Abstract
The complement system can be activated via the lectin pathway by the recognition molecules mannose-binding lectin (MBL) and the ficolins. Ficolin-2 exhibits binding against a broad range of ligands, including biomaterials in vitro, and low ficolin-2 levels are associated with increased risk of infections. Thus, we investigated the biocompatibility of the recognition molecules of the lectin pathway in two different types of cardiopulmonary bypass circuits. Bloods were drawn at five time-points before, during and postoperatively from 30 patients undergoing elective cardiac surgery. Patients were randomized into two groups using different coatings of cardiopulmonary bypass circuits, Phisio® (phosphorylcholine polymer coating) and Bioline® (albumin-heparin coating). Concentrations of MBL, ficolin-1, −2 and −3 and soluble C3a and terminal complement complex (TCC) in plasma samples were measured. Ficolin-3-mediated complement activation potential was evaluated with C4, C3 and TCC as output. There was no significant difference between the two circuit materials regarding MBL, ficolin-1 and −3. In the Bioline® group the ficolin-2 levels decreased significantly after initiation of surgery (P < 0·0001) and remained reduced throughout the sampling period. This was not seen for Phisio®-coated circuits. Ficolin-3-mediated complement activation potential was reduced significantly in both groups after start of operation (P < 0·0001), whereas soluble C3a and TCC in the samples were increased (P < 0·0001). Ficolin-2 was depleted from plasma during cardiac surgery when using heparin-coated bypass circuits and did not reach baseline level 24 h postoperation. These findings may have implications for the postoperative susceptibility to infections in patients undergoing extracorporeal circulation procedures.
Keywords: biocompatibility, cardiopulmonary bypass circuits, complement system, ficolin-2, lectin pathway
Introduction
The three ficolins, ficolin-1, -2 and -3, and mannose-binding lectin (MBL) are soluble pattern recognition molecules (PRMs), which upon binding to carbohydrate structures on, for example, foreign microorganisms or altered host cells can activate the complement system via the lectin pathway 1. Once the PRMs are bound to a ligand, the cascade is initiated by the activation of the MBL/ficolin-associated serine proteases (MASPs), which cleave and activate complement factors C4 and C2. This leads in turn to the formation of the C3 convertase, which cleaves and activates complement factor C3. Following amplification of the convertase activities and deposition of additional downstream complement components, the cascade culminates in the formation of the terminal C5b-9 complement complex (TCC) 2.
The use of biomaterials (e.g. stents, haemodialysis tubes, artificial implants, bypass circuits) in modern health technologies is expanding rapidly. This exposure of synthetic, foreign surfaces to the blood fluid and cells of the host calls for strict biocompatibility in order to avoid contact activation of the coagulation system and the complement system 3. In this study, we focused on the circuits used during cardiopulmonary bypass (CPB), where examples of some of the major complications are massive complement activation and inflammation and postoperative haemorrhage. The use of heparin coating in circuits has been reported independently to reduce both of these problems 4,5. Hence, the Bioline® circuit uses an albumin-heparin coating in order to reduce postoperative blood loss 5. Phisio® coating is produced to mimic the properties of living cells and is comprised of phosphorylcholine polymer, which has demonstrated reduced protein adsorption 6. However, the PRM of the complement system, ficolin-2, has been shown to bind to heparin 7, polysulphone haemodialysis tubes 8–10 and plasma polymerized vinyl pyrrolidone coating for biomaterials 11. The broad binding range of ficolin-2 is incompatible with the structural organization of the globular region of the molecule responsible for the ligand binding: different from the other PRMs, the fibrinogen-like domain of ficolin-2 contains four, instead of one, distinct binding grooves with distinct specificities 12. Altogether, this prompted us to investigate the biocompatibility between the recognition molecules of the lectin pathway and the routinely used Bioline® and Phisio® circuits, respectively. Samples from 30 patients undergoing elective heart surgery and randomized for the two different circuits have already been studied, but not for lectin pathway activity 13.
Materials and methods
Patients and blood samples
Patients and operation approaches have been described in detail previously 13. Thirty individuals undergoing elective cardiac surgery were randomized into two groups with 15 patients in each, to test the two different coatings of cardiopulmunary bypass circuits: Phisio® (Avant Oxygenator, Sorin-Dideco, Mirandola, Italy) and Bioline® (Quadrox oxygenator, Maquet Hirrlingen, Germany). Blood was drawn in ethylenediamine tetraacetic acid (EDTA) plasma tubes from each patient at five time-points: T0 = after induction of anaesthesia, T1 = 15 min after initiation of CPB, T2 = before terminating CPB, T3 = 2 h postoperatively and T4 = 24 h postoperatively. Samples were kept on ice for a maximum 30 min before centrifugation at 2000 g for 15 min at 4°C. The plasma was removed and stored at −80°C until analysis. The study was approved by the regional ethical committee of Oslo University Hospital, Oslo, Norway. All patients gave written informed consent.
Concentration of ficolins-1, -2, -3 and MBL
Plasma levels of ficolins-1, -2, -3 and MBL were quantified in specific sandwich enzyme-linked immunosorbent assays (ELISAs) as described previously 14–16. The assays were optimized for automated analysis in 384-well format on Biomek FX (Beckman Coulter, Fullerton, CA, USA). Briefly, Maxisorp microtitre plates (Nunc, Roskilde, Denmark) were coated with in-house-produced monoclonal mouse antibodies against the ficolins: FCN165, FCN216, FCN334, respectively, and HYB 131-01 (Bioporto, Gentofte, Denmark) against MBL. Diluted plasma samples were incubated for 3 h at room temperature, after which detection antibodies were added overnight: rabbit-α-ficolin-1 (HP9039; Hycult Biotech, Uden, the Netherlands), FCN219 biotinylated, FCN334 biotinylated and HYB 131-01 biotinylated. Secondary antibodies where coupled to horseradish peroxide (HRP) and ortho-phenylenediamine (OPD) was used as substrate for development. Finally, optical density (OD490–650 nm) was measured at 490 nm on Microplate KC4 Signature EL808.
Functional analysis of ficolins-2 and -3
Analysis of in-vitro residual complement activation mediated by ficolin-3 was detected by deposition of C4, C3 and TCC in indirect ELISA, as described previously 17. In brief, acetylated bovine serum albumin (acBSA) was used as a matrix in microtitre plates. Detection of ficolin-3 binding and downstream deposition of C4, C3 and TCC was detected with anti-ficolin-3 FCN334, rabbit anti-C4c (Dako, Glostrup, Denmark), rabbit anti-C3c (Dade-Behring, Marburg, Germany) and mouse anti-C5b-9 (Bioporto Diagnostics, Gentofte, Denmark), respectively. Ficolin-2 has been shown to bind to acBSA without mediating complement activation 18, and hence the binding of ficolin-2 to this matrix was assessed with anti-ficolin-2 FCN219. Plates were developed and read as described above. For all assays, a standard normal human serum pool was applied on each plate and set as 100% when calculating patient samples.
Classical, alternative and MBL pathway activity
Residual complement deposition capacity via the classical, lectin and alternative pathways was evaluated in ELISA as described in Seleen et al. 19, with few modifications. Wells for the classical pathway were coated with human serum albumin (HSA) and rabbit anti-HSA; mannan was used as a matrix for the MBL–lectin pathway and lipopolysaccharide (LPS) for the alternative pathway. Incubation times and latter steps were performed as described for the ficolin-3 assay and deposition of TCC was assessed as output.
C3a and soluble TCC
Plasma levels of the complement activation products C3a and soluble (s)TCC were quantified as described in Thiara et al. 13. In brief, the commercially available C3a EIA kit from Quidel Corporation (San Diego, CA, USA) was used for C3a. sTCC was measured in a sandwich ELISA with a monoclonal capture antibody against the neoepitope on C9 when built-in to the C5b-9 complex and a biotinylated anti-C6 as detection antibody. These results were mentioned briefly by Thiara et al. 13 but were not presented in detail, which is thus reported in the present paper.
Statistics
For comparison of the two circuit groups at T0, the Mann–Whitney U-test was applied. Differences between time-points within groups were analysed with the Kruskal–Wallis test. All statistical analyses were carried out with Graphpad Prism version 4·03 software.
Results
All measured values given were adjusted for haemodilution during surgery using the haematocrit value.
Level of MBL and ficolins
There was no significant difference between the two groups regarding baseline plasma levels of ficolins-1, -2, -3 and MBL at starting-time T0 (Fig. 1). Analysing the samples over time, there was a small but significant steady increase in the ficolin-1 level from T0 to T3 and then a decrease from T3 to T4 was observed (P < 0·05) (Fig. 1a). This course was observed for both Bioline® and Phisio®, with no significant difference between the groups (P > 0·05). For the ficolin-2 plasma level, there was a substantial reduction from T0 to T1 (P < 0·0001) in the Bioline® group; the concentration remained low and did not return to baseline level after 24 h (Fig. 1b). In comparison, there was no change in ficolin-2 concentration in the Phisio® group over time (P = 0·98). Ficolin-3 and MBL levels were stable throughout the surgery and there was no difference between groups (Fig. 1c,d).
Figure 1.
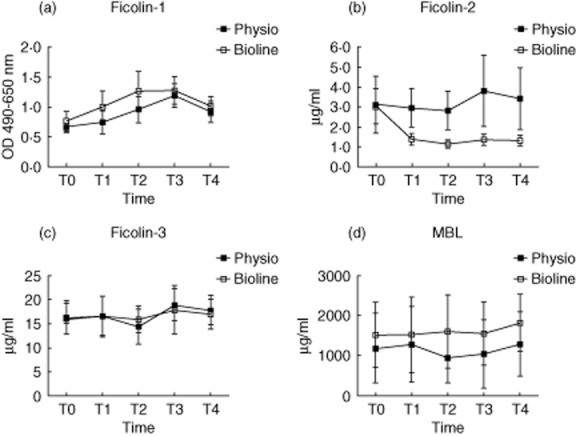
Plasma concentrations of ficolin-1 (a), ficolin-2 (b), ficolin-3 (c) and mannose-binding lectin (MBL) (d) at five predetermined time-points in patients undergoing cardiopulmonary bypass with Phisio®- (n = 15) or Bioline®-coated circuits (n = 15). There was no difference between the two groups at T0 for any of the proteins. The ficolin-2 plasma decreased significantly from T0 to T1 (P < 0·0001) in the Bioline® group and did not return to baseline level after 24 h, whereas the level was stable in Phiso® and the concentration stayed low. Bars show mean with 95% confidence intervals.
Binding capacity of ficolins-2 and -3
Based on the substantial and specific reduction in ficolin-2 in plasma, we investigated the in-vitro binding capacity of ficolins-2 and -3 to acBSA in ELISA. Consistent with the reduced concentration of ficolin-2 in plasma, the ficolin-2 binding capacity was markedly reduced in the Bioline® samples between T0 and T1 compared to Phisio® (P < 0·0001) (Fig. 2a). The binding capacity of ficolin-3 differed at T0 between the Phisio® (100·5%) and Bioline® groups (60·2%) (P = 0·02) (Fig. 2b). The difference was consistent over time and there was no change during the sampling period.
Figure 2.
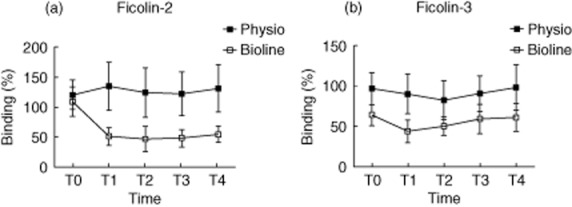
Binding capacity of ficolin-2 (a) and ficolin-3 (b) to acetylated bovine serum albumin (acBSA) at five predetermined time-points in patients undergoing cardiopulmonary bypass with Phisio®- (n = 15) or Bioline®-coated circuits (n = 15). At T0 the ficolin-3 binding capacity was significantly lower in the Bioline® group (P = 0·02). The difference was constant and no change in binding potential occurred over time in any of the groups. For ficolin-2 there was no difference at T0. However, the ficolin-2 binding capacity decreased significantly from T0 to T1 (P < 0·0001) in the Bioline® group and did not return to baseline level after 24 h, whereas the level was stable in Phiso® and the concentration stayed low. Bars show mean with 95% confidence intervals.
Complement activation capacity
In-vitro ficolin-3-mediated complement activity, measured as TCC deposition in ELISA (Fig. 3a), was also significantly lower in the Bioline® samples at starting-point T0 (P < 0·05), corresponding to the results for the ficolin-3 binding capacity. Results for ficolin-3-mediated deposition of upstream complement fragments C4 and C3 on acBSA were similar to those of TCC (data not shown). Apart from the starting-point, samples from Bioline® and Phisio® circuits showed the same trend for all complement activation capacity studies, i.e. ficolin-3–lectin pathway, MBL–lectin pathway, classical pathway and alternative pathway (Fig. 3a–d): from T0 the in-vitro complement activation capacity dropped to a minimum and increased slowly again over time. Similar to the ficolin-3 pathway, the MBL (Fig. 3b), the classical (Fig. 3c) and the alternative pathways (Fig. 3d) all displayed a significant drop in activity from T0 to T1 (P < 0·0001), but gradually returned towards baseline activity after 24 h (T4). Conversely, the in-vivo fluid phase complement activation products showed an inverse tendency with a gradual increase in C3a and sTCC with a return to baseline after 24 h (Fig. 3e–f). For both factors the plasma concentration peaked at the time for the T2 sample, and levels had normalized 24 h postoperatively. It has been documented previously that there was no difference between the two groups 13.
Figure 3.
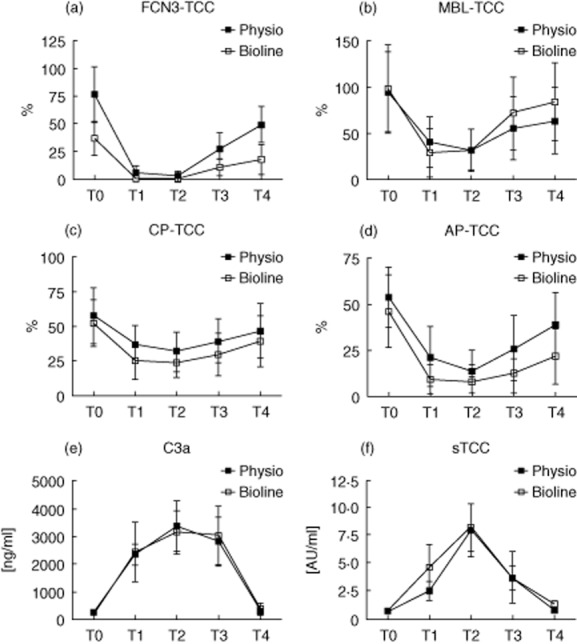
Complement activation capacity at five predetermined time-points in patients undergoing cardiopulmonary bypass with Phisio®- (n = 15) or Bioline®-coated circuits (n = 15). Deposition of terminal complement complex (TCC) via the ficolin-3 pathway (FCN3) (a), the lectin [mannose-binding lectin (MBL)] pathway (b), the classical pathway (CP) (c) and alternative pathway (AP) (d) all displayed a significant drop in activity from T0 to T1, but normalized after 24 h (T4). C3a (e) and soluble TCC (f) were at a minimum at T0 and then increased significantly to T1. For both factors the serum concentration peaked at T2, and levels had normalized 24 h postoperatively.
Discussion
The Physio® and Bioline® circuits have been investigated before and been shown to exhibit a comparable degree of in-vivo biocompatibility regarding whole-body inflammation response and complement activation 13. Another study comparing Phisio®, Bioline® and a third circuit, Softline®, found no difference in clinical output among the patients in the different groups 20. A third study only found improved results regarding CPB-related inflammation when using Bioline® compared to non-heparinized coatings 21. However, ficolin-2 was of particular interest to us because it is known to bind to several otherwise biocompatible surfaces. Besides the studies regarding ficolin-2 adsorption to haemodialysis tubing 8–10, most recently it was shown by us and others that ficolin-2 binds to the clot-activating coating, silica, used in modern-type serum tubes, resulting in a significant reduction of ficolin-2 in the sample 18,22. We also showed that even though ficolin-2 binds to acBSA when used as a ligand in ELISA, the in-vitro complement contribution from this binding is negligible 18.
With this in mind, and the observation that the complement activation in vivo and in vitro was comparable between circuits, it could be hypothesized that the physiological role of ficolin-2 is more prominent as an opsonin than inducing lectin complement pathway activation. By contrast, ficolin-3 has been shown to be the most potent among the complement activating PRMs 23. It is thus a widespread issue that ficolin-2 binds to otherwise biocompatible surfaces and the question remains as to what other surfaces could be targeted. Due to the high evolutionary conservation level of ficolin-2 24 and that no ficolin-2 deficiency has been reported to date, it is reasonable to speculate that the partial depletion found in this study could have a significant impact on patient health and outcome, at least during specific disease settings or in immune-compromised individuals; in particular considering the spectrum of opportunistic microorganisms that ficolin-2 has been reported to recognize: capsulated Staphylococcus aureus, Streptococcus pneumonia, Salmonella typhimurium, Escherichia coli, Pseudomonas aeruginosa and Aspergillus fumigatus 25–28. Furthermore, low levels of ficolin-2 have been associated with several severe respiratory diseases, e.g. chronic bronchiectasis, pulmonary Mycobacterium tuberculosis infection and susceptibility to infection during allergic inflammation of the lungs 29–31. Most recently, ficolin-2 has been shown in two independent studies to have an inhibitory effect on hepatitis C virus by binding to the envelope protein 32,33. Regrettably, there was no follow-up available on the patients in this study; thus the clinical consequences of the partial depletion of ficolin-2 have not been investigated and the impact on the health of the patients is not known.
There was no significant difference in ficolin-3 concentration in the two groups and no change was observed during the operation in either of the groups. However, an obvious limitation of this study was the difference in binding and complement activation potential of ficolin-3 between the two groups in T0. We interpret this as a consequence of the small number of individuals in each group, combined with the fact that ficolin-3 exhibits large interindividual variation 16. Overall, there was massive complement activation in both groups of patients during the operation measured as a significant increase of in-vivo fluid-phase C3a and sTCC as well as an in-vitro reduction of complement deposition capacity via ficolin-3 and the other complement activation pathways. In several studies it has now been shown that the ficolin-3 assay utilized here is a high-quality marker of in-vivo complement consumption and a fitting supplement to the existing Wielisa testing the other three pathways 17,34,35.
In conclusion, we show for the first time that the pattern recognition molecule ficolin-2 of the lectin pathway is depleted from plasma during cardiopulmonary bypass when using heparin-coated Bioline® circuits. Ficolin-2 is adsorbed to the circuit membrane presumably through a specific binding to its known ligand, heparin. The degree of downstream complement activation and clinical consequences of this finding calls for further investigation and follow-up of patients undergoing extracorporeal circulation involving heparin coating allowing ficolin-2 depletion. Another issue that remains to be addressed is the possible effect of ficolin-2 during treatment of patients with systemic heparin.
Acknowledgments
This work was supported by the Svend Andersen Research Foundation, The Danish Medical Research Council, The Novo Nordisk Research Foundation, The Research Foundation of The Capital Region of Denmark and Rigshospitalet.
Disclosures
None.
References
- Garred P, Honoré C, Ma YJ, Munthe-Fog L, Hummelshøj T. MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement. Mol Immunol. 2009;46:2737–2744. doi: 10.1016/j.molimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Mollnes TE. Complement and biocompatibility. Vox Sang. 1998;74(Suppl 2):303–307. doi: 10.1111/j.1423-0410.1998.tb05435.x. [DOI] [PubMed] [Google Scholar]
- Kreisler KR, Vance R, Cruzzavala J, Mahnken JD. Heparin-bonded cardiopulmonary bypass circuits reduce the rate of red blood cell transfusion during elective coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2005;19:608–611. doi: 10.1053/j.jvca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- De Some F, Francois K, van Oeveren W. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg. 2000;18:602–606. doi: 10.1016/s1010-7940(00)00508-x. [DOI] [PubMed] [Google Scholar]
- Gout E, Garlatti V, Smith DF. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J Biol Chem. 2010;285:6612–6622. doi: 10.1074/jbc.M109.065854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yang Y, Zhu N. Characteristics and molecular mechanism of adhesion proteins on reused hemodialysis membranes. Blood Purif. 2009;27:321–329. doi: 10.1159/000207199. [DOI] [PubMed] [Google Scholar]
- Mares J, Thongboonkerd V, Tuma Z, Moravec J, Matejovic M. Specific adsorption of some complement activation proteins to polysulfone dialysis membranes during hemodialysis. Kidney Int. 2009;76:404–413. doi: 10.1038/ki.2009.138. [DOI] [PubMed] [Google Scholar]
- Mares J, Richtrova P, Hricinova A. Proteomic profiling of blood-dialyzer interactome reveals involvement of lectin complement pathway in hemodialysis-induced inflammatory response. Proteomics Clin Appl. 2010;4:829–838. doi: 10.1002/prca.201000031. [DOI] [PubMed] [Google Scholar]
- Andersen TE, Palarasah Y, Skjødt M-O. Decreased material-activation of the complement system using low-energy plasma polymerized poly(vinyl pyrrolidone) coatings. Biomaterials. 2011;32:4481–4488. doi: 10.1016/j.biomaterials.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Garlatti V, Belloy N, Martin L. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007;26:623–633. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiara AS, Andersen VY, Videm V. Comparable biocompatibility of Phisio- and Bioline-coated cardiopulmonary bypass circuits indicated by the inflammatory response. Perfusion. 2010;25:9–16. doi: 10.1177/0267659110362822. [DOI] [PubMed] [Google Scholar]
- Munthe-Fog L, Hummelshoj T, Honoré C. Variation in FCN1 affects biosynthesis of ficolin-1 and is associated with outcome of systemic inflammation. Genes Immun. 2012;13:1–8. doi: 10.1038/gene.2012.27. [DOI] [PubMed] [Google Scholar]
- Munthe-Fog L, Hummelshøj T, Hansen BE. The impact of FCN2 polymorphisms and haplotypes on the Ficolin-2 serum levels. Scand J Immunol. 2007;65:383–392. doi: 10.1111/j.1365-3083.2007.01915.x. [DOI] [PubMed] [Google Scholar]
- Munthe-Fog L, Hummelshøj T, Ma YJ. Characterization of a polymorphism in the coding sequence of FCN3 resulting in a Ficolin-3 (Hakata antigen) deficiency state. Mol Immunol. 2008;45:2660–2666. doi: 10.1016/j.molimm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Hein E, Honoré C, Skjoedt M-O, Munthe-Fog L, Hummelshøj T, Garred P. Functional analysis of Ficolin-3 mediated complement activation. PLOS ONE. 2010;5:e15443. doi: 10.1371/journal.pone.0015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein E, Bay JT, Munthe-Fog L, Garred P. Ficolin-2 reveals different analytical and biological properties dependent on different sample handling procedures. Mol Immunol. 2013;56:406–412. doi: 10.1016/j.molimm.2013.05.233. [DOI] [PubMed] [Google Scholar]
- Seelen MA, Roos A, Wieslander J. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–198. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Reser D, Seifert B, Klein M. Retrospective analysis of outcome data with regards to the use of Phisio®-, Bioline®- or Softline®-coated cardiopulmonary bypass circuits in cardiac surgery. Perfusion. 2012;27:530–534. doi: 10.1177/0267659112454558. [DOI] [PubMed] [Google Scholar]
- Palatianos GM, Foroulis CN, Vassili MI. A prospective, double-blind study on the efficacy of the bioline surface-heparinized extracorporeal perfusion circuit. Ann Thorac Surg. 2003;76:129–135. doi: 10.1016/s0003-4975(03)00338-2. [DOI] [PubMed] [Google Scholar]
- Brady AM, Spencer BL, Falsey AR, Nahm MH. Blood collection tubes influence serum ficolin-1 and ficolin-2 levels. Clin Vaccine Immunol. 2014;21:51–55. doi: 10.1128/CVI.00607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelshoj T, Fog LM, Madsen HO, Sim RB, Garred P. Comparative study of the human ficolins reveals unique features of Ficolin-3 (Hakata antigen) Mol Immunol. 2008;45:1623–1632. doi: 10.1016/j.molimm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Garred P, Honoré C, Ma YJ. The genetics of ficolins. J Innate Immun. 2010;2:3–16. doi: 10.1159/000242419. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Endo Y, Taira S. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- Lynch NJ, Roscher S, Hartung T. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol. 2004;172:1198–1202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- Krarup A, Sørensen UBS, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–1060. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Doni A, Hummelshøj T. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem. 2009;284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DC, Chalmers JD, MacDonald SL. Stable bronchiectasis is associated with low serum L-ficolin concentrations. Clin Respir J. 2009;3:29–33. doi: 10.1111/j.1752-699X.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Luo F, Sun X, Wang Y. Ficolin-2 defends against virulent mycobacteria tuberculosis infection in vivo, and its insufficiency is associated with infection in humans. PLOS ONE. 2013;8:e73859. doi: 10.1371/journal.pone.0073859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedzynski M, Atkinson APM, St. Swierzko A. L-ficolin (ficolin-2) insufficiency is associated with combined allergic and infectious respiratory disease in children. Mol Immunol. 2009;47:415–419. doi: 10.1016/j.molimm.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ren Y, Zhang X. Ficolin-2 inhibits hepatitis c virus infection, whereas apolipoprotein E3 mediates viral immune escape. J Immunol. 2014;193:783–796. doi: 10.4049/jimmunol.1302563. [DOI] [PubMed] [Google Scholar]
- Hamed MR, Brown RJP, Zothner C. Recombinant human L-ficolin directly neutralizes hepatitis C virus entry. J Innate Immun. 2014;6:676–684. doi: 10.1159/000362209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay JT, Hein E, Sørensen SS, Hansen JM, Garred P. Pre-transplant levels of ficolin-3 are associated with kidney graft survival. Clin Immunol. 2013;146:240–247. doi: 10.1016/j.clim.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Zanier ER, Zangari R, Munthe-Fog L. Ficolin-3-mediated lectin complement pathway activation in patients with subarachnoid hemorrhage. Neurology. 2014;82:126–134. doi: 10.1212/WNL.0000000000000020. [DOI] [PubMed] [Google Scholar]


