigall VM. Pro-resolution immunological networks: binding immunoglobulin protein and other resolution-associated molecular patterns. Rheumatology (Oxf) 2010;51:780–788. doi: 10.1093/rheumatology/ker412. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Bodman-Smith MD, Brunst M, Cornell H, Panayi GS. The stress protein, BiP, stimulates human peripheral blood mononuclear cells to express an anti-inflammatory cytokine profile and to inhibit antigen presenting cell function: relevance to the treatment of inflammatory arthritis. Arthritis Rheum. 2004;50:1167–1171. doi: 10.1002/art.20134. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Vittecoq O, Panayi GS. Binding immunoglobulin protein-treated peripheral blood monocyte-derived dendritic cells are refractory to maturation and induce regulatory T-cell development. Immunology. 2009;128:218–226. doi: 10.1111/j.1365-2567.2009.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie RJ, Myers LK, Wooley PH. Treatment of murine collagen-induced arthritis by the stress protein BiP via interleukin-4-producing regulatory T cells: a novel function for an ancient protein. Arthritis Rheum. 2006;54:854–863. doi: 10.1002/art.21654. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Bodman-Smith MD, Fife MS. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001;166:1492–1498. doi: 10.4049/jimmunol.166.3.1492. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Ochiai A, Matsuno H, Panayi GS, Corrigall VM. Binding immunoglobulin protein resolves rheumatoid synovitis: a xenogeneic study using rheumatoid arthritis synovial membrane transplants in SCID mice. Arthritis Res Ther. 2011;13:R149. doi: 10.1186/ar3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang P, Liu YQ. The immunosuppressive and protective ability of glucose-regulated protein 78 for improvement of alloimmunity in beta cell transplantation 2001. Clin Exp Immunol. 2007;150:546–552. doi: 10.1111/j.1365-2249.2007.03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AM, Panayi GS, Corrigall VM. Resolution-associated molecular patterns (RAMP): RAMParts defending immunological homeostasis? Clin Exp Immunol. 2002;165:292–300. doi: 10.1111/j.1365-2249.2011.04433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Sabatino G, Lombardo A, Boccaccio C, Naldini L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13:243–260. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- Talbot GE, Waddington SN, Bales O, Tchen RC, Antoniou MN. Desmin-regulated lentiviral vectors for skeletal muscle gene transfer. Mol Ther. 2010;18:601–608. doi: 10.1038/mt.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech JT, Bainbridge T, Thompson SJ. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman-Smith MD, Corrigall VM, Berglin E. Antibody response to the human stress protein BiP in rheumatoid arthritis. Rheumatology (Oxf) 2004;43:1283–1287. doi: 10.1093/rheumatology/keh312. [DOI] [PubMed] [Google Scholar]
- Delgado M, Toscano MG, Benabdellah K. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthritis Rheum. 2008;58:1026–1037. doi: 10.1002/art.23283. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Bodman-Smith MD, Brunst M, Cornell H, Panayi GS. Inhibition of antigen-presenting cell function and stimulation of human peripheral blood mononuclear cells to express an antiinflammatory cytokine profile by the stress protein BiP: relevance to the treatment of inflammatory arthritis. Arthritis Rheum. 2004;50:1164–1171. doi: 10.1002/art.20134. [DOI] [PubMed] [Google Scholar]
- Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- Yoo S-A, You S, Yoon H-J. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med. 2012;209:871–886. doi: 10.1084/jem.20111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- Ying H, Yang L, Qiao G. Cutting edge: CTLA-4-B7 interaction suppresses Th17 cell differentiation. J Immunol. 2010;185:1375–1378. doi: 10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Cooney LA, White P. Regulation of pathogenic IL-17 responses in collagen-induced arthritis: roles of endogenous interferon-gamma and IL-4. Arthritis Res Ther. 2009;11:R158. doi: 10.1186/ar2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Ther Adv Musculoskelet Dis. 2013;5:141–152. doi: 10.1177/1759720X13485328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Fujio K, Shoda H. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179:7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- Heo Y-J, Joo Y-B, Oh H-J. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett. 2010;127:150–156. doi: 10.1016/j.imlet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Quattrocchi E, Dallman MJ, Feldmann M. Adenovirus-mediated gene transfer of CTLA-4Ig fusion protein in the suppression of experimental autoimmune arthritis. Arthritis Rheum. 2000;43:1688–1697. doi: 10.1002/1529-0131(200008)43:8<1688::AID-ANR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ijima K, Murakami M, Okamoto H. Successful gene therapy via intraarticular injection of adenovirus vector containing CTLA4IgG in a murine model of type II collagen-induced arthritis. Hum Gene Ther. 2001;12:1063–1077. doi: 10.1089/104303401750214285. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Schiff M, Luggen M. Longterm safety and efficacy of abatacept through 5 years of treatment in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor inhibitor therapy. J Rheumatol. 2012;39:1546–1554. doi: 10.3899/jrheum.111531. [DOI] [PubMed] [Google Scholar]
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He J, Chang L-J. Alteration of T cell immunity by lentiviral transduction of human monocyte-derived dendritic cells. Retrovirology. 2004;1:37. doi: 10.1186/1742-4690-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissier M, Chiocchial G, Bessisl N, Hajna J, Garotta G. Biphasic effect of interferon-γ in murine collagen-induced arthritis. Eur J Immunol. 1995;25:1184–1190. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee J, Park M-K. Interferon gamma suppresses collagen-induced arthritis by regulation of Th17 through the induction of indoleamine-2,3-deoxygenase. PLOS ONE. 2013;8:e60900. doi: 10.1371/journal.pone.0060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C-Q, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- Johansson AC, Hansson AS, Nandakumar KS, Bäcklund J, Holmdahl R. IL-10-deficient B10.Q mice develop more severe collagen-induced arthritis, but are protected from arthritis induced with anti-type II collagen antibodies. J Immunol. 2001;167:3505–3512. doi: 10.4049/jimmunol.167.6.3505. [DOI] [PubMed] [Google Scholar]