Abstract
Intravenous immunoglobulin (IVIg) reacted with a wide array of human leucocyte antigen (HLA) alleles, in contrast to normal sera, due possibly to the purification of IgG from the pooled plasma. The reactivity of IgG purified from normal sera was compared with that of native sera to determine whether any serum factors mask the HLA reactivity of anti-HLA IgG and whether IgG purified from sera can recognize the HLA types of the corresponding donors. The purified IgG, unlike native sera, mirrored IVIg reactivity to a wide array of HLA-I/-II alleles, indicating that anti-HLA IgG may be masked in normal sera – either by peptides derived from soluble HLA or by those from antibodies. A < 3 kDa peptide from the complementarity-determining region (CDR) of the Fab region of IgG (but not the HLA peptides) masked HLA recognition by the purified IgG. Most importantly, some of the anti-HLA IgG purified from normal sera – and serum IgG from a few donors – indeed recognized the HLA types of the corresponding donors, confirming the presence of auto-HLA antibodies. Comparison of HLA types with the profile of HLA antibodies showed auto-HLA IgG to the donors' HLA antigens in this order of frequency: DPA (80%), DQA (71%), DRB345 (67%), DQB (57%), Cw (50%), DBP (43%), DRB1 (21%), A (14%) and B (7%). The auto-HLA antibodies, when unmasked in vivo, may perform immunoregulatory functions similar to those of therapeutic preparations of IVIg.
Keywords: CDR peptide, HLA autoantibody, HLA reactivity, HLA typing, intravenous immunoglobulin
Introduction
Anti-human leucocyte antigen (HLA) immunoglobulin (Ig)G antibodies increase in patients waiting for organ transplantation 1. After transplantation, many patients have IgG antibodies reactive against HLA types of the allograft 2, i.e. donor-specific antibodies (DSA). Most of these patients also produce non-donor-specific HLA antibodies (NDSA), both DSA and NDSA correlating with allograft rejection 3. One of the therapeutic countermeasures developed is to administer intravenous immunoglobulin (IVIg), purified from plasma pooled from thousands of donors 1,2. In 2004, the US Food and Drug Administration (FDA) approved IVIg for lowering the incidence of anti-HLA antibodies in allograft recipients. High-dose IVIg is given to reduce the anti-HLA IgG in patients waiting for an allograft 1 and to those developing DSA post-transplant 2. IVIg is also used with several autoimmune diseases to suppress effector T and B cells involved in antibody production 4,5. Paradoxically, all available therapeutic IVIg preparations contain high levels of IgG that react to a wide array of HLA antigens 6 – possibly defeating the very purpose of reducing the levels of anti-HLA antibodies.
Perhaps IVIg recognizes a wide array of HLA alleles because the pooled sera or plasma of normal individuals from which it is derived contains naturally occurring anti-HLA IgG antibodies 7, or perhaps because of purification of the IgG from the pooled plasma. If the latter is the reason, we hypothesized that the IgG purified from normal sera should also display a similar wide variety of HLA reactivity. If so, it is essential to ascertain whether the natural IgG, purified or in native sera, recognizes corresponding donor HLA types, and it is also essential to identify the factors that mask the HLA reactivity of IgG in native sera. Identification of such masking factors may lead to a therapeutic benefit in that it may facilitate clinical minimization of the HLA reactivity of the sera of sensitized patients, allograft recipients and even patients with autoimmune diseases. In addition, elucidation of the masking factors may lead to an understanding of the innate regulation of anti-HLA antibody expression.
Accordingly, the objectives of this investigation were:
to determine whether purification and isolation of normal individual sera exposes IgG antibodies that react, like IVIg, to a wide variety of HLA alleles;
to examine whether the HLA reactivity of IgG – before or after purification from the sera – recognizes the HLA types of the corresponding donors, thus establishing the presence of auto-HLA antibodies; and
to ascertain which serum factors, possibly derived from degraded circulating HLA or IgG, mask expression of the HLA antibodies in sera.
Materials and methods
Sources of the therapeutic preparations of IVIg
Previously, four therapeutic preparations of IVIg were examined for anti-HLA-I reactivity 6. Here, we examined the same IVIg preparations for anti-HLA-II reactivity. These were: GamaSTAN™ S/D (15–18%, Lot 26NHCVI) (Telacris Biotherapeutics, Inc., Research Triangle Park, NC, USA); Sandoglobulin® (6%, Lot 4305800026) (CSL Behring, Kankakee, IL, USA); Octagam® (6%, Lot A913A8431) (Octapharma Pharmazeutika, Switzerland/Austria); and Gamunex®-C (10%, Lots 26NKLG1 and 26NKLK1) (Telacris). All IVIg was serially diluted with phosphate-buffered saline (PBS) (pH 7·2), respectively, to 1/512, 1/1024, 1/2048 and 1/4096.
Source of normal non-alloimmunized male sera
After informed consent, 5 ml of blood was drawn from each of 20 normal, non-alloimmunized male volunteers – researchers at the Terasaki Foundation Laboratory (TFL) or their family members or visiting scientists. The sera were analysed along with LABScreen® negative control serum from Lot 9 (LSNC-9) (One Lambda, Inc. Canoga Park, CA, USA), a pool of sera from eight to 10 donors. Volunteer TFL3 had received tuberculosis (TB) vaccine, and R2 had received anthrax, meningococcal C, hepatitis B and smallpox vaccines within 10 months prior to donation; TFL5 had had a titanium implant after a leg injury 6 months earlier; TFL1 had had an IVIg infusion 3 years earlier. Fresh sera were used for purification of IgG; sera and purified IgG were frozen and stored. Both fresh and thawed sera and purified IgG were analysed simultaneously. For peptide inhibition study, frozen sera of TFL5 and TFL12 were used.
IgG repertoire purified from the sera of non-alloimmunized males
To isolate IgG from the sera, the sera were passed through protein-G columns (Thermo Fisher Scientific, Rockford, IL, USA). Each serum sample was centrifuged for 5 min at 9300 g before 132 μl was applied to the protein-G agarose resin washed in PBS, pH 7·2; the mixture was incubated for 15 min, centrifuged and collected as IgG-free flow-through. Purified IgG was eluted three times, after three washes of the column with 400 μl binding buffer (PBS, pH 7·2), by adding 400 μl acidic buffer (pH 2·8) each time and recovery by centrifugation (for 1 min) in alkaline buffer (pH 8·5; 40 μl). The protein concentrations (mg/ml) in the three eluates (E1, E2, E3) were determined using a BioPhotometer (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany), calculated with standard human IgG curve. The unpurified sera were tested at 1:10 dilution, but the protein-G eluates were tested neat because they were diluted 1/10 during protein-G elution. The IgGs in the sera and eluates were tested at similar concentrations of IgG. The mean fluorescence intensity (MFI) of E1 was always low or minimal compared with that of E2 and E3.
Luminex-based immunoassay using microbeads coated with single HLA antigen
To detect IgG reactivity to HLA-I and/or -II alleles in IVIg, human sera and purified IgG, a Luminex-based immunoassay was used 6,7. Single antigen assays were carried out using xMAP® Luminex dual-laser multiplex flow cytometry (LABScan™ 100; One Lambda) 6,7. The array of various recombinant HLA alleles on the beads is listed under ‘Antibody detection products/LABScreen Single Antigen Product: HLA-II LS2A01009 (Lot 9)’ (see http://www.onelambda.com). The single recombinant HLA-Ia alleles in LS1A04, Lots 007 and 008, were also used for screening IVIg, sera, LSNC and purified IgG, as reported earlier 6,7. HLA-I and -II microbeads have built-in control beads – positive (coated with human IgG) and negative (coated with serum albumin). MFI [mean ± standard deviation (s.d.)] for each allele was recorded as a .csv file, corrected against negative control (normalized), and archived from mid-2012. Basic statistics were calculated with Excel software.
Isolation of different molecular size fragments of peptides, polypeptides and proteins from wash fluid
For molecular sieving of the peptide/polypeptide fractions in flow-through and wash fluid (WF), Millipore concentrator columns were used (EMD Millipore, Billerica, MA, USA). First, 500 μl of WF1 was transferred to an Amicon® Ultra-0·5 100 K device (Millipore ref. UFC510024), centrifuged for 5 min at 14 000 g, then 100 K of the concentrate (30 μl) was recovered and used in the studies detailed below. The rest of the filtrate was centrifuged further in an Ultra-0·5 10 K device (ref. UFC501024) and the “10 K” concentrate recovered and used (see below). Finally, the filtrate was centrifuged in an Ultra-0·5 3 K device (ref. UFC500324), centrifuged, and the 3 K-10 K concentrate recovered and used, as was the “< 3 K” filtrate.
Inhibition of HLA reactivity of IgG with different molecular sizes of peptides from the serum wash fluid 1
For inhibition of HLA reactivity of purified IgG, the concentrates obtained after molecular sieving were admixed with serum E2 or E3 and incubated 30 min at room temperature (RT) on a shaker. WF was admixed with E2 or E3 in the ratio: E : WF or PBS, 20:10, 25:5. The final volume was 30 μl, but only 20 μl of the mixture was added in a Luminex tray to 2 μl of microbeads coated with HLA. Eluates without WF, and eluates with PBS at different ratios, were used for calculating the percentage of change at each dilution. The beads were washed three times with LABScreen wash buffer. Phycoerythrin (PE)-conjugated anti-human IgG (50 μl) was added, incubated 30 min at RT and washed three times with wash buffer. The MFI was measured by Luminex.
Inhibition of HLA reactivity of purified IgG from normal serum using HLA-I and IgG-derived peptides
Sera may contain peptides derived from fragmented or proteolyzed HLA-I or HLA-II or non-HLA molecules, or from proteolyzed Fab or Fc fragments of antibodies. HLA-I may have peptides specific for HLA alleles or commonly shared peptides that are masked by β2-microglobulin (β2m). This investigation used these shared peptides, which are common to the loci of both HLA-Ia (HLA-A, HLA-B and HLA-Cw) and -Ib (HLA-E, HLA-F and HLA-G), and identified as 115QFAYDGKDY123 and 126LNEDLRSWTA135; their source, purity and characteristics were also reported earlier 8,9. In addition, the conserved complementarity-determining region (CDRII) peptide sequence of IgG was used for peptide inhibition with the recommended peptides 10–12. LifeTein® LLC (South Plainfield, NJ, USA) synthesized at 98% purity the amino acid sequence ASRNKANDYTTEYSASVKGRFIVS (Mw: 2664·2 g/mol; water-soluble) of the peptide TEPC15 (50–73), also called ‘T15H’ 10,11, and the amino acid sequence QIYPGDGDTKYNNGKFKGKATLT of the control peptide 2FIOH (50–73), which has a similar Mw and solubility.
Specific peptide inhibition was also tested with the serum E2 fraction of three different volunteers (TFL5 for HLA-I; TFL6 and TFL12 for HLA-II). Commonly shared peptide sequences of HLA and conserved CDRII peptide, with the control peptide, were used. Eluted IgG fraction (E2) (25 μl) was mixed with 5 μl of peptide at different dilutions; the final volume was 30 μl. After incubating for 30 min at RT on a shaker, 20 μl of the mixture was tested with antigen-coated microbeads. For HLA-I beads, HLA-E was used because it carries the common shared peptide sequences tested. Because loss of sensitivity is likely with multiple HLA-I allele-coated single antigen beads (n = 90), single beads of this kind were used. Sensitivity was preserved by using a mixture of HLA-II beads with the serum E2 fraction of TFL6 and TFL12, which is why observations on peptide inhibition were restricted to the most prevalent HLA-II molecules, as described in the Results. Due to the restricted availability of eluates 2 and 3 from one donor at the same time, all our observations were carried out either on the two eluates separately or from the eluates of three different sera to validate the observations.
Immunoassay using iBeads
One Lambda has generated beads with reduced amounts of β2m-free HLA (iBeads); these are regular HLA-I-coated microbeads subjected to proprietary enzymatic treatment to remove or reduce the amount of heavy chains (denatured antigens). The manufacturer provided iBeads as Felix beads for in-house use.
Murine monoclonal antibody reacting to all HLA class-II alleles
Mouse monoclonal antibody (mAb) FJ5109 (One Lambda), reactive to all HLA-II alleles, was used after being passed through a protein-G column to determine whether that caused any change in the reactivity profile.
HLA molecular typing
DNA from the donors' blood was isolated by using the QIAamp DNA Mini Kit (Qiagen Group, Valencia, CA, USA), then polymerase chain reaction (PCR)-amplified and typed using the LABType® SSO Typing Test Kit (One Lambda).
Results
Basis of the experiments
We examined the HLA reactivity of sera, and IgG purified from those sera, from 20 volunteers: 11 TFL researchers, three visiting scientists and six members of three researcher/visitor families. Serum of a 12th TFL visitor was used in a study of peptide inhibition of antibody binding to microbeads. The HLA reactivity of four IVIg preparations was also examined: GamaSTAN, Octagam, two lots of Gamunex-C and Sandoglobulin. The number of alleles examined was: 31 HLA-A, 50 HLA-B, 16 HLA-Cw, 36 HLA-DRB, 29 HLA-DQ and 26 HLA-DP.
Anti-albumin IgG: natural autoantibody in non-alloimmunized males and IVIg
IgG reactivity to HLA-I/-II alleles in IVIg and purified human IgG was measured by single antigen assay. The MFI of anti-albumin IgG in normal sera is < 150. After purification of IgG from the sera, the MFI measured several fold higher. The MFI of the purified anti-albumin IgG (at 1:10 dilution) of the donors ranged extensively; note that the seventh highest, ‘LSNC’, is not one of the 20 original donors, but the control serum: G1 (MFI, 5140), TFL3 (3477), W1 (3399), TFL4 (3237), R1 (1830), TFL5 (1549), LSNC (1394), TFL1 (1299), W2 (1186), TFL9 (1152), TFL7 (1103), T2 (910), TFL6 (808), TFL8 (778), W3 (772), TFL10 (673), R2 (663), TFL2 (633), T3 (485), TFL11 (467) and UCLA1 (213). There was no correlation between age and MFI, but the sometimes anomalous disparities will be addressed in the Discussion. The anti-albumin values were used as negative control to normalize the respective MFIs of the HLA-I/-II antibodies obtained from purified serum. Interestingly, all IVIg preparations except Sandoglobulin showed high reactivity to the albumin-coated beads, indicating the presence of differing levels of anti-albumin IgG antibodies in different preparations (data not shown). The two lots of Gamunex-C had the highest level of HLA reactivity.
Anti-HLA-II IgG: profiles before and after purification of IgG from the sera
The profile and incidence of anti-HLA-II IgG antibodies before and after purifying the IgG were examined in three cohorts of sera: the LSNC (Table 1); from different generations of non-alloimmunized, healthy males in three families (Table 2a–c); and from 11 other normal, non-alloimmunized, healthy TFL males (Table 3a–c).
Table 1.
Mean fluorescence intensity (MFI) of IgG antibodies in negative control sera [LABscreen negative serum [LSNC)] and in purified LSNC eluate 2 (E2) that reacted to human leucocyte antigen (HLA) class II alleles and albumin
| HLA class II IgG†† in LSNC before and after purification of IgG and normalization of MFI | ||||||||
|---|---|---|---|---|---|---|---|---|
| DRB | LSNC (1/10) | Normalized E2 | DQA/DQB | LSNC (1/10) | Normalized E2 | DPA/DPB | LSNC (1/10) | Normalized E2 |
| Anti-albumin | 20 | 2698 | DQA1*01:01\DQB1*03:02 | 38 | 992 | DPA1*01:03\DPB1*02:01 | 44 | 697 |
| DRB1*08:01 | 33 | 1260 | DQA1*01:01\DQB1*06:02 | 43 | 1580 | DPA1*01:03\DPB1*11:01 | 38 | 1540 |
| DRB1*09:01 | 46 | 556 | DQA1*02:01\DQB1*03:02 | 31 | 951 | DPA1*01:03\DPB1*19:01 | 54 | 2811 |
| DRB3*01:01 | 29 | 1668 | DQA1*02:01\DQB1*04:01 | 46 | 1052 | DPA1*01:05\DPB1*13:01 | 32 | 1010 |
| DRB3*03:01 | 167 | 7616 | DQA1*03:01\DQB1*03:01 | 32 | 2457 | DPA1*02:01\DPB1*13:02 | 31 | 951 |
| DQA1*03:01\DQB1*03:02 | 33 | 561 | DPA1*02:01\DPB1*15:01 | 22 | 747 | |||
| DPA1*02:01\DPB1*23:01 | 66 | 6929 | ||||||
| DPA1*02:01\DPB1*28:01 | 57 | 1499 | ||||||
The antibody with the highest MFI value for each locus is shown in bold type.
Table 2.
Mean fluorescence intensity (MFI) of serum and purified IgG from different generations of three families that reacted to human leucocyte antigen (HLA) class II alleles and albumin
| (a) HLA class II IgG antibodies in serum and in eluate 2 after purification of IgG and normalization of MFI | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | Son |
Father |
Paternal grandfather |
DQA/DQB | Son |
Father |
Paternal grandfather |
DPA/DPB | Son |
Father |
Paternal grandfather |
|||||||||
| Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | |||
| Anti-albumin | 910 | 1299 | DQA1*01:01\DQB1*03:02 | 701 | 778 | 512 | 1443 | DPA1*01:03\DPB1*02:01 | 590 | 845 | ||||||||||
| DRB1*03:02 | 555 | 649 | DQA1*01:01\DQB1*06:02 | 509 | 515 | DPA1*01:03\DPB1*03:01 | 597 | 530 | ||||||||||||
| DRB1*08:01 | 758 | 767 | 763 | DQA1*01:02\DQB1*06:02 | 797 | 1004 | 859 | DPA1*01:03\DPB1*04:01 | 572 | |||||||||||
| DRB1*09:01 | 687 | 542 | 664 | DQA1*02:01\DQB1*03:01 | 908 | 503 | DPA1*01:03\DPB1*04:02 | 1136 | 712 | |||||||||||
| DRB1*11:01 | 507 | DQA1*02:01\DQB1*03:02 | 748 | 815 | 1264 | 2440 | DPA1*01:03\DPB1*11:01 | 1014 | 694 | 1338 | 519 | 1551 | ||||||||
| DRB1*11:04 | 707 | 1026 | DQA1*02:01\DQB1*04:01 | 645 | 759 | 670 | DPA1*01:03\DPB1*19:01 | 1701 | 560 | 1520 | 1682 | |||||||||
| DRB1*13:01 | 606 | DQA1*02:01\DQB1*04:02 | 539 | DPA1*01:05\DPB1*03:01 | 572 | 697 | ||||||||||||||
| DRB1*14:02 | 660 | DQA1*03:01\DQB1*03:01 | 708 | 791 | 581 | 1148 | DPA1*01:05\DPB1*11:01 | 683 | 1089 | 1216 | 1394 | |||||||||
| DRB1*15:03 | 673 | DQA1*03:01\DQB1*03:02 | 1447 | 2125 | DPA1*01:05\DPB1*13:01 | 962 | 996 | 790 | ||||||||||||
| DRB3*01:01 | 1038 | 1102 | 1104 | DQA1*05:03\DQB1*03:01 | 1608 | 740 | DPA1*01:05\DPB1*18:01 | 1060 | ||||||||||||
| DRB3*02:02 | 508 | DQA1*05:05\DQB1*03:01 | 1523 | 765 | DPA1*01:05\DPB1*28:01 | 642 | ||||||||||||||
| DRB3*03:01 | 3436 | 467 | 3637 | 7939 | DQA1*06:01\DQB1*03:01 | 1595 | 745 | DPA1*02:01\DPB1*01:01 | 578 | 1276 | 1594 | |||||||||
| DRB4*01:01 | 786 | 710 | DPA1*02:01\DPB1*03:01 | 733 | 528 | |||||||||||||||
| DRB5*01:01 | 553 | 552 | DPA1*02:01\DPB1*05:01 | 559 | ||||||||||||||||
| DPA1*02:01\DPB1*13:01 | 1198 | 1156 | 1242 | |||||||||||||||||
| DPA1*02:01\DPB1*15:01 | 752 | 646 | 1151 | 678 | ||||||||||||||||
| DPA1*02:01\DPB1*23:01 | 3449 | 2950 | 5018 | |||||||||||||||||
| DPA1*02:01\DPB1*28:01 | 862 | 523 | 1029 | 1337 | ||||||||||||||||
| Serum of the son did not show reactivity for any of the DRB/DQA\DQB/DPA\DPB alleles. † The antibody with the highest MFI value for each locus is shown in bold type. | ||||||||||||||||||||
| (b)HLA class II IgG antibodies † in serum and in eluate 2 after purification of IgG and normalization of MFI | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | Son |
Father |
Maternal grandfather |
DQA/DQB | Son |
Father |
Maternal grandfather |
DPA/DPB | Son |
Father |
Maternal grandfather |
|||||||||
| Serum | Normalized Purified IgG | Serum | Normalized Purified IgG | Serum | Normalized Purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | |||
| Anti-albumin | 772 | 1186 | 1832 | 3399 | DQA1*01:01\DQB1*03:02 | 910 | 844 | 579 | 1566 | DPA1*01:03\DPB1*01:01 | 640 | |||||||||
| DRB1*03:02 | 501 | 1373 | 609 | DQA1*01:01\DQB1*06:02 | 533 | 604 | DPA1*01:03\DPB1*02:01 | 927 | 644 | 849 | 892 | |||||||||
| DRB1*04:01 | 513 | DQA1*01:02\DQB1*05:02 | 520 | DPA1*01:03\DPB1*11:01 | 611 | 1105 | ||||||||||||||
| DRB1*04:02 | 602 | DQA1*01:02\DQB1*06:02 | 1061 | 954 | 673 | 1914 | DPA1*01:03\DPB1*19:01 | 2256 | 1351 | 1106 | 2431 | |||||||||
| DRB1*04:03 | 717 | DQA1*01:02\DQB1*06:04 | 507 | 382 | 643 | DPA1*01:05\DPB1*03:01 | 591 | |||||||||||||
| DRB1*04:04 | 519 | DQA1*01:02\DQB1*06:09 | 546 | DPA1*01:05\DPB1*11:01 | 647 | 665 | ||||||||||||||
| DRB1*08:01 | 813 | 595 | 642 | 525 | DQA1*02:01\DQB1*03:02 | 981 | 884 | 1686 | DPA1*01:05\DPB1*13:01 | 1109 | 999 | 843 | 1402 | |||||||
| DRB1*09:01 | 590 | 956 | DQA1*02:01\DQB1*04:01 | 699 | 880 | 564 | 1060 | DPA1*02:01\DPB1*01:01 | 633 | 744 | ||||||||||
| DRB1*11:04 | 780 | DQA1*02:01\DQB1*04:02 | 508 | 537 | DPA1*02:01\DPB1*03:01 | 693 | ||||||||||||||
| DRB1*12:01 | 1608 | 834 | DQA1*03:01\DQB1*03:01 | 939 | 881 | 2185 | DPA1*02:01\DPB1*05:01 | 833 | ||||||||||||
| DRB1*12:02 | 612 | 551 | DQA1*03:01\DQB1*03:02 | 528 | 508 | 559 | DPA1*02:01\DPB1*06:01 | 1030 | ||||||||||||
| DRB1*14:02 | 671 | DQA1*03:01\DQB1*03:03 | 524 | DPA1*02:01\DPB1*10:01 | 662 | |||||||||||||||
| DRB1*14:54 | 615 | DQA1*05:01\DQB1*02:01 | 693 | DPA1*02:01\DPB1*13:01 | 1672 | 1520 | 902 | 2831 | ||||||||||||
| DRB1*15:01 | 1208 | DPA1*02:01\DPB1*14:01 | 674 | |||||||||||||||||
| DRB1*15:02 | 504 | DPA1*02:01\DPB1*15:01 | 827 | 625 | 1041 | 1226 | ||||||||||||||
| DRB1*16:01 | 714 | DPA1*02:01\DPB1*17:01 | 598 | |||||||||||||||||
| DRB1*16:02 | 519 | DPA1*02:01\DPB1*23:01 | 3251 | 3021 | 1433 | 5886 | ||||||||||||||
| DRB3*01:01 | 1022 | 825 | 1377 | 1999 | DPA1*02:01\DPB1*28:01 | 934 | 673 | 1326 | 1043 | |||||||||||
| DRB3*02:02 | 584 | |||||||||||||||||||
| DRB3*03:01 | 3013 | 552 | 2497 | 2541 | 5617 | |||||||||||||||
| DRB4*01:01 | 690 | |||||||||||||||||||
| DRB5*01:01 | 841 | |||||||||||||||||||
| Serum of the son did not show reactivity for any of the DQA\DQB/DPA\DPB alleles. † The antibody with the highest MFI value for each locus is shown in bold type. | ||||||||||||||||||||
| (c)HLA class II IgG antibodies† in serum and in eluate 2 after purification of IgG and normalization of MFI | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | Father |
1st son |
2nd son |
DQA/DQB | Father |
1st son |
2nd son |
DPA/DPB | Father |
1st son |
2nd son |
|||||||||
| Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | serum | Normalized purified IgG | Serum | Normalized purified IgG | |||
| Anti-albumin | 633 | 1830 | 663 | DQA1*01:01\DQB1*03:02 | 841 | 1162 | 815 | DPA1*01:03\DPB1*02:01 | 741 | 639 | ||||||||||
| DRB1*03:01 | 991 | 2211 | DQA1*01:01\DQB1*06:02 | 696 | 581 | DPA1*01:03\DPB1*11:01 | 1189 | 826 | 766 | |||||||||||
| DRB1*03:02 | 907 | 1834 | 4199 | DQA1*01:02\DQB1*05:02 | 525 | 847 | DPA1*01:03\DPB1*19:01 | 1392 | 2020 | 1052 | ||||||||||
| DRB1*08:01 | 834 | 1210 | 748 | DQA1*01:02\DQB1*06:02 | 1087 | 1178 | 750 | DPA1*01:05\DPB1*11:01 | 528 | 531 | 513 | |||||||||
| DRB1*09:01 | 797 | DQA1*01:02\DQB1*06:04 | 778 | DPA1*01:05\DPB1*13:01 | 963 | 1312 | 823 | |||||||||||||
| DRB1*10:01 | 574 | 1988 | 2864 | 2201 | DQA1*02:01\DQB1*03:02 | 880 | 1308 | 757 | DPA1*02:01\DPB1*05:01 | 519 | ||||||||||
| DRB1*12:01 | 705 | DQA1*02:01\DQB1*04:01 | 649 | 938 | 612 | DPA1*02:01\DPB1*13:01 | 1126 | 1991 | 1167 | |||||||||||
| DRB1*12:02 | 571 | 628 | DQA1*03:01\DQB1*02:01 | 682 | DPA1*02:01\DPB1*15:01 | 813 | 715 | 670 | ||||||||||||
| DRB1*14:02 | 558 | DQA1*03:01\DQB1*03:01 | 800 | 1325 | 588 | DPA1*02:01\DPB1*23:01 | 3891 | 5816 | 2541 | |||||||||||
| DRB3*01:01 | 1153 | 1214 | 779 | DQA1*03:01\DQB1*03:02 | 485 | 677 | DPA1*02:01\DPB1*28:01 | 1447 | 1055 | 717 | ||||||||||
| DRB3*03:01 | 4964 | 4786 | 3300 | DQA1*03:01\DQB1*03:03 | 606 | |||||||||||||||
| DRB4*01:01 | 743 | 500 | 925 | |||||||||||||||||
Sera of neither father nor sons showed reactivity for any of the DQA\DQB/DPA\DPB alleles. †The antibody with the highest MFI value for each locus is shown in bold type.
Table 3.
Mean fluorescence intensity (MFI) of serum and purified IgG of male volunteers that reacted with human leucocyte antigen (HLA) class II alleles and albumin
| (a) Anti-HLA-DRB reactivity | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA class II IgG antibodies† in serum and in eluate 2 after purification of IgG and normalization of MFI | ||||||||||||||||||||||
| DRB | TFL3 |
TFL4 |
TFL5 |
TFL6 |
TFL7 |
TFL8 |
TFL9 |
TFL10 |
TFL11 |
UCLA1 |
G1 |
|||||||||||
| Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | |
| Anti-albumin | 3477 | 3237 | 1549 | 808 | 1103 | 778 | 1 152 | 673 | 5 140 | |||||||||||||
| DRB1*01:02 | 526 | |||||||||||||||||||||
| DRB1*03:01 | 715 | 1 262 | 544 | |||||||||||||||||||
| DRB1*03:02 | 671 | 812 | 648 | 2695 | 582 | 1 826 | 606 | 1676 | ||||||||||||||
| DRB1*04:01 | 677 | 529 | 933 | 635 | ||||||||||||||||||
| DRB1*04:02 | 589 | 506 | ||||||||||||||||||||
| DRB1*04:03 | 853 | 606 | 723 | 582 | 762 | |||||||||||||||||
| DRB1*04:04 | 944 | 892 | 610 | 575 | ||||||||||||||||||
| DRB1*04:05 | 843 | 670 | ||||||||||||||||||||
| DRB1*08:01 | 863 | 713 | 706 | 548 | 1276 | 2 611 | ||||||||||||||||
| DRB1*09:01 | 939 | 613 | 662 | 1 612 | 1360 | |||||||||||||||||
| DRB1*11:01 | 721 | |||||||||||||||||||||
| DRB1*11:04 | 1117 | 1289 | 1019 | |||||||||||||||||||
| DRB1*12:02 | 523 | |||||||||||||||||||||
| DRB1*14:02 | 729 | 732 | 1034 | |||||||||||||||||||
| DRB1*14:54 | 593 | 1131 | ||||||||||||||||||||
| DRB1*15:03 | 690 | |||||||||||||||||||||
| DRB1*16:01 | 934 | 788 | ||||||||||||||||||||
| DRB3*01:01 | 1398 | 994 | 565 | 998 | 1 717 | 2128 | 630 | 4 394 | ||||||||||||||
| DRB3*02:02 | 575 | |||||||||||||||||||||
| DRB3*03:01 | 2096 | 2040 | 5013 | 5385 | 4133 | 4304 | 10 088 | 510 | 6371 | 2576 | 987 | 12 401 | ||||||||||
| DRB4*01:01 | 625 | 540 | 752 | 777 | 552 | |||||||||||||||||
| DRB5*01:01 | 628 | |||||||||||||||||||||
Sera of TFL6/8/11, UCLA1 and G1 did not show reactivity for any of the DRB alleles. †The antibody with the highest MFI value is shown in bold type.
| (b) Anti-HLA-DQ reactivity | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA class II IgG antibodies† in serum and in eluate 2 after purification of IgG and normalization of MFI | ||||||||||||||||||||||
| DQA/DQB | TFL3 |
TFL4 |
TFL5 |
TFL6 |
TFL7 |
TFL8 |
TFL9 |
TFL10 |
TFL11 |
UCLA1 |
G1 |
|||||||||||
| Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | |
| Anti-albumin | 3477 | 3237 | 1549 | 808 | 1103 | 778 | 1152 | 673 | 5140 | |||||||||||||
| DQA1*01:01\DQB1*03:02 | 1342 | 631 | 537 | 727 | 1263 | 3429 | ||||||||||||||||
| DQA1*01:01\DQB1*06:02 | 641 | 1604 | 2344 | 784 | ||||||||||||||||||
| DQA1*01:02\DQB1*05:02 | 1183 | 2439 | 3252 | 738 | ||||||||||||||||||
| DQA1*01:02\DQB1*06:02 | 567 | 538 | 1873 | 748 | 677 | 508 | 1502 | 2337 | 514 | 5181 | ||||||||||||
| DQA1*01:02\DQB1*06:04 | 935 | 1482 | 2030 | 536 | ||||||||||||||||||
| DQA1*02:01\DQB1*02:01 | 1607 | 1752 | ||||||||||||||||||||
| DQA1*02:01\DQB1*02:02 | 514 | 1793 | 2136 | |||||||||||||||||||
| DQA1*02:01\DQB1*03:01 | 543 | 1059 | 1208 | |||||||||||||||||||
| DQA1*02:01\DQB1*03:02 | 1540 | 603 | 506 | 922 | 1476 | 3429 | ||||||||||||||||
| DQA1*02:01\DQB1*04:01 | 1777 | 596 | 577 | 945 | 2362 | |||||||||||||||||
| DQA1*02:01\DQB1*04:02 | 739 | 825 | 1169 | 517 | ||||||||||||||||||
| DQA1*03:01\DQB1*02:01 | 806 | 1267 | ||||||||||||||||||||
| DQA1*03:01\DQB1*03:01 | 500 | 2170 | 606 | 620 | 1155 | 4391 | ||||||||||||||||
| DQA1*03:01\DQB1*03:02 | 656 | 758 | 1237 | 1559 | ||||||||||||||||||
| DQA1*03:01\DQB1*03:03 | 909 | 1188 | ||||||||||||||||||||
| DQA1*03:02\DQB1*03:02 | 549 | |||||||||||||||||||||
| DQA1*04:01\DQB1*02:01 | 749 | 828 | ||||||||||||||||||||
| DQA1*04:01\DQB1*04:02 | 575 | 1074 | 1199 | |||||||||||||||||||
| DQA1*05:03\DQB1*03:01 | 675 | |||||||||||||||||||||
| DQA1*05:05\DQB1*03:01 | 625 | |||||||||||||||||||||
| (c) Anti-HLA-DP reactivity | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA class II IgG antibodies† in serum and in eluate 2 after purification of IgG and normalization of MFI | ||||||||||||||||||||||
| DPA/DPB | TFL3 |
TFL4 |
TFL5 |
TFL6 |
TFL7 |
TFL8 |
TFL9 |
TFL10 |
TFL11 |
UCLA1 |
G1 |
|||||||||||
| Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | Serum | Normalized purified IgG | |
| Anti-albumin | 3477 | 3237 | 1549 | 808 | 1103 | 778 | 1152 | 673 | 5140 | |||||||||||||
| DPA1*01:03\DPB1*01:01 | 588 | 795 | ||||||||||||||||||||
| DPA1*01:03\DPB1*02:01 | 1288 | 553 | 792 | 828 | ||||||||||||||||||
| DPA1*01:03\DPB1*04:02 | 586 | 500 | ||||||||||||||||||||
| DPA1*01:03\DPB1*11:01 | 833 | 990 | 645 | 1029 | 2843 | 522 | 2846 | 627 | 726 | |||||||||||||
| DPA1*01:03\DPB1*19:01 | 793 | 1040 | 614 | 2355 | 1450 | 728 | 1382 | 1977 | 1964 | 925 | 5862 | |||||||||||
| DPA1*01:05\DPB1*03:01 | 507 | |||||||||||||||||||||
| DPA1*01:05\DPB1*11:01 | 848 | 556 | 725 | 1266 | 734 | |||||||||||||||||
| DPA1*01:05\DPB1*13:01 | 1570 | 844 | 874 | 679 | 1344 | 582 | 2082 | |||||||||||||||
| DPA1*01:05\DPB1*18:01 | 527 | |||||||||||||||||||||
| DPA1*01:05\DPB1*28:01 | 602 | 520 | ||||||||||||||||||||
| DPA1*02:01\DPB1*01:01 | 519 | 1561 | 1611 | 1034 | 572 | |||||||||||||||||
| DPA1*02:01\DPB1*03:01 | 544 | |||||||||||||||||||||
| DPA1*02:01\DPB1*05:01 | 1009 | |||||||||||||||||||||
| DPA1*02:01\DPB1*06:01 | 769 | |||||||||||||||||||||
| DPA1*02:01\DPB1*10:01 | 660 | |||||||||||||||||||||
| DPA1*02:01\DPB1*13:01 | 1037 | 2142 | 1104 | 632 | 1003 | 995 | 1604 | 763 | 7057 | |||||||||||||
| DPA1*02:01\DPB1*15:01 | 506 | 1423 | 578 | 581 | 528 | 1206 | 2815 | |||||||||||||||
| DPA1*02:01\DPB1*23:01 | 2411 | 857 | 4921 | 4419 | 2477 | 2891 | 8460 | 4706 | 2317 | 930 | 13746 | |||||||||||
| DPA1*02:01\DPB1*28:01 | 1550 | 808 | 566 | 912 | 1319 | 589 | 2168 | 667 | 2564 | |||||||||||||
Only sera of TFL3, TFL5 and TFL10 showed reactivity for any of the DPA\DPB alleles. †The antibody with the highest MFI value is shown in bold type.
| (d) Incidence of purified and normalized IgG from E2 that reacted to HLA-DRB, DQA/DQB and DPA/DPB haplotypes in all cohorts of sera examined | ||
|---|---|---|
| HLA class II | n = 20 | % |
| Albumin negative control | 17 | 85 |
| DRB | ||
| DRB1*03:02 | 9 | 45 |
| DRB1*08:01 | 15 | 75 |
| DRB1*09:01 | 10 | 50 |
| DRB3*01:01 | 17 | 85 |
| DRB3*03:01 | 20 | 100 |
| DQA/DQB | ||
| DQA1*01:01\DQB1*03:02 | 14 | 70 |
| DQA1*01:02\DQB1*06:02 | 17 | 85 |
| DQA1*02:01\DQB1*03:02 | 14 | 70 |
| DQA1*02:01\DQB1*04:01 | 13 | 65 |
| DQA1*03:01\DQB1*03:01 | 13 | 65 |
| DPA/DPB | ||
| DPA1*01:03\DPB1*02:01 | 11 | 55 |
| DPA1*01:03\DPB1*11:01 | 15 | 75 |
| DPA1*01:03\DPB1*19:01 | 18 | 90 |
| DPA1*01:05\DPB1*11:01 | 11 | 55 |
| DPA1*01:05\DPB1*13:01 | 16 | 80 |
| DPA1*02:01\DPB1*13:01 | 18 | 90 |
| DPA1*02:01\DPB1*15:01 | 15 | 75 |
| DPA1*02:01\DPB1*23:01 | 20 | 100 |
| DPA1*02:01\DPB1*28:01 | 17 | 85 |
Cohort 1
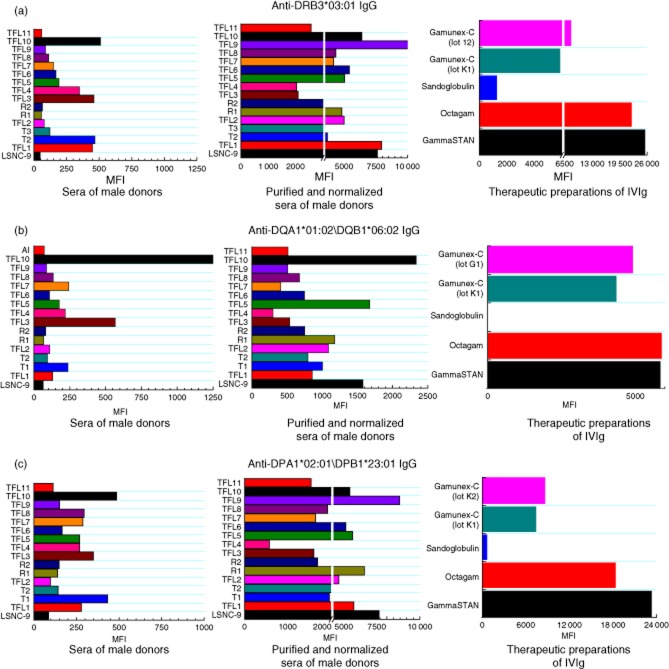
With the LSNC, four DRB, five DQA/DQB and nine DPA/DPB alleles showed high MFI after normalization of the eluate (E2). Among the DRA/DRB loci, the highest MFI was found with DRB3*03:01; among the DQA/DQB loci, with DQA1*01:02\DQB1*06:02; and among the DPA/DPB loci, with DPA1*02:01\DRB1*23:01 (Table 1).
Cohort 2
Table 2a shows the antibody profiles of TFL1, his son and grandson. The grandson's serum had no detectable levels of antibodies while there were antibodies in the sera of his father (anti-DR and anti-DP) and grandfather (anti-DQ). The MFI of antibodies for all alleles was notably higher after purification of IgG. The unpurified sera showed no reactivity to several HLA-II alleles whereas purification of the IgG resulted in strong reactivity (high MFI) against the same alleles. The most prevalent purified IgG antibodies of the grandfather, father and son were the same as those observed with LSNC (Table 1 and 2a).
Table 2b compares the HLA-II antibody profiles of TFL visitor W1, his father and maternal grandfather. Their sera contained very low levels of antibodies, but upon purification the IgG fraction of all three showed much higher reactivity. Their most prevalent purified IgG antibodies were the same as those with LSNC and family 1.
Table 2c compares the antibody profiles of TFL2 and his two sons. While their sera contained no detectable antibodies against DQA/DQB or DPA/DPB haplotypes, there were antibodies in all three sera against one or two DRB alleles. Upon purification, the IgG fraction of all three sera showed much higher reactivity to HLA-II alleles. Again, the most prevalent purified IgG antibodies were the same as with LSNC and families 1 and 2.
Cohort 3
Examination of anti-HLA-II antibodies in sera of the 11 TFL researchers or visitors in this cohort, before and after purification (Table 3a–c), confirmed that the most prevalent purified IgG antibodies in normal males are, apparently, DRB3*03:01 (Table 3a), DQA1*01:02\DQB1*06:02 (Table 3b) and DPA1*02:01\DPB1*23:01 (Table 3c). Table 3d summarizes the results of the analyses of all the cohorts, which elucidates the incidence of IgG reactivity against HLA DRB, DQA/DQB and DPA/DPB alleles. The frequency/incidence was 100% for DRB3*03:01 and for DPA1*02:01\DPB1*23:01. Other major alleles and their frequency are shown in the table.
Anti-HLA-II IgG: profiles of therapeutic IVIg
The preparations of IVIg examined included, as noted, GamaSTAN, Octagam, two lots of Gamunex-C and Sandoglobulin. Table 4 compares their titrimetric MFI profile of anti-HLA-II reactivity. Among IgG antibodies reacting to DRB alleles, reactivity to DRB3*03:01 ranked first in all IVIg preparations (Table 4a), analogous to the highest incidence of the same anti-HLA-DRB IgG purified from the sera of our cohorts. (Henceforth, ‘purified IgG’ means IgG purified from normal sera.) GamaSTAN had the highest titre of antibodies; the profiles and ranks of the other anti-HLA-DRB alleles presented in Table 4a show a striking similarity to those of our cohorts' purified IgG.
Table 4.
Titrimetric mean fluorescence intensity (MFI) values of anti-human leucocyte antigen (HLA) class II reactivity of different therapeutic preparations of IVIg
| (a) Anti-HLA-DR reactivity | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GamaSTAN (15–18%) |
Gamunex-C (10%) |
|||||||||||||
| Rank | Lot: 26NHCVI | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] | [1/2048] | [1/4096] | Lot: 26NKLK1 | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] |
| 1 | DRB3*03:01 | 23 214 | 20 352 | 13 059 | 8575 | 3017 | 1124 | 708 | DRB3*03:01 | 8665 | 6552 | 3973 | 1784 | 1466 |
| 2 | DRB1*09:01 | 8 850 | 7 136 | 2 997 | 1529 | DRB1*03:02 | 2956 | 1660 | 894 | |||||
| 3 | DRB3*01:01 | 6 931 | 4 644 | 1 069 | 1313 | DRB1*09:01 | 1900 | 1064 | 622 | |||||
| 4 | DRB1*08:01 | 3 989 | 2 146 | 810 | 703 | DRB1*08:01 | 2529 | 1034 | 523 | |||||
| 5 | DRB1*11:04 | 3 761 | 3 075 | 1 150 | 603 | DRB3*01:01 | 2241 | 1001 | 520 | |||||
| 6 | DRB1*14:02 | 3 284 | 2 609 | 1 187 | 590 | DRB1*11:04 | 1601 | 886 | ||||||
| 7 | DRB1*03:0 | 2 910 | 1 105 | 553 | 571 | DRB1*14:02 | 1668 | 861 | ||||||
| Octagam (6%) |
Gamunex-C (10%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lot: A913A8431 | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] | Lot : 26NKLG1 | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] | |||
| 1 | DRB3*03:01 | 9344 | 5024 | n/a | 1766 | 699 | DRB3*03:01 | 4640 | 4183 | 1873 | 1108 | 904 | ||
| 2 | DRB1*09:01 | 1946 | 662 | DRB1*03:02 | 2801 | 1713 | 942 | 556 | ||||||
| 3 | DRB1*03:0 | 1194 | 649 | DRB1*09:01 | 1404 | 871 | 840 | |||||||
| 4 | DRB1*11:04 | 1133 | 594 | DRB1*08:01 | 2121 | 1278 | 611 | |||||||
| 5 | DRB3*01:01 | 1102 | 574 | DRB3*01:01 | 2137 | 1228 | 550 | |||||||
| 6 | DRB1*14:02 | 1005 | 552 | DRB4*01:01 | 1430 | 907 | 505 | |||||||
| 7 | DRB1*08:01 | 970 | DRB1*03:01 | 1181 | 643 | |||||||||
| Sandoglobulin (6%) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lot: 4305800026 | [1/64] | [1/128] | [1/256] | [1/512] | ||||||||||
| 1 | DRB3*03:01 | 6197 | 4659 | 2041 | 748 | |||||||||
| (b) Anti-HLA-DQ reactivity | |||||||
|---|---|---|---|---|---|---|---|
| Rank | GamaSTAN (15–18%) |
Gamunex-C (10%) |
|||||
| Lot: 26NHCVI | [1/32] | [1/64] | Lot: 26NKLK1 | [1/32] | [1/64] | [1/128] | |
| 1 | DQA1*01:02\DQB1*06:02 | 2010 | 1177 | DQA1*01:02\DQB1*06:02 | 3546 | 2141 | 1274 |
| 2 | DQA1*01:01\DQB1*06:02 | 1918 | 1132 | DQA1*01:01\DQB1*03:02 | 3243 | 1966 | 1015 |
| 3 | DQA1*03:01\DQB1*03:01 | 1176 | 757 | DQA1*02:01\DQB1*03:02 | 3098 | 2015 | 1028 |
| 4 | DQA1*03:01\DQB1*03:02 | 1095 | 680 | DQA1*03:01\DQB1*03:01 | 2624 | 1678 | 966 |
| 5 | DQA1*01:02\DQB1*06:04 | 1261 | 770 | DQA1*03:01\DQB1*03:02 | 2488 | 1664 | 916 |
| 6 | DQA1*02:01\DQB1*03:02 | 1061 | 692 | DQA1*02:01\DQB1*04:01 | 2265 | 1678 | 739 |
| 7 | DQA1*01:02\DQB1*05:02 | 1050 | 828 | DQA1*01:01\DQB1*06:02 | 2250 | 1522 | 948 |
| Octagam (6%) |
Gamunex-C (10%) |
||||||
|---|---|---|---|---|---|---|---|
| Lot: A913A8431 | [1/32] | [1/64] | Lot: 26NKLG1 | [1/32] | [1/64] | [1/128] | |
| 1 | DQA1*01:02\DQB1*06:02 | 1007 | 613 | DQA1*01:02\DQB1*06:02 | 3661 | 1927 | 815 |
| 2 | DQA1*03:01\DQB1*03:01 | 1398 | 899 | DQA1*01:01\DQB1*03:02 | 4036 | 1755 | 735 |
| 3 | DQA1*01:01\DQB1*03:02 | 926 | 572 | DQA1*03:01\DQB1*03:01 | 3793 | 1429 | 646 |
| 4 | DQA1*02:01\DQB1*03:02 | 757 | DQA1*02:01\DQB1*03:02 | 3544 | 1654 | 679 | |
| 5 | DQA1*01:01\DQB1*06:02 | 643 | DQA1*03:01\DQB1*03:02 | 2567 | 1488 | 702 | |
| 6 | DQA1*03:01\DQB1*03:02 | 661 | DQA1*01:01\DQB1*06:02 | 2244 | 1394 | 661 | |
| 7 | DQA1*01:02\DQB1*06:04 | 713 | DQA1*03:01\DQB1*02:01 | 1497 | 911 | ||
| Sandoglobulin (6%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lot: A913A8431 | [1/2] | [1/4] | ||||||||||||||
| 1 | DQA1*05:05\DQB1*03:01 | 1102 | 625 | |||||||||||||
| 2 | DQA1*03:02\DQB1*03:02 | 1085 | 565 | |||||||||||||
| 3 | DQA1*06:01\DQB1*03:01 | 1027 | 545 | |||||||||||||
| 4 | DQA1*01:02\DQB1*06:04 | 869 | 571 | |||||||||||||
| 5 | DQA1*03:01\DQB1*03:01 | 845 | 572 | |||||||||||||
| 6 | DQA1*03:01\DQB1*03:02 | 848 | 512 | |||||||||||||
| 7 | DQA1*02:01\DQB1*03:01 | 811 | 548 | |||||||||||||
| (c) Anti-HLA-DPA\DPB reactivity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | GamaSTAN (15–18%) |
Gamunex-C (10%) |
|||||||||||||
| Lot: 26NHCVI | [1/32] | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] | [1/2048] | Lot: 26NKL (K1) | [1/32] | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] | |
| 1 | DPA1*02:01\DPB1*23:01 | 18 914 | 17 978 | 13 471 | 7736 | 4161 | 2653 | 540 | DPA1*02:01\DPB1*23:01 | 7102 | 5075 | 5315 | 2287 | 1016 | 806 |
| 2 | DPA1*01:03\DPB1*11:01 | 17 213 | 16 775 | 10 470 | 6294 | 3169 | 2471 | 1062 | DPA1*01:03\DPB1*19:01 | 4598 | 2991 | 2074 | 981 | 530 | |
| 3 | DPA1*02:01\DPB1*28:01 | 12 849 | 9 911 | 6 924 | 3738 | 1861 | 709 | DPA1*02:01\DPB1*13:01 | 4290 | 2639 | 1618 | 607 | |||
| 4 | DPA1*01:03\DPB1*19:01 | 11 616 | 9 098 | 4 312 | 2069 | 1077 | 534 | DPA1*02:01\DPB1*15:01 | 3427 | 2340 | 1408 | 549 | |||
| 5 | DPA1*02:01\DPB1*06:01 | 5 425 | 3 678 | 1 665 | DPA1*01:03\DPB1*11:01 | 1854 | 2015 | 1663 | 672 | ||||||
| 6 | DPA1*02:01\DPB1*15:01 | 3 742 | 2 167 | 1 051 | DPA1*01:05\DPB1*13:01 | 2980 | 1959 | 1288 | 564 | ||||||
| 7 | DPA1*02:01\DPB1*05:01 | 4 658 | 2 641 | 898 | DPA1*02:01\DPB1*28:01 | 2779 | 1954 | 1283 | 634 | ||||||
| Octagam (6%) |
GAMUNEX-C (10%) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lot: A913A8431 | [1/32] | [1/64] | [1/128] | [1/256] | Lot: 26NKL (G1) | [1/32] | [1/64] | [1/128] | [1/256] | [1/512] | [1/1024] | ||||
| 1 | DPA1*02:01\DPB1*23:01 | 9474 | 6105 | 2483 | 2720 | DPA1*02:01\DPB1*23:01 | 11183 | 5920 | 3610 | 1960 | 980 | 629 | |||
| 2 | DPA1*01:03\DPB1*11:01 | 7316 | 2187 | 1027 | 927 | DPA1*01:03\DPB1*19:01 | 5701 | 3370 | 1933 | 912 | |||||
| 3 | DPA1*02:01\DPB1*28:01 | 3870 | 2148 | 1025 | 524 | DPA1*01:03\DPB1*11:01 | 5551 | 3174 | 1914 | 1040 | |||||
| 4 | DPA1*01:03\DPB1*19:01 | 4018 | 2288 | 1121 | 575 | DPA1*02:01\DPB1*28:01 | 4751 | 2424 | 135 | 814 | |||||
| 5 | DPA1*01:03\DPB1*02:01 | 1563 | 1051 | 630 | DPA1*02:01\DPB1*15:01 | 4269 | 2193 | 1192 | 518 | ||||||
| 6 | DPA1*02:01\DPB1*15:01 | 1327 | 864 | DPA1*01:05\DPB1*13:01 | 3011 | 1992 | 998 | 539 | |||||||
| 7 | DPA1*02:01\DPB1*05:01 | 1290 | 944 | DPA1*02:01\DPB1*13:01 | 4622 | 2190 | 1050 | ||||||||
| Sandoglobulin (6%) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lot: 4305800026 | [1/32] | [1/64] | [1/128] | [1/256] | |||||||||||
| 1 | DPA1*02:01\DPB1*23:01 | 1098 | 3619 | 1789 | 1082 | ||||||||||
| 2 | DPA1*02:01\DPB1*28:01 | 548 | 971 | ||||||||||||
Among IgG antibodies reacting to DQA/DQB alleles, reactivity to DQA1*01:02\DQB1*06:02 ranked first in all IVIg preparations except Sandoglobulin (Table 4b), again analogous to the highest incidence of the same anti-HLA-DQA/DQB purified IgG of our cohorts, but with Gamunex-C having the highest titre of antibodies. With DPA/DPB alleles, reactivity to DPA1*01:02\DPB1*23:01 ranked first in all IVIg preparations (Table 4c) – yet again as with our cohorts' serum-derived IgG but, again, GamaSTAN now having the highest titre of antibodies.
Figure 1a–c compares the rank of HLA-II reactivity of the IgG in the four IVIg preparations with the incidence of anti-HLA-II IgG before and after purification of IgG from the sera of the study donors. Strikingly, the IgG antibodies with a high incidence of reaction to HLA-II alleles in normal individuals have a correspondingly high incidence in IVIg preparations. The rank of anti-HLA-DR was: DRB3*03:01 > DRB1*09:01 > DRB3*01:01 > DRB1*08:01. Among anti-HLA-DQ alleles, DQA1*01:02\DQB1*06:02 ranked first, and among the anti-HLA-DP it was DPA1*0201\DPB1*23:01.
Figure 1.
Comparison of the normalized mean fluorescence intensity (MFI) of human leucocyte antigen (HLA) class II immunoglobulin (Ig)G antibodies in three groups: the sera of 15 donors and the negative control [LABScreen® negative control serum (LSNC)]; the IgG in each serum sample after protein-G purification; and five different intravenous immunoglobulin (IVIg) preparations, including two lots of Gamunex-C. MFI values for the purified IgG are the mean MFI of eluates 2 and 3. The vertical white space in the middle of the bars refers to break of the x-axis, graphically generated to accommodate the entire MFI values. For all parts of this figure (a–c) the normalized trimmed mean MFI is shown for IgG reacting to the given alleles The sera were all diluted 1/10 and the IVIg diluted 1/8.
Anti-HLA-II IgG: profiles of IVIg and purified sera IgG versus mouse anti-HLA-II IgG mAb
To ensure that the high MFI was not due to an antigen load on beads, the pattern of reactivity of a mouse mAb (FJ5109) with all HLA-DRB, DQA/DQB and DPA/DPB haplotypes was examined and compared with the HLA-II reactivity of IVIg and purified IgG. Table 5 shows the most highly reactive alleles in each of the HLA-DRB, -DQA/DQB and -DPA/DPB haplotypes. Note that, in point of reactivity, almost all the alleles recognized by the highest-ranking human antibodies for each haplotype were poorly recognized by the murine mAb; conversely, high-ranking murine anti-HLA-II antibody reactivity was restricted to alleles that rank low or lowest with human anti-HLA-II IgGs. The highest-ranking human antibodies for each of the haplotypes are shown in bold type in Table 5.
Table 5.
Ranking† and reactivity of murine monoclonal antibody (mAb) FJ5109 (at 1/16 000) to human leucocyte antigen (HLA) class-II alleles
| Rank | HLA-DR | MFI | HLA-DQ | MFI | HLA-DP | MFI |
|---|---|---|---|---|---|---|
| 1 | DRB1*13:03 | 3462 | DQA1*05:01\DQB1*02:01 | 5022 | DPA1*02:01\DPB1*18:01 | 3664 |
| 2 | DRB1*15:01 | 3359 | DQA1*03:01\DQB1*02:01 | 3841 | DPA1*01:03\DPB1*02:01‡,§ | 3477 |
| 3 | DRB1*01:03 | 3317 | DQA1*03:03\DQB1*04:01 | 3679 | DPA1*01:03\DPB1*03:01 | 3425 |
| 4 | DRB1*03:01 | 3145 | DQA1*03:02\DQB1*03:02 | 3411 | DPA1*01:05 DPB1*28:01 | 3343 |
| 5 | DRB1*11:04‡ | 3091 | DQA1*01:03\DQB1*06:01 | 3305 | DPA1*02:01\DPB1*09:01 | 3325 |
| 6 | DRB1*15:02 | 3090 | DQA1*04:01\DQB1*02:01 | 3274 | DPA1*02:01\DPB1*28:01‡,§ | 3268 |
| 7 | DRB1*04:02 | 3027 | DQA1*05:05\DQB1*03:01 | 3265 | DPA1*01:03\DPB1*04:02 | 3262 |
| 8 | DRB1*01:01 | 2980 | DQA1*05:03\DQB1*03:01 | 3227 | DPA1*01:05 DPB1*03:01 | 3240 |
| 9 | DRB1*16:02 | 2965 | DQA1*06:01\DQB1*03:01 | 3223 | DPA1*02:01\DPB1*14:01 | 3168 |
| 10 | DRB5*02:02 | 2923 | DQA1*02:01\DQB1*03:03 | 3212 | DPA1*02:01\DPB1*17:01 | 3103 |
| 11 | DRB1*13:01 | 2836 | DQA1*03:02\DQB1*03:03 | 3119 | DPA1*02:01\DPB1*06:01‡ | 3096 |
| 12 | DRB1*15:03 | 2808 | DQA1*01:03\DQB1*06:03 | 2851 | DPA1*02:01\DPB1*01:01 | 3088 |
| 13 | DRB1*16:01 | 2753 | DQA1*02:01\DQB1*02:02 | 2435 | DPA1*02:01 DPB1*03:01 | 3042 |
| 14 | DRB1*04:01 | 2721 | DQA1*01:01\DQB1*05:01 | 2326 | DPA1*02:01\DPB1*05:01‡ | 3024 |
| 15 | DRB1*09:02 | 2595 | DQA1*02:01\DQB1*02:01 | 2144 | DPA1*01:03\DPB1*04:01 | 2980 |
| 16 | DRB1*07:01 | 2561 | DQA1*04:01\DQB1*04:02 | 1880 | DPA1*02:01\DPB1*10:01 | 2847 |
| 17 | DRB4*01:03 | 2552 | DQA1*01:02\DQB1*06:09 | 1867 | DPA1*01:03\PB1*01:0 | 2811 |
| 18 | DRB1*04:04 | 2538 | DQA1*01:02\DQB1*05:02‡ | 1560 | DPA1*01:05\DPB1*18:01 | 2771 |
| 19 | DRB1*12:01 | 2514 | DQA1*02:01\DQB1*04:02 | 1493 | DPA1*01:04\DPB1*18:01 | 2715 |
| 20 | DRB1*11:01 | 2502 | DQA1*02:01\DQB1*04:01§ | 1429 | DPA1*01:05\DPB1*11:01§ | 2503 |
| 21 | DRB1*01:02 | 2498 | DQA1*02:01\DQB1*03:01 | 1333 | DPA1*01:03\DPB1*19:01‡,§ | 2418 |
| 22 | DRB1*03:02‡,§ | 2494 | DQA1*03:01\DQB1*03:01‡,§ | 1063 | DPA1*01:03\DPB1*11:01‡,§ | 2307 |
| 23 | DRB1*04:05 | 2376 | DQA1*03:01\DQB1*03:03 | 726 | DPA1*02:01\DPB1*13:01‡ | 2209 |
| 24 | DRB3*02:02 | 2357 | DQA1*03:01\DQB1*03:02 | 659 | DPA1*02:01\DPB1*15:01‡,§ | 2119 |
| 25 | DRB1*08:01‡,§ | 2309 | DQA1*02:01\DQB1*03:02‡,§ | 625 | DPA1*02:01\DPB1*23:01‡,§ | 1756 |
| 26 | DRB1*04:03 | 2290 | DQA1*01:02\DQB1*06:04 | 526 | DPA1*01:05\DPB1*13:01‡,§ | 1627 |
| 27 | DRB1*10:01 | 2238 | DQA1*01:01\DQB1*03:02‡,§ | 472 | DQA1*02:01\DQB1*03:02 | 625 |
| 28 | DRB1*14:02‡ | 2214 | DQA1*01:01\DQB1*06:02 | 438 | ||
| 29 | DRB4*01:01 | 2168 | DQA1*01:02\DQB1*06:02‡,§ | 332 | ||
| 30 | DRB5*01:01 | 2046 | ||||
| 31 | DRB1*14:01 | 1586 | ||||
| 32 | DRB1*12:02 | 1281 | ||||
| 33 | DRB3*03:01‡,§ | 1272 | ||||
| 34 | DRB1*14:54 | 1138 | ||||
| 35 | DRB1*09:01‡,§ | 1099 | ||||
| 36 | DRB3*01:01‡,§ | 1077 |
† Rankings, in general, are the same as those observed for the HLA-II reactivity of intravenous immunoglobulin (IVIg) or the purified IgG of normal males. Those that are shown in bold type correspond to the HLA-II reactivity of IVIg‡ or purified human sera. Alleles with superscript ‡ are the high-ranking antibodies in IVIg. Alleles with superscript § are the high-ranking antibodies in purified normal sera. MFI = mean fluorescence intensity.
Anti-HLA-I IgG: IVIg versus normal sera IgG before and after purification
Table 6a compares the rank and incidence of MFI of anti-HLA-I IgG in IVIg with that of the same purified IgG. The MFI rankings of anti-HLA-C IgG in two different preparations of IVIg were strikingly similar, and paralleled the incidence of anti-HLA-C purified IgG. With the HLA-C locus, all seven of the listed Cw specificities had an immunogenicity score of 95%: Cw*0602, Cw*0702, Cw*1802, Cw*0401, Cw*1601, Cw*1701 and Cw*0202. The rankings of anti-HLA-B IgG in Octagam IVIg paralleled the incidence of anti-HLA-B IgG in the purified IgG. However, the rank and incidence of anti-HLA-A IgG in IVIg and in purified IgG differed considerably.
Table 6.
Rank of mean fluorescence intensity (MFI) of anti-human leucocyte antigen (HLA)-I IgG in IVIg (GamaSTAN and Octagam) and in IgG purified from the sera (eluate 2) of normal, non-alloimmunized males, and incidence of the IgG in the eluate
| (a) | GamaSTAN (1/8) |
Octagam (1/8) |
Sera (n = 20) |
||||
|---|---|---|---|---|---|---|---|
| Rank | MFI | MFI | % Incidence (E2) | ||||
| HLA-A | |||||||
| 1 | A*34:01 | 3042 | A*36:01 | 3 650 | A*24:02 | 40 | |
| 2 | A*80:01 | 2705 | A*26:01 | 3 071 | A*34:01 | 40 | |
| HLA-B | |||||||
| 1 | B*44:02 | 4301 | B*37:01 | 9 974 | B*37:01 | 65 | |
| 2 | B*82:01 | 3857 | B*82:01 | 5 026 | B*40:06 | 55 | |
| 3 | B*45:01 | 3667 | B*40:06 | 4 972 | B*82:01 | 75 | |
| 4 | B*15:16 | 3536 | B*44:02 | 3 997 | B*44:02 | 45 | |
| HLA-Cw | |||||||
| 1 | CW*06:02 | 9171 | CW*06:02 | 16 706 | CW*06:02 | 95 | |
| 2 | CW*18:02 | 7981 | CW*07:02 | 15 321 | CW*07:02 | 95 | |
| 3 | CW*17:01 | 7856 | CW*18:02 | 10 610 | CW*18:02 | 95 | |
| 4 | CW*07:02 | 7620 | CW*04:01 | 9 884 | CW*04:01 | 95 | |
| 5 | CW*02:02 | 7183 | CW*02:02 | 9 379 | CW*16:01 | 95 | |
| 6 | CW*05:01 | 6968 | CW*16:01 | 9 259 | CW*17:01 | 95 | |
| 7 | CW*04:01 | 6287 | CW*17:01 | 8 983 | CW*02:02 | 95 | |
| (b) Incidence of serum and purified IgG in normal males reacting to HLA class-I and the level (MFI) of anti-albumin IgG | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | TFL1 |
T2 |
T3 |
W1 |
W2 |
W3 |
TFL2 |
R1 |
R2 |
TFL3 |
||||||||||
| serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | |
| Anti-Albumin IgG (MFI) | 1547 | 537 | ||||||||||||||||||
| Incidence of anti-HLA-A IgG | 4 | 7 | 13 | 24 | 2 | 12 | 2 | 21 | 1 | 1 | 3 | 1 | 20 | 2 | ||||||
| MFI range 500–999 | 4 | 7 | 13 | 6 | 2 | 9 | 1 | 19 | 1 | 2 | 1 | 1 | 17 | 2 | ||||||
| 1 000–1 999 | 16 | 3 | 1 | 3 | 2 | 3 | ||||||||||||||
| 2 000–2 999 | 2 | |||||||||||||||||||
| Incidence of anti-HLA-B IgG | 2 | 13 | 3 | 1 | 16 | 39 | 39 | 2 | 21 | 28 | 1 | 8 | 2 | 8 | 31 | 2 | ||||
| MFI range 500–999 | 1 | 9 | 3 | 15 | 39 | 12 | 2 | 20 | 24 | 1 | 7 | 7 | 29 | 2 | ||||||
| 1 000–1 999 | 1 | 2 | 1 | 22 | 1 | 4 | 1 | 2 | 1 | 2 | ||||||||||
| 2 000–2 999 | 2 | 1 | 5 | |||||||||||||||||
| 3 000–3 999 | ||||||||||||||||||||
| Incidence of anti-HLA-Cw IgG | 15 | 16 | 16 | 15 | 16 | 16 | 16 | 16 | 16 | 16 | 4 | 13 | ||||||||
| MFI range 500–999 | 2 | 4 | 14 | 1 | 1 | 2 | 1 | 4 | 7 | |||||||||||
| 1 000–1 999 | 8 | 9 | 13 | 1 | 1 | 7 | 6 | 12 | 4 | 5 | 6 | |||||||||
| 2 000–2 999 | 4 | 3 | 3 | 3 | 6 | 9 | 2 | 6 | 6 | |||||||||||
| 3 000–3 999 | 1 | 4 | 2 | 2 | 3 | |||||||||||||||
| 4 000–7 000 | 8 | 3 | 2 | |||||||||||||||||
| 7 000–10 000 | ||||||||||||||||||||
| > 10 000 | ||||||||||||||||||||
| ID | TFL4 |
TFL5 |
TFL6 |
TFL7 |
TFL8 |
TFL9 |
TFL10 |
TFL11 |
UCLA1 |
G1 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | serum | Normalized/purified | |
| Anti-Albumin IgG (MFI) | 943 | |||||||||||||||||||
| Incidence of anti-HLA-A IgG | 8 | 1 | 12 | 2 | 2 | 20 | 15 | 1 | 1 | 7 | ||||||||||
| MFI range 500–999 | 5 | 1 | 12 | 2 | 2 | 8 | 4 | 1 | 1 | 4 | ||||||||||
| 1 000–1 999 | 3 | 11 | 8 | 2 | ||||||||||||||||
| 2 000–2 999 | 1 | 3 | 1 | |||||||||||||||||
| Incidence of anti-HLA-B IgG | 1 | 3 | 21 | 8 | 7 | 4 | 4 | 23 | 22 | 6 | 2 | 15 | ||||||||
| MFI range 500–999 | 1 | 3 | 17 | 7 | 6 | 16 | 10 | 6 | 2 | 7 | ||||||||||
| 1 000–1 999 | 4 | 1 | 1 | 4 | 6 | 8 | 5 | |||||||||||||
| 2 000–2 999 | 3 | 1 | 4 | 3 | ||||||||||||||||
| 3 000–3 999 | 1 | |||||||||||||||||||
| Incidence of anti-HLA-Cw IgG | 2 | 6 | 16 | 16 | 8 | 2 | 15 | 13 | 16 | 16 | 16 | 11 | 15 | |||||||
| MFI range 500–999 | 2 | 6 | 7 | 2 | 6 | 7 | 9 | 9 | 11 | 1 | ||||||||||
| 1 000–1 999 | 4 | 10 | 1 | 9 | 6 | 6 | 2 | 7 | ||||||||||||
| 2 000–2 999 | 6 | 5 | 1 | 4 | 1 | |||||||||||||||
| 3 000–3 999 | 3 | 1 | 7 | 2 | ||||||||||||||||
| 4 000–7 000 | 3 | 3 | 5 | |||||||||||||||||
| 7 000–10 000 | 6 | |||||||||||||||||||
| > 10 000 | 1 | |||||||||||||||||||
Total incidence of anti-HLA IgG is shown in bold type. TFL = Terasaki Foundation Laboratory.
Table 6b compares HLA-I reactivity of IgG before and after purification from sera. The incidence is given for anti-HLA-A/B/Cw at different ranges of MFI. Anti-HLA-I IgG antibodies are rarely observed in sera, but upon purification of IgG they are present, indicating that most already existed in the sera, but did not react with antigens until they were subjected to purification. Sera of four individuals (TFL5, TFL10, TFL3 and W1) showed reactivity to 10–98 HLA-I alleles (1–20 HLA-A, 3–39 HLA-B and 6–16 HLA-Cw). Most importantly, the level of MFI in IgG after purification and normalization was several hundredfold higher. The purified and normalized IgG from G1 showed an MFI > 10 000 for an HLA-Cw allele. Purified and normalized IgG of several donors ranged in MFI from 4000 to 7000 for HLA-Cw alleles. All these values document the existence of anti-HLA-I IgG antibodies in sera in a masked state.
Anti-HLA-I IgG: regular beads versus iBeads, distinguishing β2m-associated and β2m-free HLA heavy chains
The purified IgG from sera differed in reactivity to intact (iBeads) and heavy-chain HLA (regular beads) (Table 7), strikingly similar to the reactivity of IVIg preparations (data in [6]). Purified IgG of all the donors showed a marked increase in MFI for HLA-A on iBeads, suggesting that the purified IgG binds to intact HLA-A. The decrease in MFI of the purified IgG for HLA-B and HLA-Cw alleles suggests that the purified IgG, like different preparations of IVIg [7], binds to the β2m-free heavy chains (open conformers). The purified IgG of TFL1, TFL9 and TFL10 reacted with iBeads (containing at least one HLA-B allele) with high MFI, indicative of reactivity to intact HLA. The purified IgG of TFL3 is unique in that it reacted with HLA-B alleles only on iBeads, not regular beads; and his purified IgG, when reacting with HLA-Cw alleles, had a higher reaction to nine Cw alleles on regular beads than on iBeads; conversely, however, it had a higher reaction to nine different Cw alleles on iBeads than on regular beads. Differences in the reactivity of IgG on albumin-coated regular beads and iBeads suggest that the proprietary enzymatic treatment used to prepare iBeads affects the expression of albumin on iBeads (Table 7).
Table 7.
The reactivity (MFI) of purified IgG (eluate 2) of the sera of non-alloimmunized males to HLA-Ia and albumin differs between regular beads (rB) and iBeads (iB)
| Donor ID | A alleles (31) |
B alleles (50) |
Cw alleles (16) |
Albumin |
||||
|---|---|---|---|---|---|---|---|---|
| rB > iB | rB < iB | rB > iB | rB < iB | rB > iB | rB < iB | rB (MFI) | iB (MFI) | |
| TFL1 | 3 | 26 | 20 | 1 | 16 | 0 | < 2 K | < 1 K |
| T2 | 0 | 0 | 1 | 0 | 13 | 0 | < 0·5 | < 0·5 |
| TFL2 | 1 | 16 | 11 | 0 | 16 | 0 | < 1 K | < 0·5 |
| R1 | 5 | 15 | 17 | 0 | 16 | 0 | < 1 K | < 0·5 |
| R2 | 0 | 2 | 8 | 0 | 15 | 0 | < 0·5 | < 0·5 |
| TFL3 | 0 | 31 | 0 | 50 | 9 | 9 | < 2 K | < 0·5 |
| TFL4 | 0 | 3 | 0 | 0 | 0 | 0 | < 5 K | < 1 K |
| TFL5 | 10 | 7 | 27 | 0 | 16 | 0 | < 0·5 | < 0·5 |
| TFL6 | 1 | 14 | 10 | 0 | 16 | 0 | < 1 K | < 1 K |
| TFL7 | 0 | 2 | 0 | 0 | 14 | 0 | < 1 K | < 0·5 |
| TFL8 | 1 | 13 | 12 | 0 | 16 | 0 | < 1 K | < 1 K |
| TFL9 | 1 | 3 | 5 | 1 | 15 | 0 | < 1 K | < 1 K |
| TFL10 | 12 | 13 | 30 | 1 | 15 | 0 | < 1 K | < 1 K |
rB contains both β2-microglobulin-associated (heterodimer) and β2-microglobulin-free (open conformer) HLA molecules; iB are enzyme-treated beads predominantly contain heterodimer. TFL = Terasaki Foundation Laboratory.
Antibodies against their own HLA in the purified sera of normal males
Does IgG reacting to HLA before and after purification from the serum of each donor match the molecular HLA types of that donor? Various anti-HLA IgG antibodies in the native sera and those found after purification were indeed directed against that donor's own HLA types (Table 8), confirming the presence of anti-HLA-I/-II reactive IgGs, corresponding to the HLA alleles expressed by those individuals. For instance, serum IgG of four donors (the father in family 1, the grandfather in family 2, TFL3 and TFL10) had HLA antibodies against their own HLA type. In two of the four, the antibodies were against HLA-II alleles, and in the other two against HLA-I alleles. After purification of IgG, several sera showed reactivity to the donor's own HLA alleles. For example, DRB typing showed that TFL9 and TFL11 were homozygous for DRB3*01:01, and their purified IgG showed those antibodies against the same allele. Such matching can be seen for almost all the other donors, with autoantibodies to DRB345 appearing to be the most frequent (Table 8). Together, the data suggest that most normal non-alloimmunized males carry antibodies to their own HLA. Using an autoantibody frequency score to compare the loci we found that, of the 20 donors, six (30%) had IgG autoantibodies to HLA-A, eight (40%) to HLA-B and 16 (80%) had anti-HLA-Cw autoantibodies. Similarly, the frequency of IgG antibodies against the donor's own HLA-II was: anti-HLA DRB1 alleles (5%), anti-DRB345 (65%), anti-DQA (20%), anti-DQB (20%), anti-DPA (0%) and anti-DPB (15%).
Table 8.
Human leucocyte antigen (HLA) autoantibodies in normal sera: normalized mean fluorescence intensity (MFI) of antibodies in sera before and after purification of immunoglobulin (Ig)G corresponds to the donor HLA class I and II types of the non-alloimmunized males
| Purified and normalized IgG positive for HLA types of the individuals | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HLA class-I |
HLA class-II |
||||||||
| A | B | Cw | DRB1 | DRB345 | DQA | DQB | DPA | DPB | |
| Family 1 | |||||||||
| Paternal grandfather (TFL1) | 52:01 | 07:02 | |||||||
| Father (T1) | Reactive to homozygous 07:02 | Both serum and purified IgG reactive to homozygous 4*0101 | 01:01 | 03:02 | 02:01 | ||||
| Son (T2) | 24:02 | 40:06 | 07:02 and 08:01 | 03:01 | 03:01 | 02:01 | |||
| Family 2 | |||||||||
| Maternal grandfather (W1) | 24:02 and 31:01 | 51:01 and 52:01 | 14:02 | Serum IgG reactive to homozygous 50101 | |||||
| Father (W2) | 52:01 | 01:02 | 03:01 | 03:02 | |||||
| Son (W3) | 24:02 | Reactive to homozygous 52:01 | |||||||
| Family 3 | |||||||||
| Father (TFL2) | 58:01 | Reactive to homozygous 15:02 | 3*03:01 and 4*01:01 | 02:01 | |||||
| First son (R1) | 15:02 | 3*03:01 | |||||||
| Second son (R2) | 24:02 | 58:01 | 15:02 | 3*03:01 | |||||
| Volunteers | |||||||||
| TFL9 | 03:01 and 08:01 | 3*01:01 and 3*01:01 | |||||||
| TFL8 | 01:02 and 03:04 | 3*01:01 | |||||||
| TFL6 | Reactive to homozygous 07:02 | 4*01:01 | |||||||
| TFL10 | Both serum and purified IgG reactive to 03:02, 03:04 and 07:02 | 4*01:01 and 5*01:01 | |||||||
| TFL4 | 3*03:01 | ||||||||
| TFL5 | 11:01 and 24:02 | 02:02 | 3*01:01 | ||||||
| TFL7 | 3*03:01 | ||||||||
| TFL3 | Serum IgG reactive to 02:06 | Serum IgG reactive to 40:01 | 07:02 | 01:02 | |||||
| TFL11 | 16:01 | Reactive to homozygous 3*01:01 | |||||||
| UCLA1 | 7:02 | ||||||||
| G1 | 03:03 and 06:02 | 3:02 | |||||||
TFL = Terasaki Foundation Laboratory.
Masking efficacy of different molecular size fragments of peptides and polypeptides from wash fluid
The peptide/polypeptide fractions (> 100 K, 100-0 K, 10-3 K and < 3 K) isolated from WF1 were tested to assess their ability to mask the HLA-E reactivity of E2 and E3 from the serum of TFL5. Both eluates contained purified IgG (E2 > E3). The MFI of E2 and E3 with and without WF fractions at two different dilutions were measured and the percentage difference was calculated. Invariably, the eluate-WF mixture showed a much lower MFI than the eluate alone, indicating that the peptides or other serum factors in the WF masked the reactivity of IgG with HLA-E. All fractions inhibited the HLA-E reactivity of the eluates. The inhibition percentage of the 3 K fraction was higher than that of the 10-3 K fraction. The inhibition percentage with E2 was greater than with E3, suggesting that the serum peptides of molecular size < 3 K are capable of masking the interaction of purified IgG with HLA. Accordingly, we then performed peptide inhibition studies with different classes of peptides of molecular size < 3 K (Table 9).
Table 9.
Efficiency of different molecular size fractions obtained from the wash fluid of TFL5's serum in masking (% inhibition) the human leucocyte antigen (HLA)-E reactivity of eluates 2 and 3 of the serum
| Eluate 2 WF, after post-molecular sieving | E2 (μl) | Phosphate-buffered saline (PBS) (μl) | Normal MFI | E2 (μl) | Wash fluid (WF) (μl) | Ratio of E2/WF | Normal MFI | % inhibition |
|---|---|---|---|---|---|---|---|---|
| 20 | 10 | 2773 | 20 | 10 | (2/1) | 562 | 80 | |
| Original | 25 | 5 | 5762 | 25 | 5 | (5/1) | 1406 | 76 |
| 20 | 10 | 2773 | 20 | 10 | (2/1) | 79 | 97 | |
| > 100 K | 25 | 5 | 5762 | 25 | 5 | (5/1) | 429 | 93 |
| 20 | 10 | 2773 | 20 | 10 | (2/1) | 929 | 66 | |
| 10–100 K | 25 | 5 | 5762 | 25 | 5 | (5/1) | 1571 | 73 |
| 20 | 10 | 3746 | 20 | 10 | (2/1) | 4652 | −24 | |
| 3–10 K | 25 | 5 | 5184 | 25 | 5 | (5/1) | 6331 | −22 |
| 20 | 10 | 3746 | 20 | 10 | (2/1) | 1258 | 66 | |
| < 3 K | 25 | 5 | 5184 | 25 | 5 | (5/1) | 3600 | 31 |
| Eluate 3 WF, after post-molecular sieving | E3 (μl) | PBS (μl) | Ratio of E3/PBS | Normal MFI | E3 (μl) | Wash fluid (WF) (μl) | Ratio of E3/WF | Normal MFI | % inhibition |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 10 | (2/1) | 2064 | 20 | 10 | (2/1) | 496 | 76 | |
| Original | 25 | 5 | (5/1) | 5902 | 25 | 5 | (5/1) | 1510 | 74 |
| 20 | 10 | (2/1) | 2064 | 20 | 10 | (2/1) | 132 | 94 | |
| > 100 K | 25 | 5 | (5/1) | 5902 | 25 | 5 | (5/1) | 211 | 96 |
| 20 | 10 | (2/1) | 2064 | 20 | 10 | (2/1) | 433 | 79 | |
| 10–100 K | 25 | 5 | (5/1) | 5902 | 25 | 5 | (5/1) | 1212 | 79 |
| 20 | 10 | (2/1) | 2008 | 20 | 10 | (2/1) | 1479 | 26 | |
| 3–10 K | 25 | 5 | (5/1) | 4099 | 25 | 5 | (5/1) | 2954 | 28 |
| 20 | 10 | (2/1) | 2008 | 20 | 10 | (2/1) | 970 | 52 | |
| < 3 K | 25 | 5 | (5/1) | 4099 | 25 | 5 | (5/1) | 2988 | 27 |
Inhibition of HLA reactivity of IgG with synthetic peptides representing HLA and IgG peptides
Peptides used included those derived from HLA (commonly shared peptides) and the fragments derived from the CDRII domain. The most common shared peptides are 115QFAYDGKDY123, 117AYDGKDY123 and 126LNEDLRSWTA135 (7–9). The CDRII sequence peptide T15H (now called ‘TEPC15’) in the domain of the Fab of IgG – known to bind to naturally occurring antibodies 10–12 – is found in the sera of different strains of mice and in humans, indicating that the sequence is a conserved-idiotype determining region 10–12. Two peptides were used for the inhibition studies with the IgG fragment TEPC15: ASRNKANDYTTEYSASVKGRFIVS and the control peptide of 2FIOH; 50–73, QIYPGDGDTKYNNGKFKGKATLT.
Observations of E2 of TFL5's serum indicated that the TEPC15 peptide was the potent inhibitor of IgG reactivity to HLA-E (Fig. 2a). Neither the control peptide with the same number of amino acids nor the shared peptides of HLA-I inhibited the HLA-E binding of IgG in E2. The inhibitory potency of TEPC15 was further confirmed by the HLA-II reactivity (to DRB3*03:01 and DPA1*02:01/DPB1*23:01) of purified IgG in E2 and E3 from the serum of TFL12, and by the DRB3*03:01 reactivity of E2 from the serum of TFL6 (Fig 2b; Table 10). It appears that the peptide fragment of CDRII, TEPC15, is the potent binder of anti-HLA IgG.
Figure 2.
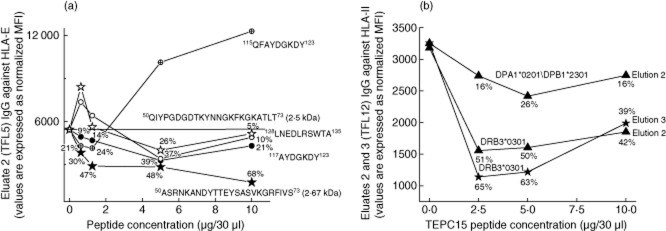
Inhibition of human leucocyte antigen (HLA) reactivity of immunoglobulin (Ig)G in eluates purified from the sera of two volunteers – Terasaki Foundation Laboratory (TFL)5 (a) and TFL12 (b) – by a < 3 K peptide sequence. (a) The peptide inhibition of eluate 2 of TFL5 used HLA-E, representing HLA class I, with peptides shared by all HLA class I alleles (115QFAYDGKDY123; 117AYDGKDY123 and 126LNEDLRSWTA135) and with IgG peptide TEPC15 (T15H; 50–73) (ASRNKANDYTTEYSASVKGRFIVS), with a peptide derived from the CDRII domain of IgG, 2FIOH (50–73) and with a control peptide (QIYPGDGDTKYNNGKFKGKATLT). (b) The peptide inhibition of eluates 2 and 3 of TFL12 were tested with HLA class II, as labelled in the figure. The IgG peptides were, as with (a), TEPC15 and 2FIOH (50–73).
Table 10.
Inhibition of human leucocyte antigen (HLA) DRB*3*03:01 reactivity of IgG in eluate 2, purified from the serum of TFL6 by a < 3 K peptide sequence
| Beads coated with DRB3*03:01 |
||||
|---|---|---|---|---|
| μl† | MFI | % decrease | ||
| Eluate | 25 | |||
| PBS | 5 | 7722 | ||
| Peptide TEPC15‡ | ||||
| 5 μg/5 μl | 5 | 2832 | 63 | |
| 2·5 μg/5 μl | 5 | 2822 | 63 | |
| 1·25 μg/5 μl | 5 | 2463 | 68 | |
| 0·625 μg/5 μl | 5 | 4132 | 46 | |
| Peptide 2F10H | ||||
| 5 μg/5 μl | 5 | 2790 | 64 | |
| 2·5 μg/5 μl | 5 | 6810 | 12 | |
† μl = total volume of the mix was 30 μl, but only 20 μl of peptide was added to wells containing beads. ‡TEPC15 is a peptide derived from the CDRII domain of IgG; 2F10H is the control peptide. PBS = phosphate-buffered saline.
Discussion
Four major findings emerged from this investigation. First is that the profile, incidence and MFIs of HLA-II IgG differ remarkably between native sera and IgG purified from the corresponding sera, indicative of the crypticity of the anti-HLA IgG antibodies in the native sera. The exposed and cryptic anti-HLA IgG in the sera of our study's normal donors helps to explain the preformed or pre-existing DSA and NDSA in the sera of transplant patients even before they receive their first kidney 13–16, liver 16–18 or lung 19 allograft. The pre-existing DSA notably correlated with graft rejection. If these correlations are valid, then an assessment of the HLA reactivity of purified IgG of patients before transplantation can predict the outcome of transplantation, play a significant role in the selection of donors, and therefore importantly benefit transplant patients.
Second is the presence of HLA IgG in sera capable of reacting to one's own HLA types both as exposed and cryptic antibodies. Indeed, this is the first and novel documentation of anti-HLA IgG autoantibodies in the sera of virtually all normal, healthy donors tested. Anti-HLA autoantibodies were found in IgG purified from all their sera, and also occurred unmasked in the sera of a few individuals. This pattern most probably prevails in the general population. The presence of a wide variety of cryptic allo-HLA antibodies warrants careful reassessment of DSA and NDSA in allograft recipients. Possibly both pre- and post-transplant DSA could be due to loss of crypticity or detachment of the masking factor from anti-HLA IgG because of tissue inflammation or injury. Clearly, the factors masking anti-HLA autoantibodies are relevant and critical for understanding the regulation of circulating HLA autoantibodies in healthy people and in patients with allografts, infection, inflammation, cancer or autoimmune diseases.
Third is the presence of the putative regulatory factors capable of masking those antibodies' expression. The findings, as presented in Fig. 2a, did not support the hypothesis that the peptide fragments of shed HLA might be masking the expression of anti-HLA IgG in the sera. Moreover, if concealed anti-HLA-I or -II antibodies are bound with their respective shed antigen, then the antigen should be present in the tissue of the blood donors, but that cannot be the primary cause of all HLA antibodies being masked because all alleles were not found in a single donor. For example, the incidence of anti-DRB3*03:01 IgG was 100%, but the antigen was not expressed by all donors.
A more likely hypothesis is that the expression of anti-HLA IgG in the sera may be masked by the fragment from degrading IgG in circulation that can bind, as does an anti-idiotypic antibody 10–12. The CDRII sequence peptide (T15H; 50–73) that occurs in the sera of different strains of mice and in humans as a consequence of natural degradation of IgG, and is considered a conserved-idiotype determining region 10–12, was a potent inhibitor of HLA reactivity of the purified IgG of three volunteers, TFL5, TFL6 and TFL12. Neither the control peptide, which had the same number of amino acids as the CDRII peptide, nor the shared peptides of HLA-I inhibited HLA binding of the purified IgG, strengthening the contention that the highly conserved CDRII peptide may be responsible for the crypticity of the anti-HLA IgG in human sera. This observation is strengthened further by the data obtained from the two eluates of the same sera. Similar investigation of the sera of patients with autoimmune diseases, infection, inflammation, cancer or transplantation is needed to evaluate the regulatory role of the serum factors masking the HLA antibodies. Specific identification of the factors masking the expression of autoantibodies may lead to a better understanding of how to regulate the pathogenic or detrimental antibodies, particularly in patients with autoimmune diseases and those waiting for allografts.
Autoantibodies to HLA-I were found in the purified sera with the frequency HLA-Cw > -B > -A. Comparison of all HLA types showed that the donors had auto-HLA antibodies in this order of frequency: DPA (80%), DQA (71%), DRB345 (67%), DQB (57%), Cw (50%), DPB (43%), DRB1 (21%), A (14%) and B (7%). It is too early to speculate on the functions of auto-HLA IgG antibodies in normal sera, except to observe that they remain concealed.
The fourth major finding is that the wide array of both allo-HLA-II and auto-HLA-II IgG antibodies isolated from normal sera was mirrored in the HLA-II reactivity and profiles of IVIg, which is purified from the pooled plasma using the Cohn–Oncley protocol with acidic ethanol, a method different from the one used in our study. Most interestingly, in all IVIg preparations, the IgG reacting to HLA-II exhibited a pattern of HLA-DRB, -DQA/DQB and -DPA/DPB reactivity similar to that of the antibodies purified and isolated from our donors' sera (Fig. 1a–c). Note especially the 100% incidence and strong reactivity of IgG reacting to DRB3*03:01 and DPA1*02:01\DPB1*23:01 (Table 3d). The most frequent HLA antibodies against DRB, DPA and DQA were found in more than 70% of the donors. Why these antibodies occur in everyone may be inexplicable; yet the ubiquity this study found makes the phenomenon seem unequivocal.
Both lots of Gamunex-C had very high titres of anti-albumin antibodies compared with the other IVIgs in this study. This should not, however, be taken as a blanket observation about other preparations or their manufacturers (indeed, GamaSTAN is made by the same manufacturer as Gamunex-C). Rather, our intent here is to show that anti-HLA IgG levels after normalization against the MFI of negative control beads, which are coated with albumin, can vary considerably from lot to lot. Because there is no method by which IVIg of different lots and different manufacturers can be compared with regard to potency, titres of anti-HLA-II IgG can be used to rank the different IVIg preparations.
The presence of a wide variety of HLA-I/-II IgG in IVIg 6,20 brings into question the validity of administering IVIg to lower the incidence of HLA antibodies in patients before and after organ transplantation. In fact, the effect of IVIg in lowering the HLA antibodies in presensitized patients 21–24 has been gainsaid by three recent clinical studies, each of which points out that IVIg is incapable of reducing HLA antibodies 25–27. None of these studies corroborates previous claims on the reduction of HLA antibody incidence (determined by calculated panel reactive antigens) in sensitized patients undergoing desensitization with high-dose IVIg. Is IVIg treatment for desensitization a clinical oxymoron?
Besides the questionable immunosuppressive effect of IVIg [34,35], as used routinely in transplant patients, there is the imminent danger of HLA-reactive IVIg preparations producing transfusion-related acute lung injury (TRALI). Variations in the IVIg preparation can make it more toxic. Stoclin et al. 28 detected HLA-I/-II IgG with broad specificity (determined by panel reactive antibody screening) in two batches of IVIg (Privigen®; CSL Behring). HLA-II antibodies were identified as causal factors for the induction of TRALI 29. Five instances of TRALI, following administration of IVIg, have been reported [30–33], with one death 30. Kopko and Holland 30 reported a case after transfusion of plasma that did not contain antibodies against HLA-I or human neutrophil antigens, but did contain anti-HLA-II IgG. Kopko et al. 31 proposed that HLA-II IgG binding to monocytes in patients with TRALI may result in activation of neutrophils, which may penetrate the endothelium of lungs to cause swelling and destruction of the endothelial lining. Indeed, in a rat lung model, Sachs 29 showed that HLA-II antibodies could induce TRALI indirectly by means of monocytes.
Documentation of anti-HLA-II IgG in alloimmunized females resulted in the avoidance of using blood from females for transfusion – avoidance that has become routine as a preventive measure against TRALI in several countries 31–33. Recently, Sachs et al. 32 stated that ‘this policy did indeed significantly reduce the incidence of TRALI both in large-scale surveillance studies and haemovigilance reports’. All this suggests that further use of IVIg for patients should be preceded by the assessment of the strength (MFI and titre) of their anti-HLA IgG and their HLA types because the detrimental agent in IVIg could be the HLA-II IgG reacting to the specific type or allele of HLA-II expressed on the immune cells. The presence of anti-HLA IgG in IVIg constitutes a distinct danger of inducing TRALI. The differences in IVIg preparations underscore the need for monitoring the strength of anti-HLA IgG as a way of standardizing IVIg.
A salient question is whether the anti-HLA-I IgG in sera and in IVIg is against open conformers (β2m-free HLA-I heavy chain polypeptide) or against intact antigen (β2m-associated with HLA-I) (Fig. 3); it was the question addressed in this study by testing with regular beads and iBeads. It appears that the IgG may react to intact HLA-A alleles and to the open conformers of HLA-B and HLA-Cw. However, in some individuals the reactivity may reverse, as noted: anti-HLA-I IgG to intact HLA may produce TRALI, whereas antibodies against open conformers may effect immunosuppression 30,34.
Figure 3.
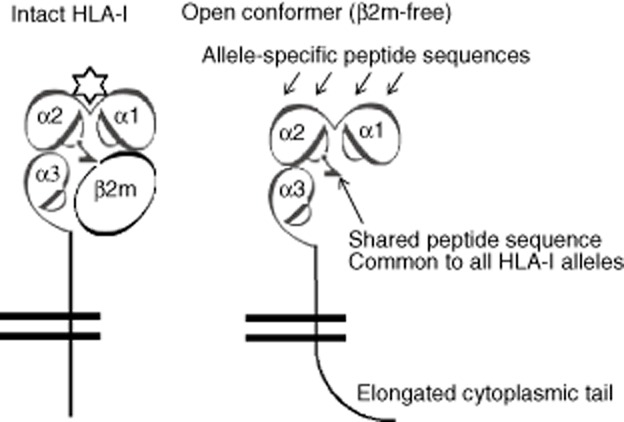
Dual expression of human leucocyte antigen (HLA) class I molecules on the cell surface of lymphocytes and other cells. The intact HLA class I occurs as HLA heavy chain (HC) polypeptides associated with β2-microglobulin (β2m). On the surface of activated lymphocytes, HLA-I occurs as open conformers (β2m-free HC). All HLA-I alleles share certain amino acid sequences (117AYDGKDY123 and 126LNEDLRSWTA135). In intact HLA, they remain cryptic due to masking by β2m. In open conformers, they are exposed to interact with antibodies. Anti-HLA-I IgG may recognize regular HLA beads coated with both β2m-associated and β2m-free HCs of HLA (open conformers) but not iBeads, on which β2m-associated intact HLA predominated.
The large differences in MFIs among the sera of our study's donors can be accounted for by considering that levels of anti-albumin antibodies can be elevated in people otherwise ‘normal’ and ‘healthy’ under disease conditions, mainly involving the liver, including persistent, undiagnosed hepatitis (especially among Asians), and by such things as transient viral infections and gastrointestinal issues. Furthermore, the anti-albumin IgG and IgM involved in the removal of altered (aged) albumin molecules from the circulation in normal sera are considered physiological autoantibodies 36. It is known that the concentration of albumin increases in liver diseases, stimulating an active synthesis of anti-albumin antibodies. These could be albumin-specific or albumin antibodies cross-reacting with other proteins such as peroxidase, or the viral proteins such as those of hepatitis B. Supporting this contention, it was shown that the levels of anti-albumin IgG autoantibodies were higher than normal in both acute and chronic hepatitis patients, but that those levels decreased to normal when the patients recovered 37. It was also shown that the circulatory pool of B cells from the majority of chronic hepatitis B surface antigen carriers contained sensitized B cells that, when stimulated, secreted IgG antibodies with specificity for human serum albumin 38.
To sum up, anti-HLA IgG antibodies in the sera of normal, healthy, non-alloimmunized males were tested against beads, each of which was coated with either one protein of one of 31 HLA-A alleles or one protein of 50 HLA-B, 16 HLA-Cw, 36 HLA-DRB, 29 HLA-DQ or 26 HLA-DP alleles. A remarkable difference exists between the reactivity of IgG before and after its purification of IgG from the sera. The profile and incidence of antibodies in the purified IgG mirrored the HLA-I 6 and -II reactivity of IVIg. Failure of IgG in the sera to react with the wide variety of HLA alleles could be due to factors masking the IgG expression in sera. A <3 kDa peptide from the CDRII domain of the Fab region of IgG inhibited the binding of purified IgG to the HLA-I/-II antigens. Some of the anti-HLA-purified IgG matched the HLA types of the corresponding donors, confirming the presence of autoantibodies reacting to HLA-I/-II. Serum IgG (without purification) in a few individuals showed the presence of such auto-HLA antibodies. The presence of HLA autoantibodies and the role of antibody-derived peptide in masking the expression of antibodies indicate that there may be a built-in regulation of HLA antibody expression in circulation.
Acknowledgments
We thank Luis Eduardo Morales-Buenrostro, Instituto Nacional de Ciencias Médicas y Nutrición, Mexico for supplying the Sandoglobulin and Octagam used in our study, and Stanley Jordan, Cedars-Sinai Hospital, Los Angeles for supplying the Gamunex-C. The study was funded by the Terasaki Foundation Laboratory, which is supported by the Terasaki Family Foundation.
Disclosure
None of the authors have any conflicts of interest.
References
- Jordan SC, Vo AA, Nast CC, Tyan D. Use of high-dose human intravenous immunoglobulin therapy in sensitized patients awaiting transplantation: the Cedars-Sinai experience. Clin Transplant. 2003;2003:193–198. [PubMed] [Google Scholar]
- Jordan SC, Toyoda M, Vo AA. Post-transplant therapy with high-dose intravenous gammaglobulin: applications to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:155–161. doi: 10.1111/j.1399-3046.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Rebellato LM, Ozawa M, Verbanac KM. Clinical and anti-HLA antibody profile of nine renal transplant recipients with failed grafts: donor-specific and non-donor-specific antibody development. Clin Transplant. 2006;2006:241–253. [PubMed] [Google Scholar]
- Shoenfeld Y, Katz U. IVIg therapy in autoimmunity and related disorders: our experience with a large cohort of patients. Autoimmunity. 2005;38:123–137. doi: 10.1080/08916930500059633. [DOI] [PubMed] [Google Scholar]
- Toubi E, Kessel A, Shoenfeld Y. High-dose intravenous immunoglobulins: an option in the treatment of systemic lupus erythematosus. Hum Immunol. 2005;66:395–402. doi: 10.1016/j.humimm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Terasaki PI, Pham T. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E antibodies that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood. 2013;121:2013–2028. doi: 10.1182/blood-2012-08-447771. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Kaneku H, El-Awar N. Antibodies to HLA-E in nonalloimmunized males: pattern of HLA-Ia reactivity of anti_HLA-E_positive sera. J Immunol. 2010;185:1935–1948. doi: 10.4049/jimmunol.1000424. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Taniguchi M, Chen CW. HLA-E monoclonal antibodies recognize shared peptide sequences on classical HLA class Ia: relevance to human natural HLA antibodies. Mol Immunol. 2010;47:1121–1131. doi: 10.1016/j.molimm.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Pham T, El-Awar N. Anti-HLA-E monoclonal Ab 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: web-tools validate the immunogenic epitopes of HLA-E recognized by the antibodies. Mol Immunol. 2011;48:423–430. doi: 10.1016/j.molimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Kaveri SV, Kang CY, Köhler H. Natural mouse and human antibodies bind to a peptide derived from a germline VH chain. Evidence for evolutionary conserved self-binding locus. J Immunol. 1990;145:4207–4213. [PubMed] [Google Scholar]
- Kang CY, Cheng HL, Rudikoff S, Kohler H. Idiotypic self binding of a dominant germline idiotype (T15). Autobody activity is affected by antibody valency. J Exp Med. 1987;165:1332–1343. doi: 10.1084/jem.165.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber-Emmons T, Kohler H. Towards a unified theory of immunoglobulin structure–function relations. Immunol Rev. 1986;90:29–48. doi: 10.1111/j.1600-065x.1986.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Lefaucheur C, Suberbielle-Boissel C, Hill GS. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- Jolly EC, Key T, Rasheed H. Preformed donor HLA-DP-specific antibodies mediate acute and chronic antibody-mediated rejection following renal transplantation. Am J Transplant. 2012;12:2845–2848. doi: 10.1111/j.1600-6143.2012.04172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A, Amrouche L, Suberbielle C. Outcome of kidney transplantations performed with preformed donor-specific antibodies of unknown etiology. Am J Transplant. 2014;14:193–201. doi: 10.1111/ajt.12512. [DOI] [PubMed] [Google Scholar]
- Leca N, Warner P, Bakthavatsalam R. Outcomes of simultaneous liver and kidney transplantation in relation to a high level of preformed donor-specific antibodies. Transplantation. 2013;96:914–918. doi: 10.1097/TP.0b013e3182a192f5. [DOI] [PubMed] [Google Scholar]
- O'Leary JG, Kaneku H, Jennings LW. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013;19:973–980. doi: 10.1002/lt.23687. [DOI] [PubMed] [Google Scholar]
- Perera MT, Silva MA, Murphy N. Influence of preformed donor-specific antibodies and C4d on early liver allograft function. Scand J Gastroenterol. 2013;48:1444–1451. doi: 10.3109/00365521.2013.845795. [DOI] [PubMed] [Google Scholar]
- Scornik JC, Zander DS, Baz MA. Susceptibility of lung transplants to preformed donor-specific HLA antibodies as detected by flow cytometry. Transplantation. 1999;68:1542–1546. doi: 10.1097/00007890-199911270-00018. [DOI] [PubMed] [Google Scholar]
- Grosse-Wilde H, Blasczyk R, Westhoff U. Soluble HLA class I and class II concentrations in commercial immunoglobulin preparations. Tissue Antigens. 1992;39:74–77. doi: 10.1111/j.1399-0039.1992.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Glotz D, Haymann JP, Sansonetti N. Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg). A potential tool for transplantation of immunized patients. Transplantation. 1993;56:335–337. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- Tyan D, Li V, Czer L. Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candi dates and transplantation with a histoincompatible organ. Transplantation. 1994;57:553–562. [PubMed] [Google Scholar]
- Jordan SC, Quartel AW, Czer LS. Posttransplant therapy using high-dose human IVIG to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanisms of action. Transplantation. 1998;66:800–805. doi: 10.1097/00007890-199809270-00017. [DOI] [PubMed] [Google Scholar]
- Montgomery RA, Zachary AA, Racusen LC. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- Nair V, Sawinski D, Akalin E. Effect of high-dose intravenous immunoglobulin on anti-HLA antibodies in sensitized kidney transplant candidates. Clin Transplant. 2012;26:E261–268. doi: 10.1111/j.1399-0012.2012.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alachkar N, Lonze BE, Zachary AA. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte antigen antibodies in highly sensitized patients. Transplantation. 2012;94:165–171. doi: 10.1097/TP.0b013e318253f7b6. [DOI] [PubMed] [Google Scholar]
- Marfo K, Ling M, Bao Y. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation. 2012;94:345–351. doi: 10.1097/TP.0b013e3182590d2e. [DOI] [PubMed] [Google Scholar]
- Stoclin A, Delbos F, Dauriat G. Transfusion-related acute lung injury after intravenous immunoglobulin treatment in a lung transplant recipient. Vox Sang. 2013;104:175–178. doi: 10.1111/j.1423-0410.2012.01645.x. [DOI] [PubMed] [Google Scholar]
- Sachs UJ, Wasel W, Bayat B. Mechanism of transfusion-related acute lung injury induced by HLA class II antibodies. Blood. 117:669–677. doi: 10.1182/blood-2010-05-286146. [DOI] [PubMed] [Google Scholar]
- Kopko PM, Holland PV. Transfusion-related acute lung injury. Br J Haematol. 2011;105:322–329. doi: 10.1111/j.1365-2141.1999.01357.x. 1999. [DOI] [PubMed] [Google Scholar]
- Kopko PM, Paglieroni TG, Popovsky MA. TRALI: correlation of antigen-antibody and monocyte activation in donor-recipient pairs. Transfusion. 2003;43:177–184. doi: 10.1046/j.1537-2995.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- Sachs UJ. A threshold model for the susceptibility to transfusion-related acute lung injury. Transfus Clin Biol. 2012;19:109–116. doi: 10.1016/j.tracli.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Rizk A, Gorson KC, Kenney L, Weinstein R. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion. 2001;41:264–268. doi: 10.1046/j.1537-2995.2001.41020264.x. [DOI] [PubMed] [Google Scholar]
- Zhu D, Ravindranath MH, Terasaki PI. Suppression allo-HLA antibodies secreted by B memory cells in vitro: IVIg versus a monoclonal anti-HLA-E IgG that mimics HLA-I reactivities of IVIg. Clin Exp Immunol. 177:464–477. doi: 10.1111/cei.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath MH, Terasaki PI, Pham T. Suppression of blastogenesis and proliferation of activated CD4+ T cells: IVIg versus novel anti-HLA-E mAbs mimicking HLA-I reactivity of IVIg. Clin Exp Immunol. 2014;178:154–177. doi: 10.1111/cei.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onica D, Calugaru A, Margineanu I. Immunochemical characterization of anti-albumin antibodies in liver diseases. Clin Immunol Immunopathol. 1983;26:223–231. doi: 10.1016/0090-1229(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Nardiello S, Pizzella T, Ronga C, Galanti B. Levels of anti-albumin autoantibodies in acute and chronic hepatitis B virus infection. Hepatogastroenterology. 1990;37(Suppl. 2):156–159. [PubMed] [Google Scholar]
- Hellström UB, Sylvan SP. Regulation of the immune response to hepatitis B virus and human serum albumin. III. Induction of anti-albumin antibody secretion in vitro by C-gene-derived proteins in peripheral B cells from chronic carriers of HBsAg. Scand J Immunol. 1992;35:53–62. doi: 10.1111/j.1365-3083.1992.tb02833.x. [DOI] [PubMed] [Google Scholar]