Abstract
Evidence exists that interleukin (IL)-10 family cytokines may be involved in the pathogenesis of rheumatoid arthritis (RA). We sought to determine whether or not these cytokines are involved in psoriatic arthritis (PsA). We conducted a prospective study on patients with PsA, RA and osteoarthritis (OA); healthy controls (HC) were also included. We analysed IL-20, IL-24 and IL-19 serum and synovial fluid (SF) levels and change of serum levels following treatment with biological agents. IL-20 serum levels were increased in PsA and RA compared with OA patients and HC and with matched SF levels. IL-24 serum levels in PsA, RA and OA patients were higher than those in HC and also with respect to matched SF in PsA. IL-19 serum levels were higher in HC and OA compared with PsA and RA patients; IL-19 SF levels were higher in PsA and RA compared with OA patients, and in PsA compared with RA patients. PsA and RA patients showed a reduction of IL-19 serum levels after biological treatment. Therefore, IL-19 seems to be involved mainly in the joint inflammation, whereas IL-20 and IL-24 appear to participate mainly in the systemic responses. These findings may further the comprehension of the contribution of these cytokines to the inflammatory response involved in chronic arthritis, as well as to the development of novel therapeutic strategies.
Keywords: IL-10 family cytokine, inflammatory arthritis, serum, synovial fluid
Introduction
In past years there have been several advances in the pathogenesis of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) that have prompted the introduction of new therapies and remarkable improvement in clinical outcomes. RA is an autoimmune disease characterized by autoantibody production, severe synovitis, synovial membrane hyperplasia, cartilage and bone destruction 1. A major role in the pathogenesis of the disease is played by RA synovial fibroblasts (RASFs), which have been recently recognized as invasive cells exhibiting multi-potent inflammatory properties rather than being relatively inert mesenchymal cells 2. One of the mechanisms by which RASFs contribute to synovial inflammation pertains to the capability of producing proteases, chemokines and cytokines, such as tumour necrosis factor (TNF) and interleukin (IL)-1β 3–5. The inhibition of TNF and, to a lesser extent, of IL-1 using biological agents proved to be a successful strategy for the treatment of RA resistant to conventional treatment, and thereafter other proinflammatory molecules became the therapeutic target for RA and other chronic inflammatory rheumatic diseases 6. The possibility of blocking IL-20 has also gathered attention 7,8, as this cytokine and its receptors (IL-20R type I and IL-20R type II) were found to be expressed in RASFs 4. More interestingly, IL-20 induced the secretion of proinflammatory mediators in RASFs and promoted RASF migration, neutrophil chemotaxis and endothelial cell proliferation, suggesting an important role in favouring local inflammation 4. IL-20 is a member of the IL-10 family, which also includes IL-19, IL-22, IL-24, IL-26, IL-28 and IL-29 9. IL-20, IL-24 and IL-19 share receptor complexes: all of them are capable of signalling through the IL-20R type I/IL-20R type II heterodimer, and both IL-20 and IL-24 can also use IL-22R and IL-20R type II, although their biological effects may differ 10. These cytokines have been shown to be up-regulated in psoriatic skin 11 and may also affect the genetic predisposition to psoriasis. In fact, the IL-19/IL-20 extended haplotype (CACCGGAA) and IL-20RA haplotype CCG showed a significant susceptibility to the disease, while other IL-20/IL-24 haplotypes (CAAAC, TGGGT and CGAGT) displayed a significant protective effect 12–14. The involvement of IL-20 and IL-24 has been investigated in only one study in patients with spondyloarthritis (SpA), including some with PsA, where both cytokines were expressed at low levels in the synovium and at higher concentrations in synovial fluid (SF) and plasma 15, while no data are available for IL-19.
The aim of our study was to investigate the contribution of IL-20, IL-24 and IL-19 to the inflammatory response in PsA. For this purpose, we first measured the levels of these cytokines both in the serum and SF in comparison with those found in patients with RA and osteoarthritis (OA). We then analysed the effect of anti-TNF therapy on the serum levels of these cytokines in a cohort of PsA and RA patients.
Materials and methods
Patients and samples
The first part of the study aimed to compare the levels of IL-20, IL-24 and IL-19 in serum and SF from patients with PsA, RA and OA. We enrolled consecutive PsA (n = 23), RA (n = 30) and OA (n = 20) patients presenting a knee joint effusion and classified according to standard criteria 16–18. SF, obtained by therapeutic arthrocentesis, was analysed focusing on white blood cell count, as well as polymorphonuclear and mononuclear cell count and percentage. We also collected blood samples from all patients and the sera, together with matched SF, were stored at −20°C until levels of IL-20, IL-24 and IL-19 were measured. We evaluated the serum/SF ratio for all the cytokines in the three groups of patients. Serum samples were also obtained from 20 healthy controls (HC). Details of patients and controls are summarized in Table 1.
Table 1.
Demographic, clinical and laboratory features of patients with knee effusion and controls
| PsA (n = 23) | RA (n = 30) | OA (n = 20) | HC (n = 20) | P | |
|---|---|---|---|---|---|
| Sex (male/female) | 16/7 | 8/22 | 13/7 | 8/12 | PsA versus RA 0·001; RA versus OA 0·007 |
| Age (years) | 44/40–56 | 53·5/45·7–65 | 59/47·5–78 | 51/44·3–65 | PsA versus OA 0·005 |
| Disease duration (months) | 96/25·5–156 | 96/21–171 | 66/12–186 | n.a. | n.s. |
| Tender joints (0–28) | 1·5/1–3·5 | 3/1–6·5 | 1/1–1 | n.a. | RA versus OA 0·01 |
| Swollen joints (0–28) | 1/1–2 | 1/1–3·5 | 1/1–1·5 | n.a. | n.s. |
| ESR (mm/h) | 16/9–33 | 32/20–40 | 12/5–24 | – | PsA versus RA 0·01; RA versus OA 0·005 |
| CRP (mg/l) | 6·5/2·25–13·5 | 10/6–40 | 2/0–4 | – | PsA versus OA 0·02; RA versus OA 0·0002 |
| DAS28 | 3·2/2·7–3·8 | 3·7/3–5 | n.a. | n.a. | n.s. |
| RF (n/%) | 0/0 | 23/76·6 | 0/0 | – | PsA versus RA < 0·0001; RA versus OA < 0·0001 |
| ACPA (n/%) | 0/0 | 25/83·3 | 0/0 | – | PsA versus RA < 0·0001; RA versus OA < 0·0001 |
| Synovial fluid | |||||
| WBC (cell/ml) | 6 750/3 238–10 180 | 7350/5450–11 650 | 550/200–2550 | n.a. | PsA versus OA < 0·0001; RA versus OA < 0·0001 |
| PMN (%) | 68/29·5–80 | 68/50–78·75 | 7·5/0–27·5 | n.a. | PsA versus OA 0·002; RA versus OA 0·0001 |
| Therapy (n/%) | |||||
| NSAIDs | 11/47·8 | 12/42·8 | 10/50 | n.a. | n.s. |
| Prednisone | 3/13·04 | 11/39·3 | 0/0 | n.a. | RA versus OA 0·001 |
| DMARDs | 10/43·4 | 16/57·1 | 0/0 | n.a. | RA versus OA < 0·0001; PsA versus OA 0·0007 |
Data are expressed as median and 25th–75th percentiles. Statistical comparisons between groups of patients and healthy controls were performed by Kruskal–Wallis analysis of variance (anova) with Dunn's post-test. Univariate comparisons between nominal variables were performed by Fisher's exact test. ACPA = anti-citrullinated protein/peptide antibodies; CRP = C-reactive protein; DAS28 = disease activity score (28 joint count, four variables, erythrocyte sedimentation rate-based); DMARDs = disease-modifying anti-rheumatic drugs; ESR = erythrocyte sedimentation rate; HC = healthy controls; n.a. = not applicable; n.s. = not significant; NSAIDs = non-steroidal anti-inflammatory drugs; OA = osteoarthritis; PMN = polymorphonuclear cells; PsA = psoriatic arthritis; RA = rheumatoid arthritis; RF = rheumatoid factor; WBC = white blood cells.
In the second part of the study we analysed the effect of anti-TNF therapy on the serum levels of these cytokines in a cohort of PsA and RA patients. We enrolled 80 patients (40 each with PsA and RA) with active disease, an inadequate response to standard disease-modifying anti-rheumatic drugs (DMARDs) and not treated previously with anti-TNF drugs. These patients commenced etanercept (50 mg weekly subcutaneously; 20 each with PsA and RA) or adalimumab (40 mg every other week subcutaneously; 20 each with PsA and RA). Disease activity score (DAS) (28 joint count, four variables, erythrocyte sedimentation rate-based; DAS28) was calculated and the clinical response was evaluated according to the EUropean League Against Rheumatism (EULAR) criteria 19. The demographic and clinical features of these patients are summarized in Table 2. Blood serum samples were collected at baseline and after 3 months from the beginning of anti-TNF treatment and stored at −20°C until cytokine levels were measured.
Table 2.
Demographic, clinical and laboratory features of psoriatic arthritis and rheumatoid arthritis patients treated with anti-tumour necrosis factor (TNF) agents
| All PsA (n = 40) | PsA ETA (n = 20) | PsA ADA (n = 20) | All RA (n = 40) | RA ETA (n = 20) | RA ADA (n = 20) | |
|---|---|---|---|---|---|---|
| Sex (male/female) | 18/22 | 11/9 | 7/13 | 22/18 | 5/15 | 17/3 |
| Age (years) | 48/43–56 | 47·5/42·2–67·2 | 48/41·5–52·5 | 59/42·7–66·7 | 60/52–69·5 | 57/39·5–64·5 |
| Disease duration (months) | 54/24–144 | 66/21–222 | 48/33–123 | 96/36–186 | 96/36–228 | 78/36–141 |
| DAS28 at baseline | 4·4/3·4–5·7 | 4·4/3·7–5·3 | 4·5/3–5·8 | 5·1/4·4–5·7 | 5·2/4·7–6·4 | 5·14/4·1–5·4 |
| DAS28 at T3 | 3·3/1·8–4·9 | 3·8/1·3–5 | 3·3/1·9–3·8 | 4·2/3·4–5·2 | 4·5/3·5–5·1 | 4·1/3·3–5·4 |
| ESR (mm/h) at baseline | 23·5/15–30·7 | 24·5/15·7–33·2 | 20/11·5–30 | 25/10–43·5 | 23/9–32 | 26/12–44·5 |
| ESR (mm/h) at T3 | 11/5–20·5 | 11·5/4–28 | 11/6·2–18·7 | 19/7·7–31 | 16·5/6·2–29·5 | 19/7·5–55·5 |
| CRP (mg/l) at baseline | 5/2–11·5 | 9·7/3·5–25 | 3/1·2–6·7 | 5·3/2·4–12 | 8·2/3·7–18·7 | 5/1·8–12 |
| CRP (mg/l) at T3 | 2·3/1–5·8 | 2·4/1–6·5 | 2/1·2–5 | 4·1/1·6–7·5 | 3·3/1·1–7·7 | 5/3·1–7·7 |
| Therapy (n/%) | ||||||
| Prednisone | 3/7·5 | 1/5 | 2/10 | 12/30 | 6/30 | 6/30 |
| DMARDs | 12/30 | 7/35 | 5/25 | 20/50 | 8/40 | 12/60 |
Data are expressed as median and 25th–75th percentiles. ADA = adalimumab; CRP = C-reactive protein; DAS28 = disease activity score 28; DMARDs = disease-modifying anti-rheumatic drugs; ESR = erythrocyte sedimentation rate; ETA = etanercept; PsA = psoriatic arthritis; RA = rheumatoid arthritis; T3 = 3 months since the onset of biological agent.
All the patients in this study were enrolled prospectively from the rheumatology out-patient clinic at Sapienza University of Rome, Italy. At recruitment, clinical evaluation included tender and swollen joint count for DAS28 calculation, while laboratory assessment included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF) and anti-citrullinated protein/peptide antibodies. Written informed consent was obtained from patients and controls and the ethics committee approved the study.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-20, IL-24, and IL-19 were measured in serum and SF samples of patients and controls. To quantify the concentration of IL-20 and IL-19, the human Quantikine IL-20 and IL-19 immunoassay kits were used, respectively, according to the manufacturer's instructions (both R&D Systems, Minneapolis, MN, USA). To quantify the concentration of IL-24, a sandwich enzyme-linked immunosorbent assay (ELISA) was performed using the Duo Set ELISA development kit (R&D Systems). Briefly, 96-well microplates were coated overnight at 4°C with 2 μg/ml mouse anti-human IL-24 (R&D Systems). After three washes using wash buffer [0·05% Tween 20 in phosphate-buffered saline (PBS)[, plates were blocked for 1 h at room temperature with 1% bovine serum albumin (BSA). After three washes using wash buffer, serial dilutions of rhIL-24 (R&D Systems) and undiluted samples were added in duplicate and incubated for 2 h at room temperature. After three washes, plates were incubated for 2 h with 400 ng/ml biotinylated goat anti-human IL-24 (R&D Systems). For detection, streptavidin–horseradish peroxidase (HRP) (R&D Systems) was added and incubated for 20 min. Plates were then washed again and tetramethylbenzidine peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, ND, USA) was added for 20 min followed by the stop solution 2NH2SO4. Absorbances were read at 450 nm using a plate reader.
Although false positive results may be caused by heterophilic antibody (such as RF) interference in the sandwich enzyme immunoassay technique, we did not use blocking agents in the serum of patients with RA based on the assumption that this non-specific effect is less relevant in monoplex relative to multiplex immunoassays 20.
Statistical analysis
Non-normally distributed variables were summarized using median with percentile ranges. Data in the longitudinal analysis during the treatment course of individual patients were evaluated with the non-parametric Wilcoxon's signed-rank test. Multiple comparisons were performed by Kruskal–Wallis analysis of variance (anova) with Dunn's post-test. Univariate comparisons between nominal variables were performed by Fisher's exact test. The significance of any correlation was determined by Spearman's rank correlation coefficient. Correlations were also calculated deleting outliers, when present. P-values < 0·05 were considered significant. All statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software, Inc., San Diego, CA, USA).
Results
IL-20, IL-24 and IL-19 in serum and synovial fluid of patients and controls
Levels of IL-20, IL-24 and IL-19 were evaluated in serum and SF of PsA, RA and OA patients and controls (Supporting information, Table S1). Serum levels of IL-20 in PsA patients were significantly higher compared with those observed in OA patients (P = 0·01) and HC (P = 0·001), while there was no significant difference between PsA and RA patients. Similarly, IL-20 serum concentration in RA patients was higher than that in OA patients (P = 0·001) and HC (P < 0·0001) (Fig. 1a). We did not detect any significant differences in IL-20 SF levels among PsA, RA and OA patients (Fig. 1a). The serum levels in both PsA and RA patients were higher than matched SF levels (P = 0·0002 and P < 0·0001, respectively) (Fig. 1a). The ratio between IL-20 serum and SF levels was not different among the three groups of patients (data not shown). In PsA patients, we found a positive correlation between IL-20 serum and SF levels (P = 0·002, r = 0·6) (Fig. 1b) and between both IL-20 SF levels and serum and SF leucocyte count (P = 0·02, r = 0·4 and P = 0·01, r = 0·5, respectively) (Fig. 1c–d). A positive correlation was also observed between IL-20 serum levels and both SF polymorphonuclear and mononuclear cell count (P = 0·02, r = 0·4 and P = 0·03, r = 0·4, respectively) (Fig. 1e–f), while a negative correlation was found between IL-20 serum levels and the age of patients (P = 0·009, r = −0·5) (Fig. 1g).
Figure 1.
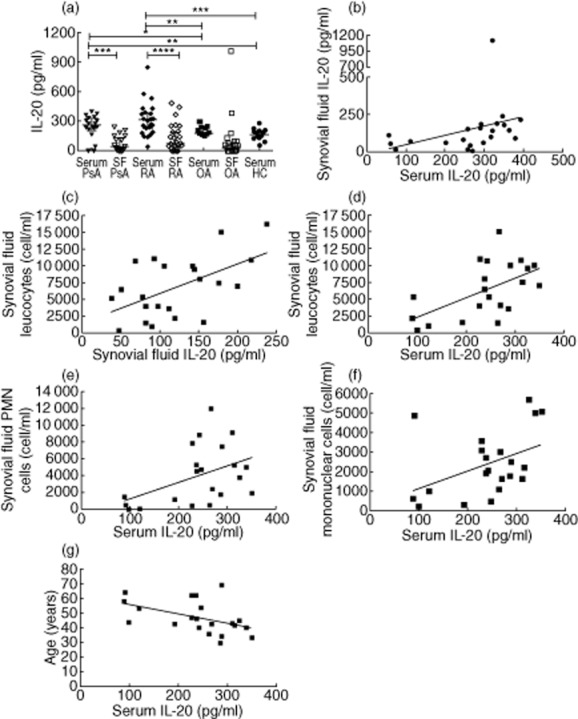
(a) Levels of interleukin (IL)-20 in serum from psoriatic arthritis (PsA) patients were higher than those in synovial fluid. IL-20 serum levels in PsA patients were higher compared with those in osteoarthritis (OA) patients and healthy controls (HC). Levels of IL-20 in serum from rheumatoid arthritis (RA) patients were higher than those in synovial fluid. IL-20 serum levels in RA patients were higher compared with OA patients and HC. Levels in PsA and RA patients were similar. Levels of IL-20 in synovial fluid from PsA, RA and OA patients were similar. (b) Positive correlation between IL-20 serum and synovial fluid levels in PsA patients (P = 0·002, r = 0·6). (c) Positive correlation between IL-20 synovial fluid levels and synovial fluid leucocyte count in PsA patients (P = 0·02, r = 0·4). (d) Positive correlation between IL-20 serum levels and synovial fluid leucocyte count in PsA patients (P = 0·01, r = 0·5). (e) Positive correlation between IL-20 serum levels and synovial fluid polymorphonuclear (PMN) cell count in PsA patients (P = 0·02, r = 0·4). (f) Positive correlation of IL-20 serum levels with synovial fluid mononuclear cell count in PsA patients (P = 0·03, r = 0·4). (g) Negative correlation of IL-20 serum levels and the age of PsA patients (P = 0·009, r = −0·5). Data in (a) are shown as scatter-plots. Each dot represents one sample. Lines represent the median level with interquartile ranges. Statistical comparisons between groups of patients and HC were performed by Kruskal–Wallis analysis of variance (anova) with Dunn's post-test. Spearman's rank-order correlation test was used in (b–g).
Serum levels of IL-24 in PsA, RA and OA patients were significantly higher than those in HC (P < 0·0002, P < 0·0003, P < 0·0001, respectively) (Fig. 2a). In SF, IL-24 levels were lower in PsA compared with those in RA (P = 0·0003) and OA patients (P < 0·0001), while the levels in RA and OA patients were similar (Fig. 2a). Higher levels of IL-24 were detected in serum compared to SF from PsA (P < 0·0001), while the opposite was true for OA patients, where levels in SF were higher than serum (P < 0·0001) (Fig. 2a). The ratio between IL-24 serum and SF levels was not different in the three groups of patients (data not shown). In RA patients, we detected a positive correlation between IL-24 SF levels and the number of tender joints (P = 0·04, r = 0·4) (Fig. 2b).
Figure 2.
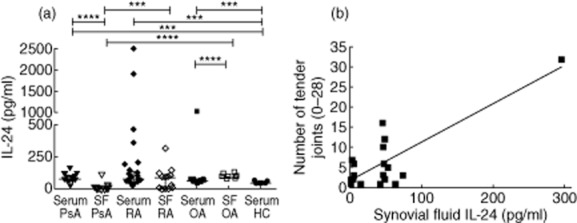
(a) Levels of interleukin (IL)-24 in serum from psoriatic arthritis (PsA), rheumatoid arthritis (RA) and osteoarthritis (OA) patients were higher than those in healthy controls (HC). Levels of IL-24 in synovial fluid from PsA patients were lower than those in RA and OA patients. IL-24 serum levels in PsA patients were higher than matched synovial fluid levels, while the opposite was true for OA patients. (b) Positive correlation of IL-24 synovial fluid levels with the number of tender joint count in RA patients (P = 0·04, r = 0·4). Data in (a) are shown as scatter-plots. Each dot represents one sample. Lines represent the median level with interquartile ranges. Statistical comparisons between groups of patients and HC were performed by Kruskal–Wallis analysis of variance (anova) with Dunn's post-test. Spearman's rank-order correlation test was used in (b).
IL-19 serum levels were significantly lower in PsA patients compared with OA patients (P = 0·0001) and HC (P < 0·0001). OA patients and HC showed comparable IL-19 serum levels. Moreover, serum levels were lower in RA compared with OA patients (P = 0·0001) and HC (P < 0·0001) (Fig. 3a). In contrast, IL-19 levels in SF of PsA patients were significantly higher than those in RA (P < 0·0001) and OA patients (P < 0·0001). Similarly, IL-19 levels in SF from RA patients were significantly higher than those in OA patients (P = 0·0005) (Fig. 3a). Higher levels were detected in SF from PsA and RA patients compared with serum levels, while in OA patients serum levels were higher than matched SF levels (P < 0·0001, P = 0·0002 and P = 0·0003, respectively) (Fig. 3a). The ratio between IL-19 serum and SF levels was higher in RA and OA compared with PsA patients (P = 0·002 and P < 0·0001, respectively). Moreover, the ratio between serum and SF was higher in OA patients than in RA patients (P < 0·0001) (Fig. 3b). We found a positive correlation between IL-19 serum and SF levels (P = 0·003, r = 0·6) in patients with PsA (Fig. 3c). In RA patients a positive correlation was observed between IL-19 SF levels and disease duration (P = 0·01, r = 0·4) (Fig. 3d), while a negative correlation was detected between IL-19 serum levels and the age of patients (P = 0·03, r = −0·4) (Fig. 3e).
Figure 3.
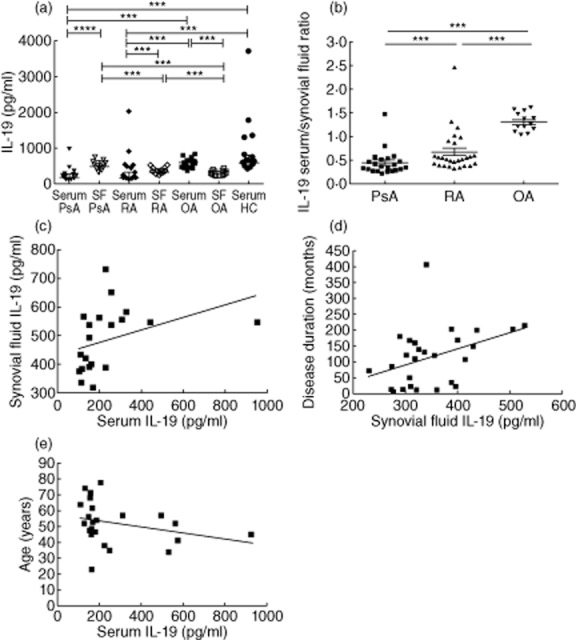
(a) Levels of interleukin (IL)-19 in serum from psoriatic arthritis (PsA) and rheumatoid arthritis (RA) patients were lower than those from osteoarthritis (OA) patients and healthy controls (HC). IL-19 serum levels from OA patients were higher than those in synovial fluid but similar to serum from HC. Levels of IL-19 in synovial fluid from PsA and RA patients were higher than those in respective serum. IL-19 synovial fluid levels in PsA patients were higher than those in RA and OA patients. IL-19 levels in the synovial fluid from RA patients were higher than those in OA patients. (b) The ratio between IL-19 serum and synovial fluid levels was higher in RA and OA patients compared with PsA patients. Moreover, the ratio between serum and SF was higher in OA patients than in RA patients. (c) Positive correlation of IL-19 serum levels with synovial fluid levels in PsA patients (P = 0·003, r = 0·6). (d) Positive correlation of IL-19 synovial fluid levels with disease duration in RA patients (P = 0·01, r = 0·4). (e) Negative correlation of IL-19 serum levels with the age of RA patients (P = 0·03, r = −0·4). Data in (a,b) are shown as scatter-plots. Each dot represents one sample. Lines represent the median level with interquartile ranges. Statistical comparisons between groups of patients and HC were performed by Kruskal–Wallis analysis of variance (anova) with Dunn's post-test. Spearman's rank-order correlation test was used in panels (c–e).
We did not find any other correlation between IL-20, IL-24 and IL-19 serum or SF levels and the other clinical and laboratory features of the patients.
All the correlations were confirmed deleting outliers when present (data not shown).
IL-20, IL-24 and IL-19 in serum of PsA and RA patients during anti-TNF treatment
Serum levels of IL-20, IL-24 and IL-19 were evaluated in PsA and RA patients before and after 3 months of treatment with etanercept or adalimumab. Analysis was performed both considering the overall population of PsA and RA patients, irrespective of the biological agent, and dividing each population into two groups depending on the treatment with etanercept or adalimumab. We did not detect any significant changes of IL-20 and IL-24 serum levels after anti-TNF treatment in PsA and RA (data not shown). In the group of RA patients we observed a positive correlation between IL-20 serum levels and ESR (P = 0·04, r = 0·6) after 3 months of etanercept treatment (Fig. 4a). A positive correlation was also found between IL-24 serum levels and CRP before and after 3 months of etanercept treatment (P = 0·005, r = 0·5 and P < 0·0001, r = 0·9, respectively) (Fig. 4b–c).
Figure 4.
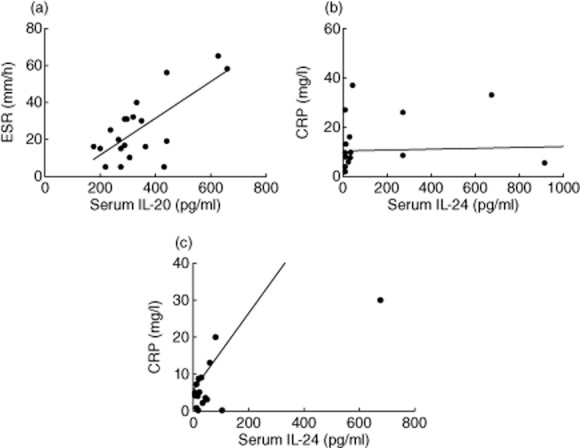
(a) Positive correlation of interleukin (IL)-20 serum levels with erythrocyte sedimentation rate levels (P = 0·04, r = 0·6) in rheumatoid arthritis (RA) patients after 3 months of etanercept treatment. (b) Positive correlation of IL-24 serum levels with C-reactive protein (CRP) levels in RA patients before etanercept treatment (P = 0·005, r = 0·5). (c) Positive correlation of IL-24 serum levels with CRP levels in RA patients after 3 months of etanercept treatment (P < 0·0001, r = 0·9). Spearman's rank-order correlation test was used in (a–c).
Of interest, RA patients showed a significant reduction of IL-19 serum levels after treatment with biological agents (P = 0·01); the same difference was observed in the group treated with adalimumab (P = 0·01) (Fig. 5a,b). Similarly, the total PsA patients displayed a reduction of IL-19 serum levels after treatment with anti-TNF agents (P = 0·01) (Fig. 5c). Furthermore, in the group of PsA patients treated with adalimumab, we observed a positive correlation between IL-19 levels and DAS28 at baseline (P = 0·03, r = 0·49) (Fig. 5d); a positive correlation was also detected at baseline between IL-19 serum levels and CRP in the group of PsA patients treated with etanercept (P = 0·0006, r = 0·7) (Fig. 5e).
Figure 5.
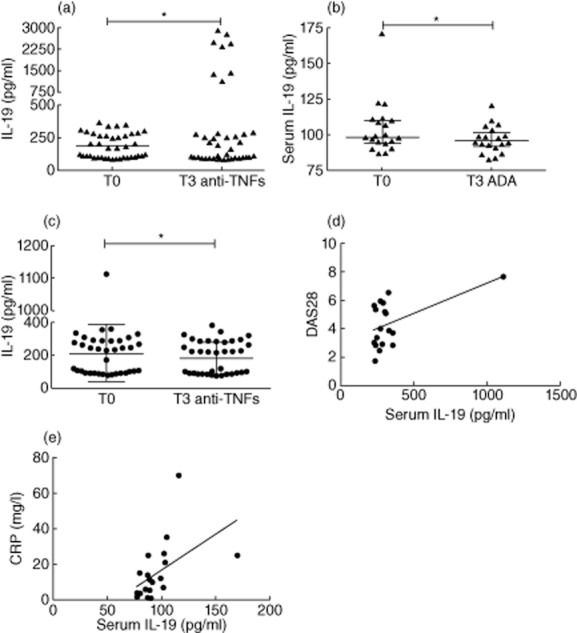
(a) Reduction of interleukin (IL)-19 serum levels in rheumatoid arthritis (RA) patients (n = 40) after 3 months (T3) of biological treatment with respect to baseline (T0). (b) Reduction of IL-19 serum levels in RA patients (n = 20) after 3 months (T3) of adalimumab treatment with respect to baseline (T0). (c) Reduction of IL-19 serum levels in psoriatic arthritis (PsA) patients (n = 40) after 3 months (T3) of biological treatment with respect to baseline (T0). (d) Positive correlation of IL-19 serum levels with disease activity score 28 (DAS28) in PsA patients before adalimumab treatment (P = 0·03, r = 0·49). (e) Positive correlation of IL-19 serum levels with CRP levels in PsA patients before etanercept treatment (P = 0·0006, r = 0·7). Data in panels (a–c) are shown as scatter-plots. Each dot represents one sample. Lines represent the median level with interquartile ranges. Statistical comparisons before and after biological treatment were performed using the Wilcoxon's signed-rank test. Spearman's rank-order correlation test was used in (d,e).
All the correlations were confirmed deleting outliers when present (data not shown).
Discussion
The results of this study indicate an interesting and distinct contribution to systemic and joint inflammation of IL-10 family cytokines (IL-20, IL-24 and IL-19) in patients with inflammatory arthritis. We evaluated both serum and SF levels and also the ratio of serum to SF levels for each cytokine. Indeed, an adequate interpretation of SF protein levels needs concurrent evaluation of serum concentration and the kinetic factors involved in local blood supply and lymphatic drainage, including microvascular permeability, interstitial properties, mode of transport, characteristics of proteins (i.e. molecular weight or radius) and synovial metabolism, which could be different for each joint 21. To normalize the protein serum concentration and the permeability of synovial membrane, the calculation of the ratio between serum and SF levels is required 22. In our study, this approach allowed us to demonstrate that IL-20 levels were increased in serum from PsA and RA patients when compared with matched SF levels. The same was observed for IL-24 in PsA patients, while the ratio between IL-19 serum and SF levels showed that this cytokine was located mainly in the SF of PsA and RA patients. Therefore, our findings sustain the view that, although IL-20, IL-24 and IL-19 belong to the same IL-10 family and share the same receptors, they have a different distribution in inflammatory arthritis, which is largely different with respect to OA patients and even more with HC. Overall, while the prevailing detection of IL-20 and IL-24 in serum from PsA and RA patients mirrors the systemic inflammatory responses, the higher levels of IL-19 in SF support an intriguing role of this cytokine in joint inflammation. The different distribution of IL-20, IL-24 and IL-19 in our study is hardly imputable to the cellular sources of the three cytokines, which are mainly shared and include monocytes, macrophages, keratinocytes, fibroblasts and T cells 8,23,24. Hence, it is feasible that their distribution reveals the different contribution to the inflammatory pathways involved in chronic arthritis. In PsA and RA, IL-20 and IL-24 may be produced outside the joint, thus leading to an increase in serum levels. However, as it has been shown that peripherally activated cells infiltrate the synovial joint in rheumatic diseases 25, we cannot exclude that cellular sources of the two cytokines may also be found within the synovial joint, which is one of their target tissues 24, thus explaining the small amount detected locally. IL-20 and IL-24 exert their biological effects, including induction of angiogenesis and apoptosis, by the activation of transcription factors in the target cells, and this may occur both systemically and locally in inflammatory arthritis 8. Differently from IL-20 and IL-24, our data suggest that IL-19 may be produced mainly within the joint in inflammatory arthritis; thus, it may be considered a novel candidate target of biological innovative therapeutics. Interestingly, not only does IL-19 appear more prone with respect to the other cytokines of IL-10 family to participate in immune reactions 26, but also has overt proinflammatory effects. These include the capability of up-regulating the production of IL-6 and TNF in monocytes 27, two cytokines strongly involved in joint inflammation.
Few data are available regarding IL-20, IL-24 and IL-19 in inflammatory arthritis, and they are especially limited for PsA. Our results on IL-20 in RA are consistent with those by Kragstrup et al. 15, showing increased plasma levels compared with OA patients, but in contrast with the findings by Hsu et al. 4. In this latter report, IL-20 SF levels overcame those in the serum, although the subjects included were not comparable to ours in terms of both number and clinical features 4.
Furthermore, we demonstrated increased IL-24 serum levels in PsA, RA and OA patients compared with HC, and in PsA patients IL-24 was represented mainly in serum rather than in SF. Kragstrup et al. found increased IL-24 serum and SF levels in SpA and RA compared with OA patients, but not between SpA and healthy subjects 15; again, the discrepancies with our results may be due to the different characteristics of the patients enrolled. In particular, our cohort of PsA patients included a high prevalence of males, due probably to the fortuitous effect of the small sample size. Moreover, we acknowledge that both PsA and RA patients showed moderate disease activity with a low tender and swollen joint count, which may have affected the results of the study. Furthermore, low and comparable levels of mRNA expression of IL-24 and IL-20 in synovial membrane biopsies from RA and OA patients were observed 15, in accordance with our observation of similar IL-24 SF levels in RA and OA subjects.
Thus far, the role of IL-19 in PsA has not been faced, and clinical associations have only been described between IL-19 and RA, psoriasis, inflammatory bowel disease and uveitis 8. Sakurai et al. detected IL-19, IL-20RI and IL-20RII mRNA in RA synovial tissue 28, while Alanara et al. reported elevated IL-19 mRNA levels in SF mononuclear cells from RA patients compared with peripheral blood mononuclear cells from RA or HC 29. Importantly, IL-19 levels were increased in RA synovial tissue with respect to OA 29. These data are similar to findings in patients with psoriasis, in which reduced IL-19 serum levels and higher expression in skin lesions than in controls were observed 30.
We also analysed IL-20, IL-24 and IL-19 serum levels in PsA and RA patients before and after 3 months of treatment with an anti-TNF. While IL-20 and IL-24 serum levels did not change following treatment with etanercept or adalimumab, we observed significantly reduced IL-19 serum levels after 3 months of biological treatment in PsA and RA patients, suggesting a feedback regulation mechanism between the two proinflammatory cytokines IL-19 and TNF 27. Recent evidence in collagen-induced arthritis showed that IL-19 moves TNF production by synovial fibroblasts, and blocking IL-19 blunts the severity of arthritis 31. These data, together with our observations, reinforce the proinflammatory role of IL-19, which is exerted mainly at local joint level and may be dampened by anti-TNF agents. However, we acknowledge that some drawbacks in our study may affect the results, including the small sample size, the moderate disease activity and concomitant treatment.
In conclusion, we observed that IL-20, IL-24 and IL-19 show different distribution in serum and SF from patients with PsA, RA and OA. In particular, IL-19 seems to be involved mainly in the joint inflammation, whereas IL-20 and IL-24 appear to participate mainly in the systemic responses. These findings may allow furthering of the contribution of the three cytokines to the inflammatory response. Functional assays should be performed to define their role more clearly in the pathogenesis of chronic arthritis.
Author contributions
R. S. and P. C. designed the research study. R. S., P. C., V. R. and A. S. performed the research and analysed the data. R. S. and M. D. F. enrolled the subjects. C. A. contributed essential reagents and tools and helped to analyse the data. R. S. and P. C. wrote the paper, which was critically revised by R. P. and G. V.
Disclosures
The authors declare no financial or commercial conflict of interest.
Supporting Information
Table S1. Serum and synovial fluid levels of interleukin (IL)-20, IL-24 and IL-19 in patients and controls.
References
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Leech MT, Morand EF. Fibroblasts and synovial immunity. Curr Opin Pharmacol. 2013;13:565–569. doi: 10.1016/j.coph.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Williams RO. Evaluation of the role of cytokines in autoimmune disease: the importance of TNFα in rheumatoid arthritis. Prog Growth Factor Res. 1992;4:247–255. doi: 10.1016/0955-2235(92)90022-a. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Li HH, Hsieh MY. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006;54:2722–2733. doi: 10.1002/art.22039. [DOI] [PubMed] [Google Scholar]
- Brennan F, Beech J. Update on cytokines in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:296–301. doi: 10.1097/BOR.0b013e32805e87f1. [DOI] [PubMed] [Google Scholar]
- Furst DE, Keystone EC, So AK. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Ann Rheum Dis. 2013;72(Suppl. 2):ii2–34. doi: 10.1136/annrheumdis-2013-203348. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Chang MS. Interleukin-20 antibody is a potential therapeutic agent for experimental arthritis. Arthritis Rheum. 2010;62:3311–3321. doi: 10.1002/art.27689. [DOI] [PubMed] [Google Scholar]
- Leng RX, Pan HF, Tao JH, Ye DQ. IL-19, IL-20 and IL-24: potential therapeutic targets for autoimmune diseases. Expert Opin Ther Targets. 2011;15:119–126. doi: 10.1517/14728222.2011.534461. [DOI] [PubMed] [Google Scholar]
- Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- Romer J, Hasselager E, Nørby PL, Steiniche T, Thorn Clausen J, Kragballe K. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine A or calcipotriol. J Invest Dermatol. 2003;121:1306–1311. doi: 10.1111/j.1523-1747.2003.12626.x. [DOI] [PubMed] [Google Scholar]
- Kõks S, Kingo K, Rätsep R, Karelson M, Silm H, Vasar E. Combined haplotype analysis of the interleukin-19 and -20 genes: relationship to plaque-type psoriasis. Genes Immun. 2004;5:662–667. doi: 10.1038/sj.gene.6364141. [DOI] [PubMed] [Google Scholar]
- Kõks S, Kingo K, Vabrit K. Possible relations between the polymorphisms of the cytokines IL-19, IL-20 and IL-24 and plaque-type psoriasis. Genes Immun. 2005;6:407–415. doi: 10.1038/sj.gene.6364216. [DOI] [PubMed] [Google Scholar]
- Kingo K, Mossner R, Rätsep R. Association analysis of IL20RA and IL20RB genes in psoriasis. Genes Immun. 2008;9:445–451. doi: 10.1038/gene.2008.36. [DOI] [PubMed] [Google Scholar]
- Kragstrup TW, Otkjaer K, Holm C. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2008;41:16–23. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Zhang W, Doherty M, Peat G. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Todd DJ, Knowlton N, Amato M. Erroneous augmentation of multiplex assay measurements in patients with rheumatoid arthritis due to heterophilic binding by serum rheumatoid factor. Arthritis Rheum. 2011;63:894–903. doi: 10.1002/art.30213. [DOI] [PubMed] [Google Scholar]
- Weinberger A, Simkin PA. Plasma proteins in synovial fluids of normal human joints. Semin Arthritis Rheum. 1989;19:66–76. doi: 10.1016/0049-0172(89)90087-5. [DOI] [PubMed] [Google Scholar]
- Killingsworth LM. Clinical applications of protein determinations in biological fluids other than blood. Clin Chem. 1982;28:1093–1102. [PubMed] [Google Scholar]
- Lebre MC, Lonckheere CL, Kraan MC. Expression of IL-20 in synovium and lesional skin of patients with psoriatic arthritis: differential response to alefacept treatment. Arthritis Res Ther. 2012;14:R200. doi: 10.1186/ar4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liang P. Interleukin-24 and its receptors. Immunology. 2005;114:166–170. doi: 10.1111/j.1365-2567.2005.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohem CL, Brezinschek RI, Wisbey H, Tortorella C, Lipsky PE, Oppenheimer-Marks N. Enrichment of differentiated CD45RBdim,CD27– memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:844–854. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- Gallagher G, Dickensheets H, Eskdale J. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- Liao YC, Liang WG, Chen FW. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J Immunol. 2002;169:4288–4297. doi: 10.4049/jimmunol.169.8.4288. [DOI] [PubMed] [Google Scholar]
- Sakurai N, Kuroiwa T, Ikeuchi H. Expression of IL-19 and its receptors in RA: potential role for synovial hyperplasia formation. Rheumatology. 2008;47:815–820. doi: 10.1093/rheumatology/ken061. [DOI] [PubMed] [Google Scholar]
- Alanara T, Karstila K, Moilanen T, Silvennoinen O, Isomaki P. Expression of IL-10 family cytokines in rheumatoid arthritis: elevated levels of IL-19 in the joints. Scand J Rheumatol. 2010;39:118–126. doi: 10.3109/03009740903170823. [DOI] [PubMed] [Google Scholar]
- Li HH, Lin YC, Chen PJ. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol. 2005;153:591–595. doi: 10.1111/j.1365-2133.2005.06665.x. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Hsieh PP, Chang MS. Interleukin-19 blockade attenuates collagen-induced arthritis in rats. Rheumatology (Oxf) 2012;51:434–442. doi: 10.1093/rheumatology/ker127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Serum and synovial fluid levels of interleukin (IL)-20, IL-24 and IL-19 in patients and controls.


